Abstract
Purpose:
AIDs may disproportionately impact specific racial groups, but autoimmune (AID) prevalence information by minority racial group is sparse for many AIDs. The objective of this analysis was to supplement previously published AID prevalence rates by providing information on race rate ratios (minority race populations compared to Caucasian populations) in the United States. Preliminary to estimating race rate ratios, contemporary US-specific, health care utilization-based AID prevalence rates and female-to-male ratios were estimated and compared to previously published AID prevalence rates.
Methods:
We used a large national electronic medical record database of 52 million individuals to estimate age-adjusted direct standardized rates for 22 AIDs for 2010 through 2016 by gender, race, and US census division. These were compared to previously published estimates.
Results:
Female-to-male ratios were comparable with published studies. Almost all observed Multiracial AID rates were significantly higher than Caucasian rates, as well as 9 of 22 AID rates observed among Native Americans and 8 of 22 AID rates estimated among African-American patients. Regional variation was noted: highest African-American systemic lupus erythematosus rates were observed in the West North Central and South Atlantic divisions, highest African-American multiple sclerosis rates in the South Atlantic and Pacific divisions, and highest Native American rheumatoid arthritis rates in the West North Central, Mountain, and Pacific divisions.
Conclusions:
Substantial AID heterogeneity exists by race and by geographic area. An important research area is further exploring factors related to heterogeneity such as potential interactions between genetic susceptibility and environmental factors.
Keywords: autoimmune disease, prevalence, North American, minority groups
1. Introduction
Autoimmune disease (AID) is a classification of pathological and molecular features encompassing medical conditions that are complex, chronic, and occasionally life-threatening. Conditions having autoimmune origins have increased over the years, and more than 80 distinct AIDs currently exist [1]. A heterogeneous group of diseases, AIDs affect a range of organ systems, with some AIDs affecting a single organ and others affecting multiple systems. A majority of AIDs have a clear adaptive immune response to an autoantigen, but not all have a well-established autoimmune etiology [2, 3]. Factors associated with higher risk of AIDs are genetic features, lifestyle characteristics (e.g., nutrition and smoking) and environmental exposures that include bacteria, viruses, and toxic levels of drug and metal exposures [4]. Additionally, multifaceted interactions between environmentally induced cellular and molecular processes and genetic susceptibility may play a role [5].
Overall, AID incidence worldwide is increasing, particularly in industrial countries like the United States (US) [6]. Collectively, AIDs are estimated to impact from 5 to 8% of Americans or 15 to 20 million individuals, depending on the number of AIDs included in estimates [2, 4]. Because many AIDs are diagnosed in children and young adults, and are chronic lifetime conditions, AIDs pose a major economic and societal burden. Pharmaceutical therapy can be a substantive portion of direct medical care cost, and the National Institutes of Health has estimated annual direct health care costs for AIDs to be in the range of $100 billion [7].
AID burden can disproportionately fall to women as AIDs are more prevalent among females [8, 9]. AIDs may also disproportionately impact specific racial groups, but sparse information exists on minority group rates in the US. While the Centers for Disease Control and Prevention (CDC) has reported that for systemic lupus erythematosus (SLE), “minority and ethnic groups, including blacks/African Americans, Hispanics/Latinos, Asians, and American Indians/Alaska Natives, are affected more than whites” [10], minimal epidemiological data has been published concerning other complex autoimmune conditions.
Obtaining reliable estimates of AID prevalence is complicated by not only the difficulty in obtaining a definitive diagnosis for many AIDs, but also by the need for large study populations given the low prevalence of many AIDs. National surveys exist, for example those by the CDC [11], to collect information about more common AIDs like rheumatoid arthritis (RA), but most rely on respondents to accurately self-report morbidities.
In 2012 Hayter and Cook provided an updated assessment regarding the prevalence, manifestations, and pathogenesis of 81 AIDS based on a review of US and worldwide clinical and epidemiological studies published 2010 and earlier [2]. Included were estimates for females as a percentage of those with an AID. Heterogeneity in rates between racial groups was not discussed in Hayter and Cook’s review [2], and in general disparities for minority groups have been understudied in regard to AIDs. The objective of our analysis was to supplement the prevalence information provided by Hayter and Cook by providing information on prevalence rates by race within the US. Using a large electronic healthcare database of patients receiving care at facilities across the US for the years 2010 to 2016, we estimated AID rate ratios by minority race group compared to Caucasian race within the US, both overall and by geographic area (census division). Preliminary to our main objective, we generated contemporary US-specific, health care utilization-based prevalence rates for AIDs and female-to-male ratios and compared those rates and ratios to the Hayter and Cook estimates.
2. Methods
We utilized Cerner Health Facts® (HF) electronic medical record (EMR) data (Cerner HealthFacts®, Cerner Corp., Kansas City, MO), which contains de-identified EMR data for approximately 16 million unique patients annually from over 600 hospitals and clinics in the US that use the Cerner medical record system and for which a data use agreement exists. Encounters may include pharmacy, clinical and microbiology laboratory, admission, and billing information from similarly accredited but affiliated patient care locations. HF has Health Insurance Portability and Accountability Act (HIPAA) compliant operating policies establishing de-identification of all data.
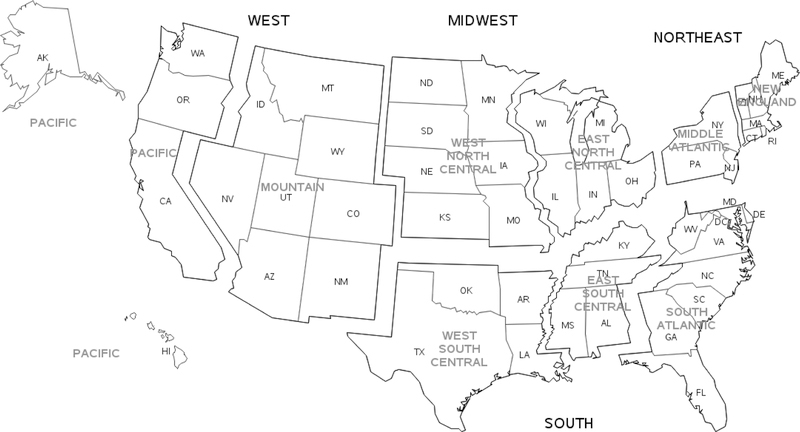
Observed AID rates were estimated over a 7-year time period from January 2010 through December 2016. Unique individuals were characterized by sex (female, male), age category (0–17, 18–44, 45–54, 55–64, 65–74, 75 and older; using an individual’s oldest age during the study period), and race (African American, Asian, Caucasian, Native American, Pacific Islander, Other [e.g., Mid-Eastern Indian], and Multiracial [more than one race reported]). Census region/division in which care was provided was also tracked (see Figure 1). To provide perspective, the distribution of races within the HF study population with known race information was compared to the US 2010 census population.
Figure 1.
US Census Regions and Divisions
Depicted on the map of the United States (US) are the four US census regions (Northeast, South, Midwest, West) and nine US census divisions.
Of Hayter and Cook’s 81 AIDs [2], 22 were included in this study with disease specific diagnosis code(s) that could be identified across the 7-year period (see Table A.1). To avoid individuals with a suspected, or “working diagnosis”, individuals with AIDs were identified based on two AID diagnosis events [12]. No other diagnostic information was required for inclusion in the analyses.
Observed rates overall, by sex, by race, and by race within each US census division were estimated per 100,000 individuals. Crude and standardized age-adjusted rates using the direct method (2000 US census), standardized age-adjusted rate ratios for females-to-males and minority racial groups vs Caucasians, and 95% confidence intervals (95% CI) were calculated using SAS procedure STDRATE [13].
This study was considered exempt by the University’s Institutional Review Board. Data analyses were conducted using SAS (Cary, NC version 9.4) statistical software.
3. Results
Over the 7-year period, among 52,246,831 unique individuals, 98.5% (n=51,463,648) had age information, 93.0% (n=48,609,646) had sex and age information, and 75.6% (n=39,516,604) had race and age information. Individuals identified as Asian or Pacific Islander were combined into one category (Asian/Pacific Islander) due to small numbers for many census divisions. The overall race distribution of the HF study population compared favorably with the US 2010 census population (Table 1).
Table 1.
Race Distribution for Health Facts Database, United States, 2010–2016; US Census Population, 2010
| Race | Health Facts Database 2010–2016 (% of population with known race) | US Census, 2010 (% of population) |
|---|---|---|
| Caucasian | 71.2 | 72.4 |
| African American | 16.0 | 12.6 |
| Asian or Pacific Islander | 2.9 | 5.0 |
| Native American | 1.0 | 1.0 |
| Multiracial | 0.7 | 2.9 |
| Other | 8.2 | 6.2 |
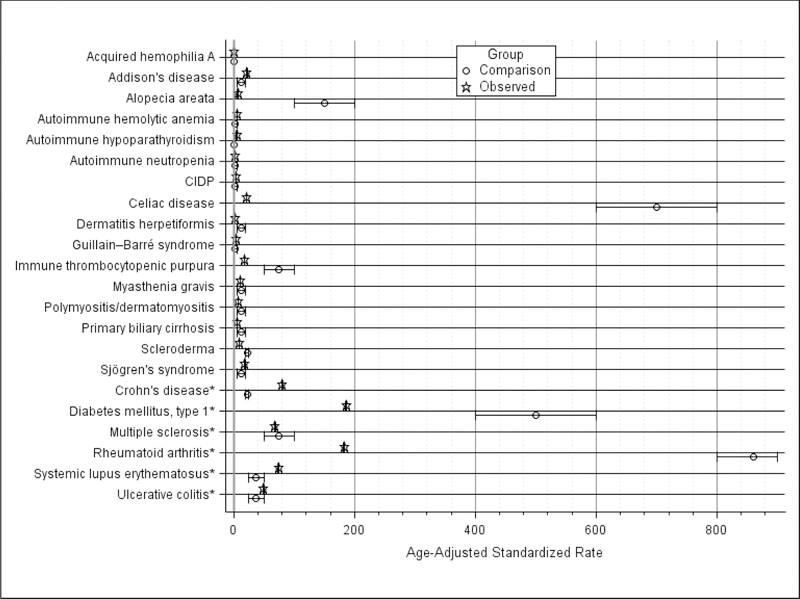
Figure 2 summarizes observed HF AID rates and comparison rates from Hayter and Cook [2]. Among observed rates, 14 of 22 were <20 per 100,000: acquired hemophilia A, alopecia areata, autoimmune hemolytic anemia, autoimmune hypoparathyroidism, autoimmune neutropenia, chronic inflammatory demyelinating polyneuropathy (CIDP), dermatitis herpetiformis, Guillain-Barre syndrome, immune thrombocytopenic purpura, myasthenia gravis, polymyositis/dermatomyositis, primary biliary cirrhosis, scleroderma, and Sjögren’s syndrome. For these 14 AIDs, 11 (78.6%) also had comparison rates <20 per 100,000, while the scleroderma comparison rate was slightly higher, and alopecia areata and immune thrombocytopenic purpura comparison rates were substantially higher. The observed rate for Addison’s disease (95% CI: 21.3, 22.0) was close to the comparison rate range of 5 to 19. The celiac disease observed rate (95% CI: 20.3, 21.1) was substantially lower than the comparison rate range of 600 to 800 per 100,000.
Figure 2.
Standardized AID Rates, United States, 2010–2016
AID, autoimmune disorders; CIDP, chronic inflammatory demyelinating polyneuropathy
* AID with observed rate of 50 per 100,000 or higher
Depicted are observed rates and 95% confidence intervals estimated from the Health Facts database population for the years 2010–2016, and the ranges for the published comparison rates.[2] All rates are per 100,000. Observed rates are age-adjusted to the 2000 US Census population.
Six AIDs had observed rates of ≥50 per 100,000 (grouped at the bottom of Figure 2). Of these, two observed rates overlapped with comparison rates: multiple sclerosis (MS) and ulcerative colitis. Two observed rates were higher than comparison rates: SLE and Crohn’s disease, while 2 observed rates were much lower: diabetes mellitus type 1 (DM1) and RA.
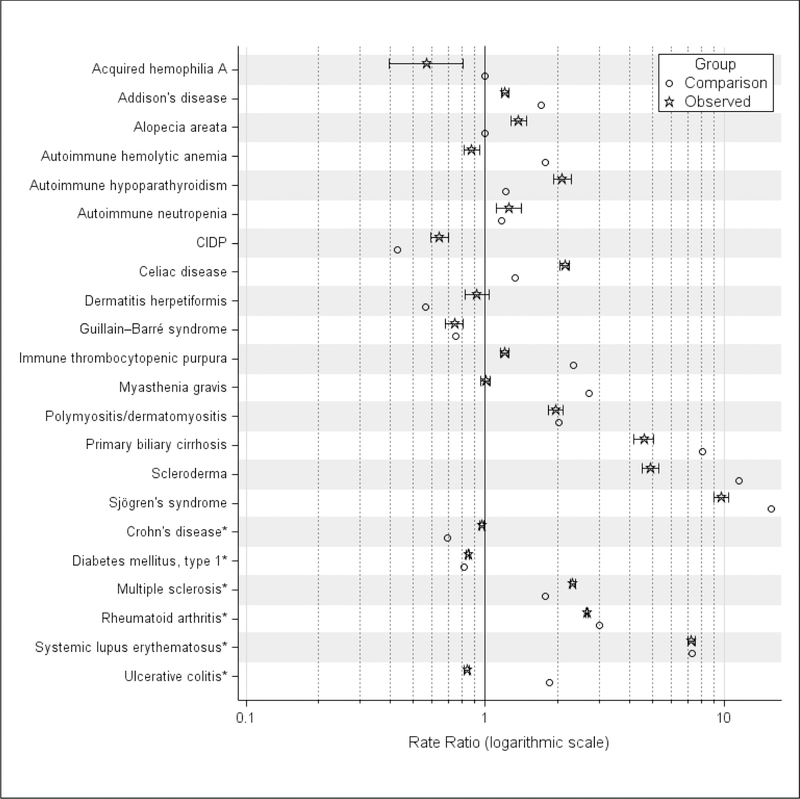
Figure 3 summarizes observed and comparison female-to-male rate ratios. Observed and comparison ratios overlapped for 5 AIDs: autoimmune neutropenia, Guillain–Barré syndrome, polymyositis/dermatomyositis, DM1, and SLE. The observed RA ratio (95% CI: 2.62, 2.70) did not overlap, but was close to the comparison ratio (3.0). Nine observed ratios were lower than comparison ratios (stated as observed 95% CI vs comparison ratio): acquired hemophilia A (0.40, 0.81 vs 1.0), Addison’s disease (1.16, 1.25 vs 1.70), autoimmune hemolytic anemia (0.81, 0.94 vs 1.78), immune thrombocytopenic purpura (1.14, 1.25 vs 2.33); myasthenia gravis (0.95, 1.05 vs 2.70); primary biliary cirrhosis (4.19, 5.03 vs 8.09); scleroderma (4.53, 5.29 vs 11.50); Sjögren’s syndrome (9.03, 10.44 vs 15.67); and ulcerative colitis (0.82, 0.86 vs 1.86). Seven observed ratios were higher than comparison ratios: alopecia areata (1.28, 1.48 vs 1.0), autoimmune hypoparathyroidism (1.92, 2.28 vs 1.22), CIDP (0.59, 0.70 vs 0.43), celiac disease (2.06, 2.24 vs 1.33), dermatitis herpetiformis (0.82, 1.04 vs 0.56), Crohn’s disease (0.95, 0.98 vs 0.69), and MS (2.27, 2.38 vs 1.78).
Figure 3.
Standardized AID Rate Ratios, Female vs Male, United States, 2010–2016
AID, autoimmune disorder; CIDP, chronic inflammatory demyelinating polyneuropathy
* AID with observed rate of 50 per 100,000 or higher
Depicted are for the comparison and observed ratios. Comparison ratios are point estimates based on female percentage reported by Hayter and Cook.[2] Observed ratios are standardized age-adjusted observed rates for female vs male estimated from the Health Facts database population for the years 2010–2016, and the associated 95% confidence intervals for the ratios.
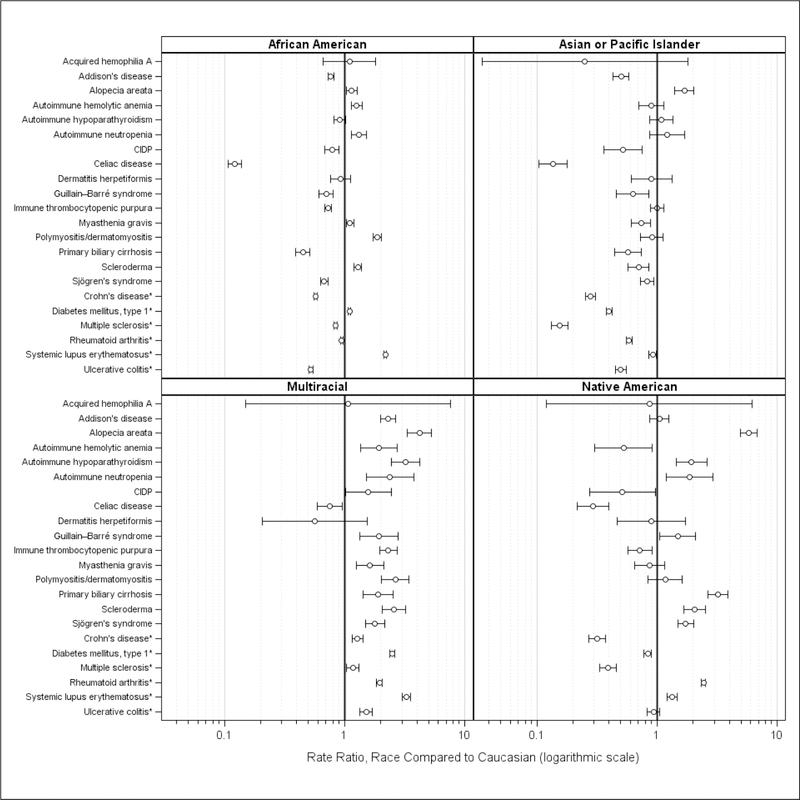
Figure 4 summarizes observed ratios for African American, Asian/Pacific Islander, Native American, and Multiracial rates compared to Caucasian rates. Large confidence intervals indicate few individuals for some AIDs and race combinations (e.g., acquired hemophilia A, dermatitis herpetiformis). For celiac disease, observed rates for all races were lower than the Caucasian rate. Multiracial rates were higher for almost all AIDs. Observed Asian/Pacific Islander rates were similar to, or lower, than Caucasian rates with the exception of alopecia areata (ratio 95% CI: 1.41, 2.02). Among African Americans, rates were doubled for polymyositis/dermatomyositis (ratio 95% CI: 1.73, 2.03) and SLE (ratio 95% CI: 2.13, 2.23). Among Native Americans, rates were doubled for autoimmune hypoparathyroidism (ratio 95% CI:1.45, 2.60), autoimmune neutropenia (ratio 95% CI:1.19, 2.94), and scleroderma (ratio 95% CI: 1.68, 2.53) and more than doubled for alopecia areata (95% CI: 4.92, 6.77), primary biliary cirrhosis (95% CI: 2.66, 3.90), and RA (95% CI: 2.34, 2.55).
Figure 4.
Standardized AID Rate Ratios, Minority Race vs Caucasian, United States, 2010–2016
AID, autoimmune disorder; CIDP, chronic inflammatory demyelinating polyneuropathy
* AID with observed rate of 50 per 100,000 or higher
Depicted are the standardized age-adjusted observed rate ratios, estimated from the Health Facts database population for the years 2010–2016, for each race vs Caucasian, and the associated 95% confidence intervals for the ratios.
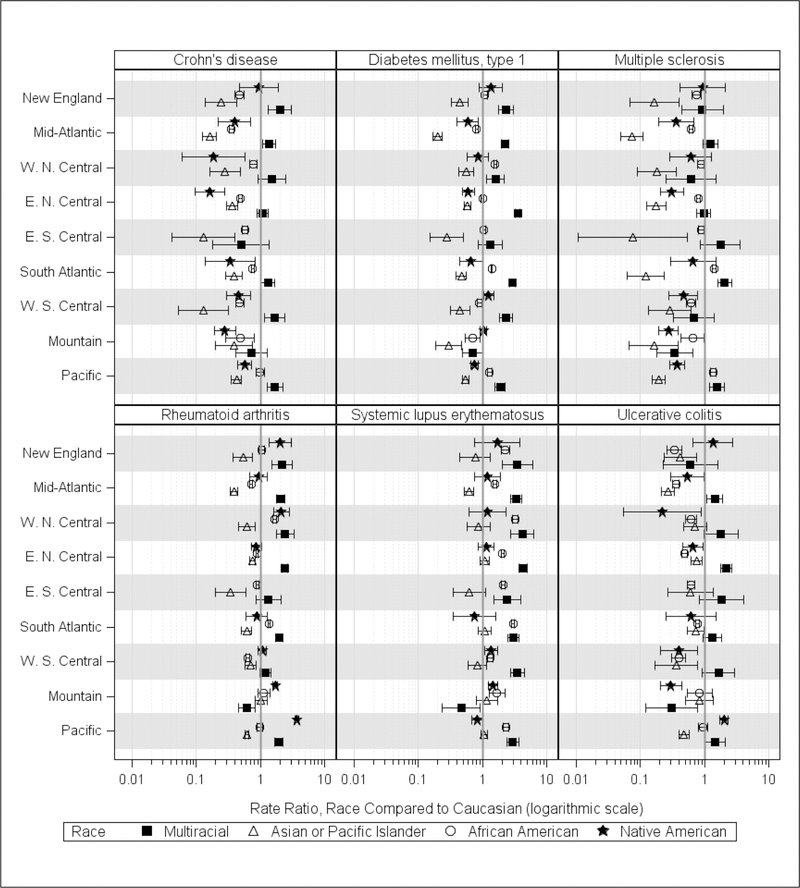
Figure 5 summarizes observed race ratios by US census division for the 6 AIDs with observed rates of ≥50 per 100,000 (see Table A.2 for specific estimated ratios). Overall, Multiracial rates tended to be higher in all divisions with the exception of the Mountain division. Three AID Multiracial ratios were consistently higher across divisions: DM1 (7 of 9), RA (6 of 9), and SLE (8 of 9). In contrast, Asian/Pacific Islander ratios were lower across all divisions, with the exception of divisions where ratios were similar: RA (Mountain division), SLE (8 divisions), and ulcerative colitis (3 divisions). For the other 2 minority race groups, African Americans and Native Americans, geographic race ratios for some AIDs exhibited variability across divisions.
Figure 5.
Standardized AID Rate Ratios for AIDs with Overall Observed Rates of 50 per 100,000 or Higher, Minority Race vs Caucasian, by Census Division, United States, 2010–2016
E, east; N, north; S, south; W, west
Depicted are the standardized age-adjusted observed rate ratios, estimated from the Health Facts database population for the years 2010–2016, for each race vs Caucasian within each US Census Division, and the associated 95% confidence intervals for the ratios. US Census Regions/Divisions and states included are: Northeast/New England (CT, ME, MA, NH, RI, VT); Northeast/Middle Atlantic (NJ, NY, PA); Midwest/West North Central (IA, KS, MN, MO, ND, SD); Midwest/East North Central (IL, IN, MI, OH, WI); South/East South Central (AL, KY, MS, TN); South/South Atlantic (DE, DC, FL, GA, MD, NC, SC, VA, WV); South/West South Central (AR, LA, OK, TX); West/Mountain (AZ, CO, ID, MT, NV, NM, UT, WY); West/Pacific (AK, CA, HI, OR, WA). There were no observations identified for MS for Native Americans in the East South Central Census Division, and only 3 or less for diabetes mellitus type 1, rheumatoid arthritis, and systemic lupus erythematosus.
African American Crohn’s disease rates were equivalent or significantly lower in all divisions, as were ulcerative colitis rates. However, African American DM1 rates were slightly higher in 3 census divisions (West North Central division, South Atlantic, and Pacific) and lower in 3 divisions (Middle Atlantic, West South Central, and Mountain West). The African American MS rate was higher in 2 divisions (South Atlantic and Pacific), and 2 divisions had a significantly higher RA rate (West North Central and South Atlantic). The African American SLE rate was significantly higher in all census divisions, and markedly higher in the West North Central (95% CI: 2.96, 3.60) and South Atlantic (95% CI: 2.86, 3.13) divisions.
Observed Native American Crohn’s disease rates were equivalent or significantly lower across divisions, and Native American ulcerative colitis rates were significantly higher in the Pacific division (95% CI 1.72, 2.32), but not in others. Native American DM1 rates were slightly higher in the West South Central division, but equivalent or lower in other divisions. No significant differences in Native American RA rates were observed for 4 divisions (Middle Atlantic, East North Central, South Atlantic, and West South Central), but 4 divisions had significantly higher rates (New England (95% CI 1.31, 3.02), West North Central (95% CI: 1.56, 2.91), Mountain (95% CI: 1.54, 1.86), and Pacific (95% CI: 3.78, 4.30). Native American SLE rates were slightly higher in 2 divisions (West South Central and Mountain) and not significantly different for other divisions.
4. Discussion
This study summarized minority race rate ratios for 22 AIDs using EMR data based on populations seeking care in facilities across the United States over a 7-year period. For many of the 22 AIDs, information on minority race prevalence, particularly among Native American populations has not been published previously. To provide context for the race rate ratios, observed standardized age-adjusted AID rates and female-to-male ratios were also calculated and compared to estimates summarized in 2012 by Hayter and Cook [2]. The majority of observed overall standardized AID rates were similar (n=15), while 5 were lower (alopecia areata, celiac disease, DM1, immune thrombocytopenic purpura, and RA) and 2 were higher (Crohn’s disease, SLE).
There are several plausible factors for differences in observed standardized AID rates. The comparison alopecia areata prevalence estimate of 150 per 100,000 was substantially higher than the observed estimate (95% CI: 6.4, 6.9). Hayter and Cook’s comparison estimate predominantly drew on one alopecia areata epidemiological study (a US population based in Minnesota) that estimated lifetime risk at 1.7% and prevalence between 100 and 200 [14]. Our study’s far lower estimate could be due to individuals with alopecia areata not seeking medical care, or to use of a diagnosis code(s) other than those utilized in this study.
For the other 6 AIDs, additional published prevalence rates lend some support for observed rate estimates. Hayter and Cook estimated celiac disease and Crohn’s disease prevalence rates at 750 and 25 per 100,000, based on US and worldwide population estimates respectively [2, 15–18]. The celiac disease estimate was previously estimated to be within a range of 500 to 1000 per 100,000 worldwide [16]. The current estimate for the US is approximately 1000 per 100,000, although among African Americans celiac disease is rare [19]. Our estimate for celiac disease (95% CI: 20.3, 21.2) was considerably lower, but as an observed rate, plausible, given that celiac disease is known to be underdiagnosed, particularly when individuals may self-treat by adopting a gluten-free diet without seeking a diagnosis, and when individuals may not receive an appropriate diagnosis who have atypical or silent symptoms of the disease [15]. Our estimate for Crohn’s disease (95% CI: 79.7, 81.3) was higher than the comparison estimate, but is in keeping with several North American studies that indicate a prevalence range of 44 to 201 per 100,000 for Crohn’s disease [20].
Our DM1 estimate (95% CI: 184.7, 187.1) was considerably lower than Hayter and Cook’s world-wide estimate of 480. There have been few prevalence studies for DM1 conducted in the US, but lower US DM1 prevalence estimates do exist. Jacobson and colleagues in 1997 stated a fairly low US prevalence of 192 [21], and a low estimate for DM1 (95% CI: 178, 187) among children and adolescents was also found in the SEARCH for Diabetes in Youth Study [22]. Incidence of DM1 has been found to be increasing [23]; for example, a study using CDC sponsored survey data for 1999–2010 estimated a higher range (95% CI: 270 to 430) [24]. Thus, there is some support for our lower US DM1 estimate, but it may also be an underestimate of overall DM1 prevalence in the US.
The comparison RA estimate, >800 per 100,000 [2], was for adults (ages >18) and is based on a 1997 US estimate of 860 among ages >16 [21]. Alternatively, our estimate includes juvenile RA and is for the total population, differences that would produce a lower prevalence estimate. In comparison, our SLE prevalence estimate (95% CI: 73.6, 75.1) was higher than the worldwide comparison range of 25–50 per 100,000, yet it is commensurate with the wide range of US prevalence rates extending from 20 to 150 per 100,000 [25–27]. Our immune thrombocytopenic purpura estimate (95% CI: 17.3, 18.1) was lower than the comparison range of 50 to 100 per 100,000, but on par with a 2012 Oklahoma population study that reported prevalence rates over 2, 5, and 10 years of 18.0, 26.7, and 33.2 per 100,000 [28].
Among the observed female-to-male rate ratios, 6 ratios were comparable to Hayter and Cook’s review, while 16 were not (7 higher, 9 lower). Though again, there are recently published estimates similar to the observed estimates. For example, more recent epidemiological reviews [29–32] support the ratios closer to 1.0 observed for 6 study AIDs – Addison’s disease, autoimmune hemolytic anemia, dermatitis herpetiformis, immune thrombocytopenic purpura, myasthenia gravis, and ulcerative colitis. More recent review ranges support higher observed ratios for 3 AIDs – alopecia areata [33], celiac disease [31, 32], and MS [29–31]; and lower observed ratios for 3 AIDs – primary biliary cirrhosis [29, 31], scleroderma [29, 31], and Sjögren’s syndrome [29–31]. It should be noted though that higher ratios may also be observed when using health care utilization data if women compared to men have more severe symptoms, potentially leading to a higher rate of seeking management, as has been found in some celiac disease studies [19].
A unique contribution of this analysis is information on heterogeneity in rates by race and by US region. Comparative rates for SLE for some minorities vs Caucasians have been previously published and these support our higher observed rate for Native Americans [34], the rate twice as high for African Americans [34], and the equivalent rate for Asian/Pacific Islanders [35]. As well, our higher observed African American scleroderma rate is supported by prior evidence that indicates an earlier age of onset and potentially more severe disease manifestations [36]. The slightly higher Native American SLE observed rate appears to be driven by higher rates in the West South Central and Mountain divisions areas in which the states of Oklahoma, Arizona, and New Mexico are home to large Tribal populations [37]. Specific SLE comparative rates have not been well reported for Native American populations, but SLE diagnosis has been found to occur at an earlier age, and diagnosed individuals to have greater disease severity, factors suggesting a higher prevalence among Native Americans [38].
Our study observed lower rates for Native American populations compared to Caucasians for celiac disease, Crohn’s disease, DM1, and MS. Prevalence rates for celiac disease in general have been found to be higher for European countries than South American countries [39]. Similarly, Nod2 mutations, mutations of the caspase-activation and recruitment domain gene 15 that are associated with higher risk of Crohn’s disease, are more common among Caucasians than among Native Americans [40]. Lower DM1 rates for Native American populations have been previously noted and one hypothesis for the lower rate is a lack of specific human leukocyte antigen (HLA) determinants [34]. Few studies have reported information on MS among Native Americans, but a recent study of US military veterans also found a lower MS rate for Native Americans [41].
Observed RA rates were higher among Multiracial (6 out of 9 divisions) and Native American populations (4 divisions). RA, along with SLE, DM1, MS, and primary biliary cirrhosis have a strong heritability association, one potential factor for higher Multiracial rates [34]. Higher rates for individuals with more than one race may also be due to environmental exposures and socioeconomic conditions interacting with population admixture, but more research is needed to identify exact contributions of these factors [42–45]. Higher RA prevalence rates were previously documented among Pima (Arizona, US); Blackfeet (Montana, US); Chippewa (Minnesota, US); Tlingit, Tsimshian, and Haida (Southeast Alaska, US) Tribal members [46]. Genetic susceptibility is hypothesized to be a main factor for higher RA rates among indigenous populations [47]; however, evidence also suggests environmental factors may play a substantive role [48, 49]. There is a growing recognition that higher rates of chronic diseases, to include AIDs, may be due to biological mechanisms that link social and environment exposure [42]. For example, individuals living in poverty may be disproportionately exposed to environmental toxicants [45], a potential factor for higher Native American rates in the Southwestern US [50].
In addition to RA, observed rates in our study for Native American populations were at least approximately double those for Caucasians for alopecia areata, primary biliary cirrhosis, scleroderma, and Sjögren’s syndrome. Few studies have investigated these conditions among Native American populations. A recent study of examining racial disparities for alopecia areas using the Nurses’ Health Study also reported higher percentages of individuals with the condition among African Americans and Other races (American Indian, Asian, Native Hawaiian, or Pacific Islander) [51]. Some evidence has been found for a higher rate of primary biliary cirrhosis among British Columbia’s First Nation’s population [52], and one study identified a substantive overlap between RA and Sjögren’s syndrome for an Oklahoma Native American population [53, 54]. Interestingly, North American studies reviewing clusters of primary biliary cirrhosis have noted a relationship between environmental exposures such as toxic waste sites and air pollution [55].
AID rates estimated for the 7-year period in this study are based on a population seen in health care facilities and not the general population. Observed rates may over-estimate disease prevalence since the denominator population may be smaller than the actual population who could have been seen for care in the facilities. Another aspect of this is that a large portion of US Native Americans who are registered members of any of the 573 federally recognized Tribes frequently rely exclusively on the federal Indian Health Service health care system, a system not included in our data, potentially leading to an underestimate of Native American prevalence rates. However, we confirmed that the percentage of Native Americans in this database was similar to the national Native American percentage providing support for credible comparative rates. The distributions of individuals with Asian and/or Pacific Islander race and of individuals with more than one race were lower in the database in comparison to the national population, and findings from this database for these populations may be limited in their generalizability to the overall US population.
An additional limitation of this study is that our identification of individuals with AID was based on the occurrence of two diagnoses. This is a simplistic algorithm for identification, and many guidelines recommend inclusion of biomarkers and/or drug treatments for greater sensitivity and positive predictive value. However, these additional screening tools are not always used in practice [56]. Lack of more complex algorithms may have resulted in prevalence overestimates for some AIDS, but since the identification criteria were applied uniformly for all included individuals, we believe there is minimal impact on the observed sex and race rate ratios estimated in this analysis.
Very large databases are necessary to study diseases with very low prevalence rates. The database utilized in this analysis included approximately 16 million individuals seen annually in healthcare facilities and provides power in estimating diseases with low prevalence, such as AIDs. Over the 7-year period approximately 50 million individuals contributed information for the overall AID rates and AID rates by sex and approximately 40 million individuals contributed information for AID rates based on race. This study has added to other studies that have reviewed differential occurrences of AIDs not only between women and men, but between individuals of different races. The health disparities involving AIDs are infrequently discussed and no reliable disease registries exist for epidemiological investigations in the US. Using large EMR databases may be a practical and necessary analytical approach to further understand differences in prevalence rates and disparities in AIDs. This could also be the best way to examine the effectiveness of therapeutic approaches and drug treatments by various groups of patients in the near future.
Supplementary Material
Highlights.
Considerable heterogeneity exists across racial groups for 22 AID rates.
Among 6 high prevalence AIDs, variation across geographic region was evident.
African American SLE rates were markedly higher in 2 out of 9 regions.
RA rates for Native Americans were significantly higher in 4 out of 9 regions.
Potential variation factors include genetic susceptibility and/or environmental exposures.
Acknowledgments and funding
This project was supported in part by University of New Mexico College of Pharmacy (UNM COP) internal funding and by the National Center for Research Resources and the National Center for Advancing Translational Science of the National Institutes of Health through grant number UL1TR00449, the University of New Mexico Clinical and Translational Science Center (UNM CTSC). Interpretations, conclusions, and/or opinions based on analyses of HF EMR data are those of the authors and do not constitute the findings, policies, or recommendations of the UNM COP or the UNM CTSC.
Abbreviations
- AID
autoimmune disease
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- CIPD
chronic inflammatory demyelinating polyneuropathy
- DM1
diabetes mellitus type 1
- EMR
electronic medical record
- HF
Health Facts
- MS
multiple sclerosis
- RA
rheumatoid arthritis
- SLE
systemic lupus erythematosus
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A is available on request from the authors.
Declarations of interest: None
REFERENCES
- [1].U.S. National Institutes of Health (NIH). Biennial Report of the Director. National Institutes of Health: Fiscal Years 2014 & 2015. https://report.nih.gov/biennialreport/ [accessed 17 January, 2019].
- [2].Hayter SM, Cook MC. Updated assessment of the prevalence, spectrum and case definition of autoimmune disease. Autoimmun Rev. 2012;11:754–65. [DOI] [PubMed] [Google Scholar]
- [3].Damoiseaux JGMC, Cohen Tervaert JW. The definition of autoimmune disease: are Koch’s postulates applicable? Netherlands J Med. 2002;60:266–8. [PubMed] [Google Scholar]
- [4].National Institutes of Health (NIH) Autoimmune Diseases Coordinating Committee. Progress in autoimmune diseases research. (Publication No. 05–5140). March 2005. https://www.niaid.nih.gov/sites/default/files/adccfinal.pdf [accessed 17 January, 2019].
- [5].Cooper GS, Gilbert KM, Greidinger EL, James JA, Pfau JC, Reinlib L, et al. Recent advances and opportunities in research on lupus: environmental influences and mechanisms of disease. Environ Health Perspect. 2008;116:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rose NR. Prediction and prevention of autoimmune disease in the 21st Century: a review and preview. Am J Epidemiol. 2016;183:403–6. [DOI] [PubMed] [Google Scholar]
- [7].American Autoimmune Related Diseases Association (AARDA). The cost burden of autoimmune disease: the latest front in the war on healthcare spending. Eastpointe, MI; 2011. http://www.diabetesed.net/page/_files/autoimmune-diseases.pdf [accessed 17 January, 2019]. [Google Scholar]
- [8].Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev. 2003;2:119–25. [DOI] [PubMed] [Google Scholar]
- [9].Thomas SL, Griffiths C, Smeeth L, Rooney C, Hall AJ. Burden of mortality associated with autoimmune diseases among females in the United Kingdom. Am J Public Health. 2010;100:2279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lim SS, Drenkard C. The Epidemiology of Lupus. In: Wallace DJ Hahn BH, editors. Dubois’ Lupus Erythematosus and Related Syndromes (Eighth Edition). Philadelphia: W.B. Saunders; 2013. p. 8–24. [Google Scholar]
- [11].Maclay JD, McAllister DA, MacNee W. Cardiovascular risk in chronic obstructive pulmonary disease. Respirology. 2007;12:634–41. [DOI] [PubMed] [Google Scholar]
- [12].Chronic Conditions Data Warehouse (CCW). CCW Condition categories algorithms (revised June 2018). https://www.ccwdata.org/web/guest/condition-categories. [accessed 17 January, 2019].
- [13].Klein RJ, Schoenborn CA. Age adjustment using the 2000 projected U.S. population. Healthy People 2010 Stat Notes. 2001:1–10. [PubMed]
- [14].Safavi KH, Muller SA, Suman VJ, Moshell AN, Melton LJ 3rd. Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628–33. [DOI] [PubMed] [Google Scholar]
- [15].Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. JAMA Int Med. 2003;163:286–92. [DOI] [PubMed] [Google Scholar]
- [16].Liu C, Crawford JM. The gastrointestinal tract. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran pathologic basis of disease. Philadelphia: Elsevier; 2004. p. 797–875. [Google Scholar]
- [17].Lakatos P. Recent trends in the epidemiology of inflammatory bowel diseases: Up or down? World J Gastroenterol. 2006;12:6102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Logan I, Bowlus CL. The geoepidemiology of autoimmune intestinal diseases. Autoimmun Rev. 2010;9:A372–A8. [DOI] [PubMed] [Google Scholar]
- [19].Ludvigsson JF, Murray JA. Epidemiology of celiac disease. Gastroenterol Clin North Am. 2019;48:1–18. [DOI] [PubMed] [Google Scholar]
- [20].Cosnes J, Gower–Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–94.e4. [DOI] [PubMed] [Google Scholar]
- [21].Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223–43. [DOI] [PubMed] [Google Scholar]
- [22].Liese A, D’Agostino R Jr, Hamman R, Kilgo P, Lawrence J, Liu L, et al. The Burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118:1510. [DOI] [PubMed] [Google Scholar]
- [23].Borchers AT, Uibo R, Gershwin ME. The geoepidemiology of type 1 diabetes. Autoimmun Rev. 2010;9:A355–A65. [DOI] [PubMed] [Google Scholar]
- [24].Menke A, Orchard TJ, Imperatore G, Bullard KM, Mayer-Davis E, Cowie CC. The prevalence of type 1 diabetes in the United States. Epidemiology. 2013;24:773–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778–99. [DOI] [PubMed] [Google Scholar]
- [26].Chakravarty EF, Bush TM, Manzi S, Clarke AE, Ward MM. Prevalence of adult systemic lupus erythematosus in California and Pennsylvania in 2000: Estimates obtained using hospitalization data. Arthritis Rheum. 2007;56:2092–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pons-Estel GJ, Alarcón GS, Scofield L, Reinlib L, Cooper GS. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum. 2010;39:257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Terrell DR, Beebe LA, Neas BR, Vesely SK, Segal JB, George JN. Prevalence of primary immune thrombocytopenia in Oklahoma. Am J Hematol. 2012;87:848–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pollard KM. Gender differences in autoimmunity associated with exposure to environmental factors. J Autoimmun. 2012;38:J177–J86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang LF, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Intern Med. 2015;278:369–95. [DOI] [PubMed] [Google Scholar]
- [31].Ji J, Sundquist J, Sundquist K. Gender-specific incidence of autoimmune diseases from national registers. J Autoimmun. 2016;69:102–6. [DOI] [PubMed] [Google Scholar]
- [32].West J, Fleming KM, Tata LJ, Card TR, Crooks CJ. Incidence and prevalence of celiac disease and dermatitis herpetiformis in the UK over two decades: population-based study. Am J Gastroenterol. 2014;109:757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fricke A, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015:397–403. [DOI] [PMC free article] [PubMed]
- [34].Seldin MF. The genetics of human autoimmune disease: a perspective on progress in the field and future directions. J Autoimmun. 2015;64:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Stojan G, Petri M. Epidemiology of systemic lupus erythematosus: an update. Curr Opin Rheumatol. 2018;30:144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gelber AC, Manno RL, Shah AA, Woods A, Le EN, Boin F, et al. Race and association with disease manifestations and mortality in scleroderma: A 20-year experience at the Johns Hopkins Scleroderma Center and review of the literature. Medicine. 2013;92:191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].The United States Census Bureau. American Indians and Alaska Natives in the United States. 2010. https://www2.census.gov/geo/maps/special/AIANWall2010/AIAN_US_2010.pdf. [accessed 14 November 2018].
- [38].Kheir JM, Guthridge CJ, Johnston JR, Adams LJ, Rasmussen A, Gross TF, et al. Unique clinical characteristics, autoantibodies and medication use in Native American patients with systemic lupus erythematosus. Lupus Sci Med. 2018;5:e000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Singh P, Arora A, Strand TA, Leffler DA, Catassi C, Green PH, et al. Global Prevalence of celiac disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16:823–36.e2. [DOI] [PubMed] [Google Scholar]
- [40].Gasche C, Nemeth M, Grundtner P, Willheim-Polli C, Ferenci P, Schwarzenbacher R. Evolution of Crohn’s disease-associated Nod2 mutations. Immunogenetics. 2008;60:115–20. [DOI] [PubMed] [Google Scholar]
- [41].Wallin MT, Culpepper WJ, Coffman P, Pulaski S, Maloni H, Mahan CM, et al. The Gulf War era multiple sclerosis cohort: age and incidence rates by race, sex and service. Brain. 2012;135:1778–85. [DOI] [PubMed] [Google Scholar]
- [42].Bagby SP, Martin D, Chung ST, Rajapakse N. From the Outside In: Biological mechanisms linking social and environmental exposures to chronic disease and to health disparities. Am J Public Health. 2019;109:S56–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Araki Y, Mimura T. The histone modification code in the pathogenesis of autoimmune diseases. Mediators Inflamm. 2017;2017:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ellis JA, Kemp AS, Ponsonby A-L. Gene–environment interaction in autoimmune disease. Expert Rev Mol Med. 2014;16:e4. [DOI] [PubMed] [Google Scholar]
- [45].Anaya J-M, Ramirez-Santana C, Alzate MA, Molano-Gonzalez N, Rojas-Villarraga A. The autoimmune ecology. Front Immunol. 2016;7. [DOI] [PMC free article] [PubMed]
- [46].Peschken CA, Esdaile JM. Rheumatic diseases in North America’s indigenous peoples. Semin Arthritis Rheum. 1999;28:368–91. [DOI] [PubMed] [Google Scholar]
- [47].Hurd K, Barnabe C. Systematic review of rheumatic disease phenotypes and outcomes in the Indigenous populations of Canada, the USA, Australia and New Zealand. Rheumatol Int. 2017;37:503–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hewagama A, Richardson B. The genetics and epigenetics of autoimmune diseases. J Autoimmun. 2009;33:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Parks CG, de Souza Espindola Santos A, Barbhaiya M, Costenbader KH. Understanding the role of environmental factors in the development of systemic lupus erythematosus. Best Pract Res Clin Rheumatol. 2017;31:306–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lewis J, Hoover J, MacKenzie D. Mining and Environmental Health Disparities in Native American Communities. Curr Environ Health Rep. 2017;4:130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Thompson JM, Park MK, Qureshi AA, Cho E. Race and alopecia areata amongst US women. J Investig Dermatol Symp Proc. 2018;19:S47–S50. [DOI] [PubMed] [Google Scholar]
- [52].Arbour L, Field L, Ross P, Erikson A, Yoshida E. The mystery of primary biliary cirrhosis in British Columbia’s First Nations people. Int J Circumpolar Health. 2004;63:185–8. [DOI] [PubMed] [Google Scholar]
- [53].Gaddy JR, Vista ES, Robertson JM, Dedeke AB, Roberts VC, Klein WS, et al. Rheumatic disease among Oklahoma Tribal populations: a cross-sectional study. J Rheumatol. 2012;39:1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Scofield RH, Fogle M, Rhoades ER, Harley JB. Rheumatoid arthritis in a United States Public Health Service Hospital in Oklahoma: serologic manifestations in rheumatoid arthritis vary among tribal groups. Arthritis Rheum. 1996;39:283–6. [DOI] [PubMed] [Google Scholar]
- [55].Gross RG, Odin JA. Recent advances in the epidemiology of primary biliary cirrhosis. Clin Liver Dis. 2008;12:289–303; viii. [DOI] [PubMed] [Google Scholar]
- [56].Giacomelli R, Afeltra A, Alunno A, Bartoloni-Bocci E, Berardicurti O, Bombardieri M, et al. Guidelines for biomarkers in autoimmune rheumatic diseases - evidence based analysis. Autoimmun Rev. 2019;18:93–106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.