Abstract
This study developed a method for simultaneous determination of 13 elements of Semen Cuscutae (quercitrin, quercetin, hyperoside, caffeic acid, chlorogenic acid, luteolin, apigenin, kaempferol, isoquercitrin, cryptochlorogenic acid, isorhamnetin-3-O-glucoside, astragalin, and rutin) in rat plasma using high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) in the negative MRM mode. The analytes were analyzed with CORTECS®C18 column (4.6 × 150 mm, 2.7 μm) with mobile phases consisting of 0.1% formic acid in water (A) and acetonitrile (B). The intra- and interday precision of the target compounds were expressed as relative standard deviation (RSD) in the range of 0.5%–10.4%, and the accuracy of the target compounds was expressed as relative error (RE) not exceeding ±14.5% for all analytes. In the meantime, the extraction recovery of the target compounds in plasma samples ranged from 87.4% to 106.2% and matrix effect from 81.0% to 115.5%. The established method was successfully accomplished for the pharmacokinetic study of the analytes in rat plasma samples following oral administration of Semen Cuscutae extract, and the pharmacokinetic parameters of seven compounds were obtained.
1. Introduction
Semen Cuscutae (Longxuzi, Tusizi), the dry mature seed, belongs to Cuscuta australis R.Br. or Cuscuta chinensis Lam. of Convolvulaceae family [1, 2]. It was first recorded in the “Shen Nong's Herbal” as an upper grade drug [1]. As a well-known traditional Chinese medicine, Semen Cuscutae has numerous pharmacological functions, such as regulating the body's endocrine system, nourishing the liver and kidney, improving eyesight, and preventing miscarriage [3, 4]. It has also been reported to have neuroprotective, hepatoprotective, antioxidative, osteoblastogenic, and immunomodulatory properties and to have positive effects on chronic prostatitis [5–7]. There are many natural active ingredients in Semen Cuscutae, which includes flavonoids, lignans, polysaccharides, alkaloids, and other chemicals [1, 6, 8], with flavonoids and phenolic compounds being the predominantly bioactive constituents [9, 10].
Traditional Chinese medicine has been used in the treatment of diseases for thousands of years in China [11]. However, due to the complex composition of traditional Chinese medicine, its safety and effectiveness are still in doubt worldwide [10]. Qualitative and quantitative analysis of its components in traditional Chinese medicine has certain significance. In the recent years, the chemical studies on Semen Cuscutae have mainly focused on qualitative analysis of its major components using different analytical methods, such as HPLC, LC-MS/MS, and so on [12], but the pharmacokinetic studies of Semen Cuscutae were rarely reported. In the latest research, Zhang et al. [13] established the kidney-deficiency model on rats to expound the pharmacokinetic differences of six renoprotective compounds from Semen Cuscutae between normal and kidney-deficiency rats. The metabolism process in vivo of six flavonoids were clarified and showed prospective results in their study. However, it is insufficient to study the pharmacokinetics of only one kind of component, and other active ingredients from Semen Cuscutae need to be further investigated too.
As we know, study on the pharmacokinetics of traditional Chinese medicine can elucidate its material basis and explain the mechanism of drug absorption, distribution, metabolism, and excretion in vivo [14]. Therefore, in our study, we simultaneously separated and determined 13 elements from Semen Cuscutae extract by a selective HPLC-MS/MS method and analyzed their pharmacokinetics data. This research would contribute to the understanding of the metabolism of these elements, as well as the mechanism of action of Semen Cuscutae.
2. Material and Methods
2.1. Analytical Standards and Reagents
Methanol and acetonitrile (HPLC pure grade) were purchased from Fisher Co., Ltd. Formic acid was of chromatographic purity obtained from ROE Co., Ltd. Ultrapure water for the HPLC-MS/MS analysis was purified by Milli-Q water purification system (Millipore, Milford, MA, USA). Hyperoside, caffeic acid, rutin, chlorogenic acid, luteolin, apigenin, kaempferol, isoquercitrin, cryptochlorogenic acid, isorhamnetin-3-O-glucoside, astragalin, and liquiritin (internal standards, IS) were obtained from Chengdu Must Bio-Technology Co., Ltd (Chengdu, China). Quercitrin and quercetin were purchased from National Institutes for Food and Drug Control. Semen Cuscutae was purchased from Anguo, Hebei province. The structures of 13 compounds are displayed in Figure 1.
Figure 1.
Chemical structures of 13 components. (a) Hyperoside. (b) Caffeic acid. (c) Rutin. (d) Chlorogenic acid. (e) Isoquercitrin. (f) Isorhamnetin-3-O-glucoside. (g) Astragalin. (h) Apigenin. (i) Luteolin. (j) Kaempferol (k) Quercetin. (l) Cryptochlorogenic acid. (m) Quercitrin.
Sprague–Dawley rats (SPF, 200 ± 10 g, male) were purchased from HFK Laboratory Animal Technology Co., Ltd (license number: SCXK 2014-0004).
2.2. Instruments and Experimental Conditions
The electrospray ionization (ESI) source was used to connect the HPLC-MS/MS system consisting of an Agilent 1200 high-performance liquid chromatography equipped with an Agilent 6430 series triple quadrupole mass spectrometer (Agilent Technologies, USA). The target compounds and IS were separated on the CORTECS®C18 column (4.6 × 150 mm, 2.7 μm) with mobile phases consisting of 0.1% formic acid in water (A) and acetonitrile (B) at a flow rate of 0.3 mL/min. The gradient elution method as follows: 0–5 min, 25%–75% B; 5–7 min, 75%–95% B; 7–12 min, 95%–95% B. And the column balance procedure was 12–13 min, 95%–25% B; 13–17 min, 25%–25% B. The column temperature was maintained at 30°C and the injection volume set at 5 μL. The data obtained were processed using Mass Hunter workstation software (Agilent Technologies, USA).
Quantification was achieved in the negative multiple reaction monitoring mode (MRM). Parameters were set as follows: capillary temperature 250°C, drying gas flow 9 L/min, and nebulizing gas pressure at 20 psi. The parameters in mass spectrometry analysis were set as listed in Table 1.
Table 1.
The mass spectrometry of 13 compounds and IS.
| Compound | Ion masses | Formula | Retention time | Precursor ion | Product ion | Fragment ions | CE (V) |
|---|---|---|---|---|---|---|---|
| Caffeic acid | 180.157 | C9H8O4 | 6.57 | 179.1 | 135.0 | 135.0 | 12 |
| Apigenin | 270.237 | C15H10O5 | 10.85 | 269.0 | 117.0 | 116.9, 151.2 | 34 |
| Luteolin | 286.236 | C15H10O6 | 10.23 | 285.1 | 132.8 | 132.8 | 29 |
| Kaempferol | 286.236 | C15H10O6 | 10.99 | 285.1 | 187.1 | 93.1, 117.2, 205.0 | 25 |
| Quercetin | 302.236 | C15H10O7 | 10.33 | 300.9 | 151.0 | 197.8, 179.1, 151.0 | 15 |
| Cryptochlorogenic acid | 354.309 | C16H18O9 | 5.33 | 353.1 | 173.2 | 191.0 | 11 |
| Chlorogenic acid | 354.309 | C16H18O9 | 5.32 | 353.0 | 191.3 | 191.3 | 11 |
| Quercitrin | 448.377 | C21H20O11 | 8.53 | 447.0 | 299.9 | 299.9 | 22 |
| Astragalin | 448.377 | C21H20O11 | 8.22 | 477.1 | 284.1 | 284.1 | 25 |
| Isoquercitrin | 464.376 | C21H20O12 | 6.68 | 462.9 | 300.0 | 300.0 | 24 |
| Hyperoside | 464.376 | C21H20O12 | 6.73 | 463.1 | 300.0 | 300.0, 271.2 | 31 |
| Isorhamnetin-3-O-glucoside | 478.403 | C22H22O12 | 8.36 | 476.9 | 313.8 | 300.9, 178.9, 150.9 | 10 |
| Rutin | 610.518 | C27H30O16 | 5.81 | 609.2 | 300.1 | 300.1, 272.3 | 34 |
| Liquiritin (IS) | 418.39 | C21H22O9 | 7.19 | 416.9 | 255.0 | 179.0 | 17 |
2.3. Semen Cuscutae Extract Preparation
3 kg of Semen Cuscutae were weighed accurately, crushed and sifted with No. 4 sieve, and extracted three times using continuous reflux method by 80% ethanol (v/v) in the volume of 15 L, 12 L, 10 L for 2 h, 1.5 h, and 1 h, respectively. The extraction was then filtered and mixed. The ethanol mixture was concentrated with reduced pressure and dried by vacuum to obtain the Semen Cuscutae extract. The herb extract was crushed into powder form and stored in a desiccator until analysis. The extract contains quercitrin, quercetin, hyperoside, caffeic acid, rutin, chlorogenic acid, luteolin, apigenin, kaempferol, isoquercitrin, cryptochlorogenic acid, isorhamnetin-3-O-glucoside, and astragalin 0.2, 88.9, 841.6, 69.1, 4.1, 452.8, 7.4, 1.0, 351.3, 834.8, 47.3, 168.8, and 461.7 μg/g, respectively.
2.4. Preparation of Calibration Standards, QC, and IS Solutions
Stock solutions of quercitrin, quercetin, hyperoside, caffeic acid, rutin, chlorogenic acid, luteolin, apigenin, kaempferol, isoquercitrin, cryptochlorogenic acid, isorhamnetin-3-O-glucoside, astragalin, and liquiritin (IS) were prepared in methanol at 100 μg/mL. The mixed stock solutions were further obtained by mixing the stock samples together and diluted with an appropriate volume of methanol.
The calibration curve of quercitrin, quercetin, hyperoside, caffeic acid, chlorogenic acid, luteolin, apigenin, kaempferol, isoquercitrin, cryptochlorogenic acid, isorhamnetin-3-O-glucoside, astragalin (1, 2, 5, 10, 25, 50, 100, 250 ng/mL), and rutin (2, 4, 10, 20, 50, 100, 200, 500 ng/mL) were prepared by adding the respective mixed stock solutions and 20 μL of liquiritin (IS, 200 ng/mL) into 100 μL blank plasma.
Quality control (QC) samples included low, middle, and high concentrations, prepared with the appropriate mixed stock solutions and blank plasma sample to meet the desired concentration. All the solutions were stored at −4°C.
2.5. Treatment of Plasma
20 μL of methanol, 20 μL of IS (liquiritin, 200 ng/mL) and 20 μL of formic acid were added to 100 μL of plasma sample and then vortex-mixed. The mixture was extracted with 800 μL of acetonitrile by vortexing for 3 min. After centrifugation at 14000 rpm for 10 min, the supernatant was transferred to a clean glass tube and dried with nitrogen. The residue was reconstituted in 100 μL of methanol and then centrifuged at 14000 rpm for 10 min. 5 μL of supernatant was injected into the LC–MS/MS system for analysis.
2.6. Method Validation
Specificity, linearity, lower limit of quantitation (LLOQ), precision, accuracy, extraction recovery, matrix effect, and stability for the method were validated based on the guidelines published by regulatory authorities [15].
2.6.1. Specificity
The specificity was investigated by analyzing the chromatography of blank plasma samples from six different rats to determine whether the endogenous substances in the sample would affect the quantitative analysis of each component.
2.6.2. Linearity and LLOQ
The linearity was achieved by spiking rat plasma with the mixed standard solution and IS in a series of concentrations. The calibration curves were constructed with peak-area ratio of analyte to IS (y) against concentration of the calibration standard (x), with 1/x2 as the weighing factor. LLOQ was evaluated according to the lowest concentration of standard curve at which the signal-to-noise ratio (S : N) was about 10 : 1.
2.6.3. Precision and Accuracy
Intra- and interday precision were obtained by determining QC samples at three concentration levels, i.e., low, middle, and high (consisting of 2, 25, and 250 ng/mL of hyperoside, caffeic acid, chlorogenic acid, luteolin, apigenin, kaempferol, isoquercitrin, cryptochlorogenic acid, isorhamnetin-3-O-glucoside, astragalin, quercitrin, quercetin, and 4, 50, and 500 ng/mL for rutin, respectively). All plasma samples were performed in six replicates at three concentrations. Relative standard deviations were used to determine precision, and accuracy was evaluated by RE.
2.6.4. Matrix Effect and Extraction Recovery
The extraction recovery was assessed by comparing the peak areas obtained from pretreatment procedures samples with those from postextracted spiked samples. The matrix effect of the 13 analytes was determined by comparing the peak area obtained from postextracted spiked samples to that from pure standards solutions. All the extraction recovery and matrix effect experiments were evaluated in six replicates with three concentration levels.
2.6.5. Stability
The stability of the 13 analytes was evaluated by QC samples in different processing and storage conditions, including short-term stability (putting the analytes in ambient temperature for 4 h and storing analytes in autosampler for 12 h after treatment), long-term stability (storing the analytes at −70°C for 21 days) and subjecting analytes to three freeze-thaw cycles. All stability experiments were tested in six parallels with low, middle, and high concentrations.
2.7. Pharmacokinetic Studies
Six Sprague–Dawley rats (SPF, 200 ± 10 g, male) were purchased from HFK Laboratory Animal Technology Co., Ltd. The rats were housed in a standard laboratory condition (12 h dark-light cycle; temperature was 25°C ± 2°C and humidity was kept 50 ± 5%) and fed standard dry pellet diet and water for one week for acclimatization. Before the experiments, the rats were in fasting state for 12 h, with free access to water. The Semen Cuscutae extracts were dissolved in 0.5% carboxymethyl cellulose-sodium, prepared into suspension and gavage to the rats at a dose of 13 g/kg. Approximately 200 μL of rat blood samples was collected from the orbital venous plexus at 0, 0.03, 0.08, 0.17, 0.25, 0.5, 1, 2, 4, 6, 8, 10, 12, 24, 36, and 48 h after oral administration into 1.5 mL heparinized centrifuge tube. After centrifugation at 7000 rpm for 10 min immediately, the supernatant was collected into a new centrifuge tube and stored at −70°C until analysis.
2.8. Data Analysis
Pharmacokinetic parameters (Cmax, t1/2, Tmax, AUC(0-tn), and AUC(0-∞)) were obtained using the software “Drug and Statistics 3.0” (DAS 3.0) (Medical College of Wannan, China).
3. Results and Discussion
3.1. Method Development
In order to improve sensitivity and shorten analysis time, we tested different columns and various mobile phase systems. Comparing different columns such as CORTECS®C18 column (4.6 mm × 150 mm, 2.7 μm), Xbridge™C18 column (2.1 mm × 150 mm, 3.5 μm), and Xbridge™C18 column (4.6 mm × 50 mm, 2.5 μm), and various mobile phase systems such as acetonitrile-water or 0.1% formic acid in water and methanol-water or 0.1% formic acid in water, we found that the sensitivity and signal response of compounds analyzed with CORTECS®C18 column (4.6 mm × 150 mm, 2.7 μm) and acetonitrile-water containing 0.1% formic acid were more satisfactory.
The standard solutions of the target compounds and IS were infused into the instrument separately, both positive and negative ion modes were compared to optimize mass conditions. The results showed that all analytes were better eluted under negative ionization mode. Optimized precursor-to-production transitions were observed at 447.0 ⟶ 299.9 for quercitrin, 300.9 ⟶ 151.0 for quercetin, 463.1 ⟶ 300.0 for hyperoside, 179.1 ⟶ 135.0 for caffeic acid, 609.2 ⟶ 300.1 for rutin, 353.0 ⟶ 191.3 for chlorogenic acid, 285.1 ⟶ 132.8 for luteolin, 269.0 ⟶ 117.0 for apigenin, 285.1 ⟶ 187.1 for kaempferol, 462.9 ⟶ 300.0 for isoquercitrin, 353.1 ⟶ 173.2 for cryptochlorogenic acid, 476.9 ⟶ 313.8 for isorhamnetin-3-O-glucoside, 447.1 ⟶ 284.1 for astragalin, and 416.9 ⟶ 255.0 for liquiritin (IS).
3.2. Sample Preparation
In our study, three methods were attempted at disposing the plasma sample, namely, liquid-liquid extraction (LLE) with ethyl acetate, protein precipitation (PPT) with acetonitrile, and protein precipitation (PPT) with methyl alcohol. The results showed that the method of PPT with acetonitrile showed higher extraction efficiency, lower matrix effect, and simpler operational flow. Meeting the requirements of this experiment in determining biological samples, protein precipitation (PPT) with acetonitrile was employed in this study for sample preparation.
3.3. Method Validation
3.3.1. Specificity
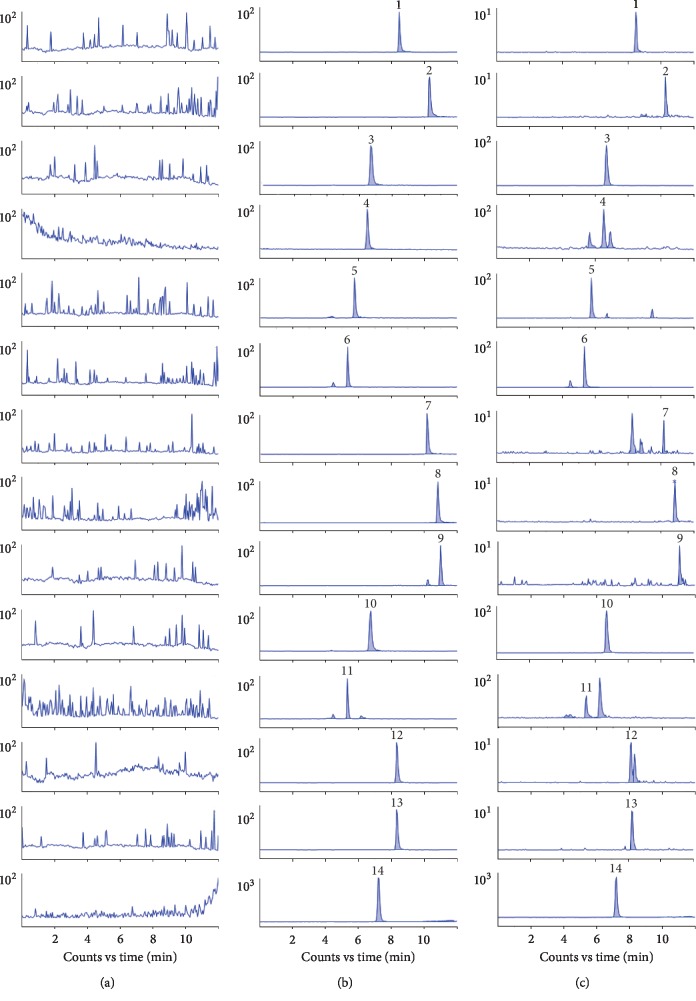
Specificity was evaluated by testing blank plasma, plasma samples with target compounds, and plasma samples after oral administration of Semen Cuscutae extract from six different rats. The retention time of quercitrin, quercetin, hyperoside, caffeic acid, rutin, chlorogenic acid, luteolin, apigenin, kaempferol, isoquercitrin, cryptochlorogenic acid, isorhamnetin-3-O-glucoside, astragalin, and liquiritin (IS) were 8.53, 10.33, 6.73, 6.57, 5.81, 5.33, 10.23, 10.85, 10.99, 6.68, 5.32, 8.36, 8.22, and 7.19 min, respectively. The chromatograms as shown in Figure 2 suggested no interfering peaks from the endogenous matrix in rat blood sample.
Figure 2.
MRM chromatograms of 13 analytes. Quercitrin (1). Quercetin (2). Hyperoside (3). Caffeic acid (4). Rutin (5). Chlorogenic Acid (6). Luteolin (7). Apigenin (8). Kaempferol (9). Isoquercitrin (10). Cryptochlorogenic Acid (11). Isorhamnetin-3-O-glucoside (12). Astragalin (13). IS (14). (a) Blank plasma; (b) blank plasma spiked with the analytes and IS; (c) plasma sample collected at 0.25 h after oral administration of Semen Cuscutae extract.
3.3.2. Linearity and Sensitivity
The data of the linear equation, correlation coefficients, linearity ranges, and LLOQ of all target compounds are listed in Table 2. The correlation coefficients of all analytes were greater than 0.9903, indicating that the 13 analytes in the plasma sample had good linearity in the corresponding concentration range. LLOQ with S/N ratio >10 ranged from 1–2 ng/mL of the 13 analytes, showing that the above method developed is suitable for quantitative pharmacokinetic studies.
Table 2.
Calibration curves, correlation coefficients, linear ranges, and LLOQ of the analytes.
| Compound | Calibration curves | Correlation coefficients (r) | Linear range (ng/mL) | LLOQ (ng/mL) |
|---|---|---|---|---|
| Quercitrin | Y = 0.6491X + 0.0086 | 0.9925 | 1–250 | 1 |
| Quercetin | Y = 0.5980X + 0.0026 | 0.9967 | 1–250 | 1 |
| Hyperoside | Y = 0.8758X + 0.0098 | 0.9961 | 1–250 | 1 |
| Caffeic acid | Y = 0.8239X + 0.0030 | 0.9986 | 1–250 | 1 |
| Rutin | Y = 0.2459X + 0.0138 | 0.9920 | 2–500 | 2 |
| Chlorogenic acid | Y = 0.1467X + 0.0038 | 0.9914 | 1–250 | 1 |
| Luteolin | Y = 0.6678X + 0.0025 | 0.9936 | 1–250 | 1 |
| Apigenin | Y = 0.7207X + 0.0017 | 0.9929 | 1–250 | 1 |
| Kaempferol | Y = 0.0250X − 0.0001 | 0.9952 | 1–250 | 1 |
| Isoquercitrin | Y = 0.8758X + 0.0098 | 0.9960 | 1–250 | 1 |
| Cryptochlorogenic acid | Y = 0.1038X + 0.0003 | 0.9903 | 1–250 | 1 |
| Isorhamnetin-3-O-glucoside | Y = 0.5362X + 0.0036 | 0.9942 | 1–250 | 1 |
| Astragalin | Y = 0.6307X + 0.0052 | 0.9962 | 1–250 | 1 |
3.3.3. Precision and Accuracy
In this experiment, all results of the intra- and interday precision and accuracy were analyzed at three different concentration levels, including low, medium, and high concentrations in six replicates, as displayed in Table 3. Intra- and interday RSD for the analytes ranged from 0.5%–10.4% and 0.8–7.9%, respectively. RE of accuracy did not exceed ±14.5% for all analytes. The results implied that this method is reliable and accurate for the study of the above target compounds in rat plasma.
Table 3.
Precision and accuracy of 13 analytes in rat plasma (n = 6).
| Compounds | Spiked concentration (ng/mL) | Intraday | Interday | ||||
|---|---|---|---|---|---|---|---|
| Measured concentration (ng mL−1) | Accuracy (RE, %) | Precision (RSD, %) | Measured concentration (ng mL−1) | Accuracy (RE, %) | Precision (RSD, %) | ||
| Quercitrin | 2 | 1.94 ± 0.05 | −3.0 | 2.6 | 1.95 ± 0.05 | −2.5 | 2.6 |
| 25 | 27.65 ± 1.75 | 10.6 | 6.3 | 26.16 ± 0.94 | 4.6 | 3.6 | |
| 250 | 253.47 ± 4.06 | 1.4 | 1.6 | 264.45 ± 10.46 | 5.8 | 4.0 | |
| 2 | 1.99 ± 0.10 | −0.5 | 5.0 | 2.02 ± 0.04 | 1.0 | 2.0 | |
| Quercetin | 25 | 26.60 ± 1.02 | 6.4 | 3.8 | 25.56 ± 0.68 | 2.2 | 2.7 |
| 250 | 262.38 ± 5.97 | 5.0 | 2.3 | 252.55 ± 3.26 | 1.0 | 1.3 | |
| 2 | 1.98 ± 0.12 | −1.0 | 6.1 | 2.05 ± 0.05 | 2.5 | 2.4 | |
| Hyperoside | 25 | 28.58 ± 0.33 | 14.3 | 1.2 | 28.21 ± 0.39 | 12.8 | 1.4 |
| 250 | 277.08 ± 12.08 | 10.8 | 4.4 | 270.47 ± 4.99 | 8.2 | 1.8 | |
| Caffeic acid | 2 | 2.14 ± 0.06 | 7.0 | 2.8 | 2.00 ± 0.06 | −0.1 | 3.0 |
| 25 | 25.92 ± 1.78 | 3.7 | 6.9 | 25.83 ± 0.47 | 3.3 | 1.8 | |
| 250 | 257.37 ± 5.90 | 2.9 | 2.3 | 250.56 ± 2.29 | 0.2 | 0.9 | |
| Rutin | 4 | 4.24 ± 0.17 | 6.0 | 4.0 | 4.16 ± 0.13 | 4.0 | 3.1 |
| 50 | 55.09 ± 2.26 | 10.2 | 4.1 | 49.67 ± 1.34 | −0.7 | 2.7 | |
| 500 | 494.24 ± 8.21 | −1.2 | 1.7 | 498.04 ± 5.47 | −0.4 | 1.1 | |
| Chlorogenic acid | 2 | 1.98 ± 0.03 | −1.0 | 1.5 | 1.90 ± 0.15 | −5.0 | 7.9 |
| 25 | 26.10 ± 2.72 | 4.4 | 10.4 | 26.28 ± 0.73 | 5.1 | 2.8 | |
| 250 | 254.48 ± 7.30 | 1.8 | 2.9 | 254.24 ± 4.74 | 1.7 | 1.9 | |
| 2 | 2.18 ± 0.14 | 9.0 | 6.4 | 1.96 ± 0.05 | −2.0 | 2.6 | |
| Luteolin | 25 | 26.98 ± 1.74 | 7.9 | 6.4 | 25.97 ± 1.16 | 3.9 | 4.5 |
| 250 | 256.22 ± 6.42 | 2.5 | 2.5 | 255.18 ± 3.16 | 2.1 | 1.2 | |
| Apigenin | 2 | 2.12 ± 0.14 | 6.0 | 6.6 | 2.03 ± 0.09 | 1.5 | 4.4 |
| 25 | 27.22 ± 1.31 | 8.9 | 4.8 | 25.60 ± 0.48 | 2.4 | 1.9 | |
| 250 | 258.13 ± 10.48 | 3.3 | 4.1 | 261.87 ± 13.20 | 4.7 | 5.0 | |
| Kaempferol | 2 | 2.03 ± 0.06 | 1.5 | 3.0 | 2.05 ± 0.10 | 2.5 | 4.9 |
| 25 | 25.92 ± 0.84 | 3.7 | 3.2 | 25.43 ± 0.58 | 1.7 | 2.3 | |
| 250 | 253.01 ± 7.68 | 1.2 | 3.0 | 266.49 ± 14.27 | 6.6 | 5.4 | |
| Isoquercitrin | 2 | 2.02 ± 0.07 | 1.0 | 3.5 | 2.00 ± 0.07 | 0.1 | 3.5 |
| 25 | 28.62 ± 0.62 | 14.5 | 2.2 | 28.29 ± 1.06 | 13.2 | 3.7 | |
| 250 | 272.50 ± 4.96 | 9.0 | 1.8 | 273.47 ± 8.62 | 9.4 | 3.2 | |
| Cryptochlorogenic acid | 2 | 2.03 ± 0.06 | 1.5 | 3.0 | 2.00 ± 0.08 | 0.1 | 4.0 |
| 25 | 25.92 ± 0.84 | 3.7 | 3.2 | 25.61 ± 1.03 | 2.4 | 4.0 | |
| 250 | 253.01 ± 7.68 | 1.2 | 3.0 | 251.30 ± 1.99 | 0.5 | 0.8 | |
| Isorhamnetin-3-O-glucoside | 2 | 1.99 ± 0.07 | −0.5 | 3.5 | 2.02 ± 0.05 | 1.0 | 2.5 |
| 25 | 26.18 ± 0.85 | 4.7 | 3.2 | 25.53 ± 0.58 | 2.1 | 2.3 | |
| 250 | 254.51 ± 1.60 | 1.8 | 0.6 | 250.00 ± 3.23 | 0.0 | 1.3 | |
| Astragalin | 2 | 1.95 ± 0.05 | −2.5 | 2.6 | 2.00 ± 0.04 | −0.1 | 2.0 |
| 25 | 26.19 ± 0.93 | 4.8 | 3.6 | 25.07 ± 0.39 | 0.3 | 1.6 | |
| 250 | 248.93 ± 1.24 | −0.4 | 0.5 | 253.07 ± 2.55 | 1.2 | 1.0 | |
3.3.4. Extraction Recovery and Matrix Effect
The results of extraction recovery and matrix effect are given in Table 4. The extraction recoveries of the analytes in rat plasma sample at three concentration levels ranged from 87.4% to 106.2%. The matrix effects of the target compounds were in the range of 81.0–115.5%. The data showed that the process of the experiment is efficient and there was no significant matrix effect observed for the plasma sample tested.
Table 4.
Extraction recoveries and matrix effects of the analytes (n = 6).
| Compounds | Concentration (ng/mL) | Extraction recovery (%) | RSD (%) | Matrix effect (%) | RSD (%) |
|---|---|---|---|---|---|
| Quercitrin | 2 | 97.3 ± 5.1 | 5.2 | 105.8 ± 4.8 | 4.5 |
| 25 | 97.5 ± 2.8 | 2.9 | 102.1 ± 2.4 | 2.4 | |
| 250 | 87.7 ± 2.0 | 2.3 | 106.7 ± 4.3 | 4.0 | |
| Quercetin | 2 | 89.9 ± 7.3 | 8.1 | 115.5 ± 11.6 | 10.0 |
| 25 | 98.7 ± 3.8 | 3.9 | 93.5 ± 7.2 | 7.7 | |
| 250 | 87.7 ± 1.8 | 2.1 | 97.0 ± 4.3 | 4.4 | |
| Hyperoside | 2 | 103.9 ± 4.4 | 4.2 | 101.4 ± 6.2 | 6.1 |
| 25 | 102.3 ± 6.3 | 6.2 | 97.1 ± 5.7 | 5.9 | |
| 250 | 101.7 ± 0.6 | 0.6 | 81.1 ± 2.9 | 3.6 | |
| Caffeic acid | 2 | 98.8 ± 7.1 | 7.2 | 101.0 ± 4.5 | 4.5 |
| 25 | 102.0 ± 2.9 | 2.8 | 86.9 ± 11.2 | 12.9 | |
| 250 | 96.2 ± 4.8 | 5.0 | 87.4 ± 3.0 | 3.4 | |
| Rutin | 4 | 103.2 ± 4.6 | 4.5 | 103.3 ± 3.9 | 3.8 |
| 50 | 92.0 ± 3.7 | 4.0 | 95.3 ± 7.2 | 7.6 | |
| 500 | 90.7 ± 5.2 | 5.7 | 85.9 ± 10.7 | 12.5 | |
| Chlorogenic acid | 2 | 103.6 ± 5.1 | 4.9 | 97.9 ± 9.1 | 9.3 |
| 25 | 97.8 ± 2.0 | 2.0 | 98.2 ± 6.1 | 6.2 | |
| 250 | 95.4 ± 1.3 | 1.4 | 85.1 ± 6.4 | 7.5 | |
| Luteolin | 2 | 106.2 ± 4.5 | 4.2 | 95.3 ± 6.0 | 6.3 |
| 25 | 95.3 ± 3.5 | 3.7 | 103.9 ± 9.9 | 9.5 | |
| 250 | 87.4 ± 2.6 | 3.0 | 97.4 ± 2.4 | 2.5 | |
| Apigenin | 2 | 99.1 ± 6.7 | 6.8 | 95.4 ± 4.5 | 4.7 |
| 25 | 91.4 ± 2.6 | 2.8 | 81.0 ± 2.3 | 2.8 | |
| 250 | 88.5 ± 1.5 | 1.7 | 88.7 ± 2.1 | 2.4 | |
| Kaempferol | 2 | 99.1 ± 5.2 | 5.2 | 105.6 ± 15.2 | 14.4 |
| 25 | 94.7 ± 4.2 | 4.4 | 96.6 ± 3.8 | 3.9 | |
| 250 | 91.4 ± 1.7 | 1.9 | 85.9 ± 1.0 | 1.2 | |
| Isoquercitrin | 2 | 104.3 ± 2.2 | 2.1 | 97.3 ± 2.7 | 2.8 |
| 25 | 104.7 ± 6.7 | 6.4 | 93.9 ± 2.3 | 2.4 | |
| 250 | 101.8 ± 2.6 | 2.6 | 83.2 ± 2.1 | 2.5 | |
| Cryptochlorogenic acid | 2 | 94.7 ± 4.9 | 5.2 | 94.5 ± 7.6 | 8.0 |
| 25 | 94.0 ± 3.6 | 3.8 | 111.2 ± 1.7 | 1.5 | |
| 250 | 90.8 ± 3.1 | 3.4 | 81.9 ± 3.1 | 3.8 | |
| Isorhamnetin-3-O-glucoside | 2 | 99.3 ± 5.4 | 5.4 | 109.9 ± 3.8 | 3.5 |
| 25 | 96.4 ± 5.2 | 5.4 | 99.8 ± 5.0 | 5.0 | |
| 250 | 88.8 ± 2.5 | 2.8 | 102.3 ± 3.4 | 3.3 | |
| Astragalin | 2 | 97.9 ± 5.1 | 5.2 | 115.1 ± 4.9 | 4.3 |
| 25 | 98.7 ± 3.9 | 4.0 | 98.7 ± 1.2 | 1.2 | |
| 250 | 87.6 ± 3.9 | 4.5 | 103.8 ± 4.9 | 4.7 |
3.3.5. Stability
Stability data of the 13 analytes in different conditions are listed in Table 5. All analytes subjected to different processing and storage conditions had an acceptance criterion in the range of 1.1%–11.1% for QC sample at three concentration levels. The results suggested that the analytes had a satisfactory stability for storage and analytical process.
Table 5.
Stability of all analytes in rat plasma (n = 6).
| Compounds | Spiked concentration (ng/mL) | Room temperature for 4h | Three freeze-thaw | Autosampler for 12h | −70°C for 21 days | ||||
|---|---|---|---|---|---|---|---|---|---|
| Measured (ng/mL) | RSD (%) | Measured (ng/mL) | RSD (%) | Measured (ng/mL) | RSD (%) | Measured (ng/mL) | RSD (%) | ||
| Quercitrin | 2 | 2.1 ± 0.1 | 4.8 | 2.3 ± 0.1 | 4.3 | 2.1 ± 0.1 | 4.8 | 2.0 ± 0.1 | 5.0 |
| 25 | 26.3 ± 0.7 | 2.7 | 26.9 ± 1.5 | 5.6 | 26.4 ± 0.9 | 3.4 | 26.5 ± 0.5 | 1.9 | |
| 250 | 252.5 ± 9.1 | 3.6 | 261.4 ± 10.3 | 3.9 | 253.1 ± 4.7 | 1.9 | 247.9 ± 5.3 | 2.1 | |
| Quercetin | 2 | 2.2 ± 0.1 | 4.5 | 2.0 ± 0.1 | 5.0 | 2.3 ± 0.1 | 4.3 | 1.9 ± 0.1 | 5.3 |
| 25 | 26.2 ± 0.7 | 2.7 | 27.3 ± 0.6 | 2.2 | 26.6 ± 0.7 | 2.6 | 25.6 ± 0.9 | 3.5 | |
| 250 | 254.9 ± 11.8 | 4.6 | 253.2 ± 5.0 | 2.0 | 245.3 ± 11.6 | 4.7 | 260.0 ± 6.2 | 2.4 | |
| Hyperoside | 2 | 2.0 ± 0.1 | 5.0 | 2.1 ± 0.1 | 4.8 | 1.8 ± 0.1 | 5.6 | 1.9 ± 0.1 | 5.3 |
| 25 | 23.0 ± 1.2 | 5.2 | 26.2 ± 1.5 | 5.7 | 23.6 ± 0.7 | 3.0 | 26.4 ± 1.8 | 6.8 | |
| 250 | 244.4 ± 14.3 | 5.9 | 254.7 ± 9.7 | 3.8 | 230.2 ± 13.1 | 5.7 | 256.4 ± 15.8 | 6.2 | |
| Caffeic acid | 2 | 2.1 ± 0.1 | 4.8 | 2.1 ± 0.1 | 4.8 | 2.2 ± 0.1 | 4.5 | 2.0 ± 0.1 | 5.0 |
| 25 | 27.9 ± 1.0 | 3.6 | 27.0 ± 1.3 | 4.8 | 28.1 ± 0.8 | 2.8 | 26.0 ± 0.6 | 2.3 | |
| 250 | 272.5 ± 8.9 | 3.3 | 255.7 ± 3.9 | 1.5 | 262.6 ± 7.4 | 2.8 | 250.2 ± 5.1 | 2.0 | |
| Rutin | 4 | 3.9 ± 0.2 | 5.1 | 4.0 ± 0.1 | 2.5 | 4.0 ± 0.2 | 5.0 | 4.0 ± 0.1 | 2.5 |
| 50 | 56.3 ± 2.3 | 4.1 | 57.7 ± 1.0 | 1.7 | 49.8 ± 2.7 | 5.4 | 52.7 ± 3.9 | 7.4 | |
| 500 | 505.7 ± 13.0 | 2.6 | 513.1 ± 7.4 | 1.4 | 489.3 ± 14.2 | 2.9 | 494.4 ± 8.8 | 1.8 | |
| Chlorogenic acid | 2 | 2.1 ± 0.1 | 4.8 | 2.1 ± 0.1 | 4.8 | 2.1 ± 0.1 | 4.8 | 2.1 ± 0.1 | 4.8 |
| 25 | 27.7 ± 1.6 | 5.8 | 28.1 ± 1.0 | 3.6 | 26.6 ± 1.6 | 6.0 | 26.6 ± 1.2 | 4.5 | |
| 250 | 258.4 ± 15.7 | 6.1 | 268.6 ± 15.8 | 5.9 | 253.1 ± 6.0 | 2.4 | 253.6 ± 7.7 | 3.0 | |
| Luteolin | 2 | 2.2 ± 0.1 | 4.5 | 2.0 ± 0.1 | 5.0 | 2.1 ± 0.1 | 4.8 | 2.0 ± 0.1 | 5.0 |
| 25 | 26.5 ± 0.5 | 1.9 | 26.8 ± 1.6 | 6.0 | 25.6 ± 1.0 | 3.9 | 27.4 ± 0.9 | 3.3 | |
| 250 | 256.7 ± 11.5 | 4.5 | 261.1 ± 8.2 | 3.1 | 244.9 ± 6.3 | 2.6 | 257.6 ± 5.1 | 2.0 | |
| Apigenin | 2 | 2.2 ± 0.1 | 4.5 | 2.0 ± 0.1 | 5.0 | 2.1 ± 0.2 | 9.5 | 2.1 ± 0.1 | 4.8 |
| 25 | 26.4 ± 1.2 | 4.5 | 25.7 ± 2.0 | 7.8 | 25.0 ± 1.2 | 4.8 | 26.5 ± 1.3 | 4.9 | |
| 250 | 249.3 ± 12.8 | 5.1 | 262.2 ± 16.0 | 6.1 | 248.5 ± 4.9 | 2.0 | 257.8 ± 8.2 | 3.2 | |
| Kaempferol | 2 | 2.2 ± 0.2 | 9.1 | 2.0 ± 0.2 | 10.0 | 2.1 ± 0.2 | 9.5 | 2.0 ± 0.1 | 5.0 |
| 25 | 24.8 ± 0.3 | 1.2 | 26.2 ± 1.6 | 6.1 | 25.4 ± 2.5 | 9.8 | 25.7 ± 0.8 | 3.1 | |
| 250 | 247.5 ± 6.3 | 2.5 | 252.6 ± 7.5 | 3.0 | 254.0 ± 4.9 | 1.9 | 257.2 ± 6.4 | 2.5 | |
| Isoquercitrin | 2 | 1.8 ± 0.2 | 11.1 | 2.1 ± 0.1 | 4.8 | 1.9 ± 0.1 | 5.3 | 1.9 ± 0.1 | 5.3 |
| 25 | 22.8 ± 1.4 | 6.1 | 26.2 ± 1.8 | 6.9 | 24.0 ± 0.5 | 2.1 | 25.9 ± 1.8 | 6.9 | |
| 250 | 247.2 ± 14.7 | 5.9 | 248.9 ± 5.1 | 2.0 | 238.3 ± 7.2 | 3.0 | 263.6 ± 10.2 | 3.9 | |
| Cryptochlorogenic acid | 2 | 2.0 ± 0.1 | 5.0 | 2.0 ± 0.1 | 5.0 | 2.1 ± 0.1 | 4.8 | 2.0 ± 0.1 | 5.0 |
| 25 | 27.0 ± 1.8 | 6.7 | 26.2 ± 0.3 | 1.1 | 27.3 ± 0.6 | 2.2 | 25.5 ± 1.2 | 4.7 | |
| 250 | 260.4 ± 3.3 | 1.3 | 248.9 ± 6.5 | 2.6 | 251.2 ± 9.7 | 3.9 | 244.4 ± 7.4 | 3.0 | |
| Isorhamnetin-3-O-glucoside | 2 | 2.0 ± 0.1 | 5.0 | 2.0 ± 0.1 | 5.0 | 2.1 ± 0.1 | 4.8 | 2.1 ± 0.1 | 4.8 |
| 25 | 27.1 ± 1.3 | 4.8 | 27.1 ± 1.5 | 5.5 | 26.9 ± 1.0 | 3.7 | 26.2 ± 0.6 | 2.3 | |
| 250 | 259.9 ± 5.5 | 2.1 | 255.0 ± 5.7 | 2.2 | 251.5 ± 5.4 | 2.1 | 249.5 ± 7.0 | 2.8 | |
| Astragalin | 2 | 2.1 ± 0.1 | 4.8 | 2.1 ± 0.1 | 4.8 | 2.1 ± 0.1 | 4.8 | 2.0 ± 0.1 | 5.0 |
| 25 | 27.6 ± 1.2 | 4.3 | 27.2 ± 0.8 | 2.9 | 27.3 ± 1.0 | 3.7 | 26.1 ± 1.2 | 4.6 | |
| 250 | 248.1 ± 4.3 | 1.7 | 258.6 ± 8.5 | 3.3 | 254.6 ± 4.6 | 1.8 | 251.5 ± 8.7 | 3.5 | |
3.4. Pharmacokinetic Application
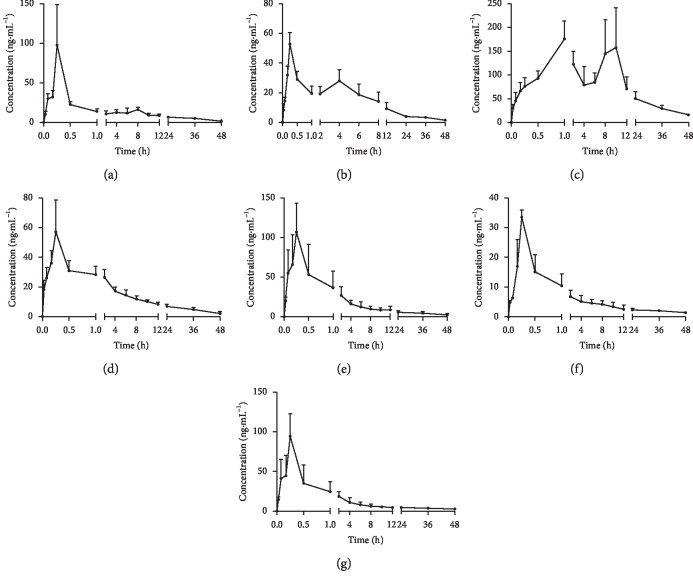
The validated method was successfully applied to a pharmacokinetic study of orally administered Semen Cuscutae extract (13.0 g/kg) in rats with the determination of 13 active ingredients of Semen Cuscutae in rat plasma. The corresponding pharmacokinetic parameters are listed in Table 6. Mean plasma concentration-time profiles of target compounds were illustrated in Figure 3.
Table 6.
Pharmacokinetic parameters of 7 analytes after oral administration of Semen Cuscutae extract (n = 6).
| Compounds | T max (h) | C max (ng/mL) | t 1/2 (h) | AUC(0-tn) (h·ng/mL) | AUC(0-∞) (h·ng/mL) |
|---|---|---|---|---|---|
| Hyperoside | 0.17 ± 0.07 | 29.81 ± 12.78 | 0.36 ± 0.23 | 684.47 ± 406.92 | 753.93 ± 423.71 |
| Caffeic acid | 0.28 ± 0.04 | 33.66 ± 8.43 | 11.60 ± 0.92 | 613.06 ± 290.85 | 651.94 ± 291.89 |
| Rutin | 10.00 ± 0.03 | 157.68 ± 84.08 | 7.70 ± 0.90 | 4463.92 ± 2274.52 | 4793.86 ± 2243.02 |
| Chlorogenic acid | 0.22 ± 0.06 | 35.51 ± 7.30 | 1.02 ± 0.67 | 994.54 ± 654.39 | 1000.96 ± 645.80 |
| Isoquercitrin | 0.21 ± 0.07 | 52.61 ± 7.55 | 0.49 ± 0.05 | 838.79 ± 518.04 | 949.49 ± 594.15 |
| Isorhamnetin-3-O-glucoside | 0.30 ± 0.01 | 19.51 ± 9.80 | 1.23 ± 0.26 | 171.70 ± 49.21 | 256.76 ± 74.37 |
| Astragalin | 0.24 ± 0.09 | 58.18 ± 28.53 | 0.45 ± 0.26 | 370.23 ± 258.63 | 440.81 ± 291.89 |
Figure 3.
Mean plasma concentration-time curves of hyperoside, caffeic acid, rutin, chlorogenic acid, isoquercitrin, isorhamnetin-3-O-glucoside, and astragalin after oral administration of Semen Cuscutae extract at a single dose of 13 g/kg to SD rats (mean ± SD, n = 6). (a) Hyperoside. (b) Caffeic acid. (c) Rutin. (d) Chlorogenic acid. (e) Isoquercitrin. (f) Isorhamnetin-3-O-glucoside. (g) Astragalin.
In our experiment, 6 ingredients, namely, quercitrin, quercetin, apigenin, kaempferol, luteolin, and cryptochlorogenic acid were found to be of low content in vivo, and their concentrations after 15 minutes of intragastric administration were lower than the LLOQ. Therefore, complete pharmacokinetic curves of the above 6 compounds were not obtained. In the previous studies, researchers have explored the pharmacokinetics rules of kaempferol and quercetin [16, 17]. Compared with our study, the dose of oral administration was significantly higher than ours, and caused by the low oral bioavailability of them, we could just detect them at a few time points, and a whole mean plasma concentration-time curve could not be obtained. Hence, their pharmacokinetic parameters were not further discussed.
The Tmax of hyperoside, chlorogenic acid, isoquercitrin, isorhamnetin-3-O-glucoside, and astragalin was less than 1 h. These results showed that the 5 ingredients were absorbed quickly in vivo. The elimination half-life (t1/2) of caffeic acid and rutin was more than 7.5 h, suggesting that caffeic acid and rutin are present in the body for a longer time, and may exert continuous therapeutic action, enhancing clinical efficacy. Similar pharmacokinetic trends have been reported in previous studies [18, 19]. In addition to this, the t1/2 of hyperoside, astragalin, isoquercitrin, chlorogenic acid, and sorhamnetin-3-O-glucoside was less than 1.5 h, indicating that the above compounds were eliminated quickly after intragastric administration of Semen Cuscutae extract.
In the previous study of our research group, we have established a HPLC-MS/MS method to explore the pharmacokinetic rule of rutin from mulberry leaves [16]. The results were different from this study. In this research, we found that rutin had an isomeride from Semen Cuscutae extract, that is, the isomeride with a high concentration. However, its plasma concentration was low. So, we guessed this isomeride may transform into rutin after oral administration in vivo. Thus, in the pharmacokinetic application, Cmax of rutin is higher than that of other components.
4. Conclusions
A validated and selective method of HPLC-MS/MS for simultaneous quantification of 13 compounds of Semen Cuscutae was established in this study, and the pharmacokinetics of Semen Cuscutae extract in rats was investigated. We found that the content of quercitrin, quercetin, apigenin, kaempferol, luteolin, and cryptochlorogenic acid were at a lower level in vivo after oral administration, which could only be detected at a few time points. Meanwhile, other compounds except caffeic acid and rutin had shorter elimination half-life. The pharmacokinetic parameters indicate the metabolism rate of these elements and may provide references for further research of Semen Cuscutae.
Acknowledgments
This study was supported by National Natural Science Foundation of China (81673824) and National Major Science and Technology Major Projects of China (2018ZX01031301).
Contributor Information
Huizi Ouyang, Email: huihui851025@163.com.
Jun He, Email: hejun673@163.com.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Ethical Approval
The study was approved by the Laboratory Animal Ethics Committee of Tianjin University Traditional of Chinese Medicine (TCM-LAEC20180052).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Yang S., Xu X., Xu H., et al. Purification, characterization and biological effect of reversing the kidney-yang deficiency of polysaccharides from Semen Cuscutae. Carbohydrate Polymers. 2017;175(1):249–256. doi: 10.1016/j.carbpol.2017.07.077. [DOI] [PubMed] [Google Scholar]
- 2.Lin M.-K., Yu Y.-L., Chen K.-C., et al. Kaempferol from Semen Cuscutae attenuates the immune function of dendritic cells. Immunobiology. 2011;216(10):1103–1109. doi: 10.1016/j.imbio.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Yang J., Wang Y., Bao Y., Guo J. The total flavones from Semen Cuscutae reverse the reduction of testosterone level and the expression of androgen receptor gene in kidney-yang deficient mice. Journal of Ethnopharmacology. 2008;119(1):166–171. doi: 10.1016/j.jep.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 4.Wang J. N., Li X. R., Gao L. Study on extraction process of tannins from Semen Cuscutae and their anti-papilloma activity. African Journal of Traditional, Complementary, and Alternative Medicines. 2013;10(3):469–474. doi: 10.4314/ajtcam.v10i3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim E.-Y., Kim E. K., Lee H.-S., et al. Protective effects of cuscutae semen against dimethylnitrosamine-induced acute liver injury in sprague-dawley rats. Biological & Pharmaceutical Bulletin. 2007;30(8):1427–1431. doi: 10.1248/bpb.30.1427. [DOI] [PubMed] [Google Scholar]
- 6.Yang S., Xu H. F., Zhao B. S., et al. The difference of chemical components and biological activities of the crude products and the salt-processed product from Semen Cuscutae. Evidence-Based Complementary and Alternative Medicine. 2016;2016:9. doi: 10.1155/2016/8656740.8656740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang S. Y., Jung H. W., Lee M.-Y., Lee H. W., Chae S. W., Park Y.-K. Effect of the semen extract of Cuscuta chinensis on inflammatory responses in LPS-stimulated BV-2 microglia. Chinese Journal of Natural Medicines. 2014;12(8):573–581. doi: 10.1016/s1875-5364(14)60088-1. [DOI] [PubMed] [Google Scholar]
- 8.Wang J., Tan D., Wei G., et al. Studies on the chemical constituents of Cuscuta chinensis. Chemistry of Natural Compounds. 2016;52(6):1133–1136. doi: 10.1007/s10600-016-1886-y. [DOI] [Google Scholar]
- 9.Ye M., Li Y., Yan Y. N., Liu H. W., Ji X. H. Determination of flavonoids in Semen Cuscutae by RP-HPLC. Journal of Pharmaceutical and Biomedical Analysis. 2002;28(3-4):621–628. doi: 10.1016/s0731-7085(01)00672-0. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y., Xiong H., Xu X., et al. Compounds identification in Semen Cuscutae by ultra-high-performance liquid chromatography (UPLCs) coupled to electrospray ionization mass spectrometry. Molecules. 2018;23(5):p. 1199. doi: 10.3390/molecules23051199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X., Ma B., Zhang Q., et al. Comparative pharmacokinetics with single substances and Semen Cuscutae extract after oral administration and intravenous administration Semen Cuscutae extract and single hyperoside and astragalin to rats. Analytical Methods. 2014;6(18):p. 7250. doi: 10.1039/c4ay00437j. [DOI] [Google Scholar]
- 12.Li Y., Ye M., Liu H. W., Ji X. H., Yan Y. N. Characterization and analysis of Semen Cuscutae by capillary gas chromatography and gas chromatography–mass spectrometry. Journal of Separation Science. 2002;25(4):255–259. doi: 10.1002/1615-9314(20020301)25:4<255::aid-jssc255>3.0.co;2-c. [DOI] [Google Scholar]
- 13.Zhang W., Fu Z. T., Xie Y., Duan Z. , W., Wang Y., Fan R. H. High resolution UPLC-MS/MS method for simultaneous separation and determination of six flavonoids from Semen Cuscutae extract in rat plasma: application to comparative pharmacokinetic studies in normal and kidney-deficient rats. Natural Product Research. 2018:1–6. doi: 10.1080/14786419.2018.1511556.1511556 [DOI] [PubMed] [Google Scholar]
- 14.Tang S.-Q., Chen Y.-H., Chen X.-P., Zhang X.-D., Huang W. In vivo effect of guiding-herb radix platycodonis and radix cyathulae on paeoniflorin pharmacokinetics of XueFu ZhuYu tang in rats. African Journal of Traditional, Complementary and Alternative Medicines. 2017;14(4):289–296. doi: 10.21010/ajtcam.v14i4.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US FDA. Guidance for Industry: Bioanalytical Method Validation. Silver Spring, MD, USA: US FDA; 2001. [Google Scholar]
- 16.He J., Feng Y., Ouyang H.-z., et al. A sensitive LC-MS/MS method for simultaneous determination of six flavonoids in rat plasma: application to a pharmacokinetic study of total flavonoids from mulberry leaves. Journal of Pharmaceutical and Biomedical Analysis. 2013;84:189–195. doi: 10.1016/j.jpba.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z.-P., Sun J., Chen H.-X., et al. Comparative pharmacokinetics and bioavailability studies of quercetin, kaempferol and isorhamnetin after oral administration of ginkgo biloba extracts, ginkgo biloba extract phospholipid complexes and ginkgo biloba extract solid dispersions in rats. Fitoterapia. 2010;81(8):1045–1052. doi: 10.1016/j.fitote.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 18.Shi P. Y., Yang C. L., Su Y., Huang L. Y., Lin X. H., Yao H. Simultaneous determination of five phenolic acids and four flavonoid glycosides in rat plasma using HPLC-MS/MS and its application to a pharmacokinetic study after a single intravenous administration of kudiezi injection. Molecules. 2019;24(1):p. 64. doi: 10.3390/molecules24010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kammalla A. K., Ramasamy M. K., Chintala J., Dubey G. P., Agrawal A., Kaliappan I. Comparative pharmacokinetic interactions of quercetin and rutin in rats after oral administration of European patented formulation containing hipphophae rhamnoides and co-administration of quercetin and rutin. European Journal of Drug Metabolism and Pharmacokinetics. 2015;40(3):277–284. doi: 10.1007/s13318-014-0206-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.