Abstract
We aimed to study the effects of Citrus aurantium (C. aurantium) on renal functions in cisplatin-induced nephrotoxicity in rats. The study involved male Wistar rats weighing 250–300 g that were fed and kept under standard conditions. Rats were randomly divided into control, cisplatin administered, C. aurantium 200 mg/kg, and C. aurantium 400 mg/kg groups. Cisplatin was administered at 5 mg/kg i.p. once at the start of study to induce nephrotoxicity. Blood and urine samples were obtained at alternative days for analysis. The body weight and urine output were monitored at regular intervals. Plasma and urinary sodium, potassium, and creatinine levels were measured at the end of study duration. Absolute excretion of sodium and potassium; sodium to potassium ratio; kidney weights; fractional excretion of sodium and potassium; and absolute creatinine clearance were determined to analyze the effects of C. aurantium. Histopathological changes of kidney tissues were studied to determine relevant effects. The results indicate that cisplatin lowered the total body weights while raising the urinary output and kidney weights, reversed by C. aurantium both dose and time dependently. Similarly, C. aurantium markedly normalized plasma, urinary sodium, potassium, and creatinine levels. Cisplatin-induced absolute sodium clearance, absolute potassium clearance, absolute creatinine clearance, sodium to potassium ratio, and fractional excretion of sodium and potassium were significantly reversed by C. aurantium. Histopathological analysis showed notable improvement in C. aurantium administered groups as compared to cisplatin-induced group. Study suggests that C. aurantium possesses excellent nephroprotective effects against cisplatin-induced toxicity.
1. Introduction
Cis-diamminedichloroplatinum (II) is inorganic platinum which is widely used as a potent chemotherapeutic drug [1]. It is an alkylating agent that reacts with DNA to form interstrand cross-links and intrastrand bifunctional N-7 DNA adducts at d(GpG) and d(ApG) [2]. Thus far it has shown its effectiveness in testicular cancer, ovarian cancer, cervical cancer, breast cancer, bladder cancer, head and neck cancer, esophageal cancer, lung cancer, germ cell tumors, lymphomas, and sarcomas, brain tumors, and neuroblastoma [3]. Despite its large-scale effectiveness in malignancies, cisplatin-induced (CIN) toxicities remain a single-most limiting factor in its use in cancer therapies. It causes toxicities of gastrointestinal [4], renal [5], neurological [6], hepatic [7], and haematological systems [8], even when administered at normal doses. Despite a wide range of side effects, nephrotoxicity remains a prominent reason for its discontinuation in malignancies as its prevalence touches one-third of treated patients with dose-limiting effects [9, 10]. For instance, moderate to severe nephrotoxicity was observed in 25%–33% of patients at the dose of 50–75 mg/m2 [11]. Even at much lower doses of 20 mg/m2 IV for five days, most of the patients (50%–70%) suffered moderate to severe nephrotoxicity [12]. Expectedly, incidence of nephrotoxicity increased as cisplatin dose was raised [13]. Clinically, it appears nearly after 10 days of treatment. Due to its nephrotoxic potential, it is generally restrained to patients having creatinine clearance (CrCl) > 60 Ml/min. There are other risk factors like co-treatment with other nephrotoxins such as aminoglycosides, NSAIDs, and streptozocin [14] that adds to the already raised chances of cisplatin-induced nephrotoxicity.
A numbers of mechanisms are reported to be responsible for CIN, among which overproduction of reactive oxygen species (ROS) and vasoconstriction in the renal microvasculature are reported accountable for the cisplatin-induced renal tubular injury. Furthermore, S3 segment of the proximal tubule on the external band of the outer medulla is selectively injured by cisplatin. Beyond that, generation of reactive nitrogen species (NOS) have also been reported in CIN which incite alterations in the operation and structure of lipid peroxidation and chemical cleavage of DNA and proteins [15].
There are fewer therapies that can be offered to tolerate the cisplatin-induced toxicity. The standard approach is to reduce the doses of cisplatin in combination with IV hydration before and after cisplatin administration which significantly decreases cisplatin half-life, urinary cisplatin concentration, and proximal tubule transit time [16–18]. Slow infusion rate and concomitant administration of mannitol can also reduce the severity of cisplatin-induced toxicity [19]. Moreover, antioxidant therapies are indicated in the literature to reduce the intensity of CIN and make it more tolerable for cancer patients. One such robust study presented by Nematbakhsh et al. demonstrated that selenium and vitamin E which are antioxidants are effective in curtailing oxidative toxicity of cisplatin [20]. There are other evidences which suggest that ROS and mitochondrial damage is at least partially responsible for CIN [21, 22].
C. aurantium is a traditional plant used for centuries to treat indigestion, diarrhea dysentery, constipation, and dry cough [23, 24]. It is mentioned in South American folk medicine as therapeutic agent for insomnia, anxiety, and epilepsy [25]. Research has also highlighted their pharmacological effects as an antioxidant, cardioprotective, antiproliferative, anticancer, and hypolipidemic agent [26–29]. Due to its antioxidative and anticancer potentials, it becomes a natural therapeutic agent for CIN. Hence, this study is designed to evaluate the pharmacological potential of C. aurantium in CIN.
2. Materials and Methods
2.1. Animals
Wistar albino rats, weighing 250–300 g, were kept in the experimental research laboratory, in the Islamia University of Bahawalpur, under 12 h light/dark cycles. The standard humidity (45–65%) and temperature (22–24°C) conditions were maintained. All the mice were provided with water and standard pellet diet ad libitum. Approvals of all the experimental protocols were taken from the Ethical Review Committee, Islamia University of Bahawalpur.
2.2. Materials
Chemicals of analytical grade used for research purpose included cisplatin (Pfizer Laboratory LTD), 2, 2-diphenyl, 1-picrylhydrazyl (DPPH), formalin, ketamine (Indus pharma Lahore), xylazine (prix pharmaceutical, Lahore), ether, aqueous ethanol, picric acid, sodium hydroxide, and trichloroacetic acid which all are of analytical grade.
2.3. Preparation of Extract
The peels of citrus aurantium were separated, dried under shade for 15 days, and powdered in a blender. It was then grinded by an electric grinder and soaked in a mixture of 60% ethanol for three days with shaking and agitation occasionally. Obtained residue is then evaporated under reduced pressure and at temperature of 30–40°C by using a rotary evaporator. Semisolid residue obtained was then kept in the refrigerator till further analysis.
2.4. Experimental Protocol
Rats were randomly divided into four groups containing six animals each. Group-I was kept untreated and received normal saline via oral route for 21 days. Group-II was considered intoxicated group administered only with cisplatin at 5 mg/kg i.p. on day 1. Group-III and Group-IV were treated with Citrus Aurantium extract at the oral doses of 200 mg/kg and 400 mg/kg once daily, respectively, for 21 days in the presence of cisplatin-induced toxicity.
2.5. Blood Samples and Metabolic Data Collection
For the measurement of sodium and potassium levels (metabolic data) in all the experimental groups, urine was sampled on 0, 7th, 14th, and 21st days of the study. Rats were kept in metabolic cages for 24 hours with free access to tap water. Water intake and urine output were measured regularly. For the estimation of creatinine, sodium, and potassium levels, samples were kept at −30°C. The flame photometer (Sherwood model 410, UK) was used to measure the levels of sodium and potassium in plasma and urine samples. For the measurement of sodium and potassium in plasma, samples were diluted as 1 : 200. Identical dilution was made to estimate potassium levels in urine but for the sodium level, the 1 : 1000 dilution was used. Creatinine was measured using the spectrophotometer method at the wave length of 520 nm.
2.6. Histopathology
A section of the kidney was fixed in 10% V/V neutral-buffered formalin and then processed for dehydration by passing them through pools of ethanol having different concentrations. Then, paraffin blocks were prepared and 5 μm thick sections were cut for staining with hematoxylin and eosin (H&E).
2.7. Statistical Analysis
The values are shown as mean ± SEM of 6 animals in each group. The results are evaluated by using one-way ANOVA followed by Bonferroni post hoc test and then compared with the normal control group. The results are mainly considered significant (∗) if p < 0.05, more significant (∗∗) if p < 0.01, and highly significant (∗∗∗) if p < 0.001.
3. Results
3.1. C. aurantium Effects on Physical Features of Cisplatin-Administered Rats
Cisplatin notably reduced the body weights and pattern continued till the end of study duration. It is worth noticing that the control group followed the natural trend as body weights kept growing till the end of study. C. aurantium at its highest dose was able to significantly curtail the weight loss (Table 1). Similarly cisplatin increased the daily urinary output, while C. aurantium at both doses controlled the urinary output (Table 2). It was noticed that kidney weight was incomparably elevated in the animals associated with the cisplatin group when contrasted with normal control. Co-administrating C. aurantium extract at different doses with cisplatin caused slight decrease in kidney weight (Table 3).
Table 1.
Effect of crude extract of C. aurantium on body weight in rats treated with cisplatin.
| Days of observation | ||||
|---|---|---|---|---|
| Groups | 0th day | 7th day | 14th day | 21st day |
| Control | 241 ± 7.5 | 261 ± 8.0 | 328 ± 8.7 | 310 ± 8.2 |
| Cisplatin | 245 ± 8.0 | 187 ± 9.2∗∗∗ | 198 ± 11∗∗∗ | 163 ± 8.4∗∗∗ |
| Cisplatin + C. aurantium (200 mg/kg) | 261 ± 8.0∗∗ | 220 ± 7.9∗∗∗ | 241 ± 8.3∗∗∗ | 229 ± 11∗∗∗ |
| Cisplatin + C. aurantium (400 mg/kg) | 263 ± 8.2∗∗ | 229 ± 5.6∗∗∗ | 282 ± 8.5∗∗∗ | 270 ± 9.3∗∗∗ |
Statistical analysis was done through one-way analysis of variance (ANOVA) trailed by Bonferroni post hoc test for all groups in respective days. The results are considered significant (∗) if p < 0.005. ∗∗p < 0.05, ∗∗∗p < 0.05. Results were compared in a column with the respective control values.
Table 2.
Effect of crude extract of C. aurantium on urine output in rats treated with cisplatin.
| Days of observation | ||||
|---|---|---|---|---|
| Groups | 0th day | 7thday | 14thday | 21stday |
| Control | 7.2 ± 1.8 | 7.0 ± 1.8 | 7.5 ± 1.7 | 6.3 ± 1.1 |
| Cisplatin | 7.0 ± 1.8 | 31 ± 2.2∗∗∗ | 31 ± 3.0∗∗∗ | 33 ± 1.5∗∗∗ |
| Cisplatin + C. aurantium (200 mg/kg) | 8.0 ± 1.7∗∗ | 23 ± 1.8∗∗∗ | 24 ± 8.3∗∗∗ | 22 ± 1.2∗∗∗ |
| Cisplatin + C. aurantium (400 mg/kg) | 7.2 ± 1.6∗∗ | 15 ± 1.4∗∗∗ | 16 ± 1.6∗∗ | 17 ± 0.88∗∗∗ |
Statistical analysis was done through one-way analysis of variance (ANOVA) trailed by Bonferroni post hoc test for all groups in respective days. The results are considered significant (∗) if p < 0.005. ∗∗p < 0.05, ∗∗∗p < 0.05. Results were compared in a column with the respective control values.
Table 3.
Effect of crude extract of C. aurantium on kidney weights.
| Groups | Kidney weight |
|---|---|
| Normal control | 0.41 ± 0.047 |
| Cisplatin | 1.7 ± 0.088∗∗∗ |
| Cisplatin + C. aurantium (200 mg/kg) | 1.1 ± 0.044 |
| Cisplatin + C. aurantium (400 mg/kg) | 0.72 ± 0.042 |
Statistical analysis was done through one-way analysis of variance (ANOVA) trailed by Bonferroni post hoc test for all groups in respective days. The results are considered significant (∗) if p < 0.005. ∗∗∗p < 0.05. Results were compared in a column with the respective control values.
3.2. C. aurantium Normalizes the Plasma and Urinary Sodium and Potassium Levels of Cisplatin-Administered Rats
Cisplatin-induced rats reduced the plasma sodium levels down to 103 mEq/L as compared to the control group which remained at 139 mEq/L. Comparatively, C. aurantium raised the cisplatin-induced suppression of plasma sodium at increasing doses. Plasma potassium also followed similar pattern and cisplatin-inhibited levels were brought to normal by C. aurantium (Table 4). On the contrary, urinary sodium and potassium levels were notably increased, which were expectedly brought down by C. aurantium (Table 5).
Table 4.
Effect of crude extract of C. aurantium on plasma sodium and potassium levels in rats treated with cisplatin.
| Days of observation | |||||
|---|---|---|---|---|---|
| Parameter | Groups | 0th day | 7th day | 14th day | 21st day |
| Plasma sodium (mEq/L) | Control | 139 ± 6.0 | 179 ± 5.9 | 178 ± 4.2 | 139 ± 6.1 |
| Cisplatin | 146 ± 4.9 | 94 ± 8.0∗∗∗ | 106 ± 6.3∗∗∗ | 103 ± 6.6∗∗∗ | |
| Cisplatin + C. aurantium (200 mg/kg) | 144 ± 4.8∗∗ | 123 ± 5.9∗∗∗ | 130 ± 4.9∗∗∗ | 122 ± 7.1∗∗∗ | |
| Cisplatin + C. aurantium (400 mg/kg) | 149 ± 4.2∗∗∗ | 150 ± 5.5∗∗∗ | 155 ± 5.7∗∗∗ | 144 ± 6.4 | |
| Plasma potassium (mEq/L) | Control | 5.9 ± 0.34 | 5.5 ± 0.45 | 6.1 ± 0.33 | 5.9 ± 0.27 |
| Cisplatin | 5.7 ± 0.17 | 1.6 ± 0.20∗∗∗ | 2.2 ± 0.27∗∗∗ | 2.2 ± 0.28∗∗∗ | |
| Cisplatin + C. aurantium (200 mg/kg) | 5.7 ± 0.42 | 3.1 ± 0.20∗∗∗ | 3.4 ± 0.24∗∗∗ | 3.6 ± 0.17∗∗∗ | |
| Cisplatin + C. aurantium (400 mg/kg) | 5.8 ± 0.23 | 4.3 ± 0.19∗∗∗ | 4.7 ± 0.29∗∗∗ | 4.7 ± 0.33∗∗∗ | |
Statistical analysis was done through one-way analysis of variance (ANOVA) trailed by Bonferroni post hoc test for all groups in respective days. The results are considered significant (∗) if p < 0.005. ∗∗p < 0.05, ∗∗∗p < 0.05. Results were compared in a column with the respective control values.
Table 5.
Effect of crude extract of C. aurantium on urinary sodium and potassium levels in rats treated with cisplatin.
| Days of observation | |||||
|---|---|---|---|---|---|
| Parameter | Groups | 0th day | 7th day | 14th day | 21st day |
| Urinary sodium (mEq/24 h) | Control | 172 ± 4.0 | 180 ± 9.2 | 190 ± 7.3 | 170 ± 8.4 |
| Cisplatin | 169 ± 4.5 | 300 ± 11∗∗∗ | 303 ± 11∗∗∗ | 347 ± 17∗∗∗ | |
| Cisplatin + C. aurantium (200 mg/kg) | 172 ± 8.0 | 261 ± 8.0∗∗∗ | 267 ± 7.8∗∗∗ | 296 ± 11∗∗∗ | |
| Cisplatin + C. aurantium (400 mg/kg) | 164 ± 6.2∗∗∗ | 218 ± 8.3∗∗∗ | 228 ± 8.7∗∗∗ | 247 ± 7.8∗∗∗ | |
| Urinary potassium (mEq/24 h) | Control | 3.8 ± 0.35 | 3.0 ± 0.25 | 3.3 ± 0.35 | 3.5 ± 0.19 |
| Cisplatin | 3.4 ± 0.36∗∗∗ | 4.3 ± 0.19∗∗∗ | 4.7 ± 0.29∗∗∗ | 5.1 ± 0.34∗∗∗ | |
| Cisplatin + C. aurantium (200 mg/kg) | 3.5 ± 0.26∗∗∗ | 3.1 ± 0.20 | 3.4 ± 0.24 | 3.6 ± 0.17 | |
| Cisplatin + C. aurantium (400 mg/kg) | 3.3 ± 0.51∗∗∗ | 1.6 ± 0.20∗∗∗ | 2.2 ± 0.27∗∗∗ | 2.8 ± 0.32∗∗∗ | |
Statistical analysis was done through one-way analysis of variance (ANOVA) trailed by Bonferroni post hoc test for all groups in respective days. The results are considered significant (∗) if p < 0.005. ∗∗∗p < 0.05. Results were compared in a column with the respective control values.
3.3. C. aurantium Regulates Plasma and Urinary Creatinine Levels
The cisplatin-induced animal group showed raised plasma creatinine levels as compared to control. However C. aurantium at both of its studied doses remarkably reduced the plasma creatinine levels (Figure 1). On the contrary, cisplatin-induced urinary creatinine was increased by C. aurantium (Figure 1). It may be plausible to comment that increased excretion of creatinine leads to lower plasma levels of creatinine.
Figure 1.
Effects of C. aurantium on plasma (a) and urinary (b) creatinine levels. The values are mean ± SEM (n = 6). Statistical analysis was done through one-way analysis of variance (ANOVA) trailed by Bonferroni post hoc test for all groups in respective days. The results are considered significant (∗) if p < 0.005. ∗p < 0.05 vs. normal control, &p < 0.05 vs. cisplatin, and #p < 0.05 vs. cisplatin + C. aurantium on corresponding days.
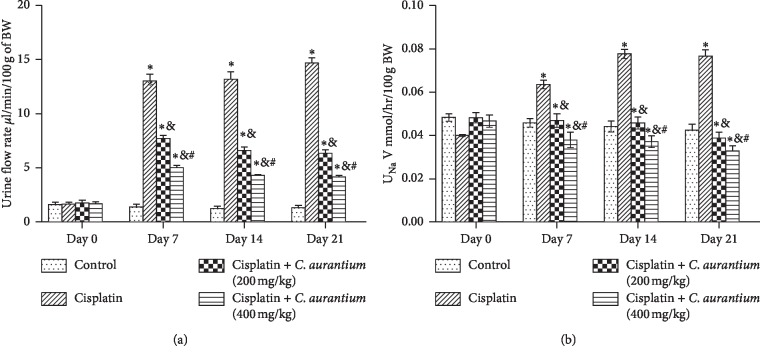
3.4. C. aurantium Reduces Absolute Sodium Excretion (UNaV)
C. aurantium significantly reduces absolute sodium excretion both dose and time dependently. Maximum sodium excretion is recorded at 21st day which C. aurantium was able to reverse at both 200 mg/kg and 400 mg/kg doses (Figure 2(b)). Correspondingly, urinary flow rate was also reduced following a similar pattern. Effects of C. aurantium were most pronounced at 400 mg/kg at 21st day (Figure 2(a)).
Figure 2.
Demonstration of urine flow rate (a); absolute sodium excretion (UNaV) (b). The values are mean ± SEM (n = 6). Statistical analysis was done through one-way analysis of variance (ANOVA) followed by Bonferroni post hoc test for all groups in respective days. The results are considered significant (∗) if p < 0.005. ∗p < 0.05 vs. normal control, &p < 0.05 vs. cisplatin, and #p < 0.05 vs. cisplatin + C. aurantium (400 mg/kg) on respective days.
3.5. Effects of C. aurantium on Absolute Creatinine and Absolute Potassium Clearance
C. aurantium notably restored the cisplatin-induced lower absolute creatinine clearance. The effects at the dose of 400 mg/kg were more pronounced after 21st day as compared to other dose groups, signifying both dose and time dependent effects (Table 6). Similarly, absolute potassium excretion increased by cisplatin, which was brought to almost normal levels by C. aurantium at 400 mg/kg (Table 7).
Table 6.
Effect of crude extract of C. aurantium on absolute creatinine clearance in rats treated with cisplatin.
| Days of observation | |||||
|---|---|---|---|---|---|
| Parameter | Groups | 0th day | 7th day | 14th day | 21st day |
| Absolute creatinine clearance | Control | 0.27 ± 0.024 | 0.42 ± 0.016# | 0.40 ± 0.011 | 0.86 ± 0.024 |
| Cisplatin | 0.26 ± 0.036∗ | 0.33 ± 0.019∗∗∗ | 0.31 ± 0.014∗∗∗ | 0.40 ± 0.028∗∗ | |
| Cisplatin + C. aurantium (200 mg/kg) | 0.26 ± 0.028∗∗ | 0.49 ± 0.015∗∗∗ | 0.53 ± 0.037∗∗∗ | 0.66 ± 0.054 | |
| Cisplatin + C. aurantium (400 mg/kg) | 0.29 ± 0.037∗∗∗ | 0.61 ± 0.030∗∗∗ | 0.73 ± 0.031∗∗∗ | 1.2 ± 0.096∗ | |
Statistical analysis was done through one-way analysis of variance (ANOVA) trailed by Bonferroni post hoc test for all groups in respective days. The results are considered significant (∗) if p < 0.005. ∗p < 0.05, ∗∗p < 0.05, ∗∗∗ indicates p < 0.05. Results were compared in a column with the respective control values.
Table 7.
Effect of crude extract of C. aurantium on absolute potassium excretion in rats treated with cisplatin
| Days of observation | |||||
|---|---|---|---|---|---|
| Parameter | Groups | 0 day | 7th day | 14th day | 21st day |
| Absolute excretion of potassium | Control | 0.0062 ± 0.00070 | 0.0039 ± 0.00087 | 0.0029 ± 0.00027 | 0.0024 ± 0.00048 |
| Cisplatin | 0.0055 ± 0.00089∗∗∗ | 0.0057 ± 0.00042∗∗∗ | 0.0061 ± 0.00037 | 0.0067 ± 0.00092∗∗∗ | |
| Cisplatin + C. aurantium (200 mg/kg) | 0.0055 ± 0.0096∗∗∗ | 0.0043 ± 0.00069∗∗∗ | 0.0041 ± 0.00063 | 0.042 ± 0.00055∗∗∗ | |
| Cisplatin + C. aurantium (400 mg/kg) | 0.0057 ± 0.0071∗∗∗ | 0.0023 ± 0.00045∗∗∗ | 0.0018 ± 0.00051 | 0.034 ± 0.00057∗∗∗ | |
Statistical analysis was done through one-way analysis of variance (ANOVA) trailed by Bonferroni post hoc test for all groups in respective days. The results are considered significant (∗) if p < 0.005. ∗∗∗p < 0.05. Results were compared in a column with the respective control values.
3.6. Effects of C. aurantium on Urinary Na2+/K+ Ratio and Fractional Excretion of Sodium and Potassium
The urinary Na2+/K+ ratio was most notably enhanced by cisplatin as compared to control and treatment groups. C. aurantium at 200 mg/kg and 400 mg/kg both dose and time dependently reversed the effects of cisplatin. After 21st day of treatment, the Na/K ratio was determined at 20 for cisplatin group as compared to 6 for C. aurantium at 400 mg/kg (Table 8). Similarly, fractional excretion of sodium and potassium was also increased by cisplatin to 26 and 0.69, respectively. C. aurantium brought fractional excretion of sodium and potassium back to 13 and 0.35, respectively (Table 9).
Table 8.
Effect of crude extract of C. aurantium on urinary sodium to potassium ratio in rats treated with cisplatin.
| Days of observation | |||||
|---|---|---|---|---|---|
| Parameter | Groups | 0th day | 7th day | 14th day | 21st day |
| Urinary Na2+/K+ ratio | Control | 4.7 ± 0.56 | 3.4 ± 0.48 | 3.3 ± 0.45 | 3.7 ± 0.44∗∗∗ |
| Cisplatin | 5.1 ± 0.47∗ | 16 ± 1.3∗∗∗ | 19 ± 0.83∗∗∗ | 20 ± 3.7∗∗∗ | |
| Cisplatin + C. aurantium (200 mg/kg) | 6.0 ± 0.68∗∗∗ | 11 ± 1.4∗∗∗ | 8.3 ± 0.67∗∗∗ | 10 ± 1.2∗∗∗ | |
| Cisplatin + C. aurantium (400 mg/kg) | 4.7 ± 0.42 | 5.7 ± 0.61∗∗∗ | 4.6 ± 0.43∗∗∗ | 6 ± 0.81∗∗∗ | |
Statistical analysis was done through one-way analysis of variance (ANOVA) trailed by Bonferroni post hoc test for all groups in respective days. The results are considered significant (∗) if p < 0.005. ∗p < 0.05, ∗∗∗p < 0.05. Results were compared in a column with the respective control values.
Table 9.
Effect of crude extract of C. aurantium on fractional excretion of potassium and sodium in rats treated with cisplatin.
| Days of observation | |||||
|---|---|---|---|---|---|
| Parameter | Groups | 0th day | 7th day | 14th day | 21st day |
| Fractional excretion of potassium | Control | 3.8 ± 0.48 | 5.6 ± 1.4 | 10 ± 2.3 | 9.2 ± 1.6 |
| Cisplatin | 3.8 ± 0.29 | 17 ± 3.2∗∗∗ | 24 ± 3.5∗∗∗ | 26 ± 2.8∗∗∗ | |
| Cisplatin + C. aurantium (200 mg/kg) | 3.6 ± 0.23∗∗∗ | 12 ± 2.0∗∗ | 19 ± 2.6∗∗∗ | 19 ± 2.1∗∗∗ | |
| Cisplatin + C. aurantium (400 mg/kg) | 3.7 ± 0.48∗∗∗ | 8.3 ± 1.8∗∗∗ | 14 ± 2.3∗∗∗ | 13 ± 1.6∗∗∗ | |
| Fractional excretion of sodium | Control | 0.28 ± 0.036 | 0.30 ± 0.06 | 0.33 ± 0.09 | 0.30 ± 0.05 |
| Cisplatin | 0.26 ± 0.044 | 0.77 ± 0.25∗∗∗ | 0.61 ± 00.16∗∗∗ | 0.69 ± 0.09∗∗∗ | |
| Cisplatin + C. aurantium (200 mg/kg) | 0.27 ± 0.07∗∗ | 0.62 ± 0.22∗∗∗ | 0.49 ± 0.11∗∗∗ | 0.53 ± 0.06∗∗∗ | |
| Cisplatin C. aurantium (400 mg/kg) | 0.32 ± 0.14∗∗ | 0.43 ± 0.12∗∗∗ | 0.03 ± 0.009∗∗∗ | 0.35 ± 0.05∗∗∗ | |
Statistical analysis was done through one-way analysis of variance (ANOVA) trailed by Bonferroni post hoc test for all groups in respective days. The results are considered significant (∗) if p < 0.005. ∗∗p < 0.05, ∗∗∗p < 0.05. Results were compared in a column with the respective control values.
3.7. Histopathological Analysis of Kidney
To observe impact of disparate doses of C. aurantium, a kidney from every animal was anatomized out for histopathological investigation. The control group showed the intact bowman's capsule, proximal and distal convoluted tubule. No capillary congestion, hemorrhage, and interstitial damage are seen in this group. The cisplatin group indicated extreme tubular and glomerular degeneration alongside putrefaction when contrasted to the control group. While the treatment groups at different doses (200 mg/kg and 400 mg/kg) of CA extract demonstrated checked capacity to keep the cisplatin prompted epithelial damage. 400 mg/kg group showed many glomeruli that are intact with bowman's capsule, and less interstitial damage was seen in proximal and distal convoluted tubules (Figure 3).
Figure 3.
Histopathological sections of the rat kidney. Control (a); cisplatin (b); cisplatin + C. aurantium + 200 mg/kg (c); cisplatin + C. aurantium + 400 mg/kg (d).
4. Discussion
The renal system is a routine victim of xenobiotic due to its ability to remove concentrated toxins. The load on the kidney that leads to serious complications is increased by the development of nephrotoxicity [30]. Cisplatin is a widely used inorganic platinum-based potent chemotherapeutic drug which is a great success in the war of cancer [3]. Cisplatin is now being used in the treatment of solid organ malignancies that include the lung, ovarian, testicular, bladder, colorectal, and head and neck cancers [31]. Nephrotoxicity was reported in the earlier clinical trials undergoing cisplatin chemotherapy [32]. Nephrotoxicity is initiated by changes in renal hemodynamics followed by acute, mainly proximal tubular impairment. After 72 hours of treatment with cisplatin, distal and proximal tubular reabsorptive capacities are compromised. Five-day clinical treatment with 20 mg cisplatin/m2 per day caused notable reduction in decrease in 51Cr-EDTA clearance. At higher doses of 40 mg/m2 day, GFR is severely compromised which remained blunted for up to 2 years even after termination of cisplatin [32]. High-dose administration of cisplatin has showed tendency to cause proteinuria [33]. Proteinuria chiefly belonging to tubular and glomerular origin and tend to occur between treatment cycles. The processes by which cisplatin causes nephrotoxicity are complex and are mediated by various cellular processes that includes electrolyte imbalance and wasting [34], abnormal creatinine clearance [35], oxidative stress [36], apoptosis [37], and inflammation [38].
The medical history has proved that indigenous plants are natural barriers against ailments and serves as a healing reservoir. C. aurantium is a natural herb indigenous to Southeast Asia, Bahamas, United States, and Spain. It is consumed as a fruit and has been linked with treatment of various diseases as described in Introduction. The study aims to evaluate the pharmacological potential of C. aurantium in cisplatin-induced nephrotoxicity.
Nephrotoxicity was observed by several renal function parameters which was also a contributing factor in mortality rate. Weight loss was observed due to gastrointestinal toxicity and by reduced ingestion of food. Progressive weight loss of animals in the cisplatin-treated group has been closely linked to poor feed, increased catabolism, physiological imbalance, or mental stress [39]. As per previous results, our study also showed weight loss in cisplatin-induced group, which was brought back by C. aurantium both dose and time dependently. It is suspected that gastrointestinal damage and increased ingestion of food are responsible for C. aurantium antianorexic effects. Similarly, the increase in kidney weight was observed in groups treated with cisplatin correlated with edema or inflammation due to cisplatin-induced tubular necrosis [40]. The weight reduction of the kidney was observed significantly in animals treated with C. aurantium which might be due to its anti-inflammatory action [41]. Furthermore, it was observed that the urine volume is increased in animals treated with cisplatin which is related with cisplatin-induced nonoligouric acute renal failure. Cisplatin induces increase in urine output due to reduction in the gene expression of aquaporins and their density in the proximal tubule. The decrease in urine output was observed in groups that received C. aurantium as it was also reported previously that increase in urine volume was seen in rats having nephrotoxicity caused by cisplatin [42].
CIN causes debilitation of cell membrane pumps such as sodium potassium pumps which results in reduced reabsorption of sodium eventually raising its concentration in urine [43]. The transport system abnormality may result in hypernatremia and hyperkalemia. In this study, hypernatriuria and hyperkaliuria were presented in cisplatin-treated groups. Administration of C. aurantium along with cisplatin causes reduction in levels of sodium and potassium near to normal values as compared to the intoxicated group. Similarly, there was a decrease in sodium and potassium levels in plasma in groups treated with cisplatin as compared to the control group, while C. aurantium dose groups brought plasma, sodium, and potassium levels near to normal values.
The study additionally showed the increase in plasma creatinine level and lowered creatinine clearance in cisplatin-treated groups as compared to the control group. This outcome evidently shows the reduction in ability of kidney to filter waste products or to conserve cations abundantly. The alteration in electrolytes and decrease in activity of Na–K ATPase are associated with cisplatin nephrotoxicity which are demonstrated by hyponatremia and hypokalaemia [44]. Expectedly C. aurantium reversed the cisplatin-induced changes in creatinine level and creatinine clearance. Our results corresponds with the earlier findings showing lower creatinine clearance and higher creatinine levels in plasma when induced with cisplatin [45].
It was also observed that renal markers such as absolute excretion of sodium and potassium, fractional excretion of sodium cisplatin and potassium, urine to sodium potassium ratio, and absolute creatinine clearance values showed notable disturbance in cisplatin-induced group. C. aurantium reversed the values to near normalcy as it is evident in results section. Our results are in line with the previous findings that showed the disturbances in abovementioned parameters [46, 47].
Nephrotoxicity in experimental animals can be affirmed by assessing pathological symptoms such as, for example, tubular degeneration, putrefaction, intertubular drain, desquamation, presence of hyaline casts in tubules, and blockage and swelling in glomerulus. In our study, treatment with cisplatin caused extreme tubular and glomerular degeneration alongside putrefaction when contrasted to the control group. These alterations were reduced in groups that are treated with C. aurantium, thus showing its protective effect. It was observed that improvement of cisplatin-incited renal damage was more notable in rats treated with 400 mg/kg of C. aurantium. This study leaves a scientific gap for the isolation of C. aurantium components and its further determination of mechanism in CIN.
5. Conclusion
It may be concluded that C. aurantium has the ability to counter the nephrotoxic potential of cisplatin at the experimental dose of 5 mg/kg i.p. We recommend further studies to ascertain the exact mechanism of action as well as to identify the potential active constituent exhibiting the nephroprotective potential.
Acknowledgments
The study was supported by the Department of Pharmacy, the Islamia University of Bahawalpur.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Rui Wang and Waseem Hassan designed the study. Omer Iqbal performed the experiments and compiled the data. Hammad Ahmed analyzed the data. Faiz ud Din Ahmad and Qaiser Jabeen wrote the manuscript. Rui Wang and Waseem Hassan coordinated the project preparation of the final version of the manuscript.
References
- 1.Crona D. J., Faso A., Nishijima T. F., McGraw K. A., Galsky M. D., Milowsky M. I. A systematic review of strategies to prevent cisplatin-induced nephrotoxicity. The Oncologist. 2017;22(5):609–619. doi: 10.1634/theoncologist.2016-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go R. S., Adjei A. A. Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. Journal of Clinical Oncology. 1999;17(1):p. 409. doi: 10.1200/jco.1999.17.1.409. [DOI] [PubMed] [Google Scholar]
- 3.Dasari S., Bernard Tchounwou P. Cisplatin in cancer therapy: molecular mechanisms of action. European Journal of Pharmacology. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shahid F., Farooqui Z., Khan F. Cisplatin-induced gastrointestinal toxicity: an update on possible mechanisms and on available gastroprotective strategies. European Journal of Pharmacology. 2018;827:49–57. doi: 10.1016/j.ejphar.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Gómez-Sierra T., Eugenio-Pérez D., Sánchez-Chinchillas A., Pedraza-Chaverri J. Role of food-derived antioxidants against cisplatin induced-nephrotoxicity. Food and Chemical Toxicology. 2018;120:230–242. doi: 10.1016/j.fct.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Gispen W. H., Hamers F. P. T., Vecht C. J., Jennekens F. G. I., Neyt J. P. ACTH/MSH like peptides in the treatment of cisplatin neuropathy. The Journal of Steroid Biochemistry and Molecular Biology. 1992;43(1–3):179–183. doi: 10.1016/0960-0760(92)90205-w. [DOI] [PubMed] [Google Scholar]
- 7.Omar H. A., Mohamed W. R., Arab H. H., Arafa E.-S. A. Tangeretin alleviates cisplatin-induced acute hepatic injury in rats: targeting MAPKs and apoptosis. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0151649.e0151649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohno S., Strebel F., Stephens L., et al. Haematological toxicity of carboplatin and cisplatin combined with whole body hyperthermia in rats. British Journal of Cancer. 1993;68(3):469–474. doi: 10.1038/bjc.1993.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sastry J., Kellie S. J. Severe neurotoxicity, ototoxicity and nephrotoxicity following high-dose cisplatin and amifostine. Pediatric Hematology and Oncology. 2005;22(5):441–445. doi: 10.1080/08880010590964381. [DOI] [PubMed] [Google Scholar]
- 10.Boulikas T. Poly (ADP-ribose) synthesis in blocked and damaged cells and its relation to carcinogenesis. Anticancer Research. 1992;12(3):885–898. [PubMed] [Google Scholar]
- 11.Madias N. E., Harrington J. T. Platinum nephrotoxicity. The American Journal of Medicine. 1978;65(2):307–314. doi: 10.1016/0002-9343(78)90825-2. [DOI] [PubMed] [Google Scholar]
- 12.Lippman A. J., Helson C, Helson L, Krakoff I. H. Clinical trials of cis-diamminedichloroplatinum (NSC-119875) Cancer Chemotherapy Reports. 1973;57(2):191–200. [PubMed] [Google Scholar]
- 13.Higby D. J., Wallace H. J., Jr., Holland J. F. Cis-diamminedichloroplatinum (NSC-119875): a phase I study. Cancer Chemotherapy Reports. 1973;57(4):459–463. [PubMed] [Google Scholar]
- 14.Ahmad F. U. D., Sattar M. A., Rathore H. A., et al. Exogenous hydrogen sulfide (H2S) reduces blood pressure and prevents the progression of diabetic nephropathy in spontaneously hypertensive rats. Renal Failure. 2012;34(2):203–210. doi: 10.3109/0886022x.2011.643365. [DOI] [PubMed] [Google Scholar]
- 15.Yao X., Panichpisal K., Kurtzman N., Nugent K. Cisplatin nephrotoxicity: a review. The American Journal of the Medical Sciences. 2007;334(2):115–124. doi: 10.1097/maj.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- 16.Hayes D. M., Cvitkovic E., Golbey R. B., Scheiner E., Helson L., Krakoff I. H. High dose cis-platinum diammine dichloride: amelioration of renal toxicity by mannitol diuresis. Cancer. 1977;39(4):1372–1381. doi: 10.1002/1097-0142(197704)39:4<1372::aid-cncr2820390404>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 17.Frick G. A., Ballentine R, Driever C. W, Kramer W. G. Renal excretion kinetics of high-dose cis-dichlorodiammineplatinum (II) administered with hydration and mannitol diuresis. Cancer Treatment Reports. 1979;63(1):13–16. [PubMed] [Google Scholar]
- 18.Hayati F., Hossainzadeh M, Shayanpour S, Abedi-Gheshlaghi Z, Beladi Mousavi S. S. Prevention of cisplatin nephrotoxicity. Journal of Nephropharmacology. 2016;5(1):57–60. [PMC free article] [PubMed] [Google Scholar]
- 19.Blachley J. D., Hill J. B. Renal and electrolyte disturbances associated with cisplatin. Annals of Internal Medicine. 1981;95(5):628–632. doi: 10.7326/0003-4819-95-5-628. [DOI] [PubMed] [Google Scholar]
- 20.Nematbakhsh M., Nasri H. The effects of vitamin E and selenium on cisplatin-induced nephrotoxicity in cancer patients treated with cisplatin-based chemotherapy: a randomized, placebo-controlled study. Journal of Research in Medical Sciences. 2013;18(7):626–627. [PMC free article] [PubMed] [Google Scholar]
- 21.Santos N. A., Bezerra C. S, Martins N. M, Curti C, Bianchi M. L, Santos A. C. Hydroxyl radical scavenger ameliorates cisplatin-induced nephrotoxicity by preventing oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Cancer Chemotherapy and Pharmacology. 2008;61(1):145–155. doi: 10.1007/s00280-007-0459-y. [DOI] [PubMed] [Google Scholar]
- 22.Sheu S.-S., Nauduri D., Anders M. W. Targeting antioxidants to mitochondria: a new therapeutic direction. Biochimica et Biophysica Acta (BBA)—Molecular Basis of Disease. 2006;1762(2):256–265. doi: 10.1016/j.bbadis.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Chen R., Qi Q.-L., Wang M.-T., Li Q.-Y. Therapeutic potential of naringin: an overview. Pharmaceutical Biology. 2016;54(12):3203–3210. doi: 10.1080/13880209.2016.1216131. [DOI] [PubMed] [Google Scholar]
- 24.Wu Q., Li R., Soromou L. W., et al. p-Synephrine suppresses lipopolysaccharide-induced acute lung injury by inhibition of the NF-κB signaling pathway. Inflammation Research. 2014;63(6):429–439. doi: 10.1007/s00011-014-0715-7. [DOI] [PubMed] [Google Scholar]
- 25.Pimenta F. C. F., Alves M. F., Pimenta M. B. F., et al. Anxiolytic effect of Citrus aurantium L. On patients with chronic myeloid leukemia. Phytotherapy Research. 2016;30(4):613–617. doi: 10.1002/ptr.5566. [DOI] [PubMed] [Google Scholar]
- 26.Prouillet C., Mazière J.-C., Mazière C., Wattel A., Brazier M., Kamel S. X. D. Stimulatory effect of naturally occurring flavonols quercetin and kaempferol on alkaline phosphatase activity in MG-63 human osteoblasts through ERK and estrogen receptor pathway. Biochemical Pharmacology. 2004;67(7):1307–1313. doi: 10.1016/j.bcp.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka Y., Kirita M., Miyata S., et al. O-methylated theaflavins suppress the intracellular accumulation of triglycerides from terminally differentiated human visceral adipocytes. Journal of Agricultural and Food Chemistry. 2013;61(51):12634–12639. doi: 10.1021/jf404446h. [DOI] [PubMed] [Google Scholar]
- 28.Tenore G. C., Manfra M., Stiuso P., et al. Polyphenolic pattern and in vitro cardioprotective properties of typical red wines from vineyards cultivated in Scafati (Salerno, Italy) Food Chemistry. 2013;140(4):803–809. doi: 10.1016/j.foodchem.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 29.Cirmi S., et al. Anticancer potential of citrus juices and their extracts: a systematic review of both preclinical and clinical studies. Frontiers in Pharmacology. 2017;8:p. 420. doi: 10.3389/fphar.2017.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pazhayattil G. S., Shirali A. C. Drug-induced impairment of renal function. International Journal of Nephrology and Renovascular Disease. 2014;7:457–468. doi: 10.2147/IJNRD.S39747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X., Wu Y., Dong H., Zhang C., Zhang Y. Platinum-based agents for individualized cancer treatment. Current Molecular Medicine. 2013;13(10):1603–1612. doi: 10.2174/1566524013666131111125515. [DOI] [PubMed] [Google Scholar]
- 32.Daugaard G. Cisplatin nephrotoxicity: experimental and clinical studies. Danish Medical Bulletin. 1990;37(1):1–12. [PubMed] [Google Scholar]
- 33.Broomhead J. A., Fairlie D. P., Whitehouse M. W. cis-Platinum (II) amine complexes: some structure-activity relationships for immunosuppressive, nephrotoxic and gastrointestinal (side) effects in rats. Chemico-Biological Interactions. 1980;31(1):113–132. doi: 10.1016/0009-2797(80)90144-1. [DOI] [PubMed] [Google Scholar]
- 34.Lee Y. K., Shin D. M. Renal salt wasting in patients treated with high-dose cisplatin, etoposide, and mitomycin in patients with advanced non-small cell lung cancer. The Korean Journal of Internal Medicine. 1992;7(2):118–122. doi: 10.3904/kjim.1992.7.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montoya J., et al. Renal function of cancer patients “fit” for Cisplatin chemotherapy: physician perspective. Gulf Journal of Oncology. 2014;1(16):64–72. [PubMed] [Google Scholar]
- 36.Pratibha R., Sameer R., Rataboli P. V., Bhiwgade D. A., Dhume C. Y. Enzymatic studies of cisplatin induced oxidative stress in hepatic tissue of rats. European Journal of Pharmacology. 2006;532(3):290–293. doi: 10.1016/j.ejphar.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Henkels K. M., Turchi J. J. Cisplatin-induced apoptosis proceeds by caspase-3-dependent and -independent pathways in cisplatin-resistant and -sensitive human ovarian cancer cell lines. Cancer Research. 1999;59(13):3077–3083. [PubMed] [Google Scholar]
- 38.Humanes B., Camaño S., Lara J. M., et al. Cisplatin-induced renal inflammation is ameliorated by cilastatin nephroprotection. Nephrology Dialysis Transplantation. 2017;32(10):1645–1655. doi: 10.1093/ndt/gfx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alhadeff A. L., Holland R. A., Zheng H., Rinaman L., Grill H. J., De Jonghe B. C. Excitatory hindbrain-forebrain communication is required for cisplatin-induced anorexia and weight loss. The Journal of Neuroscience. 2017;37(2):362–370. doi: 10.1523/jneurosci.2714-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Y., Ma H., Shao J., et al. A role for tubular necroptosis in cisplatin-induced AKI. Journal of the American Society of Nephrology. 2015;26(11):2647–2658. doi: 10.1681/asn.2014080741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen C.-Y., Jiang J.-G., Huang C.-L., Zhu W., Zheng C.-Y. Polyphenols from blossoms of Citrus aurantium L. var. amaraEngl. Show significant anti-complement and anti-inflammatory effects. Journal of Agricultural and Food Chemistry. 2017;65(41):9061–9068. doi: 10.1021/acs.jafc.7b03759. [DOI] [PubMed] [Google Scholar]
- 42.Hosseinian S., et al. The protective effect of Nigella sativa against cisplatin-induced nephrotoxicity in rats. Avicenna Journal of Phytomedicine. 2016;6(1):44–54. [PMC free article] [PubMed] [Google Scholar]
- 43.Stakisaitis D., Dudeniene G, Jankūnas R. J, Grazeliene G, Didziapetriene J, Pundziene B. Cisplatin increases urinary sodium excretion in rats: gender-related differences. Medicina. 2010;46(1):45–50. [PubMed] [Google Scholar]
- 44.Kubala M., Geleticova J., Huliciak M., Zatloukalova M., Vacek J., Sebela M. Na+/K+-ATPase inhibition by cisplatin and consequences for cisplatin nephrotoxicity. Biomedical Papers. 2014;158(2):194–200. doi: 10.5507/bp.2014.018. [DOI] [PubMed] [Google Scholar]
- 45.Yajima A., et al. Construction of a model for predicting creatinine clearance in Japanese patients treated with cisplatin therapy. Anticancer Research. 2015;35(5):2909–2914. [PubMed] [Google Scholar]
- 46.Goulding N. E., Johns E. J. Neural regulation of the kidney function in rats with cisplatin induced renal failure. Frontiers in Physiology. 2015;6:p. 192. doi: 10.3389/fphys.2015.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martinez F., Deray G., Dubois M., et al. Comparative nephrotoxicity of carboplatin and cisplatin in euvolemic and dehydrated rats. Anticancer Drugs. 1993;4(1):85–90. doi: 10.1097/00001813-199302000-00011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.