Abstract
Hypertension continues to be global pandemic with huge mortality, morbidity, and financial burden on the health system. Unfortunately, most patients with hypertension would eventually require two or more drugs in combination to achieve their target blood pressure (BP). To this end, emergence of more potent antihypertensive drugs is a welcome sign. Angiotensin receptor blockers (ARBs) are cornerstones of hypertension management in daily practice. Among all ARBs, azilsartan is proven to be more potent in most of the head-to-head trials till date. Azilsartan is the latest ARB approved for hypertension with greater potency and minimal side effects. This review highlights the role of azilsartan in management of hypertension in the current era.
1. Introduction
Hypertension continues to be a global health pandemic causing huge mortality and morbidity. It contributes for almost half of CVD and stroke deaths [1, 2]. Unfortunately, it is no more a disease of western world and in South Asia itself, almost one third of population is suffering from hypertension [3]. To compound the situation, the recent NHANES data peg the disease treatment rates at only 71–80%, whereas the overall control rates are dismal at 45–50% [4]. More importantly, a majority of them would require two or more drug combinations to achieve their blood pressure goals [5, 6].
Drugs targeting the renin-angiotensin-aldosterone system (RAAS) are cornerstone of the management of hypertension. Four classes of molecules make it to the list of RAAS blockers: angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), mineralocorticoid receptor antagonist, and direct renin inhibitors (DRI) (Figure 1). Aldosterone antagonists are primarily reserved for resistant hypertension, whereas major trials of DRI did not meet their primary end points. Hence, RAAS modulators in daily practice of hypertension include ACEi and ARB. Because of a favourable side effect profile, many practitioners choose ARB over ACEi as first-line therapy.
Figure 1.
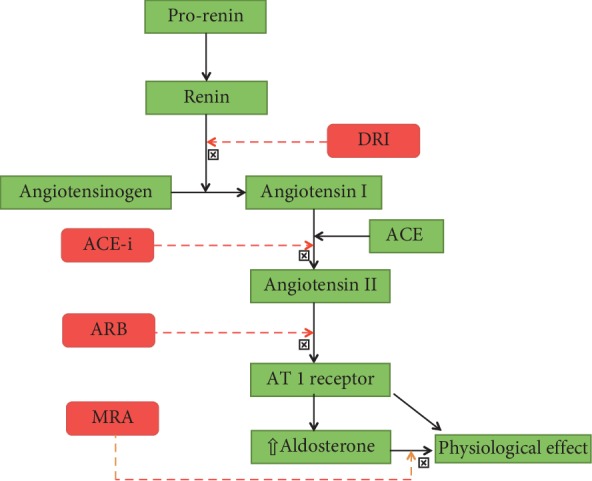
Drugs acting on renin angiotensin aldosterone system. ACE, angiotensin-converting enzyme; ACE-i, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; DRI, direct renin inhibitor; MRA, mineralocorticoid receptor antagonist; and AT1, angiotensin 1.
2. Evolution of ARBs in Hypertension
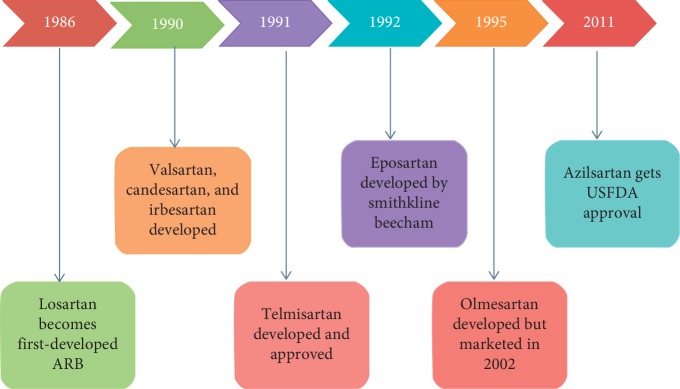
ARBs act via inhibiting the angiotensin II type 1 receptor and decreasing RAAS-associated adverse effects. The first ARB which was approved for hypertension was losartan, way back in 1986 [7]. Till March 2018, Food and Drug Administration (FDA) approved 8 ARBs for various indications. In chronological order the list includes losartan, valsartan,candesarten, irbesartan,eposartan, telminsartan, olmesartan, and azilsartan, being the latest addition (Figure 2).
Figure 2.
Milestones of development of various ARB's. Eprosartan was another ARB developed in 1992 by Glaxo Smithkline but not marketed in our country though approved by USFDA; losartan got USFDA approval only in 1995.
As ARBs cause dose-dependent decrease in peripheral resistance, it decreases the effect of aldosterone on the kidney and peripheral vasculature including decrease in smooth muscle vascular tone. ARBs have been successfully used in management of hypertension, coronary heart disease, heart failure, chronic kidney disease, and other miscellaneous conditions. Because of difference in affinity for the angiotensin receptor and other mechanisms, various ARBs differ in their pharmacokinetics and pharmacodynamics in human body. Major trials of different ARBs approved in our country for treating hypertension are LIFE (Losartan), ROADMAP (Olmesartan), VALUE (Valsartan), and ON TARGET (Telmisartan) [8–11] (see Table 1).
Table 1.
Pivotal trials of ARB's and their key findings.
| ARB | Major trials | Number of patients | Year | Major findings |
|---|---|---|---|---|
| Losartan | LIFE [8] | 9193 | 2002 | Losartan prevents more cardiovascular morbidity and death than atenolol for similar reduction in blood pressure and is better tolerated. Losartan seems to confer benefits beyond reduction in blood pressure |
|
| ||||
| Telmesartan | ONTARGET [9] | 25,620 | 2008 | Telmisartan was equivalent to ramipril in patients with vascular disease or high-risk diabetes and was associated with less angioedema. The combination of the two drugs was associated with more adverse events without an increase in benefit |
|
| ||||
| Olmesartan | ROADMAP [10] | 1147 | 2015 | Additive treatment with an angiotensin receptor blocker, olmesartan, did not improve clinical outcome in hypertensive patients with chronic heart failure (CHF) treated with angiotensin-converting enzyme (ACE) inhibitors, β-blockers, or both |
|
| ||||
| Valsartan | VALUE [11] | 15,425 | 2004 | The hypothesis with equivalent amount of blood pressure control, valsartan would reduce cardiac morbidity and mortality more than amlodipine in hypertensive patients with high cardiovascular risk could not be proved Unequal reductions in blood pressure might account for differences between the groups in cause-specific outcomes. The findings emphasise the importance of prompt blood-pressure control in hypertensive patients with high cardiovascular risk |
3. Azilsartan: ARB with a Difference
Azilsartan medoxomil (development code: TAK-491) has been developed by Takeda Global Research & Development Centre, Inc., U.S. and got FDA approval in February 2011 for treatment in hypertension in adults [12]. Azilsartan is now worldwide approved for hypertension either as a prodrug (Azilsartan medoxomil) or primary compound.
4. Mechanism of Action, Pharmacodynamics, and Pharmacokinetics
Azilsartan medoxomil is a prodrug which is hydrolysed in the gastrointestinal tract before getting absorbed in the system. Azilsartan acts against angiotensin II in a dose-dependent manner. After administration of azilsartan to healthy subjects, plasma angiotensin I and II concentrations increased, while plasma renin activity increased while plasma aldosterone concentrations decreased. Azilsartan does not cause any clinical significant effects on serum sodium or potassium. After oral administration, bioavailability of azilsartan medoxomil is approximately 60% with peak plasma concentration reached within 1.5 to 3 hours. There is no food interaction on bioavailability of azilsartan [13].
Azilsartan is closely related to candesartan with greater potency and prolonged duration of action as compared with other ARBs. Unlike candesartan which must be orally administrated as the prodrug (candesartan cilexetil) for better bioavailability, azilsartan is equally effective as either ester prodrug (azilsartan medoxomil) or primary compound itself. Azilsartan contains an oxo-oxadiazole ring which is not found in any of clinically approved ARBs, which makes azilsartan less acidic and more lipophilic than others.
5. Comparison with Other Sartans: Clinical Evidence
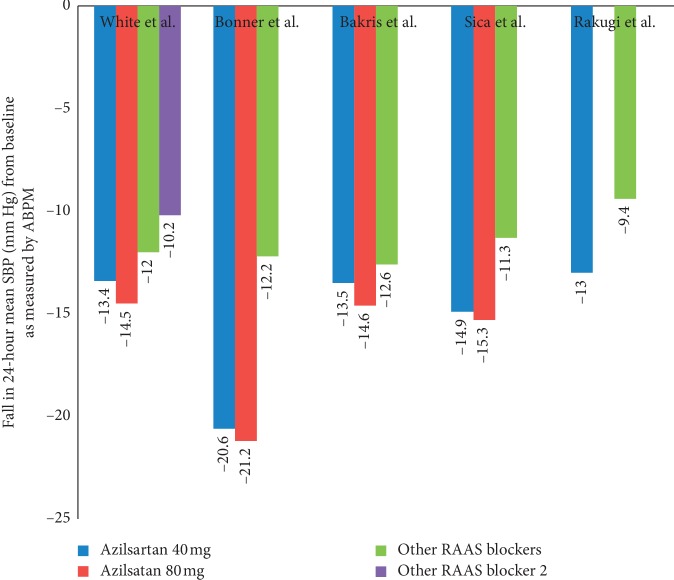
All major head-to-head randomized controlled trials indicate that azilsartan exhibits more potent antihypertensive action than any other drugs in its class. This potent antihypertensive action includes better clinical systolic blood pressure (SBP), diastolic blood pressure (DBP), and 24 hour ambulatory blood pressure (Figure 3, Table 2).
Figure 3.
Head-to-head comparison of azilsartan and other RAAS blockers in clinical studies for reduction in 24-hour mean systolic blood pressure (SBP, as measured by ambulatory BP monitoring) from baseline. RAAS blockers used as a comparator arm in various studies were as follows: White et al., valsartan 320 mg (purple bar) and olmesartan 40 mg (green bar); Bonner et al., ramipril 10 mg; Bakris et al., olmesartan 40 mg; Sica et al., valsartan 320 mg; Rakugi et al., candesartan.
Table 2.
Major azilsartan studies and their results.
| Major trials/studies of azilsartan | |||||||
|---|---|---|---|---|---|---|---|
| Design | Number of patients | Inclusion criteria | Duration | Dose | Primary outcome | Results | |
| Sica et al. [13] | RCT, double blinded, placebo controlled | 984 | SBP 150–180 mm Hg and 24-hour mean SBP 130–170 mm Hg | 24 weeks | Azilsartan 40 or 80 mg OD vs. valsartan 320 mg OD | Change in 24-hour mean SBP by ABPM from baseline | Azilsartan 40 mg (−14.9) and 80 mg (−15.3) significantly improved 24-hour mean SBP (−11.3) p < 0.0001 |
|
| |||||||
| Bakris et al. [14] | RCT, double blinded, placebo controlled | 1275 | SBP 150–180 mm Hg or 24-hour mean SBP 130–170 mm Hg | 6 weeks | Azilsartan 20, 40, 80 mm Hg OD vs. olmesartan 40 mg OD vs. placebo | Change in 24-hour mean SBP by ABPM from baseline | Azilsartan 80 mg (−14.6) significantly improved mean SBP vs. olmesartan (−12.6) (p=0.038) 40 mg dose was noninferior to olmesartan |
| White et al. [15] | RCT, double blinded, placebo controlled | 1291 | SBP 150–180 mm Hg and 24-hour mean SBP 130–170 mm Hg | 6 weeks | Azilsartan 40, 80 mg OD vs. olmesartan 40 mg OD vs. valsartan 320 mg OD | Change in 24-hour SBP by ABPM from baseline | Azilsartan 80 mg (−14.5) significantly improved mean SBP more than olmesartan (−11.7) and valsartan (−10.2). Azilsartan 40 mg (−13.4) noninferior to olmesartan |
|
| |||||||
| Rakugi et al. [16] | RCT, double blinded, placebo controlled | 622 | Grade I-II essential hypertension | 16 weeks | Azilsartan 20–40 mg OD vs. candesartan 8–12 mg OD | Change in sitting SBP, DBP, and ABPM | Azilsartan significantly improved DBP (−12.4) vs. candesartan (−9.8) (p=0.0003) and SBP azilsartan (-21.8) vs. candesartan (−17.5) (p < 0.0001) |
|
| |||||||
| Gitt et al. (EARLY registry) [17] | Prospective, observational, national, multicenter registry | 3849 | >18 year, essential hypertension | 12 months | Azilsartan 40 and 80 mg vs. ACE-inhibitor (mainly ramipril 10 mg) | Change in clinic SBP, DBP, and ABPM | Azilsartan 40 and 80 mg reduced both clinic systolic BP and mean ambulatory systolic BP significantly more than ramipril at a dose of 10 mg. Clinic SBP −20.6 + −0.9 with 40 mg and −21.2 ± 0.9 with 80 mg Vs. ramipril −12.2 ± 0.9 |
|
| |||||||
| Takagi et al. [18] | Meta-analysis | 6152 | Essential hypertension | — | Azilsartan 40 mg vs. control | Change in SBP and DBP | SBP reduction difference −4.2 mm Hg; DBP reduction difference −2.58; SBP (ABPM) −3.33 mm Hg; DBP (ABPM) −2.12 mm Hg (p < 0.0001) |
|
| |||||||
| Kario et al. [19] | RCT | 668 | Stage I and II hypertension∗ | 8 weeks | Azilsartan 20 mg vs. amlodipine 5 mg | Sleep ABPM | Among those >60 years, similar control rate of sleep BP, despite a trend favouring amlodipine (35% vs. 30%) |
In a randomized, double-blinded, placebo-controlled trial, Sica et al. compared azilsartan medoxomil and valsartan in primary hypertension using ambulatory and clinic BP measurement [13]. In the trial, azilsartan 40 mg (−14.9 mm Hg) and 80 mg (−15.3 mm Hg) significantly improved 24-hour mean SBP than valsartan (−11.3 mm Hg; p < 0.001).
Bakris et al. compared azilsartan medoxomil with olmesartan medoxomil in 1275 primary hypertension patients. Azilsartan 80 mg (−14.6 mm Hg) significantly improved mean SBP versus olmesartan (−12.6 mm Hg; p=0.038), whereas azilsartan 40 mg (−13.5 mm Hg) dose was noninferior to olmesartan. In this study, azilsartan was well tolerated and more efficacious at its maximal dose than the highest dose of olmesartan medoxomil [14].
White et al. compared azilsartan 40–80 mg with valsartan 320 mg and olmesartan 40 mg in a double-blinded, placebo-controlled RCT [15]. Trial results revealed that azilsartan 80 mg (−14.5) significantly improved mean SBP more than olmesartan (−11.7) and valsartan (−10.2). Azilsartan medoxomil at 40 mg (−13.4 mm Hg) was also noninferior to 40 mg of olmesartan (−1.4 mm Hg).
Another RCT comparing azilsartan 20–40 mg versus candesartan 8–12 mg by Rakugi et al. showed significantly improved DBP (−12.4 vs. −9.8; p=0.0003) and SBP (−21.8 vs. −17.5; p < 0.0001) with azilsartan as well as 24 hour ambulatory blood pressure [16].
The prospective, observational, multicentre EARLY registry in Germany compared patients initiated on monotherapy comprising either azilsartan or an ACE-inhibitor [17]. The results revealed that azilsartan medoxomil provided statistically significant albeit small improvement in blood pressure control. More patients on azilsartan attained blood pressure targets vis-a-vis ACE inhibitors (61.1% vs. 56.4%; p < 0.05).
Finally, Takagi et al. performed a meta-analysis which included a total of 6152 patients from 7 randomized-controlled trials with azilsartan [18]. The pooled analysis suggested a significant reduction in BP changes among patients randomized to 40 mg of azilsartan versus control therapy (clinic SBP: −4.20 mm Hg; 95% CI: −6.05 to −2.35 mm Hg; p < 0.00001; clinic DBP: −2.58 mm Hg; 95% CI: −3.69 to −1.48 mm Hg; p < 0.00001; 24-h mean SBP: −3.33 mm Hg; 95% CI: −4.74 to −1.93 mm Hg; p < 0.00001; and 24-h mean DBP: −2.12 mm Hg; 95% CI: −2.74 to −1.49 mm Hg; p < 0.00001). Meta-analysis concluded that in patients with hypertension, azilsartan therapy resulted in greater BP reduction.
6. Azilsartan in Combination Therapy
There are a few studies available in the literature comparing azilsartan-based combination therapies and most of them have used azilsartan in combination with chlorthalidone. In the largest such study, Cushman et al. compared azilsartan (40/80 mg) and chlorthalidone combination with olmesartan (40 mg) and hydrochlorothiazide combination [20]. They enrolled 1071 patients with stage 2 hypertension and evaluated mean ABPM (Systolic BP) pressure at 12 weeks. Azilsartan-based combination therapies lowered systolic BP (ABPM) better than olmesartan-based regimens (p < 0.001 for all comparisons, Figure 4).
Figure 4.
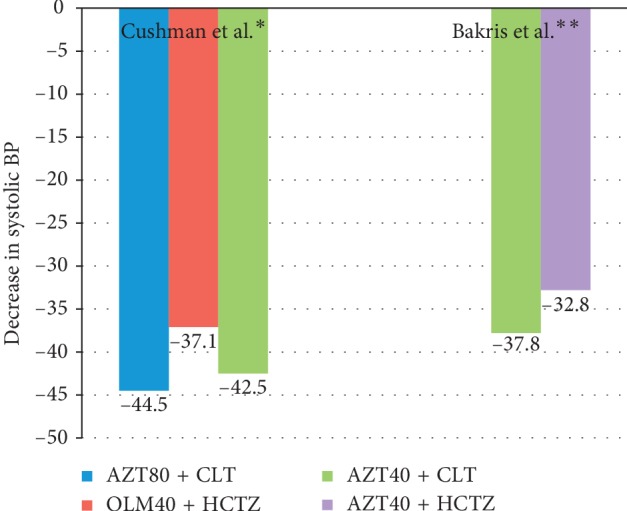
Clinical studies of head-to-head comparison of combination therapy with azilsartan for reduction in mean systolic blood pressure from the baseline. ∗24-hour systolic BP measured by ABPM; ∗∗clinic systolic BP used; AZT, azilsartan; CLT, chlorthalidone; HCTZ, hydrochlorothiazide; and OLM, olmesartan.
Similarly, Bakris et al. compared an azilsartan plus chlorthalidone regimen with azilsartan with hydrochlorothiazide in 609 patients with moderate-to-severe hypertension [21]. The dose of azilsartan was 40 mg while diuretic doses were titrated from 12.5 mg to 25 mg. The fall in clinical systolic BP from the baseline was higher in the chlorthalidone-based regimen (–37.8 mm Hg vs. –32.8 mm Hg, respectively, p < 0.001).
Interestingly, the blood pressure-lowering efficacy was maintained across both white and black races as demonstrated by Ferdinand et al. [22]. In this pooled analysis from two RCTs, azilsartan-based combination as well as monotherapy resulted in better BP control among both black and whites alike when pitted against an olmesartan-based regimes.
7. Azilsartan: Effects beyond Blood Pressure Control
Because of its inverse agonistic effects, azilsartan has potential effects beyond BP control which include amelioration of deleterious effects of angiotensin II such as cardiac hypertrophy, fibrosis, insulin resistance, and stabilization of coronary plaques [23].
In patients with heart failure with preserved ejection fraction (HfpEF), azilsartan improved parameters of diastolic function of left ventricle [24]. In their study of fifteen patients with HfpEF by Sakomoto et al., mitral annular E/e' ratio on echocardiography decreased with azilsartan therapy at six months while there was no change with candesartan treatment. This was despite comparable with reductions in blood pressure with both drugs. Azilsartan also decreased heart rate in the study while candesartan did not.
Azilsartan also improved endothelial dysfunction better than amlodipine as assessed by flow-mediated dilatation in brachial artery. In a group of twenty four hypertensive patients, 3 months of azilsartan therapy achieved superior flow-mediated dilatation, higher plasma renin activity, and lower plasma aldosterone levels [25]. Azilsartan therapy was also associated with improvement in arterial stiffness parameters (assessed by carotid-femoral pulse wave velocity) at 6 months [26].
In the CHAOS study published by Sezai et al., the effect of azilsartan and olmesartan on plasma renin activity, aldosterone II, and angiotensin in patients with essential hypertension after cardiac surgery was studied [27]. Apart from the primary endpoint, CHAOS study also included left ventricular mass index (LVMI), estimated glomerular filtration rate (eGFR), and urinalysis as secondary end point. The plasma renin levels were not different between the groups but aldosterone and angiotensin II levels were lower with olmesartan arm. There was no difference in two groups in terms of eGFR and urinalysis. LVMI was significantly lower in the olmesartan group than in the azilsartan group (p < 0.0001).
A cost effectiveness analysis between various ARB-based combination therapy has been published [28]. Azilsartan plus chlorthalidone combination therapy proved to have maximal incremental cost effectiveness followed by losartan plus hydrochlorthiazide-based therapy. Because of increased efficacy, azilsartan-based group was dominant despite an increment in price.
8. Side Effect of Drug
The various side effects of the drug seen in clinical studies include dizziness (8.9%), increased serum creatinine (3.6%), fatigue (2%), diarrhoea (2%), hypotension (1.7%), and syncope (0.3%) [12, 29]. Hypotension was the commonest cause of drug discontinuation with monotherapy, whereas raised serum creatinine and dizziness were most abundant causes in combination with chlorthalidone. Other side effects reported by the manufacturer include fatigue, muscle spasm, nausea, and abnormalities in hemogram. Increases in serum creatinine were often transient and related to a large fall in blood pressure. They were exacerbated by old age (>75 years) as well as coadministration of diuretics. On a similar note, manufacturers warn that volume and salt-depleted individuals are more prone to hypotensive effects of the drug. As with other ARBs, it should not be administered during pregnancy.
9. Current Indications
ARB has the best patient satisfaction profile (as assessed by the lowest rate of treatment discontinuation) among contemporary drugs [30]. With plethora of data establishing the superiority of azilsartan in controlling BP, it can be recommended that wherever blood pressure is not controlled on combination therapy with or without ARBs, adding azilsartan or replacing other ARBs with azilsartan can be an acceptable approach. For de novo hypertension too, azilsartan is an attractive option. However, for patients whose blood pressure is already well controlled by other ARBs, it is not imperative to switch to azilsartan. The drug is listed as the first-line ARB in the recent ACC/AHA 2017 Hypertension guidelines [5]. Azilsartan was also used in the pivotal SPRINT trial which has redefined blood pressure goals [31]. Based on the data from the available clinical trials, the dose equivalence between azilsartan and other ARBs is summarized in Table 3.
Table 3.
Dose equivalence of azilsartan with other sartans based on available data.
10. Conclusion
Hypertension is a global pandemic with huge morbidity and mortality. Unfortunately, the awareness and control rates remain dismal even in the western world. Azilsartan is the latest ARB to be added to the armamentarium of hypertension (Figure 5). It has emerged as a potent ARB which has demonstrated superior BP control in both monotherapy and combination therapy not only against other ARBs but also against other class of antihypertensive agents. The drug becomes the first-line choice in patients whose BP is not at goal despite combination therapy. In de novo hypertension, ARB is often the first-line choice due to their better tolerability and potency. In this scenario, it is the discretion of the treating physician to initiate any ARB of his choice but the wealth of data discussed above makes azilsartan an attractive first-line ARB.
Figure 5.
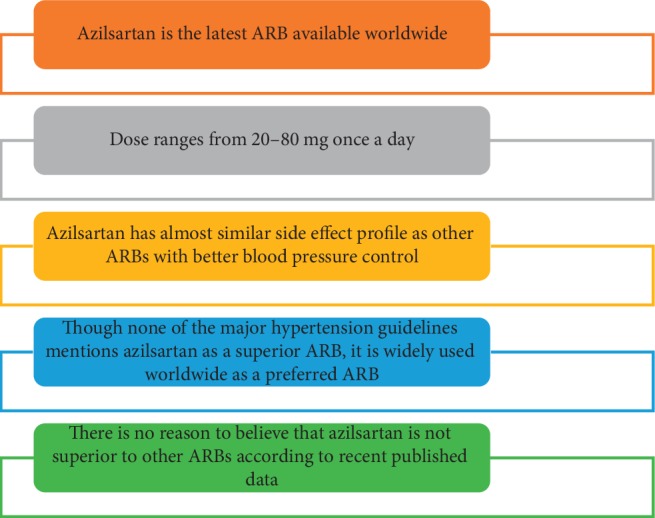
Take home messages.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Lewington S., Clarke R., Qizilbash N., et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. The Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 2.Lim S. S., Vos T., Flaxman A. D., et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. The Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prabhakaran D., Jeemon P., Ghosh S., et al. Prevalence and incidence of hypertension: results from a representative cohort of over 16,000 adults in three cities of South Asia. Indian Heart Journal. 2017;69(4):434–441. doi: 10.1016/j.ihj.2017.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Center for Health Statistics (U.S.) Health, United States, 2013: With Special Feature on Prescription Drugs. Hyattsville, MD, USA: National Center for Health Statistics (U.S.); 2014. [PubMed] [Google Scholar]
- 5.Whelton P. K., Carey R. M., Aronow W. S., et al. 2017. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2018;71(6):1269–1324. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 6.Gradman A. H., Basile J. N., Carter B. L., Bakris G. L. Combination therapy in hypertension. The Journal of Clinical Hypertension. 2011;13(3):146–154. doi: 10.1111/j.1751-7176.2010.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Timmermans P. B., Duncia J. V., Carini D. J., et al. Discovery of losartan, the first angiotensin II receptor antagonist. Journal of Human Hypertension. 1995;9(5):S3–S18. [PubMed] [Google Scholar]
- 8.Dahlöf B., Devereux R. B., Kjeldsen S. E., et al. Cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. The Lancet. 2002;359(9311):995–1003. doi: 10.1016/s0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 9.Yusuf S., Teo K. K., Pogue J., et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. New England Journal of Medicine. 2008;358(15):1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 10.Haller H., Ito S., Izzo J. L., et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. New England Journal of Medicine. 2011;364(10):907–917. doi: 10.1056/nejmoa1007994. [DOI] [PubMed] [Google Scholar]
- 11.Julius S., Kjeldsen S. E., Weber M., et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. The Lancet. 2004;363(9426):2022–2031. doi: 10.1016/s0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 12.Edarbi (Azilsartan Medoxomil), Prescribing Information. Atlanta, GA, USA: Arbor pharmaceuticals LLC; 2016. http://www.edarbi.com/media/pdf/EDARBI-PI.pdf. [Google Scholar]
- 13.Sica D., White W. B., Weber M. A., et al. Comparison of the novel angiotensin II receptor blocker azilsartan medoxomil vs. valsartan by ambulatory blood pressure monitoring. The Journal of Clinical Hypertension. 2011;13(7):467–472. doi: 10.1111/j.1751-7176.2011.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakris G. L., Sica D., Weber M., et al. The comparative effects of azilsartan medoxomil and olmesartan on ambulatory and clinic blood pressure. The Journal of Clinical Hypertension. 2011;13(2):81–88. doi: 10.1111/j.1751-7176.2010.00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White W. B., Weber M. A., Sica D., et al. Effects of the angiotensin receptor blocker azilsartan medoxomil versus olmesartan and valsartan on ambulatory and clinic blood pressure in patients with stages 1 and 2 hypertension. Hypertension. 2011;57(3):413–420. doi: 10.1161/hypertensionaha.110.163402. [DOI] [PubMed] [Google Scholar]
- 16.Rakugi H., Enya K., Sugiura K., Ikeda Y. Comparison of the efficacy and safety of azilsartan with that of candesartan cilexetil in Japanese patients with grade I-II essential hypertension: a randomized, double-blind clinical study. Hypertension Research. 2012;35(5):552–558. doi: 10.1038/hr.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gitt A. K., Bramlage P., Potthoff S. A., et al. Azilsartan compared to ACE inhibitors in anti-hypertensive therapy: one-year outcomes of the observational EARLY registry. BMC Cardiovascular Disorders. 2016;16(1):p. 56. doi: 10.1186/s12872-016-0222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takagi H., Mizuno Y., Niwa M., Goto S.-N., Umemoto T. A meta-analysis of randomized controlled trials of azilsartan therapy for blood pressure reduction. Hypertension Research. 2014;37(5):432–437. doi: 10.1038/hr.2013.142. [DOI] [PubMed] [Google Scholar]
- 19.Kario K., Hoshide S. Age-related difference in the sleep pressure-lowering effect between an angiotensin II receptor blocker and a calcium channel blocker in Asian hypertensives. Hypertension. 2015;65(4):729–735. doi: 10.1161/hypertensionaha.114.04935. [DOI] [PubMed] [Google Scholar]
- 20.Cushman W. C., Bakris G. L., White W. B., et al. Azilsartan medoxomil plus chlorthalidone reduces blood pressure more effectively than olmesartan plus hydrochlorothiazide in stage 2 systolic hypertension. Hypertension. 2012;60(2):310–318. doi: 10.1161/hypertensionaha.111.188284. [DOI] [PubMed] [Google Scholar]
- 21.Bakris G. L., Sica D., White W. B., et al. Antihypertensive efficacy of hydrochlorothiazide vs. chlorthalidone combined with azilsartan medoxomil. The American Journal of Medicine. 2012;125(12):1229.e1–1229.e10. doi: 10.1016/j.amjmed.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 22.Ferdinand K. C., Bakris G. L., Cushman W. C., et al. Comparison of effectiveness of azilsartan medoxomil and olmesartan in blacks versus whites with systemic hypertension. The American Journal of Cardiology. 2018;122(9):1496–1505. doi: 10.1016/j.amjcard.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Arumugam S., Sreedhar R., Thandavarayan R. A., et al. Angiotensin receptor blockers: focus on cardiac and renal injury. Trends in Cardiovascular Medicine. 2016;26(3):221–228. doi: 10.1016/j.tcm.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Sakamoto M., Asakura M., Nakano A., et al. Azilsartan, but not candesartan improves left ventricular diastolic function in patients with hypertension and heart failure. International Journal of Gerontology. 2015;9(4):201–205. doi: 10.1016/j.ijge.2015.06.003. [DOI] [Google Scholar]
- 25.Nishida T., Ayaori M., Shiotani K., et al. Beneficial effect of azilsartan and amlodipine on endothelial function in hypertensive patients. Atherosclerosis. 2016;252:p. e53. doi: 10.1016/j.atherosclerosis.2016.07.404. [DOI] [Google Scholar]
- 26.Zheleznova E., Zhernakova Y., Chazova I., Blinova N., Rogosa A. Beneficial effects of azilsartan medoxomil on target organs in patients with hypertension and metabolic syndrome. Journal of Hypertension. 2018;36:p. e193. doi: 10.1097/01.hjh.0000539535.24921.ed. [DOI] [Google Scholar]
- 27.Sezai A., Osaka S., Yaoita H., et al. Changeover trial of azilsartan and olmesartan comparing effects on the renin-angiotensin-aldosterone system in patients with essential hypertension after cardiac surgery (CHAOS study) Annals of Thoracic and Cardiovascular Surgery. 2016;22(3):161–167. doi: 10.5761/atcs.oa.16-00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiu-Ugalde J., Vargas J. A., Asbun-Bojalil J., et al. Pharmacoeconomic analysis of azilsartan medoxomil + chlortalidone in patients with arterial hypertension: comparison with valsartan + hydrochlorothiazide, telmisartan + hydrochlorothiazide, losartan + hydrochlorothiazide and irbesartan + hydrochlorothiazide. Value in Health. 2014;17(3):p. A113. doi: 10.1016/j.jval.2014.03.659. [DOI] [Google Scholar]
- 29.Edarbyclor (Prescribing Information) Atlanta, GA, USA: Arbor Pharmaceuticals LLC; 2016. http://www.edarbi.com/media/pdf/EDARBYCLOR-PI.pdf. [Google Scholar]
- 30.Thomopoulos C., Parati G., Zanchetti A. Effects of blood-pressure-lowering treatment in hypertension. Journal of Hypertension. 2016;34(10):1921–1932. doi: 10.1097/hjh.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 31.Wright J. T., Jr., Williamson J. D., Whelton P. K., et al. A randomized trial of intensive versus standard blood-pressure control. New England Journal of Medicine. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]