Abstract
Background
Salinity is one of the major abiotic constraints that hinder health and quality of crops. Conversely, halotolerant plant growth-promoting rhizospheric (PGPR) bacteria are considered biologically safe for alleviating salinity stress.
Results
We isolated halotolerant PGPR strains from the rhizospheric soil of Artemisia princeps, Chenopodium ficifolium, Echinochloa crus-galli, and Oenothera biennis plants; overall, 126 strains were isolated. The plant growth-promoting traits of these isolates were studied by inoculating them with the soil used to grow soybean plants under normal and salt stress (NaCl; 200 mM) conditions. The isolates identified as positive for growth-promoting activities were subjected to molecular identification. Out of 126 isolates, five strains—Arthrobacter woluwensis (AK1), Microbacterium oxydans (AK2), Arthrobacter aurescens (AK3), Bacillus megaterium (AK4), and Bacillus aryabhattai (AK5)—were identified to be highly tolerant to salt stress and demonstrated several plant growth-promoting traits like increased production of indole-3-acetic acid (IAA), gibberellin (GA), and siderophores and increased phosphate solubilization. These strains were inoculated in the soil of soybean plants grown under salt stress (NaCl; 200 mM) and various physiological and morphological parameters of plants were studied. The results showed that the microbial inoculation elevated the antioxidant (SOD and GSH) level and K+ uptake and reduced the Na+ ion concentration. Moreover, inoculation of these microbes significantly lowered the ABA level and increased plant growth attributes and chlorophyll content in soybean plants under 200 mM NaCl stress. The salt-tolerant gene GmST1 was highly expressed with the highest expression of 42.85% in AK1-treated plants, whereas the lowest expression observed was 13.46% in AK5-treated plants. Similarly, expression of the IAA regulating gene GmLAX3 was highly depleted in salt-stressed plants by 38.92%, which was upregulated from 11.26% to 43.13% upon inoculation with the microorganism.
Conclusion
Our results showed that the salt stress-resistant microorganism used in these experiments could be a potential biofertilizer to mitigate the detrimental effects of salt stress in plants via regulation of phytohormones and gene expression.
1. Introduction
Salinization is a devastating environmental stress factor that greatly limits plant growth and productivity [1]. About 800 million hectares of earth's land has been recognized as having a high salinity level globally [2]; this includes >15% land of the arid and semiarid regions and approximately 40% of the world's irrigated land [3]. Recently, Etesami and beattie [4] reported that salinity stress decreases the productivity of important cereal crops like wheat, maize, rice, and barley by up to 70% in different areas. Both direct and indirect effects of soil salinity inhibit the growth and productivity of crops. The direct adverse effects of salinity stress on plants include osmotic stress induced on root surface, compromised water acquisition, and toxic ion (e. g., Na+) accumulation in plant cells, which lead to nutrient deficiency and growth retardation of different plants [5–10]. Furthermore, even indirect effects of salinity stress greatly hamper the activities of various beneficial microbes present in the rhizosphere and decrease organic matter accumulation [11].
Salinity is a major limitation to the growth of several different agriculture crops all over the world, and various approaches have been applied to mitigate the adverse effects of salinity stress. Recently, various eco-friendly techniques were proposed and applied to mitigate the harmful effects of salt stress on various crops, including soybean. To achieve such an environment-friendly solution, it is necessary to isolate and identify plant growth-promoting microbes that can enhance soybean growth during salt stress. Salinity stress induces different biochemical and molecular effects in plants through reactive oxygen species (ROS) production, which could be manifested in the form of anatomical and physiological changes [12]. High concentrations of ROS are extremely harmful and lead to unwanted effects on plants at a cellular level, such as chlorophyll degradation, cell membrane oxidation, and the eventual cell death [13]. The detoxification of high ROS levels in plants is achieved by the development of different antioxidant enzymes. In connection to this, the well-known enzymes, such as superoxide dismutase (SOD) and reduced glutathione (GSH), have been reported for their ability to eradicate free radicals produced during salinity stress on the plants [13]. Various studies have shown a marked increase in the antioxidant defense system, adaptability, and survival of plants in a stress-inducing environment [14]. Higher concentration of salt (NaCl) causes ionic imbalance, which leads to osmotic stress and ionic toxicity [15]. Since Na+ and K+ play a major role in plant physiology during salt stress conditions, expulsion or efflux of Na+ and influx of K+ are the most important strategies to alleviate salinity stress in plants. The influx of sodium ion concentration affects low-affinity potassium uptake by the roots and causes water deficiency, thus causing an osmotic effect that has a devastating effect on plant growth, such as ceasing photosynthesis, low stomatal conductance, unusual transpiration rate, and reduced chlorophyll concentration [16].
Among crops, soybean is one of the most important and staple legume crop providing high oil (18%) and protein (38%) contents. It is moderately tolerant to salinity stress [17, 18], with 20%–50% reduction in the production under high salinity stress [19]. Thus, salinity stress significantly affects all the developmental stages from seed germination to seed yield [20]. During soybean development, salt stress reduces plant biomass, chlorophyll content, the number of internodes, the number of pods, and seed quality [20–22]. The availability of the whole genome sequence of soybean improved our understanding of the basic mechanisms of how salinity affects gene expression and regulation [20]. Salt stress-tolerance genes, such as auxin-resistant 1 (GmLAX) and soybean salt tolerance 1 (GmST1), are greatly involved in ABA signaling and mitigating ROS stress; these genes are also known as salt stress-responsive genes [17, 20, 23, 24]. Gm-ST1 encodes cation/proton exchanger family members and is responsible for conferring NaCl stress tolerance to plants at different stages, such as seedling and adult stage [17, 20]. Additionally, the gene expression of Gm-ST1 transgenic lines also increased ABA sensitivity and decreased ROS production under prolonged salt stress conditions [17, 25]. Similarly, GmLAX, encoding a putative auxin influx carrier, is also involved in abiotic stress responses and plays a very prominent role in different aspects of plant growth and development; for example, it has an important role in vascular development and regulation of auxin uptake or transport [26]. Researchers have already reported that GmLAX also plays an important role in regulation of vascular development, xylem differentiation, and regulation of lateral root growth [27–29].
A number of approaches were used to address the negative impact of salinity stress, including gypsum application, recombinant DNA technology approach, and traditional breeding, because planting salt-tolerant crop varieties had limited successes despite significant efforts [18, 30–33]. An alternative strategy to alleviate salinity stress is the application of halotolerant bacteria that enhance crop growth under salt stress conditions [30, 34]. The most commonly used halotolerant PGPR bacteria are Acinetobacter, Azotobacter, Bacillus sp., Serratia sp., Pseudomonas sp., and Rhizobium sp., which enhance plant growth by nitrogen fixation, promote inorganic phosphate solubilization, and promote siderophore and phytohormone production [35–39]. Similarly, a number of different reports have revealed that halotolerant microbes enhance the growth and development of various crops (rice, wheat, maize, tomato, soybean, lettuce, cotton, pepper, and canola) under both normal and salinity stress conditions [1, 12, 25, 40–43]. Thus, in the present study, we aimed to isolate and characterize halotolerant PGPR bacteria for mitigating salinity stress damages in the growth and development of soybean plants. We also elucidated the role of K+ and Na+ translocation, stress hormone (ABA), and antioxidants (GSH and SOD). Additionally, the expression of the candidate salt stress-responsive genes GmLAX3 and GmST1 was evaluated in soybean plants.
2. Materials and Methods
2.1. Isolation and Screening of PGPR Bacteria and Their Capabilities
During sampling, we isolated a number of different bacterial strains from the near vicinity of different plants inhabiting sand dunes at Pohang beach with a latitude of 36°7′56.2″N and a longitude of 129°23′55.1″E, Republic of Korea. The detailed method of Khan et al. [25] was followed for the isolation of PGP rhizospheric bacteria from the rhizospheric soil (1 g) of O. biennis L., A. princeps Pamp, C. ficifolium Smith, and E. crus-galli. The plant roots were excised along with rhizospheric soil, placed into 10 mL of sterile 0.9% NaCl solution, and vortexed for 10 min to detach the associated rhizospheric bacteria. After serial dilutions (10−1 until 10−9), the samples were inoculated on LB agar medium (tryptone, 10 g; yeast extract, 5 g; NaCl, 10 g; and agar, 1.5%; pH 7.0) and incubated (28°C) till the appearance of bacterial colonies; then, they were observed for the morphological characteristics.
Before bioassay assessment and molecular identification, the pure culture of selected isolates was screened for phosphate solubilization and siderophore and indole acetic acid (IAA) production. For siderophore production, the detailed methods of Khan et al. and Louden et al. [25, 44] were followed using chrome azurol S blue agar media. Each bacterial isolate was spot inoculated and incubated at 28°C and then analyzed for the appearance of orange halos in contrast to the blue background for five consecutive days. The detailed method of Katznelson and Bose [45] was assessed for phosphate solubilization using trypticase soy agar (TSA) medium supplemented with Ca3(PO4)2. Each bacterial isolate was spot inoculated and incubated at 28°C for 5 days until the formation of transparent “halos” around each colony. The method of Patten and Glickk [46] was used to detect the bacterial IAA in culture broth. The supernatant (1 mL) of each bacterial isolate was added in 1 mL of Salkowski reagent (50 mL of 35% HClO4 and 1 mL of 0.5 M FeCl3) for 30 min in dark condition. Visual assessment of the change in pink color indicated IAA production.
2.2. Bioassay Assessment and Molecular Identification of Bacterial Isolates
Based on multiple PGP traits, a total of seven isolates were capable of multiple traits in different media. Hence, the selected isolates were subjected to further analysis on Waito-C (GA-deficient) rice seedlings [47, 48]. Seeds of Waito-C (stored in desiccators at 4°C) were surface sterilized by soaking in 10 mL of 75% ethanol for 2 min, followed by 1% sodium hypochlorite (NaClO) treatment for 1 min. Then, they were thoroughly washed five times with sterilized distilled water. Sterilized seeds were treated for 24 h with bacterial isolates (109 CFU/mL) in a shaking incubator. Conversely, autoclaved double-distilled water was used for untreated seeds used as control. The test seeds were grown over an autoclaved filter paper, which were soaked in 1.5 mL of Hoagland solution for 14 consecutive days under controlled environmental conditions (14 h/10 h light/dark cycle; temperature, 28°C/24°C) at a relative humidity of approximately 70% and light intensity of 250 μmol m−2s−1. Bacterial isolates that enhanced the growth and development of rice seedlings were evaluated for further experiments. The selected isolates after screening experiments on Waito-C rice were identified through 16S rDNA gene amplification using 27F (5′-AGA GTT TGA TC(C/A) TGG CTC AG-3′) and 1492R (5′-CGG (T/C)TA CCT TGT TAC GAC TT-3′) primers, and BLAST search tool of NCBI (http://blast.ncbi.nlm.nih.gov) was used for sequence alignment. Similarly, GenBank (database/EzTaxon) was used to determine the nucleotide sequence homology of the targeted bacterial isolate, and MEGA v. 6.1 was used for phylogenetic analysis by constructing a neighbor joining (NJ) using 16S rDNA gene sequences from selected and related strains [49].
2.3. Quantification of In Vitro IAA and GAs in Bacterial Culture through GCMS-SIM
The bacterial strains were grown in LB media (10 g tryptone, 5°g yeast extract, and 10°g NaCl with a pH of 7.0) for 72 h, centrifuged at 5000g for 10 min, and filtered through a 45 μm filter. The isolated culture filtrate (CF) was analyzed for different types of IAAs and GAs through gas chromatography-mass spectrometry under selected ion monitoring mode (GC/MS SIM). The detailed method of Ullah et al. [50] was used for IAA analysis. To measure IAA concentration in the broth, the peak areas of IAA were compared to the known standard using GC/MS SIM. For the extraction and quantification of GA contents, the detailed method of Khan et al. [51, 52] was followed. GA internal standards ((17, 17-2H2) GA1, GA3, GA4, GA7, GA8, GA9, GA12, GA19, GA20, GA24, and GA36) were added to CF before performing column chromatography. All extracts were passed through a C18 column (90–130 μm; Alltech, USA) to obtain different fractions. For each type of GA, 1 μL aliquot was injected into the GC/MS column. The amounts of GAs (GA1, GA3, GA4, GA7, GA8, GA9, GA12, GA19, GA20, GA24, and GA36) in CF were calculated from the peak-area ratios, and retention time was determined using hydrocarbon standards.
2.4. Quantification of Organic Acids
The organic acid was quantified using the method of Kang et al. [53] and Khan et al. [47]. Briefly, the bacterial culture in the LB medium was centrifuged at 5000g for 20 min. The culture supernatant was adsorbed using Sep-Pak C18 cartridge (Waters, Milford, MA, USA) and filtered through a 0.45 μm cellulose acetate membrane filter. The samples were analyzed through high-performance liquid chromatography (HPLC) (Waters 600, Milford, MA, USA) using a PL Hi-Plex H column (7.7 × 300 mm, Waters Co., Milford, MA, USA), detector refractive index (RI) (Waters 410, Milford, MA, USA), and 5 mM H2SO4 as the solvent in distilled water. The flow rate was set to 0.6 mL min−1 with 65°C oven temperature and 20 μL injection volume.
2.5. Pot Experiment
In the present study, we used soybean seeds of cv. Pungsannamul were collected from Kyungpook National University's Genetic Resource Centre, Republic of Korea. All the seeds were surface sterilized with 2.5% sodium hypochlorite for 20 min, followed by treatment with 70% ethanol for 30 s; then, they were washed three times with deionized double-distilled water and subjected to germination. Uniformly germinated seedlings were selected and transferred to sterilized pots filled with autoclaved soil. We used specific soil prepared by Punong Co., Ltd., Korea, which comprised perlite (11%), cocopeat (68%), zeolite (8%), NO3− (∼0.205 mg g−1), NH4+ (∼0.09 mg g−1), P2O5 (∼0.35 mg g−1), and K2O (∼0.1 mg g−1). The selected seedlings were grown in a growth chamber: 14 h/10 h light/dark cycle; temperature, 28°C/24°C; relative humidity, 60%–70%; and light intensity, 1000 μE m−2 s−1 from sodium lamps. The seedlings were irrigated with autoclaved distilled water as required. There were seven treatments in this experiment, including control-untreated rice seedlings, control-NaCl (treated with 150°mM NaCl), and five treatments with five different bacterial isolates. However, the seedlings treated with NaCl were also inoculated with bacterial isolates for evaluating their combined effects on plant growth and development. After stress treatment, the plants were directly subjected to liquid nitrogen and stored at −80°C for further analysis. Finally, different plant growth attributes were recorded, and for chlorophyll content measurement, Minolta SPAD-502 (Konica Minolta, Japan) was used. Fresh samples were used for gene expression analysis, and lyophilized samples were used for other analyses.
2.6. Molecular Analysis to Understand Transcript Involved in Salinity Stress
The RNA was extracted using the protocol described by Chan et al. [54]. The leaf tissues of soybean seedlings were treated with liquid nitrogen to grind them to form a fine powder of the leaf sample. In brief, 5 μg of extracted RNA was used for preparing cDNA (SuperScript® III, Invitrogen, USA). The cDNA (1 μL) was subjected to 30 cycles in PCR machine using Taq DNA polymerase (New England Biolabs, Ipswich, MA, USA). The specific candidate gene primers GmST1 (forward: 5′TCTAGAATGGCGTTTGTTGCAGC CATG3′; reverse: 5′GAGCTCTCATAAGGTTCGGGGATCCTTTC3′) and GmLAX3 (forward: 5′CTGGCAGGGTTTTGCATTAT3′; reverse: 5′GCCTGTGCATTTCATAGCAA3′) along with actin primers (as a reference) were used for evaluating target gene amplification.
2.7. Elemental Analysis
The detailed method of Kang et al. [39] was used for elemental analysis of the plants. The lyophilized (0.5 g) crushed powder of plant samples was soaked in 0.5 M HCl and rinsed through double-distilled water before oven drying. The sample was treated with a mixture of nitric acid, sulfuric acid, and perchloric acid (10 : 1 : 4, v/v/v). The digested sample obtained was then analyzed by inductively coupled plasma mass spectrometry (Optima 7900DV Perkin-Elmer, Waltham, MA, USA).
2.8. Abscisic Acid Analysis
The method described by Kang et al. and Qi et al. [39, 55] was followed to extract and quantify ABA. Briefly, 0.5 g of powder sample was extracted with 95% isopropanol: 5% acetic acid solution; then, filtrate and standard ABA (20 ng/mL) were added to the mixture. The extracts were dried and methylated by adding diazomethane for GC/MS-SIM analysis (6890N network GC system, and 5973 network mass selective detector; Agilent Technologies, Palo Alto, CA, USA). For quantification, the Lab-Base data system software (Thermo Quest, Manchester, UK) was used to monitor responses to ions of m/e 162 and 190 for Me-ABA and of m/e 166 and 194 for Me-[2H6]-ABA.
2.9. Analysis of Antioxidant Enzymes
The detailed method of Marklund and Marklund [56] was adapted for the SOD activity assay. Briefly, leaf samples (100 mg) were homogenized with 0.01 M phosphate buffer at pH 7.0 and centrifuged (17,000g/4°C/15 min). The supernatant was used as a crude enzyme extract and passed through a reaction mixture containing Tris-HCl buffer (2 mL), pH 8.2, double-distilled water (2 mL), and 2 mM pyrogallol (0.5 mL). The absorption of the assay mixture and blank (lacking pyrogallol or tissue homogenate) was measured at 470 nm using a spectrophotometer (Shimadzu, Kyoto, Japan) at 180 s intervals. The data were expressed as units/mg of protein. To determine the reduction in glutathione concentration, each sample (500 mg) was treated with 2 mL of 10% trichloroacetic acid and centrifuged at 10,000 rpm for 15 min at 4°C. The resulting supernatant (1 mL) was combined with 0.5 mL of Ellman's reagent and 3 mL of 15 mM sodium phosphate buffer (pH 7.4) and was incubated for 5 min at 30°C. The absorbance was measured at 412 nm using a spectrophotometer [57, 58].
2.10. Statistical Analysis
The present study was conducted in a completely randomized design, wherein each treatment had 10 replications. All statistical analyses, including DMRT analysis, were performed using Statistic Analysis System (SAS 9.1), and for graphical presentation, GraphPad Prism software (version 5.0, San Diego, California, USA) was used.
3. Results
3.1. PGPR Bacterial Isolates Screening for Siderophore Production, Phosphate Solubilization, and Indole-3-Acetic Acid Production
Initially, we isolated a total of 126 rhizobacterial strains from four plants (A. princeps, C. ficifolium, O. biennis, and E. crus-galli). The roots of E. crus-galli revealed 46 rhizospheric bacterial isolates, which was the highest number among the four plant species, followed by C. ficifolium with 32 rhizospheric bacteria, A. princeps with 26 rhizospheric bacteria, and O. biennis with 22 rhizospheric bacteria (Supplementary ).
For the assessment of plant growth-promoting (PGP) traits, different morphological and biochemical tests were conducted. In a colorimetric assay for IAA production, a total of 39 bacterial isolates displayed positive results using Salkowski reagent. However, about 13 rhizospheric bacterial isolates revealed siderophores on CAS agar medium, whereas 14 isolates showed phosphate solubilization capability on PVK medium (Supplementary (a) and (b)).
3.2. Bioassay Assessment and Molecular Identification of Bacterial Isolates
Through bioassay assessment, it was found that seven isolates were capable to represent multiple PGP traits in different media. Hence, these isolates were selected, and their PGP roles were further studied through inoculation on gibberellin-deficient rice mutant Waito-C seedlings. A total of five selected bacterial isolates on Waito-C rice revealed significantly increased growth and fresher biomass than other screened isolates and control plants (Supplementary (c)). These bacterial isolates were evaluated for additional traits.
For molecular identification and phylogenetic analysis of the isolates (05), the 16S rRNA genes were amplified and sequenced and compared against a database of known 16S rRNA sequences. The sequences were then submitted to NCBI to get accession numbers (Figure 1). Our analysis revealed that the rhizospheric bacteria AK1, AK2, AK3, AK4, and AK5 showed sequence identity with Arthrobacterwoluwensis, Microbacterium oxydans, Arthrobacter aurescens, Bacillus megaterium, and Bacillus aryabhattai, respectively. Additionally, the neighbor-joining (NJ) method was used to construct a phylogenetic tree for 16S with MEGA 6 after sequence alignment using Clustal W (version 7.222). The results revealed that AK1 and AK3 exhibited a high level of 16S rRNA sequence identity (99%) with A. woluwensis and A. aurescens. Similarly, AK2 formed a clade with M. oxydans, while AK4 and AK5 formed clades with B. megaterium and B. aryabhattai (Figure 1).
Figure 1.
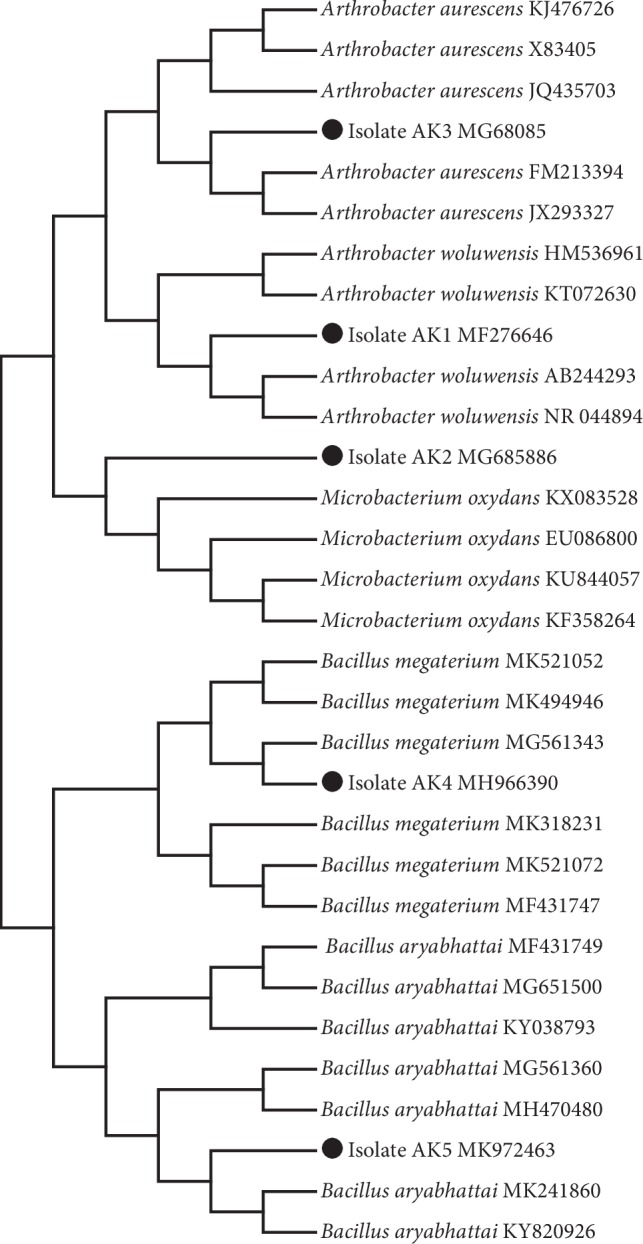
Phylogenetic analysis of rhizospheric bacterial strains isolated from the root rhizosphere of different plants Artemisia princeps (Korean mugwort), Chenopodium ficifolium (nettle-leaved goosefoot), Oenothera biennis (evening star), and Echinochloa crus-galli (cockspur grass).
3.3. Phytohormones and Organic Acid Quantification in the Culture Broth of Selected Isolates
The culture filtrate of rhizospheric bacterial isolates was tested for quantifying phytohormones, such as IAAs and GAs, using GC/MS SIM. Interestingly, all of the selected strains were able to produce IAA in a significant amount (Table 1). The bacterial isolate of A. woluwensis AK1 produced the highest amount (4.87 ± 0.7 μg mL−1) of IAA, followed by M. oxydans AK2 and A. aurescens AK3, which produced 2.78 ± 0.52 and 2.9 ± 0.72 μg mL−1, and B. aryabhattai AK5 and B. megaterium AK4, which produced 1.08 ± 0.06 μg mL−1 and 0.13 ± 0.7 μg mL−1. Moreover, both active and nonactive GAs were also evaluated in the culture filtrates (Table 1). Our results revealed that GA20 was present in the CF culture of all isolates in a range of 1.4 ± 0.00 ng mL−1 to 0.04 ± 0.9 ng mL−1. Functionally active GAs included GA4 (3.14 ± 0.2 ng mL−1, 1.58 ± 0.8 ng mL−1, 1.55 ± 0.06 ng mL−1, and 1.4 ± 0.6 ng mL−1) which was detected in CF of B. megaterium AK4, M. oxydans AK2, A. woluwensis AK1, and A. aurescens AK3 isolates, respectively.
Table 1.
Quantification of IAA and gibberellins produced by rhizospheric bacterial isolates.
| IAA | GA1 | GA4 | GA5 | GA7 | GA8 | GA12 | GA15 | GA19 | GA20 | GA24 | GA36 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. woluwensis AK1 | 4.87 ± 0.73A | ND | 1.55 ± 0.06A | ND | ND | 0.045 ± 0.04D | 0.755 ± 0.08B | ND | ND | 0.393 ± 0.03C | ND | ND |
| M. oxydans AK2 | 2.78 ± 0.52B | ND | 1.58 ± 0.8B | ND | 0.455 ± 0.6C | 0.05 ± 0.04D | 7.415 ± 0.1A | 0.265 ± 0.08C | 0.02 ± 0.04D | 0.485 ± 0.09C | ND | 0.36 ± 0.09C |
| A. aurescens AK3 | 2.69 ± 0.72B | 0.52 ± 0.6D | 1.4 ± 0.1C | ND | ND | 8.32 ± 0.11A | 0.43 ± 0.09DE | ND | 0.401 ± 0.9DE | 0.12 ± 0.8E | ND | ND |
| B. megaterium AK4 | 0.13 ± 0.03D | ND | 3.147 ± 0.2A | ND | ND | 0.27 ± 0.09A | ND | ND | ND | 1.4 ± 0.03C | ND | 0.385 ± 0.04B |
| B. aryabhattai AK5 | 1.08 ± 0.1B | 2.5 ± 0.21A | ND | 0.07 ± 0.01C | ND | 0.08 ± 0.07C | ND | ND | ND | 0.04 ± 0.01D | ND | ND |
Each data point is the mean of at least three replicates. Error bars represent standard errors. The bars represented with different letters are significantly different from each other as evaluated by DMRT.
In this manner, GA1 was detected in A. aurescens AK3 and B. megaterium AK5 (0. 5 ± 0.6 ng mL−1and 2.5 ± 0.11 ng mL−1, respectively), whereas GA7 was detected in M. oxydans AK2 (0.45 ± 0.6 ng mL−1). Conversely, inactive types of GAs present in the CF culture of the isolates were GA5, GA8, GA12, GA15, GA19, GA24, and GA36, and the highest amount of GA12 was detected in M. oxydans AK2 (7.32 ± 0.11 ng mL−1). Only GA5, GA19, and GA24 were detected in B. aryabhattai AK5 (0.07 ± 0.01 ng mL−1), M. oxydans AK2 (0.02 ± 0.04 ng mL−1), and A. aurescens AK3 (0.175 ± 0.8 ng mL−1) (Table 1).
Organic acid analysis revealed that the CF of the selected isolates produced malic acid, quinic acid, succinic acid, lactic acid, formic acid, acetic acid, butyric acid, and gallic acid (Table 2). Our results also showed that the CF of all strains contained quinic acid and acetic acid. The highest amounts of quinic acid and acetic acid were observed in A. aurescens AK3 (2.62 ± 0.9 ng mL−1) and M. oxydans AK2 (5.51 ± 0.9 ng mL−1), respectively. Only lactic acid and gallic acid were detected in the culture filtrate of M. oxydans AK2 (1.87 ± 0.4 ng mL−1) and B. megaterium AK4 (0.04 ± 0.1 ng mL−1) (Table 2). Malic acid was detected in M. oxydans AK2 (4.62 ± 0.8 ng mL−1) and A. aurescens AK3 (2.36 ± 0.6 ng mL−1). Butyric acid was detected in trace amounts in the CF of A. woluwensis AK3 (0.33 ± 0.5 ng mL−1) and in the highest amount in the CF of M. oxydans AK2 (2.51 ± 0.8 ng mL−1).
Table 2.
Types and quantity of organic acids in the culture broth of rhizospheric bacteria.
| Malic acid | Quinic acid | Succinic acid | Lactic acid | Acetic acid | Butyric acid | Gallic acid | |
|---|---|---|---|---|---|---|---|
| Arthrobacter woluwensis AK1 | ND | 1.53 ± 0.7AB | 2.58 ± 0.7A | ND | 2.38 ± 0.8A | 0.33 ± 0.5B | ND |
| Microbacterium oxydans AK2 | 4.62 ± 0.8A | 1.58 ± 0.6C | 2.75 ± 0.8B | 1.87 ± 0.4C | 5.51 ± 0.9A | 2.51 ± 0.8B | ND |
| Arthrobacter aurescens AK3 | 2.36 ± 0.6A | 2.62 ± 0.9A | 0.94 ± 0.7B | ND | 0.61 ± 0.5B | ND | ND |
| Bacillus megaterium AK4 | ND | 0.05 ± 01B | ND | ND | 1.64 ± 02A | ND | 0.04 ± 01B |
| Bacillus aryabhattai AK5 | ND | 0.06 ± 01B | ND | ND | 0.31 ± 02A | ND | ND |
Each data point is the mean of at least three replicates. Error bars represent standard errors. The bars represented with different letters are significantly different from each other as evaluated by DMRT.
3.4. Ameliorative Effect of Bacterial Isolates in Alleviating Salinity Stress
The selected rhizospheric strains were evaluated for salinity stress alleviation effects in soybean plants, which revealed some interesting results under 200 mM of NaCl stress. The bacterial isolates greatly mitigated the adverse effects of salinity stress and significantly influenced soybean growth, biomass, and chlorophyll content compared to NaCl-stressed plants (Table 3; Figure 2). In our results, a significant decrease in shoot length (30.24%) and root length (36.09%) was observed in 200 mM NaCl-stressed soybean plants compared with control-unstressed plants. However, with the combined inoculation of rhizospheric bacteria, significant increases in shoot length from 7.55% to 23.52% and root length from 11.92% to 31.17% were observed. Significant increases in shoot length were observed with A. woluwensis AK1 (23.52%) treatment and M. oxydans AK2 (17.89%) treatment, whereas the bacterial strains A. woluwensis AK1 and A. aurescens AK3 significantly increased root length up to 31.17% and 24.99%, respectively.
Table 3.
Influence of rhizospheric bacteria on growth attributes and chlorophyll content of soybean plants grown with/without NaCl.
| Shoot length (cm) | Root length (cm) | Shoot fresh weight (mg) | Root fresh weight (mg) | Shoot dry weight (mg) | Root dry weight (mg) | SPAD (C.C) | |
|---|---|---|---|---|---|---|---|
| Control | 139.73 ± 3.60A | 251.00 ± 3.60A | 12.56 ± 0.65A | 7.66 ± 0.55A | 1.86 ± 0.03A | 0.60 ± 0.10A | 46.83 ± 3.61A |
| NaCl (200 mM) | 97.46 ± 1.85F | 160.40 ± 3.41F | 3.74 ± 0.35E | 2.30 ± 0.22D | 0.51 ± 0.01F | 0.17 ± 0.03C | 24.03 ± 3.10D |
| Arthrobacter woluwensis AK1 | 120.40 ± 2.62B | 210.40 ± 3.41B | 7.73 ± 0.50B | 4.60 ± 0.31B | 1.40 ± 0.11B | 0.37 ± 0.03B | 39.23 ± 2.54B |
| Microbacterium oxydans AK2 | 114.9 ± 2.15C | 195.23 ± 2.15C | 6.13 ± 0.41C | 4.93 ± 0.25B | 1.10 ± 0.10C | 0.30 ± 0.02B | 35.70 ± 3.05BC |
| Arthrobacter aurescens AK3 | 112.03 ± 1.95CD | 200.50 ± 2.68C | 5.43 ± 0.35CD | 3.63 ± 0.31C | 0.82 ± 0.06E | 0.37 ± 0.22B | 35.63 ± 3.55BC |
| Bacillus megaterium AK4 | 104.83 ± 1.55E | 179.53 ± 3.32E | 5.23 ± 0.35D | 3.53 ± 0.30C | 0.98 ± 0.04D | 0.29 ± 0.20B | 32.06 ± 3.51C |
| Bacillus aryabhattai AK5 | 110.56 ± 1.53D | 185.30 ± 2.26D | 4.33 ± 0.25E | 3.73 ± 0.25C | 0.83 ± 0.04E | 0.29 ± 0.03B | 30.86 ± 3.12C |
Each data point represents the mean of three replicates ± standard errors. The different letters represent data points that are significantly different from each other as evaluated by DMRT analysis.
Figure 2.
Effects of selected rhizospheric bacterial isolates on the growth attributes of soybean plants under NaCl concentrations (200 mM).
Similarly, fresh and dry weights were significantly higher for plants treated with bacterial isolates compared to salt-treated soybean plants. NaCl stress (200 mM) decreased the fresh shoot and root weights (70% and 69%) and dry shoot and root weights (72.45% and 71.11%, respectively) compared with control soybean plants. However, compared with salt-stressed soybean plants, plants treated with these strains showed significantly greater increases in fresh shoot weight from 15.65% to 106.39%, fresh root weight (53% to 114.49%), dry shoot weight (61.03% to 172.72%), and dry root weight (67.63% to 118.87%) (Table 3; Figure 2).
We also evaluated the effect of NaCl stress in the presence and absence of selected rhizospheric bacterial isolates on the chlorophyll content. The chlorophyll content was found to be significantly decreased in NaCl-stressed plants by up to 48.63% when compared with control plants. However, plants treated with these bacterial strains showed a prominent increase in chlorophyll content of soybean from 28.43% to 63.24% as compared to salt-treated plants (Table 3; Figure 2). Hence, the prolific effect of rhizospheric bacterial isolates mitigated the adverse effects of NaCl stress and promoted root/shoot growth and chlorophyll content.
3.5. Endogenous ABA Content of the Soybean Plants
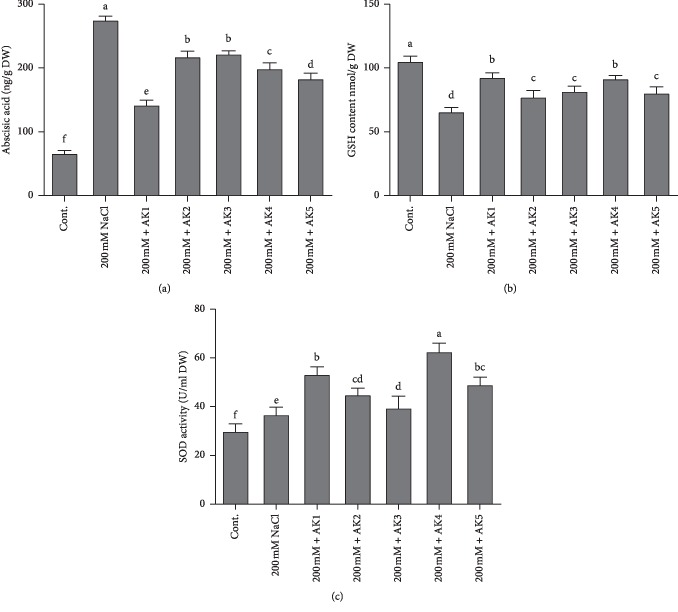
The ABA level was significantly elevated to 275 ± 5.5 ng/g in salt-stressed plants compared with 66.5 ± 4.09 ng/g in control-unstressed soybean plants. However, the amount of endogenous ABA content under 200 mM salt stress was greatly reduced because of inoculation of rhizospheric bacteria (Figure 3(a)). The ABA level was most significantly decreased by the bacterial strain A. woluwensis AK1 (142.5 ± 7.05 ng/g), followed by B. aryabhattai AK5 (183.66 ± 8.50 ng/g). The fluctuation in the ABA level leads to different traits, such as the opening and closure of stomata, which affects the physiological responses of soybean and demonstrates the enhanced stress-mitigating capability of rhizospheric bacteria-treated plants, which showed a reduction in the level from 222.5 ± 10.50 ng/g to 142.5 ± 7.05 ng/g, compared with salt-stressed soybean plants, which had a level of 275 ± 5.5 ng/g (Figure 3(a)).
Figure 3.
Endogenous abscisic acid (ABA), reduced glutathione (GSH), and superoxide dismutase (SOD) activity quantification in rhizospheric bacteria inoculated on soybean plants. (a) shows ABA, (b) shows the amount of GSH, and (c) shows SOD activity under normal and stressful conditions. Each data point is the mean of at least three replicates. Error bars represent standard errors. The bars represented with different letters are significantly different from each other as evaluated by DMRT.
3.6. Regulation of Antioxidant Enzyme during NaCl Stress
Under normal growth conditions, salt-stressed soybean plants showed a 37.42% decrease in GSH biosynthesis compared with normal control soybean plants. However, the amount of GSH content under 150 mM salt stress greatly increased because of inoculation of selected rhizospheric bacterial strains. Compared with salt-stressed soybean plants, bacteria-inoculated plants showed a significant increase in the level of the key antioxidant GSH from 17.58% to 40.89%. Among the bacteria-inoculated soybean plants, the highest GSH content was observed in A. woluwensis AK1-inoculated plants (92.92 ± 5.20 ng/g), whereas the lowest GSH content was in M. oxydans AK2 (77.55 ± 6.71 ng/g) (Figure 3(b)).
Furthermore, SOD analysis results revealed an increase in SOD activity observed in soybean plants exposed to salt stress and those treated with a combined inoculation of bacterial strains compared with normal control plants. The results showed that soybean plants treated with 200 mM NaCl showed enhanced SOD activity (22.80%). However, a combined inoculation of salt and selected bacterial strains remarkably increased SOD activity (31.90% to 108.95%). Among the bacteria-inoculated soybean plants, the highest GSH content was observed in B. megaterium AK4 inoculated plants (108.95%), whereas the lowest GSH content was noted in A. aurescens AK3-inoculated plants (31.90%) (Figure 3(c)).
3.7. Role of Bacterial Isolates in Ion Uptake during NaCl Stress of Soybean
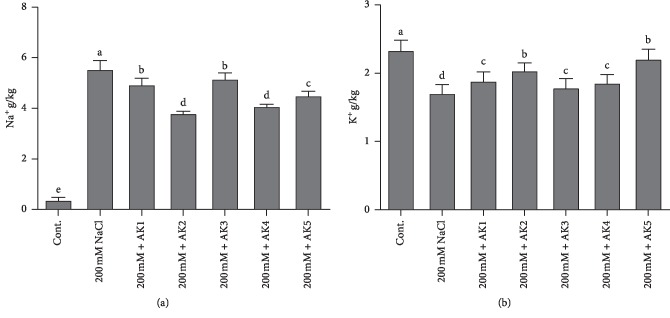
ICP-MS analysis of the Na+ content was performed in control and salt-treated plants with combined inoculation of rhizospheric-inoculated soybean plants. The results showed that NaCl stress (200 mM) increased the Na+ content in soybean plants. However, soybean plants inoculated with bacterial strains significantly decreased the Na+ content to a range of 6.83%–31% (Figure 3(a)). The highest reduction in the Na+ content was to 6.83%, observed in plants inoculated with A. aurescence AK3, whereas the least reduction was to 31%, observed in plants inoculated with M. oxydans AK2, compared with 200 mM NaCl-stressed soybean plants (Figure 4(a)).
Figure 4.
Evaluation of ICP analysis for Na+ and K+ contents in soybean shoots. (a) Na+ uptake and (b) K+ contents. Each data point is the mean of at least three replicates. Error bars represent standard errors. The bars represented with different letters are significantly different from each other as evaluated by DMRT.
ICP analysis of K+ showed a significant decrease in K+ concentrations (24.78%) in soybean plants under 200 mM NaCl stress compared with control plants. However, the K+ content under 200 mM NaCl stress greatly increased because of bacterial inoculation. The bacterial strain B. aryabhattai AK5 significantly increased K+ content (25.56%) followed by M. oxydans AK2 (15.92%). Among bacteria-inoculated plants, the lowest K+ concentration was observed in A. aurescence AK3 (1.76%)-inoculated soybean plants compared with 200 mM NaCl-stressed plants (Figure 4(b)).
3.8. Gene Expression during NaCl Stress of Soybean Plants
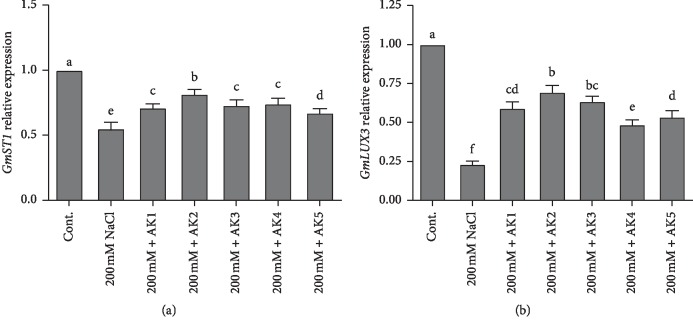
GmST1 is a soybean salt tolerance gene that functions in salt stress tolerance, and its protein product functions to decrease the production of ROS. Thus, we investigated the expression and regulation of GmST1 under high NaCl stress (200 mM) with and without the presence of selected rhizospheric strains inoculated to soybean plants using qRT-PCR. Our results revealed that salt stress significantly decreased the expression of GmST1 up to 44.99% compared with unstressed control soybean plants. However, salt stress with combined inoculation of rhizospheric bacteria augmented the expression of GmST1 up to 18.99% to 32.99% (Figure 5(a)). Compared with NaCl-stressed soybean, the highest expression of GmST1 was observed in M. oxydans AK2 (18.99%)-inoculated soybean plants while lowest expression was observed in B. aryabhattai AK5 (32.99%)-inoculated soybean plants.
Figure 5.
Relative expression of GmST1 and GmLUX3 genes in soybean plants. The values were calculated relative to those of actin gene expression and are means of three replicates. Error bars represent standard errors. The bars represented with different letters are significantly different from each other as evaluated by DMRT analysis.
Similarly, GmLAX3 plays an important role and may be the most promising candidate gene for improving soybean adaptability against salinity stress. The expression of GmLAX3 in NaCl-stressed plants decreased 76.66% compared with control soybean plants. Our results revealed that the upregulation of GmLAX3 gene significantly increased soybean resistance to NaCl stress plant inoculated with selected rhizospheric strain. The expression level of GmLAX3 increased from 30.33% to 51.33% in soybean plants with combined inoculation of bacterial strains compared with NaCl stress (200 mM) plants. Furthermore, the highest expression of GmLAX3 was observed in M. oxydans AK2 (43.13%)-inoculated plants, whereas the lowest expression was in B. megaterium AK4-inoculated plants and B. aryabhattai AK5-inoculated plants (16.6% and 17.30%), respectively (Figure 5(b)).
4. Discussion
Salinity adversely affects the plant growth by exerting osmotic ionic stresses, primarily due to elevated Na+ levels in the soil, which drive out the water from the plant cell, thus affecting the turgor pressure, leaf area, chlorophyll metabolism, and photosynthetic activity [59]. Since salt stress-resistant PGPR are recognized as potential stress relievers in plants due to their ability to reduce the Na+/K+ ratio. In the present study, the application of Arthrobacter woluwensis (AK1), Microbacterium oxydans (AK2), Arthrobacter aurescens (AK3), Bacillus megaterium (AK4), and Bacillus aryabhattai (AK5) significantly enhanced the K+/Na+ ratio in plants and enhanced the PGP characteristics, including photosynthetic activity (Table 3). NaCl stress exposure (200 mM) significantly reduced shoot and root lengths and fresh and dry weights of soybean plants compared to controls (unstressed plants). Similarly, previous reports showed that NaCl stress affected the growth of sweet sorghum [40], groundnut [12], okra [41], pepper [42], wheat [43], and soybean [39, 60]. The selected rhizospheric strains greatly mitigated the adverse effects of salinity stress and significantly enhanced soybean growth, biomass, and chlorophyll content compared with NaCl-stressed plants (Table 3; Figure 2). Recently, plant growth-promoting rhizospheric bacteria have been used to alleviate salt stress and improve crop production [25, 39, 58, 61–66]. Thus, here we describe how the findings of our results provide an insight on advancing the strategy to counter salinity stress.
The interaction of plants and microbes occurs in the rhizosphere due to rhizo-deposition, which includes several chemical compounds exudated from the plant root, such as organic acids and phytohormones [67]. Organic acids are also considered an important source of carbon and rich in energy. Our results revealed that cultured filtrates of selected isolates produced malic acid, quinic acid, succinic acid, lactic acid, formic acid, acetic acid, butyric acid, and gallic acid (Table 2). Previous reports showed that different bacteria produce various types of organic acids [25, 46, 66, 68]. In addition, organic acid enhances the degree and rate of metal dissolution, increases pH homeostasis, and promotes plant growth [69]. Eventually, these aspects influence chemical signals between root and microbes and promote the microbial community and support its functional role in plants [70, 71]. Furthermore, the microbes that produce organic acids have an important role in the solubilization of mineral substances. One of the most accepted examples is the mineralization of phosphate [72]. Phosphate-solubilizing PGPR reportedly produce organic acids that facilitate the uptake of P as well as essential nutrients from the soil [73]. The microbes used in the current experiment have an innate ability to produce organic acids (Table 2) and enhance phosphate solubilizing activity (Supplementary ). These microbial strategies may have influenced the growth and development of the plant. Similar results have been reported by several authors where organic acid and phosphate solubilizing bacteria mitigated abiotic stress by regulating phytohormones and antioxidants [25, 39, 46, 66, 68]. The combination of IAA production ability, phosphorous solubilization, and siderophore production of rhizospheric bacteria is greatly beneficial to plant rhizospheric soil as it mitigates the adverse effects of salinity stress [42, 74, 75].
The microbes used in the current study actively participated in IAA, GA, OA (organic acid), and siderophore production and phosphate solubilization. The application of bacterial inoculants to plant roots is expected to contain sufficient amount of growth regulators to influence future plant growth and development [76, 77]. There is the possibility that more hormones and other bioactive secondary metabolites are readily available to the plant for absorption and contribution in the root growth, cell elongation, tissue differentiation, and plant growth. It has been reported that the IAA produced by rhizosphere bacteria will increase the length and root surface of plants, thus offering them better access to nutrients available in the soil [78]. Similarly, various researchers revealed that IAA-producing bacteria significantly enhance plant growth under saline stress conditions [79, 80]. In connection to this, our bacterial isolates also produced IAA and greatly mitigated the adverse effects of NaCl stress on soybean plants (Table 1). Similarly, microorganisms that produced GAs play a key role in plant growth promotion and mitigating salt stress [39, 66]. Our selected bacterial isolates produced different bioactive and nonbioactive GAs (Table 1) and mitigated salinity stress in soybean plants. Many previous reports have shown the ameliorative effects of GAs on plant growth under abiotic stress [25, 39, 66]. It is considered that although there are several forms of GA, biologically active forms are limited to GA1, GA3, and GA4 [46]. These biologically active GA forms promote plant growth by reducing stress hormones like ABA [81]. As plants suspect stress, they regulate stress hormones like ABA through active chemical signals, which lead to increased sensitivity of plants for stomatal conductance [82]. As microbial interaction mitigates the stress effects by reducing the ABA content [83], similar results were observed in our study where selected rhizospheric bacterial inoculates resulted in decreased ABA content and increased plant growth parameters (Table 3; Figures 2 and 3(a)). Similarly, salinity stress leads to the formation of ROS, which cause cellular toxicity and damage to cell structures in plants [84, 85]. However, antioxidant enzymes, such as superoxide dismutase (SOD), peroxidase, glutathione reductase, and catalase, which protect the plant against cellular stress, remove free radicals and scavenge excess ROS. In the present study, the rhizospheric bacteria-inoculated plants revealed increases in SOD and GSH contents (Figures 3(b) and 3(c)). Similar results were reported for lettuce, potato, and okra [41, 86], wherein PGPB enhanced the activities of different ROS-scavenging enzymes under increasing salinity stress.
When microbes are introduced in the roots, they alter toxic ion uptake and modify the physical barriers around the rhizosphere by the formation of extensive rhizosheaths through the production of exopolysaccharides, reduction of foliar accumulation of toxic ions, and improvements in the nutritional status of both macro and micronutrients [30]. The greater accessibility of plants to nutrients is particularly due to changes in rhizospheric pH due to organic acid secretion and chelation through siderophore production [87]. This leads to the maintenance of water homeostasis and osmolyte accumulation and stimulates carbohydrate metabolism and transport to maintain source-sink relations that avoid photo-inhibition during the osmotic state of salinity; furthermore, this reduces Na+ toxicity and ultimately promotes plant growth [30]. Salt stress causes higher accumulation of Na+, which competes with K+-binding proteins and decreases protein synthesis. Na+ expulsion and K+ influx is the most important plant strategy for relieving salinity stress [88]. The present results showed that salt stress-inoculated soybean plants had higher Na+ and decreased K+ levels (Figure 4). However, the inoculation of selected rhizospheric strains resulted in decreased Na+ and increased K+ concentrations (Figure 4). Similarly, it was reported that Na+ expulsion and K+ influx can be restricted to roots of various plants (maize and soybean) using different bacterial strains [43, 89, 90].
The identification of salt tolerance genes is of great importance to develop sustainable agriculture practices. Several crop plants can be genetically engineered for agricultural practice in a salt-stressed environment. Furthermore, genome-wide transcriptomic analysis in soybean revealed that a number of hormone-related genes were differentially expressed in shoots and roots under salinity and drought stresses [91]. The candidate genes, such as GmLAX3 and GmST1, for cation antiporters and salt tolerance have been identified in soybean. GmST1 gene reportedly reduces the toxic effect of ROS production and enhances ABA sensitivity. GmLAX3 has a similar role in the context of IAA regulation. Results from the present study showed that salinity stress down-regulated the expression of GmST1 (Figure 5(a)). However, the inoculation of rhizospheric bacterial strains stimulated the expression of GmST1 in soybean plants exposed to salt stress (Figure 5(a)). The expression of GmST1 was regulated through an ABA-dependent pathway and decreased production of ROS during salt stress. Similar results of GmST1 overexpression showed strong tolerance in Arabidopsis to salinity stress [20], making it a potential candidate gene for genetic engineering of salt-tolerant plants. Furthermore, GmLAX3 is among the key genes involved in salt stress response in plants [24, 25]; it encodes a multimembrane spanning transmembrane protein and functions in auxin uptake and intercellular auxin flow [24]. The present results revealed that GmLAX3 expression was down-regulated under salinity stress. However, rhizospheric bacteria stimulated the expression of GmLAX3 in soybean plants exposed to salt stress (Figure 5(b)). As reported previously, the overexpression of GmLAX3 enhanced salt stress tolerance of plants [24, 25, 92].
5. Conclusion
The use of PGPR could be an efficient way to confer salinity stress resistance in crop plants. Presently, the ameliorative role of PGPR was evaluated in soybean plants under salt stress conditions. The experimental data revealed that salt stress-resistant PGPR—A. woluwensis (AK1), M. oxydans (AK2), A. aurescens (AK3), B. megaterium (AK4), and B. aryabhattai (AK5)—greatly helped in the recovery of soybean plants by producing bioactive metabolites which activated antioxidants (GSH and SOD), modulated phytohormones (ABA), maintained osmotic balance by suppressing Na+ and promoting K+ ion uptake, and regulated salt tolerance (GmST1) and IAA-mediating (GmLAX3) genes. Hence, the present research supports and takes further the notion of using halotolerant PGPR to develop eco-friendly biofertilizers for enhanced growth and quality yield of crop plants grown under salinity stress.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1A6A1A05011910).
Abbreviations
- PGPR:
Plant growth rhizospheric bacteria
- IAA:
Indole-3-acetic acid
- GA:
Gibberellin
- ROS:
Reactive oxygen species
- SOD:
Superoxide dismutase
- GSH:
Reduced glutathione (GSH)
- GmLAX:
Auxin resistant 1
- GmST1:
Soybean salt tolerance 1
- PGP:
Plant growth-promoting
- GC/MS SIM:
Gas chromatography-mass spectrometry under selected ion monitoring mode
- HPLC:
High-performance liquid chromatography
- ICP:
Inductively coupled plasma
- CF:
Culture filtrate.
Data Availability
All data generated or analyzed during this study are included in this article.
Disclosure
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
MAK, AA, MI, and S. Ali conducted the experiments; RJ and KMK conducted qRT-PCR analysis; S. Asaf and ALK helped in writing the manuscript; and IJL designed, supervised, and financed the research.
Supplementary Materials
Table S1: description of plant species, rhizospheric bacteria isolation, and the number of yielded isolates with individual/multiple plant growth-promoting characteristics. Figure S1 Siderophores and phosphate solubilization activity on PVK and CAS medium are shown. (a) Capability of siderophores production, (b) Phosphate solubilization activity, and (c) Growth promotion of Waito-C rice using rhizospheric bacteria.
References
- 1.Hashem A., Abd Allah E. F., Alqarawi A. A., Al-Huqail A. A., Wirth S., Egamberdieva D. The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardii under salt stress. Frontiers in Microbiology. 2016;7:p. 1089. doi: 10.3389/fmicb.2016.01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnawal D., Bharti N., Maji D., Chanotiya C. S., Kalra A. ACC deaminase-containing Arthrobacter protophormiae induces NaCl stress tolerance through reduced ACC oxidase activity and ethylene production resulting in improved nodulation and mycorrhization in Pisum sativum. Journal of Plant Physiology. 2014;171(11):884–894. doi: 10.1016/j.jplph.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Orhan F. Alleviation of salt stress by halotolerant and halophilic plant growth-promoting bacteria in wheat (Triticum aestivum) Brazilian Journal of Microbiology. 2016;47(3):621–627. doi: 10.1016/j.bjm.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Etesami H., Beattie G. A. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Frontiers in Microbiology. 2018;9:p. 148. doi: 10.3389/fmicb.2018.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egamberdieva D., Davranov K., Wirth S., Hashem A., Abd_Allah E. F. Impact of soil salinity on the plant-growth - promoting and biological control abilities of root associated bacteria. Saudi Journal of Biological Sciences. 2017;24(7):1601–1608. doi: 10.1016/j.sjbs.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan J., Peng F., Xue X., et al. The growth promotion of two salt-tolerant plant groups with PGPR inoculation: a meta-analysis. Sustainability. 2019;11(2):p. 378. doi: 10.3390/su11020378. [DOI] [Google Scholar]
- 7.Ruiz-Lozano J. M., Porcel R., Azcon C., Aroca R. Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: new challenges in physiological and molecular studies. Journal of Experimental Botany. 2012;63(11):4033–4044. doi: 10.1093/jxb/ers126. [DOI] [PubMed] [Google Scholar]
- 8.Assaha D. V. M., Ueda A., Saneoka H., Al-Yahyai R., Yaish M. W. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Frontiers in Physiology. 2017;8:p. 509. doi: 10.3389/fphys.2017.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu J.-K. Plant salt tolerance. Trends in Plant Science. 2001;6(2):66–71. doi: 10.1016/s1360-1385(00)01838-0. [DOI] [PubMed] [Google Scholar]
- 10.Munns R. Comparative physiology of salt and water stress. Plant, Cell and Environment. 2002;25(2):239–250. doi: 10.1046/j.0016-8025.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- 11.Waśkiewicz A., Muzolf-Panek M., Goliński P. Phenolic content changes in plants under salt stress. In: Ahmad P., Azooz M. M., Prasad M. N. V., editors. Ecophysiology and Responses of Plants under Salt Stress. New York, NY, USA: Springer New York; 2013. pp. 283–314. [Google Scholar]
- 12.Ambede J. G., Netondo G. W., Mwai G. N., Musyimi D. M. NaCl salinity affects germination, growth, physiology, and biochemistry of Bambara groundnut. Brazilian Journal of Plant Physiology. 2012;24(3):151–160. doi: 10.1590/s1677-04202012000300002. [DOI] [Google Scholar]
- 13.Sharma P., Jha A. B., Dubey R. S., Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany. 2012;2012:26. doi: 10.1155/2012/217037.217037 [DOI] [Google Scholar]
- 14.Osman H. S. Enhancing antioxidant-yield relationship of pea plant under drought at different growth stages by exogenously applied glycine betaine and proline. Annals of Agricultural Sciences. 2015;60(2):389–402. doi: 10.1016/j.aoas.2015.10.004. [DOI] [Google Scholar]
- 15.Hanin M., Ebel C., Ngom M., Laplaze L., Masmoudi K. New insights on plant salt tolerance mechanisms and their potential use for breeding. Frontiers in Plant Science. 2016;7:p. 1787. doi: 10.3389/fpls.2016.01787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keisham M., Mukherjee S., Bhatla S. Mechanisms of sodium transport in plants-progresses and challenges. International Journal of Molecular Sciences. 2018;19(3):p. 647. doi: 10.3390/ijms19030647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan R., Qu Y., Guo Y., et al. Salinity tolerance in soybean is modulated by natural variation inGmSALT3. The Plant Journal. 2014;80(6):937–950. doi: 10.1111/tpj.12695. [DOI] [PubMed] [Google Scholar]
- 18.Jin R., Tester M. Mechanisms of salinity tolerance. Annual Review of Plant Biology. 2008;59(1):651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 19.Papiernik S. K., Grieve C. M., Lesch S. M., Yates S. R. Effects of salinity, imazethapyr, and chlorimuron application on soybean growth and yield. Communications in Soil Science and Plant Analysis. 2005;36(7-8):951–967. doi: 10.1081/css-200050280. [DOI] [Google Scholar]
- 20.Ren S., Lyle C., Jiang G.-l, Penumala A. Soybean salt tolerance 1 (GmST1) reduces ROS production, enhances ABA sensitivity, and abiotic stress tolerance in Arabidopsis thaliana. Frontiers in Plant Science. 2016;7(445) doi: 10.3389/fpls.2016.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu K. X., Cao B. H., Feng X. P., He Y., Jiang D. A. Photosynthetic response of salt-tolerant and sensitive soybean varieties. Photosynthetica. 2009;47(3):381–387. doi: 10.1007/s11099-009-0059-7. [DOI] [Google Scholar]
- 22.Phang T.-H., Shao G., Lam H.-M. Salt tolerance in soybean. Journal of Integrative Plant Biology. 2008;50(10):1196–1212. doi: 10.1111/j.1744-7909.2008.00760.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhou L., He H., Liu R., Han Q., Shou H., Liu B. Overexpression of GmAKT2 potassium channel enhances resistance to soybean mosaic virus. BMC Plant Biology. 2014;14(1):p. 154. doi: 10.1186/1471-2229-14-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chai C., Wang Y., Valliyodan B., Nguyen H. T. Comprehensive analysis of the soybean (Glycine max) GmLAX auxin transporter gene family. Frontiers in Plant Science. 2016;7:p. 282. doi: 10.3389/fpls.2016.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan M. A., Ullah I., Waqas M., et al. Halo-tolerant rhizospheric Arthrobacter woluwensis AK1 mitigates salt stress and induces physio-hormonal changes and expression of GmST1 and GmLAX3 in soybean. Symbiosis. 2019;77(1):9–21. doi: 10.1007/s13199-018-0562-3. [DOI] [Google Scholar]
- 26.Péret B., Swarup K., Ferguson A., et al. AUX/LAX genes encode a family of auxin influx transporters that perform distinct functions during Arabidopsis development. The Plant Cell. 2012;24(7):2874–2885. doi: 10.1105/tpc.112.097766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang K., Benková E., Swarup R., et al. The auxin influx carrier LAX3 promotes lateral root emergence. Nature Cell Biology. 2008;10(8):946–954. doi: 10.1038/ncb1754. [DOI] [PubMed] [Google Scholar]
- 28.Levesque H., Ma T., Wang X., et al. OsAUX1 controls lateral root initiation in rice (Oryza sativa L.) Plant, Cell & Environment. 2015;38(11):2208–2222. doi: 10.1111/pce.12467. [DOI] [PubMed] [Google Scholar]
- 29.Fàbregas N., Formosa-Jordan P., Confraria A, et al. Auxin influx carriers control vascular patterning and xylem differentiation in Arabidopsis thaliana. PLoS Genetics. 2015;11(4) doi: 10.1371/journal.pgen.1005183.e1005183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dodd I. C., Perez-Alfocea F. Microbial amelioration of crop salinity stress. Journal of Experimental Botany. 2012;63(9):3415–3428. doi: 10.1093/jxb/ers033. [DOI] [PubMed] [Google Scholar]
- 31.Schubert S., Neubert A., Schierholt A., Sümer A., Zörb C. Development of salt-resistant maize hybrids: the combination of physiological strategies using conventional breeding methods. Plant Science. 2009;177(3):196–202. doi: 10.1016/j.plantsci.2009.05.011. [DOI] [Google Scholar]
- 32.Joshi R., Mangu V. R., Bedre R., et al. Salt adaptation mechanisms of halophytes: improvement of salt tolerance in crop plants. In: Pandey G. K., editor. Elucidation of Abiotic Stress Signaling in Plants: Functional Genomics Perspectives. Vol. 2. New York, NY, USA: Springer New York; 2015. pp. 243–279. [Google Scholar]
- 33.Krishna G., Singh B. K., Kim E.-K., Morya V. K., Ramteke P. W. Progress in genetic engineering of peanut (Arachis hypogaea L.)-A review. Plant Biotechnology Journal. 2015;13(2):147–162. doi: 10.1111/pbi.12339. [DOI] [PubMed] [Google Scholar]
- 34.Saharan B., Nehra V. Plant growth promoting rhizobacteria: a critical review. Life Sciences and Medicine Research. 2011;21:1–30. [Google Scholar]
- 35.Park Y.-G., Mun B.-G., Kang S.-M., et al. Bacillus aryabhattai SRB02 tolerates oxidative and nitrosative stress and promotes the growth of soybean by modulating the production of phytohormones. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0173203.e0173203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaidi A., Khan M., Ahemad M., Oves M. Plant growth promotion by phosphate solubilizing bacteria. Acta microbiologica et immunologica Hungarica. 2009;56(3):263–284. doi: 10.1556/amicr.56.2009.3.6. [DOI] [PubMed] [Google Scholar]
- 37.Nadeem S. M., Ahmad M., Naveed M., Imran M., Zahir Z. A., Crowley D. E. Relationship between in vitro characterization and comparative efficacy of plant growth-promoting rhizobacteria for improving cucumber salt tolerance. Archives of Microbiology. 2016;198(4):379–387. doi: 10.1007/s00203-016-1197-5. [DOI] [PubMed] [Google Scholar]
- 38.Dimkpa C., Weinand T., Asch F. Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant, Cell & Environment. 2009;32(12):1682–1694. doi: 10.1111/j.1365-3040.2009.02028.x. [DOI] [PubMed] [Google Scholar]
- 39.Kang S.-M., Radhakrishnan R., Khan A. L., et al. Gibberellin secreting rhizobacterium, Pseudomonas putida H-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiology and Biochemistry. 2014;84:115–124. doi: 10.1016/j.plaphy.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Almodares A., Hadi M. R., Dosti B. The effects of salt stress on growth parameters and carbohydrates contents in sweet sorghum. Research Journal of Environmental Sciences. 2008;2(24):298–304. doi: 10.3923/rjes.2008.298.304. [DOI] [Google Scholar]
- 41.Habib S. H., Kausar H., Saud H. M. Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. BioMed Research International. 2016;2016:10. doi: 10.1155/2016/6284547.6284547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hahm M.-S., Son J.-S., Hwang Y.-J., Kwon D.-K., Ghim S.-Y. Alleviation of salt stress in pepper (Capsicum annum L.) plants by plant growth-promoting rhizobacteria. Journal of Microbiology and Biotechnology. 2017;27(10):1790–1797. doi: 10.4014/jmb.1609.09042. [DOI] [PubMed] [Google Scholar]
- 43.Nadeem S. M., Zahir Z. A., Naveed M., Asghar H. N., Arshad M. Rhizobacteria capable of producing ACC-deaminase may mitigate salt stress in wheat. Soil Science Society of America Journal. 2010;74(2):533–542. doi: 10.2136/sssaj2008.0240. [DOI] [Google Scholar]
- 44.Louden B. C., Lynne A. M., Haarmann D. Use of blue agar CAS assay for siderophore detection. Journal of Microbiology & Biology Education. 2011;12(1):51–53. doi: 10.1128/jmbe.v12i1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katznelson H., Bose B. Metabolic activity and phosphate-dissolving capability of bacterial isolates from wheat roots, rhizosphere, and non-rhizosphere soil. Canadian Journal of Microbiology. 1959;5(1):79–85. doi: 10.1139/m59-010. [DOI] [PubMed] [Google Scholar]
- 46.Lee K.-E., Adhikari A., Kang S.-M., et al. Isolation and characterization of the high silicate and phosphate solubilizing novel strain Enterobacter ludwigii GAK2 that promotes growth in rice plants. Agronomy. 2019;9(3):p. 144. doi: 10.3390/agronomy9030144. [DOI] [Google Scholar]
- 47.Khan M. A., Asaf S., Khan A. L., et al. Alleviation of salt stress response in soybean plants with the endophytic bacterial isolate Curtobacterium sp. SAK1. Annals of Microbiology. 2019;69(8):797–808. doi: 10.1007/s13213-019-01470-x. [DOI] [Google Scholar]
- 48.Shahzad R., Waqas M., Khan A. L., et al. Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiology and Biochemistry. 2016;106:236–243. doi: 10.1016/j.plaphy.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ullah I., Khan A. R., Park G.-S., et al. Analysis of phytohormones and phosphate solubilization in Photorhabdus spp. Food Science and Biotechnology. 2013;22(1):25–31. doi: 10.1007/s10068-013-0044-6. [DOI] [Google Scholar]
- 51.Khan A., Hamayun M., Kang S.-M., et al. Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: an example of Paecilomyces formosus LHL10. BMC Microbiology. 2012;12(1):p. 3. doi: 10.1186/1471-2180-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khan M. A., Hamayun M., Iqbal A., et al. Gibberellin application ameliorates the adverse impact of short-term flooding on Glycine max L. Biochemical Journal. 2018;475(18):2893–2905. doi: 10.1042/bcj20180534.BCJ20180534 [DOI] [PubMed] [Google Scholar]
- 53.Kang S. M., Khan A. L., Hamayun M., et al. Acinetobacter calcoaceticus ameliorated plant growth and influenced gibberellins and functional biochemicals. Pakistan Journal of Botany. 2012;44:365–372. [Google Scholar]
- 54.Chan C.-X., Teo S.-S., Ho C.-L., Othman R. Y., Phang S.-M. Optimisation of RNA extraction from gracilaria changii (gracilariales, rhodophyta) Journal of Applied Phycology. 2004;16(4):297–301. doi: 10.1023/b:japh.0000047782.20940.de. [DOI] [Google Scholar]
- 55.Qi Q., Rose P. A., Abrams G. D., Taylor D. C., Abrams S. R., Cutler A. J. (+)-Abscisic acid metabolism, 3-ketoacyl-coenzyme a synthase gene expression, and very-long-chain monounsaturated fatty acid biosynthesis inBrassica napus embryos. Plant Physiology. 1998;117(3):979–987. doi: 10.1104/pp.117.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. European Journal of Biochemistry. 1974;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 57.Ellman G. L. Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 58.Asaf S., Khan A. L., Khan M. A., Imran Q. M., Yun B.-W., Lee I.-J. Osmoprotective functions conferred to soybean plants via inoculation with Sphingomonas sp. LK11 and exogenous trehalose. Microbiological Research. 2017;205:135–145. doi: 10.1016/j.micres.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 59.Negrão S., Schmöckel S. M., Tester M. Evaluating physiological responses of plants to salinity stress. Annals of Botany. 2017;119(1):1–11. doi: 10.1093/aob/mcw191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khan M. A., Asaf S., Khan A. L., et al. Rhizobacteria AK1 remediates the toxic effects of salinity stress via regulation of endogenous phytohormones and gene expression in soybean. Biochemical Journal. 2019;476(16):2393–2409. doi: 10.1042/bcj20190435.BCJ20190435 [DOI] [PubMed] [Google Scholar]
- 61.Nakbanpote W., Panitlurtumpai N., Sangdee A., Sakulpone N., Sirisom P., Pimthong A. Salt-tolerant and plant growth-promoting bacteria isolated from Zn/Cd contaminated soil: identification and effect on rice under saline conditions. Journal of Plant Interactions. 2014;9(1):379–387. doi: 10.1080/17429145.2013.842000. [DOI] [Google Scholar]
- 62.Marulanda A., Azcón R., Chaumont F., Ruiz-Lozano J. M., Aroca R. Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta. 2010;232(2):533–543. doi: 10.1007/s00425-010-1196-8. [DOI] [PubMed] [Google Scholar]
- 63.Mayak S., Tirosh T., Glick B. R. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiology and Biochemistry. 2004;42(6):565–572. doi: 10.1016/j.plaphy.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 64.Siddikee M. A., Glick B. R., Chauhan P. S., Yim W. j., Sa T. Enhancement of growth and salt tolerance of red pepper seedlings (Capsicum annuum L.) by regulating stress ethylene synthesis with halotolerant bacteria containing 1-aminocyclopropane-1-carboxylic acid deaminase activity. Plant Physiology and Biochemistry. 2011;49(4):427–434. doi: 10.1016/j.plaphy.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 65.Timmusk S., Abd El-Daim I. A., Copolovici L., et al. Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: enhanced biomass production and reduced emissions of stress volatiles. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0096086.e96086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim M.-J., Radhakrishnan R., Kang S.-M., et al. Plant growth promoting effect of Bacillus amyloliquefaciens H-2-5 on crop plants and influence on physiological changes in soybean under soil salinity. Physiology and Molecular Biology of Plants. 2017;23(3):571–580. doi: 10.1007/s12298-017-0449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turner T. R., James E. K., Poole P. S. The plant microbiome. Genome Biology. 2013;14(6):p. 209. doi: 10.1186/gb-2013-14-6-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vyas P., Gulati A. Organic acid production in vitro and plant growth promotion in maize under controlled environment by phosphate-solubilizing fluorescent Pseudomonas. BMC Microbiology. 2009;9(1):p. 174. doi: 10.1186/1471-2180-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones D. L., Dennis P. G., van Hees P. A. W., van Hees P. Organic acid behavior in soils - misconceptions and knowledge gaps. Structure and Functioning of Cluster Roots and Plant Responses to Phosphate Deficiency. 2003;248(1-2):31–41. doi: 10.1023/a:1022304332313. [DOI] [Google Scholar]
- 70.Babalola O. O. Beneficial bacteria of agricultural importance. Biotechnology Letters. 2010;32(11):1559–1570. doi: 10.1007/s10529-010-0347-0. [DOI] [PubMed] [Google Scholar]
- 71.Kamilova F., Kravchenko L. V., Shaposhnikov A. I., Azarova T., Makarova N., Lugtenberg B. Organic acids, sugars, andl-tryptophane in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Molecular Plant-Microbe Interactions. 2006;19(3):250–256. doi: 10.1094/mpmi-19-0250. [DOI] [PubMed] [Google Scholar]
- 72.Waqas M., Khan A. L., Lee I.-J. Bioactive chemical constituents produced by endophytes and effects on rice plant growth. Journal of Plant Interactions. 2014;9(1):478–487. doi: 10.1080/17429145.2013.860562. [DOI] [Google Scholar]
- 73.Deubel A., Merbach W., Buscot F., Varma A. Influence of Microorganisms on Phosphorus Bioavailability in Soils. Berlin, Germany: Springer; 2005. Microorganisms in soils: roles in genesis and functions; pp. 177–191. [Google Scholar]
- 74.Gupta S., Pandey S. ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French bean (Phaseolus vulgaris) plants. Frontiers in Microbiology. 2019;10(1506) doi: 10.3389/fmicb.2019.01506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kadmiri I. M., Chaouqui L., Azaroual S. E., Sijilmassi B., Yaakoubi K., Wahby I. Phosphate-solubilizing and auxin-producing rhizobacteria promote plant growth under saline conditions. Arabian Journal for Science and Engineering. 2018;43(7):3403–3415. doi: 10.1007/s13369-017-3042-9. [DOI] [Google Scholar]
- 76.Bashan Y., de-Bashan L. E., Prabhu S. R., Hernandez J.-P. Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998–2013) Plant and Soil. 2014;378(1-2):1–33. doi: 10.1007/s11104-013-1956-x. [DOI] [Google Scholar]
- 77.Glick B. R. Plant growth-promoting bacteria: mechanisms and applications. Scientifica (Cairo) 2012;2012:15. doi: 10.6064/2012/963401.963401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang J., Kloepper J. W., Ryu C.-M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends in Plant Science. 2009;14(1):1–4. doi: 10.1016/j.tplants.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 79.Numan M., Bashir S., Khan Y., et al. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: a review. Microbiological Research. 2018;209:21–32. doi: 10.1016/j.micres.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 80.Bianco C., Defez R. Medicago truncatula improves salt tolerance when nodulated by an indole-3-acetic acid-overproducing Sinorhizobium meliloti strain. Journal of Experimental Botany. 2009;60(11):3097–3107. doi: 10.1093/jxb/erp140. [DOI] [PubMed] [Google Scholar]
- 81.Zhang H.-J., Zhang N., Yang R.-C., et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4interaction in cucumber (Cucumis sativus L.) Journal of Pineal Research. 2014;57(3):269–279. doi: 10.1111/jpi.12167. [DOI] [PubMed] [Google Scholar]
- 82.de Ollas C., Dodd I. C. Physiological impacts of ABA-JA interactions under water-limitation. Plant Molecular Biology. 2016;91(6):641–650. doi: 10.1007/s11103-016-0503-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Radhakrishnan R., Lee I.-J. Regulation of salicylic acid, jasmonic acid and fatty acids in cucumber (Cucumis sativus L.) by spermidine promotes plant growth against salt stress. Acta Physiologiae Plantarum. 2013;35(12):3315–3322. doi: 10.1007/s11738-013-1364-0. [DOI] [Google Scholar]
- 84.Pang C.-H., Wang B.-S. Oxidative stress and salt tolerance in plants. In: Lüttge U., Beyschlag W., Murata J., editors. Progress in Botany. Heidelberg, Germany: Springer Berlin Heidelberg; 2008. pp. 231–245. [Google Scholar]
- 85.Das K., Roychoudhury A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Frontiers in Environmental Science. 2014;2(53) doi: 10.3389/fenvs.2014.00053. [DOI] [Google Scholar]
- 86.Gururani M. A., Upadhyaya C. P., Strasser R. J., Yu J. W., Park S. W. Evaluation of abiotic stress tolerance in transgenic potato plants with reduced expression of PSII manganese stabilizing protein. Plant Science. 2013;198:7–16. doi: 10.1016/j.plantsci.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 87.Pérez-Alfocea F., Albacete A., Ghanem M. E., Dodd I. C. Hormonal regulation of source— sink relations to maintain crop productivity under salinity: a case study of root-to-shoot signalling in tomato. Functional Plant Biology. 2010;37(7):592–603. doi: 10.1071/fp10012. [DOI] [Google Scholar]
- 88.Shabala S., Cuin T. A. Potassium transport and plant salt tolerance. Physiologia Plantarum. 2008;133(4):651–669. doi: 10.1111/j.1399-3054.2007.01008.x. [DOI] [PubMed] [Google Scholar]
- 89.Ashraf M., Hasnain S., Berge O., Mahmood T. Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biology and Fertility of Soils. 2004;40(3):157–162. doi: 10.1007/s00374-004-0766-y. [DOI] [Google Scholar]
- 90.Vaishnav A., Jain S., Kasotia A., Kumari S., Gaur R. K., Choudhary D. K. Effect of nitric oxide signaling in bacterial-treated soybean plant under salt stress. Archives of Microbiology. 2013;195(8):571–577. doi: 10.1007/s00203-013-0902-x. [DOI] [PubMed] [Google Scholar]
- 91.Song L., Prince S., Valliyodan B., et al. Genome-wide transcriptome analysis of soybean primary root under varying water-deficit conditions. BMC Genomics. 2016;17(1):p. 57. doi: 10.1186/s12864-016-2378-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rahman A. Auxin: a regulator of cold stress response. Physiologia Plantarum. 2013;147(1):28–35. doi: 10.1111/j.1399-3054.2012.01617.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: description of plant species, rhizospheric bacteria isolation, and the number of yielded isolates with individual/multiple plant growth-promoting characteristics. Figure S1 Siderophores and phosphate solubilization activity on PVK and CAS medium are shown. (a) Capability of siderophores production, (b) Phosphate solubilization activity, and (c) Growth promotion of Waito-C rice using rhizospheric bacteria.
Data Availability Statement
All data generated or analyzed during this study are included in this article.