Abstract
Phages, viruses that prey on bacteria, are the most abundant and diverse inhabitants of the Earth. Temperate bacteriophages can integrate into the host genome and, as so-called prophages, maintain a long-term association with their host. The close relationship between host and virus has significantly shaped microbial evolution and phage elements may benefit their host by providing new functions. Nevertheless, the strong activity of phage promoters and potentially toxic gene products may impose a severe fitness burden and must be tightly controlled. In this context, xenogeneic silencing (XS) proteins, which can recognize foreign DNA elements, play an important role in the acquisition of novel genetic information and facilitate the evolution of regulatory networks. Currently known XS proteins fall into four classes (H-NS, MvaT, Rok and Lsr2) and have been shown to follow a similar mode of action by binding to AT-rich DNA and forming an oligomeric nucleoprotein complex that silences gene expression. In this review, we focus on the role of XS proteins in phage-host interactions by highlighting the important function of XS proteins in maintaining the lysogenic state and by providing examples of how phages fight back by encoding inhibitory proteins that disrupt XS functions in the host. Sequence analysis of available phage genomes revealed the presence of genes encoding Lsr2-type proteins in the genomes of phages infecting Actinobacteria. These data provide an interesting perspective for future studies to elucidate the impact of phage-encoded XS homologs on the phage life cycle and phage-host interactions.
Keywords: Xenogeneic silencer, H-NS, Lsr2, Actinobacteria, phage-host interaction
“You have a grand gift for silence, Watson. It makes you quite invaluable as a companion.”
(Sherlock Holmes)
Introduction
Phages, viruses that prey on bacteria, represent the most abundant biological entities on this planet and are a major driver of horizontal gene transfer (HGT). Phages are not only present as infectious particles in the environment but are also found as integrated elements (prophages) within the genomes of their bacterial hosts. In some cases, DNA of viral origin accounts for up to 20% of an organism’s entire genome [1–3]. Some of this DNA originates from fully functional prophages, which are capable of undergoing a lytic life cycle. However, a considerable part is made up of prophage-like elements, including phage remnants left after incomplete excision events, cryptic (degenerated) prophages or other genetic material acquired by HGT. In fact, this genetic material has significantly shaped microbial evolution due to the development of mutually beneficial interactions between prophage and host [4]. Nevertheless, the safe integration of viral elements into bacterial genomes demands stringent regulation of phage gene expression.
Upon integration into the host genome, a functional phage can exit the prophage state and enter the lytic cycle, which is typically triggered by severe DNA damage that activates the cellular SOS response. Even under non-inducing conditions, cells may encounter spontaneous DNA damage [5], leading to the SOS-dependent induction of prophages in a small fraction of the lysogenic population [6, 7]. Originally, this spontaneous prophage induction (SPI) was considered a potentially detrimental process, but recent research in the fields of microbial biofilms, host-pathogen interaction and population dynamics emphasizes that SPI is an important contributor to the social behavior of microbes [6]. In a recent study, we quantified SPI in populations of C. glutamicum using reporter promoter fusions. While we observed a positive correlation between SPI and the DNA damage (SOS) response, a significant fraction of the cases also occurred in an SOS-independent manner [8, 9]. Thus, the molecular factors influencing SPI in single individuals and how the host modulates its frequency remain largely unknown.
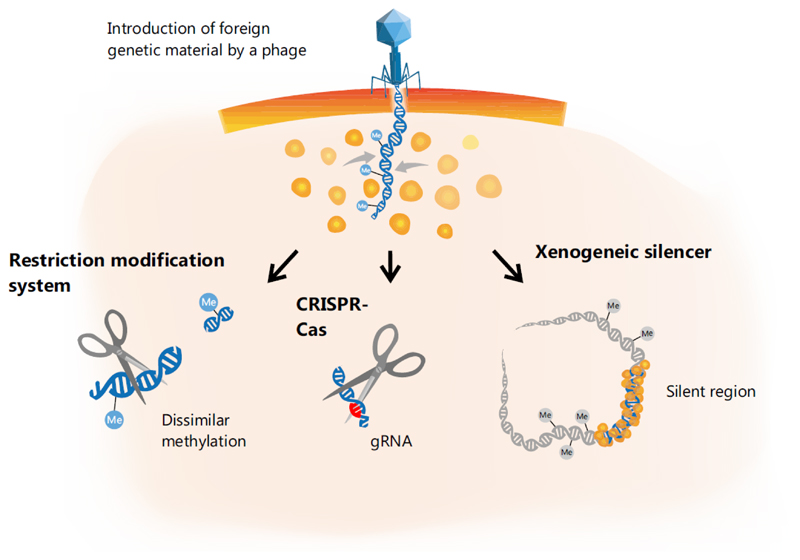
Compared to point mutations or genomic rearrangements, HGT allows bacteria to acquire new traits much more rapidly, but the downside of this medal is that the new information is encoded on foreign genetic material [10, 11]. The encounter with xenogeneic (foreign) DNA is a ‘high risk - high gain’ situation: While the acquisition provides the potential for the fast gain of new beneficial traits, the activity of selfish genetic elements or bacterial viruses (bacteriophages) represents a perpetual threat to bacterial cells. Gene expression from xenogeneic material can strongly impair cellular fitness by sequestering RNA polymerase [12, 13], by producing toxic proteins and, in the case of phages, by causing cell lysis. With more than 1024 productive viral infections on earth [14], the activity of bacteriophages plays a vital role in HGT, which is also reflected by the variety of phage defense mechanisms encoded in bacterial genomes, with restriction modification (RM) systems and CRISPR-Cas being among the most prominent mechanisms [15]. With the expansion of the phage genomic space, many more examples of phage defense systems have been described and have been covered in a number of recent reviews [16, 17]. In contrast to the destructive mode of action of RM and CRISPR-Cas systems, where nucleases are employed to wipe out incoming foreign DNA [18], xenogeneic silencing (XS) represents a mechanism promoting tolerance of foreign genetic material [19, 20]. The mechanism of XS is based on the activity of small, nucleoid-associated proteins (NAPs) that recognize and bind foreign, AT-rich DNA stretches and silence gene expression due to the formation of a tight nucleoprotein complex [19] (Figure 1). By this means, XS proteins provide an important basis for the safe acquisition of new genetic material and foster evolutionary network expansion [21]. Hitherto, all known XS proteins fall into one of four different classes: H-NS in Proteobacteria [19, 22], MvaT/U in Pseudomonas species [23], Rok in Bacillus subtilis [24] and Lsr2 in Actinobacteria [25]. Despite the low sequence similarity between different silencers, these proteins appear to fulfill very similar functions in their respective host, and examples of cross-complementation have been found for Lsr2, MvaT and H-NS [9, 23, 25].
Figure 1. Xenogeneic silencing of foreign DNA.
Microbial cells have evolved a variety of different defense mechanisms to deal with viral DNA and to counteract potential detrimental effects. Schematically included examples are the restriction modification (RM) systems and CRISPR-Cas. In contrast, xenogeneic silencing proteins are able to recognize foreign, AT-rich DNA and form an oligomeric nucleoprotein complex that silences gene expression at the particular target regions [19].
Several recent studies focused, in particular, on the impact of H-NS on bacterial genome evolution and network expansion (for recent reviews, see [19, 20, 26]). Given the mosaic-like structure of bacterial genomes, (pro-)phages apparently account for a significant fraction of bacterial strain diversification [1]. In a recent study, we provided the first example of a prophage-encoded Lsr2-like protein functioning as an essential silencer of a cryptic prophage in the actinomycete Corynebacterium glutamicum. Considering the generic role of XS proteins in the silencing of foreign DNA, it is reasonable to assume that these proteins play an important role and may adopt different functions in phage-host regulatory interaction. In this review, we summarize the literature focusing on the role of XS proteins on phage-host interactions, including examples of phage-mediated counter-silencing as a defense strategy during infection. Furthermore, we provide a comprehensive bioinformatics analysis of bacterial and phage genomes revealing that genes encoding Lsr2-like proteins are ubiquitously found in the genomes of actinobacteriophages, suggesting an adoption of XS function in the lifestyles of virulent and temperate phages.
Xenogeneic silencing: Recognition and binding to foreign, AT-rich DNA
The basis for XS is provided by the domain organization of the XS proteins, which is remarkably similar among the different classes. Typically, these proteins exhibit a small size of <15 kDa and consist of an N-terminal oligomerization domain and a C-terminal DNA-binding domain [20, 27]. All silencers have the common feature of preferentially binding DNA regions that are more AT rich than the host genome. This feature appears to be the basis for the targeting of foreign elements since the vast majority of exogenous DNA has been found to be more AT rich than the host core genome [19, 28, 29].
High-resolution structural analysis of the C-termini of H-NS and Lsr2 resulted in the identification of the ‘prokaryotic AT-hook’ with the ’Q/RGR’ motif, which is reminiscent of the ‘AT-hook’ motif found in eukaryotic HMG-I(Y) proteins [30]. AT-sequences lack the exocyclic 6-amino group, which consequently allows a deeper interaction with protein side-chain residues. In addition, the narrower minor groove of AT-rich sequences harbors a surface with a higher electronegative potential (than mixed or GC-rich sequences), enabling a stronger interaction with positively charged residues. From the evolutionary point of view, it is astonishing that although H-NS and Lsr2 do not share any structural similarity, these proteins show the same binding mechanism. In contrast, MvaT/U proteins, found in Pseudomonas species, lack the AT-hook motif but instead recognize target DNA via a so-called ‘AT-pincer’ motif consisting of a conserved lysine residue and a downstream KGGN motif interacting with the minor groove [31]. Recently, Duan et al. elucidated how the Bacillus silencer Rok distinguishes between host and foreign DNA [27]. The authors could show that Rok directly binds to the minor groove of AT-rich sequences in a novel mode that, so far, has not been described for any other winged helix protein. Using in vitro protein binding microarrays and comparative genome analysis, the authors concluded that Rok preferentially recognizes a few distinct AT-rich DNA motifs present in horizontally acquired regions, which are significantly underrepresented in Bacillus host genomes [27]. However, for the other XS proteins, no sequence-specific recognition has been observed. This characteristic has been, most intensively, studied for H-NS, where AT-rich regions were suggested to function as initial nucleation regions [32]. In a more recent study, a single-molecule counting approach revealed that nucleation sites are crucial for recruiting H-NS molecules [33].
Xenogeneic silencing: Formation of the nucleoprotein complexes
However, binding to DNA is itself not sufficient for XS proteins to fulfill their function. Protein multimerization is an essential step that is required to enable silencing of the target regions. Generally, it is assumed that after the initial nucleation, additional silencing molecules are recruited and concurrently spread along adjacent AT-rich regions (filament formation) [32]. Finally, the high-order oligomerization between distal silencer-DNA complexes leads to strong condensation and DNA compaction that enables silencing of target gene expression [19]. In the current literature, three main mechanisms for silencing are suggested: (i) Promoter occlusion, where the filamentous nucleoprotein complex prevents the binding of the RNA polymerase [19, 34], (ii) trapping of the RNA polymerase, blocking promoter escape upon binding [34] and, (iii) ρ-dependent transcriptional termination that occurs during the pausing of RNA polymerase [35].
Multimerization of XS proteins is facilitated by the N-terminal domains. This effect has been shown, experimentally, for H-NS-, MvaT- and Lsr2-like proteins [9, 36–38] and was suggested for Rok [24]. Primarily, the mechanism behind this protein oligomerization was widely studied using H-NS and high-resolution approaches, like atomic force microscopy (AFM), electron microscopy and single-molecule magnetic tweezers experiments [37, 39, 40]. Depending on the concentration of the divalent cations (Mg2+ or Ca2+), AFM studies reported either a bridging or stiffened mode of the bound DNA stretches [40, 41]. These two different modes were also found for MvaT and Lsr2 [36, 38, 42, 43], underlining a common silencing mechanism among the silencers and explain why these XS proteins are partly able to complement each other in the abovementioned cross-complementation experiments [9, 25]. Nevertheless, the dissimilar AT compositions of host genomes and the differences in DNA binding underline the specialization of the particular XS protein to the requirements in the particular host background.
For H-NS- and MvaT-like silencers, it was shown that XS proteins may form silencing nucleoprotein complexes by interacting with other NAPs. Efficient protein oligomerization is, however, still a prerequisite for the establishment of a silencing nucleoprotein complex. In particular, the small proteins Hha and its paralog YdgT (Cnu) were shown to structurally resemble the N-terminal domain of H-NS and are therefore capable of forming heteromeric complexes [44]. Further studies based on transcriptomics revealed that the binding of Hha to H-NS polymers is required for the efficient repression of a subset of the H-NS regulon [45]. At this point, it is worthwhile to mention that Cnu represents an oriC binding protein that binds within a DnaA binding box and has been suggested to contribute to optimal oriC activity [46]. In B. subtilis, a regulative dependency between the replication initiation protein DnaA and the silencer Rok was recently reported [47], evincing an interesting link between bacterial replication and this XS protein.
In the genomes of E. coli and Salmonella Typhimurium, paralogs of H-NS, such as StpA, are encoded and are capable of forming heterodimers that in turn can interact again with the aforementioned small proteins Hha/YdgT [44]. Recently, the impact of Hha and StpA on the binding mode of the H-NS protein was investigated by in vitro biochemical and biophysical experiments. With their high-resolution approach, Boudreau et al., nicely demonstrated how StpA and Hha modify H-NS-polymeric filaments to increase transcriptional pausing and provided evidence showing that the mixed nucleoprotein complexes (consisting of Hha/H-NS or StpA/H-NS, etc.) differentially affect gene regulation [48]. Thus, these experiments further suggest that interactions with paralogs or accessory proteins likely specify the silencing characteristics also depending on the local concentrations of the respective proteins. The formation of heteromeric silencer complexes was also reported for MvaT and its paralog MvaU in Pseudomonas aeruginosa [49]. In P. putida, MvaT-like proteins were shown to control distinct regulons revealing a functional specialization of these proteins [50–52]. Similar scenarios are also conceivable for Lsr2-like proteins, since the genomes of several Actinobacteria, like Streptomyces coelicolor A(3), S. venezuelae ATCC 10712 and M. smegmatis MC2; 155, encode more than one copy of Lsr2-like proteins (based on a BLAST search with the sequence of Lsr2 (Rv3597c) and default parameters, e-value < 1 × 10-5). The genome compaction of XS proteins resembles the compaction by heteromeric nucleoprotein complexes formed by histones and DNA in eukaryotic cells. Hence, it is reasonable to assume that XS proteins, or in general NAPs, are targets of posttranslational modification (PTM) enzymes. In a very recent review, this topic was elucidated by Dilweg and Dame [53]. Interestingly, the authors reported approximately 29 PTMs for H-NS from E. coli; these PTMs were not experimentally investigated, but the physiological implication for DNA condensation and/or silencing was discussed [53].
Silencing of prophages in bacteria
The safe integration of viral elements into bacterial genomes demands stringent regulation of expression from highly active phage promoters to avoid the production of potentially toxic proteins. The function of XS proteins thereby provides a basis for integrating foreign genes into host regulatory networks by XS and counter-silencing (the latter is discussed in the next section).
For silencers of all four groups, an influence on the regulation of phage genes has been observed (Table 1). However, this effect is often noted only incidentally in these studies [9, 54–56]. Interestingly, the essentiality of a particular XS-encoding gene strongly depends on the genetic setup of the particular strain as the induction of mobile genetic elements, such as prophages, may lead to cell death. In the case of the Gram-positive actinomycete Corynebacterium glutamicum, the Lsr2-like protein CgpS (C. glutamicum prophage silencer) was shown to be essential due to its function as a silencer of the cryptic but still inducible prophage CGP3 [9]. Counteracting of CgpS activity led to prophage induction and regional (in situ) replication at the CGP3 locus [57]. In contrast to other XS proteins described so far, CgpS is encoded on the CGP3 prophage itself and appears to act mainly as a silencer of CGP3 gene expression. Genome-wide profiling of CgpS confirmed the binding of this XS protein to AT-rich sequences in the CGP3 element. As a matter of fact, the essentiality of the cgpS gene is linked to the presence of CGP3, and C. glutamicum strains lacking the prophage do not require CgpS. Interestingly, integration of a second genomic copy of cgpS significantly reduced the fraction of spontaneously induced cells, emphasizing the role of XS proteins in the modulation of SPI in bacterial populations (Frunzke and Pfeifer, unpublished). Additionally, for Mycobacterium tuberculosis, which is also a member of the Actinobacteria, genome-wide binding studies conducted with a Lsr2-like protein not only confirmed the binding to prophage regions (and other mobile genetic elements (MGEs)) [9, 38] but also revealed several additional targets in the host genome involved in virulence and immunogenicity [38]. Interestingly, in Bacillus subtilis, the Rok protein was also shown to bind prophage genes and to be involved in the control of phage gene expression, as is the case for genes of the prophage SPβ [24, 58]. A further interesting example involves E. coli H-NS, where a link between H-NS activity and enhanced biofilm formation was recently demonstrated. Here, H-NS was shown to repress the cryptic prophage Rac. Derepression of Rac resulting from hns deletion led to prophage induction and cell lysis in a toxin-dependent manner [56]. Moreover, in Shewanella oneidensis, H-NS was also reported to be involved in prophage induction during cold adaptation [59]. Additionally, the H-NS orthologs MvaT and MvaU were shown to be essential due to the silencing of prophage elements in P. aeruginosa strains [54, 55]. The depletion of silencers caused increased phage gene expression, the formation of infectious phage particles and cell lysis. Remarkably, only mutants impaired in phage production were capable of compensating the double deletion of mvaT and mvaU [54, 55]. An overview of the studies showing the influence of XS proteins on the control of phage gene expression is provided in Table 1.
Table 1. Silencing of phage elements in bacterial genomes.
| Type of silencer | Host strain | GC of host (%) | Prophage-like element | Length (kb) of phage | GC (%) of phage | Reference |
|---|---|---|---|---|---|---|
| H-NS |
E. coli K-12 BW25113 |
50.8 | Rac (cryptic) | 23.1 | 47.1 | [56] |
|
S. onediensis MR-1 |
45.9 | CP4So (cryptic) | 36 | 43 | [59] | |
| MvaT |
P. aeruginosa PAO1 |
66.6 | Filamentous phage Pf4 | 15.7 | 58.7 | [54, 55] |
| Rok | B. subtilis168 | 43.5 | Prophage region 4 | 8 | 35.8 | [24] |
| Prophage region 5 | 20.7 | 37.5 | ||||
| Prophage region 6 | 34.8 | 36.1 | ||||
| SPβ | 134.4 | 34.6 | ||||
| Lsr2 |
C. glutamicum ATCC 13032 |
53.8 | Cryptic prophage CGP1 | 13.5 | 47.1 | [9] |
| Cryptic prophage CGP3 | 186.0 | 48.4 | ||||
|
M. tuberculosis H37Rv |
65.6 | Prophage region 1, Rv1573-1588c, (Rv1582c) | 10.5 (1.4*) | 66.2 (62.5*) | [38] | |
| Prophage region 2, Rv2645-2664, (Rv2658-2659c) | 12.3 (1.5*) | 66.2 (63.5*) | ||||
In case of M. tuberculosis also the bound genes were considered and are indicated in brackets.
How to overcome silencing?
The formation of a nucleoprotein complex nucleating at AT-rich regions is a prerequisite for XS. Different studies focusing on counter-silencing mechanisms in various species have revealed that upon activation of gene expression, XS proteins are not released from their target DNA; instead, remodeling of the XS-DNA complex enables RNA polymerase to bind and activate transcription. Different counter-silencing mechanisms are mainly based other proteins binding in the upstream promoter region, thereby counteracting XS silencing. For instance, this mechanism has been nicely demonstrated by synthetic counter-silencing approaches, where operator sequences of specific transcription factors (TFs) were inserted in the upstream promoter region to counter-silence gene expression upon binding of the particular TF [60]. Several further studies demonstrated that different host-encoded TFs have been coopted – in the course of evolution - to act as counter-silencers. Examples include the response regulator PhoP, an essential activator of Salmonella virulence [21], the AraC-family TF ToxT of Vibrio cholerae [61], LeuO from S. enterica [62], and the two MarR-type regulators RovA and SlyA of S. enterica and Yersinia pseudotuberculosis, which were shown to antagonize H-NS-dependent silencing of horizontally acquired genes [63, 64]. In a recent study, we also could show that the MarR-type regulator MalR of C. glutamicum, which controls genes involved in stress-responsive cell envelope remodeling, binds to several regions within the CGP3 prophage and is able to counteract SOS-dependent prophage induction (manuscript submitted, BIORXIV/2019/544056).
An alternative route for counter-silencing lies in the interference between XS proteins belonging to the same protein family. An interesting example has been provided for the unusual H-NS paralog Ler, which functions as a regulator of pathogenicity islands (locus of enterocyte effacement, LEE) in enteropathogenic (EPEC) and enterohemorrhagic (EHEC) E. coli strains [65–68]. Structural analysis emphasized that its function as a counter-silencer lies in differences in protein oligomerization as both H-NS and Ler bind to AT-rich regions [69]. Ler shows two different modes of DNA interaction: At low concentrations, Ler is able to increase DNA folding and wraps DNA; otherwise, with increasing concentration, Ler binds DNA in an unwrapped mode where Ler increases the rigidity of DNA similarly to the nucleoprotein filament formed by H-NS [69]. At these high concentrations, Ler displaces H-NS from the bound DNA and therefore overcomes the silencing of target regions. A further interesting example is provided with H-NST, a truncated derivative of H-NS lacking the DNA-binding domain. This XS protein was found to antagonize H-NS in enteropathogenic (EPEC) and uropathogenic E. coli by interfering with its oligomerization domain [70, 71]. Remarkably, this principle of silencer interference can be harnessed to study the function of essential XS proteins. Overproduction of the N-terminal oligomerization domain of the Lsr2-type silencer CgpS was used to counteract CgpS silencing in vivo in the Actinobacterium C. glutamicum [9]. Interference between XS proteins was further demonstrated in this study as the expression of other mycobacterial Lsr2 genes as well as introduction of E. coli H-NS led to XS interference at AT-rich regions, resulting in prophage induction. These findings are also supported by a bioinformatics analysis showing that different classes of silencers do not occur in the same species [72].
Altogether, these examples provide important insights how silencing and counter-silencing facilitate the expansion of regulatory networks in bacteria [21]. In the following, we will focus on mechanisms employed by phages to counteract XS proteins in an ongoing arms race between the phage and the host. A few studies, discussed below, already suggest a variety of different mechanisms used by phages to gain control. One example is provided by the 5.5 protein of the E. coli phage T7, which is able to antagonize H-NS function upon phage infection [73]. By interfering with the central oligomerization domain of H-NS [37], the 5.5 protein blocks H-NS from forming high-order oligomers, leading to counter-silencing of H-NS-silenced genes [74]. Another example has been reported with the Mip protein (MvaT inhibiting protein), encoded by the LUZ24 phage of P. aeruginosa [75]. In 2015, Wagemans et al. showed that Mip is able to inhibit the binding of the nucleoid-structuring silencer MvaT to DNA and that Mip and MvaT coprecipitate in pulldown assays. However, the exact mechanism of MvaT inhibition by Mip is not completely understood, yet.
In the case of E. coli T4 phage, two different proteins were reported to interfere with H-NS silencing. The protein MotB is a DNA-binding protein that co-purifies with H-NS as well as with the H-NS homolog StpA. Deletion of the motB gene led to a decreased burst size [76]. The T4 protein Arn represents an interesting example of a phage-encoded DNA mimic protein and was shown to directly interact with E. coli H-NS [77]. While Arn was originally described as an inhibitor of the McrBC restriction enzyme, structural analysis revealed that the shape of the protein mimics the shape and charge of double-stranded DNA, and the authors highlight this DNA mimicry as a mechanistic basis for interfering with the function of DNA-binding proteins, like H-NS [77]. Interestingly, the DNA mimic proteins Ocr of the phage T7 and ArdA of the plasmid Collb-P9 were also reported to antagonize H-NS in a similar way [78].
Finally, the direct interference with TFs or other proteins likely does not represent the only way to fight off XS. In a very recent study, Kronheim et al. highlighted the important role of small molecules secreted by bacterial hosts as weapons against phage infection [79]. A link between these compounds and XS proteins does not necessarily exist, but a few examples suggest that small natural compounds – other than proteins – may also counteract XS. One class of compounds is represented by polyamides containing a biaryl motif. These polyamides especially target the minor groove of AT-rich DNA sequences and manipulate their topology [80]. In their study, Brucoli et al. therefore suggest an effect of these chemical compounds on XS. A further example is the antiasthma drug zafirlukast, which was shown to inhibit the DNA-binding ability of Lsr2 in M. tuberculosis and M. smegmatis [81]. This compound was found to inhibit the growth of both mycobacterial strains and led to clarification of the bacterial cultures after three days. A direct interaction between zafirlukast and Lsr2 was revealed by MALDI-TOF analysis. However, we suggest that such interactions are specific for the particular protein since, in our hands, zafirlukast does not counteract the silencing mediated by the Lsr2-like protein CgpS (unpublished data).
Silencers in actinobacteriophages
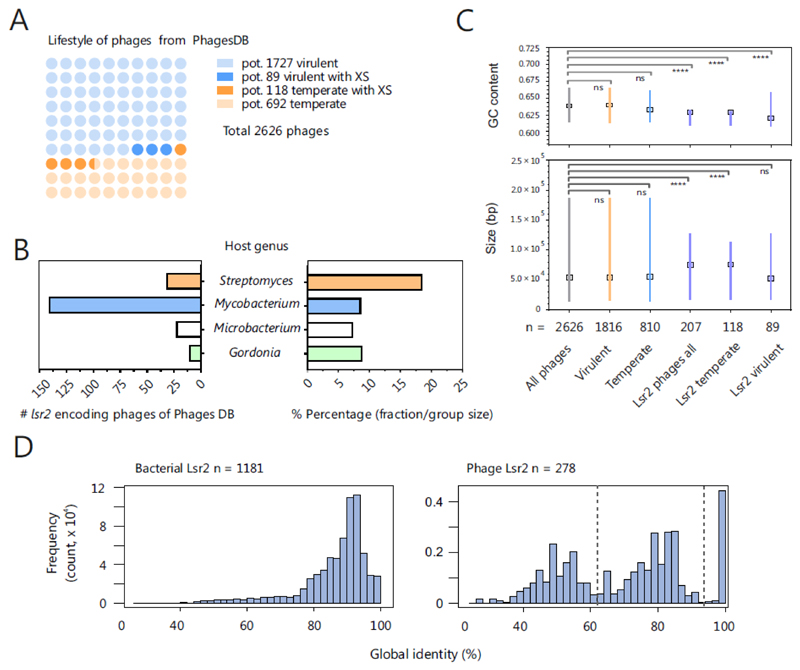
With the Lsr2-like silencer CgpS, we recently provided the first example of a prophage-encoded XS protein [9]. We showed that this protein is crucial for silencing of phage gene expression to maintain the lysogenic state of the large cryptic prophage CGP3 on which it is encoded. Thus far, in the current literature, only one publication, which was based on metagenomics, has reported the presence of an H-NS-like gene in a phage genome [82]. To evaluate how commonly XS-encoding genes are found in phage genomes, we screened phage databases for these genes. Using the actinobacteriophage database PhagesDB [83], we obtained >300 hits (blastp, e-value < 0.005) for Lsr2-like proteins. No phages encoding the other types of silencers, H-NS, MvaT or Rok, were found in the genomes of phages infecting actinobacteria, which is not surprising, as members of this bacterial class harbor only Lsr2-like proteins. Thus, we extended the screening to the Virus-Host database (>2500 bacteriophages) [84]. Strikingly, we could not obtain a single hit for H-NS-, MvaT- or Rok-like proteins in >1000 Proteobacterium phages and in >600 phages that infect Firmicutes. These findings illustrate that the function of Lsr2-like proteins has clearly been adopted by actinobacteriophages and that genes are commonly transferred by phages between GC-rich Actinobacteria. In an initial evaluation, we used PHACTS [85] to allocate the >2600 actinophages into temperate (>800) and virulent phages (>1800) and found Lsr2-like proteins in both groups (Figure 2A, Table S1). Taking into account the respective group sizes (virulent > temperate), Lsr2-like proteins are approximately three times more frequent in temperate than virulent phages (Figure 2A). An overview of their respective hosts evinced four different genera, Gordonia, Microbacterium, Mycobacterium and Streptomyces, in which the mycobacteriophages represent the largest group of Lsr2-encoding phages with 141 members (Figure 2B). However, mycobacteriophages are strongly overrepresented in PhagesDB (>1600). When considering the overall group sizes of the respective hosts, lsr2 genes are most likely to be found in genomes of Streptomyces phages (18.6 %, 32 of 172) (Figure 2B). Remarkably, comparisons of the GC contents and the genome sizes of Lsr2-encoding phages showed that the genomes were significantly more AT-rich and larger, especially for phages with a putative temperate lifestyle (Figure 2C). In addition, within the Lsr2 group, genomes of virulent phages exhibit a higher variation with respect to GC content and genome size (Figure 2C). Furthermore, we performed secondary structure predictions to compare phage- and host-encoded silencer proteins (within the respective group) by their global pairwise identity. While bacterial Lsr2 proteins are highly conserved, we identified a strong variability in terms of the predicted secondary structure within phage-encoded Lsr2-like proteins (Figure 2D). Taken together, these findings suggest that phage-encoded Lsr2-like proteins have different functions, presumably based on the phage lifestyle.
Figure 2. Lsr2-like proteins are encoded on actinobacteriophage genomes.
A. Distribution of Lsr2-encoding phages is shown among temperate and virulent actinophages. Based on the genomes downloaded from the actinobacteriophage database PhagesDB [83], coding sequences were predicted by prodigal [101] and the lifestyles of phages were predicted using PHACTS [85]. The temperate lifestyle was assigned if the mean minus the standard deviation of the calculated probability was >0.5. Otherwise, a virulent lifestyle was assumed. By this approach, 1816 (1727 corresponding to light blue and 89 corresponding to blue balls) were predicted to be virulent and 810 (692 light orange and 118 corresponding to orange balls), to be temperate (out of 2626 phages, downloaded 19.10.2018). A blastp search (default parameters, e-value < 0.005) revealed 207 phages encoding Lsr2-like proteins, of which 89 (blue balls) are virulent and 118 temperate phages (orange balls). Phages containing more than one gene encoding an Lsr2-like protein were counted only once; hits found in draft genomes were excluded. B. Overview of the host genus of Lsr2-encoding phages. On the left side, the absolute numbers are indicated. The right side of plot shows the proportion of Lsr2-encoding phages among all phages for the respective host genus. C GC contents and sizes of temperate, virulent and silencer encoding phages were compared with reference group (all phages) by the Kruskal-Wallis test. The medians are indicated with boxes. In the GC-content plot, the lines represent the interquartile ranges, whereas in the size plot, the lines indicate the range of the minimal and maximal values. D. Global pairwise secondary structure identity between Lsr2 sequences encoded in bacterial (left side) and phage genomes (right side). The identity of Lsr2 structure within bacterial genomes is relative high (mean ~87%) compared to the phage encoded Lsr2 sequences (mean ~70%). Furthermore, the distribution of the phage encoded Lsr2 structure identity evinces a higher diversity by pointing to the existence of particular pairs with high identity to each other and low to the rest of the data set.
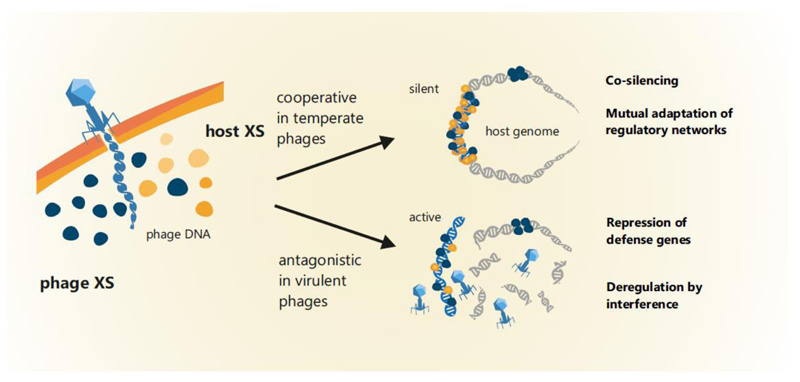
A hypothesis derived from stealth plasmids (discussed in the section below) and emphasized by the example of CgpS [9] is that XS proteins are involved in maintaining the lysogenic state, thereby presenting a mechanism of mutual adaptation and integration into host regulatory networks (Figure 3). In this scenario XS proteins would work in conjunction with the endogenous phage repression system, stabilizing lysogeny and minimizing the costs for harboring the temperate phage. Furthermore, it is conceivable that XS proteins may influence the lytic-lysogenic decision during phage infection, but this has not yet been addressed experimentally. In virulent phages, silencer proteins may inherit completely different roles. In addition to affecting multiple other targets, XS proteins were also found to repress genes encoding different phage defense systems, including CRISPR-cas genes [86] and an RM system [9]. Furthermore, interference of XS proteins, which are not capable of forming functional heteromeric complexes, may lead to a deregulation of phage genes and counteracting the silencing of phage genes by the host XS. We tested this hypothesis in our previous study where expression of E. coli hns as well as other genes encoding mycobacterial Lsr2 proteins led to activation of the CgpS-silenced prophage [9]. Hence, we suggest that virulent phages might employ XS homologs as a weapon to interfere with host defense systems (Figure 3). In addition, XS proteins or, in general, NAPs are also used to organize and structure the genome during replication cycles. Hence, it is likely that phages, especially with ‘larger’ genomes, will benefit from efficient DNA packaging proteins that facilitate optimized phage replication and production. Moreover, it is conceivable that phage-encoded silencers may contribute to compaction of the host genome. Here, an analogous example is given by the eukaryotic dinoflagellates. It is suggested that virus-like proteins, termed Dinoflagellate/Viral NucleoProteins (DVNPs), fulfill the functions of histones by packaging the genomes. This is supported by a recent study in which it was shown that heterologous produced DVNPs outcompete histones in Saccharomyces cerevisiae by causing toxic effects [87]. Although the dinoflagellate genomes encode histone proteins, the histone expression is strongly reduced, and it is assumed that they provide only regulatory functions [88]. Moreover, based on phylogenetics, it is hypothesized that the histone depletion occurred simultaneously with the acquisition of DVNPs from large-genome viruses (genome size up to 560 kb) and with massive genome expansion [89].
Figure 3. Model for the functions of phage-encoded silencers depending on the phage lifestyle.
Examples like the Lsr2-like silencer CgpS demonstrated that XS proteins may play an important role in maintaining the lysogenic state. Depending on the XS repertoire or the particular host strain, (pro-)phage-encoded XS proteins may also cooperate with the host-encoded protein to form heteromeric complexes. The function of phage-encoded silencers has not been studied experimentally and therefore remains subject to speculation. Nevertheless, it can be postulated that virulent phages might employ XS-like proteins as a weapon to interfere with host XS proteins or to repress other host defense mechanisms.
Stealth silencers encoded on plasmids
Usually, the introduction of new MGEs, such as plasmids, imposes high fitness costs on the host organism [90]. The magnitude of the costs depends on many factors, such as plasmid-specific characteristics (replication, plasmid reception, integration & conjugation, encoded traits), expression level of plasmid-borne genes and the genetic background of the host organism [90]. Interestingly, it is assumed that the main burden arises from the expression of plasmid-encoded genes that comes from transcription, translation, or the interactions between plasmid- and host-encoded proteins [91]. One way to reduce the cost is to use ‘stealth genes’, particularly genes encoding silencer proteins. The H-NS-like protein Sfh was one of the first characterized plasmid encoded stealth proteins. Doyle et al. investigated the costs of the AT-rich pSf-R27 plasmid and evinced a significant biosynthetic burden in the absence of sfh [92]. Therefore, the authors concluded that these proteins are quite useful in infiltrating a new host by reducing the metabolic burden to a minimum. Strikingly, transcriptome and genome-wide binding analysis revealed that the regulons from the chromosomally- and plasmid-encoded H-NS-like silencers are completely different [93–95], although the proteins are closely related. While plasmid-encoded variants typically exhibit a specific and narrow target spectrum (mostly with a focus on HGT-acquired regions), the host H-NS is known to act as a global regulator modulating the expression of both HGT-acquired and core genes [94, 95]. The basis for this selectivity was addressed by chimeric protein fusions, and the experimental results suggest a correlation between higher flexibility of the linker-domain (connecting the N-terminal oligomerization part to the C-terminal DNA-binding domain) and a decrease in selectivity due to stable binding to broader ranges of DNA geometries [96]. Astonishingly, in a more recent study, a known H-NS target, the gadAB operon, was examined in Shigella flexneri. Here, reduced expression was observed in the presence of the pSf-R27 plasmid, leading to reduced acid resistance and showing that a plasmid- and host- encoded silencer also co-regulate core genes [97].
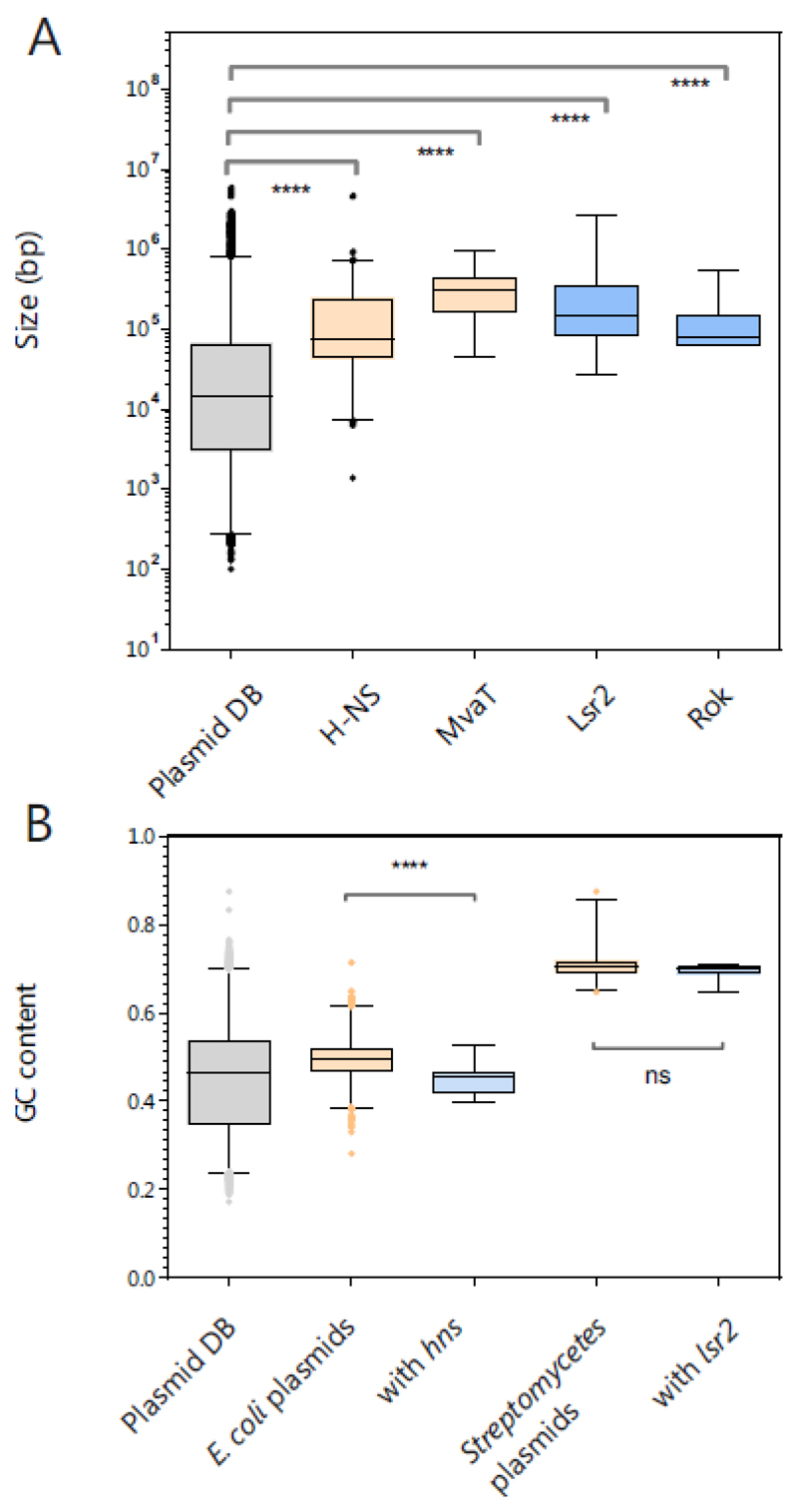
Bioinformatics analyses revealed that genes encoding H-NS- and MvaT-like silencers are overrepresented on large plasmids. In addition, H-NS-encoding plasmids are found to be more AT-rich than other NAP-harboring plasmids [98]. To also evaluate the distribution of Lsr2- and Rok-like silencers, we performed a BLASTp search (e-value < 0.005) using a plasmid database that we retrieved from the NCBI nucleotide database (>24000 plasmid sequences, source database RefSeq, Figure 4A). Here, we identified 408 hits for H-NS-like proteins, 35 for MvaT-like proteins, 63 for Lsr2-like proteins and 18 for Rok-like proteins. A comparison of the average sizes revealed that silencer-encoding plasmids are at least five times larger than the average plasmid sequence deposited in the database (Figure 4A). Furthermore, H-NS-encoding E. coli plasmids displayed a significantly higher AT content (96 of the 408 H-NS plasmids) (Figure 4B). This trend was, however, not observed for GC-rich Streptomycetes (Figure 4B) or P. aeruginosa and B. subtilis plasmids (data not shown), but the sequence data in these databases feature a strong bias, and only a few sequences are available for some species (e.g. n=13 for Streptomycetes plasmids). In line with previous studies, our findings emphasize that especially large plasmids appear to benefit from DNA-organizing proteins and that self-silencing may represent a strategy for host infiltration harnessed by phages and plasmids alike.
Figure 4. Distribution of silencer-encoding genes on plasmids.
A. Overall, 24197 sequences for plasmids were retrieved from the NCBI nucleotide database using RefSeq as the source data base (Filter criteria: Bacteria, genomic DNA, Plasmid, RefSeq on 29.10.2018). Via a local blastp search (e-value < 0.005) with the amino acid sequences of H-NS (WP_001287378.1), MvaT (WP_003093888.1), Lsr2 (WP_003419513.1) and Rok (WP_003232378.1), approximately 408, 35, 63 and 18 hits, respectively, were found. The sizes were compared in a boxplot with ranges from 1-99% and evaluated by Kruskal-Wallis tests. B. GC content of the plasmids, including all sequences, E. coli plasmids (n = 2600), with hns (n=95) and Streptomycetes plasmids (n=147) with lsr2 (13) were compared in a boxplot (range 1-99%, evaluated by Kruskal-Wallis tests).
Future perspectives
In conclusion, several recent studies highlight the important role of XS proteins in phage-host interactions, e.g., by silencing expression of genomically integrated prophages or phage remnants. The first examples, like the T7 5.5 protein or Mip [74, 75] encoded by a Pseudomonas phage, demonstrate that counteracting XS represents an important aspect of lytic infection. As illustrated by the examples of Lsr2-like proteins encoded by various actinophages, the function of XS proteins apparently has been adopted by phages as well. Nevertheless, many gaps remain in the prokaryotic and phage sequence space as for the majority of prokaryotic phyla, no XS protein has been identified so far. Furthermore, it is striking that while homologs of H-NS, MvaT, Rok and Lsr2 are encoded by plasmids, only the function of Lsr2-like proteins has been employed by phages. Considering the high sequence variability of phage-encoded silencers, these proteins most likely perform many different functions depending on the particular lifestyle of the phage, which needs to be addressed in future studies.
Supplementary files
Table 2. Counteracting xenogeneic silencing by phages or other MGEs.
| Counter-actor | Mechanism | Host | Host XS | Phage or MGE | Source |
|---|---|---|---|---|---|
| H-NST | H-NST represents a truncated version of H-NS consisting of the oligomerization domain. H-NST interferes with the correct oligomerization of the native H-NS protein and therefore the correct function. | E. coli; Uro-pathogenic strain CFT073 and entero-pathogenic strain E2348/69 | H-NS | CFT073: UPEC-specific island inserted at serU, E2348/69: EPEC-specific island at asnW |
[70] |
| T7 protein 5.5 | Protein 5.5 is able to interact with the central oligomerization domain and hinders H-NS from forming high-order oligomers | E. coli e.g. BL21 (DE3) | H-NS | Phage T7 (virulent) | [73, 74] |
| Ler | Ler is a DNA-binding H-NS homologue that is able to increase DNA rigidity similar to the nucleoprotein filament formed by H-NS. This binding leads to a displacement of H-NS and counter-silencing. | E. coli (enteropathogenic strain E2348/69) | H-NS | Horizontally acquired pathogenicity island (LEE1) | [65–67, 69] |
| Mip | The MvaT-inhibiting protein (Mip) coprecipitates with MvaT and was shown to inhibit the DNA-binding of MvaT. | P. aerigunosa PAO1 | MvaT | Phage LUZ24 (virulent) | [75] |
| MotB | MotB copurifies with H-NS and StpA. Deletion of the motB gene leads to decreased burst size of T4. Hence, a counter-acting ability against H-NS was suggested. | E. coli | H-NS | Phage T4 (virulent) | [76] |
| Arn | Arn acts as DNA mimicking protein (mimicking the charge and structure of dsDNA) and is able to bind H-NS. Arn binding could be shown to interfere with the binding of H-NS to target regions. | E. coli e.g. BL21 (DE3) | H-NS | Phage T4 (virulent) | [77, 78] |
| Ocr | Ocr is a DNA mimicking protein that mimics B-form DNA. It was shown that Ocr is able to counter-silence H-NS-silenced promoters. | E. coli (i.a. C600) | H-NS | Phage T7 (virulent) | [78, 99] |
| ArdA | The crystallization of the ArdA dimer led to the assumption that ArdA is a DNA mimic proteins. Further, it could be shown that increased amounts of ArdA leads to a counter-silencing of H-NS silenced promoters in vivo. | multiple organisms | H-NS | Plasmid Collb-P9 | [78, 100] |
Acknowledgments
For financial support, we thank the German Research Foundation (SPP 1617, grant FR2759/2-2) and the European Research Council (ERC-StG-2017, grant 757563).
We thank Larissa Kever (Institute for Bio- and Geosciences 1, IBG-1) for support in graphic design of the the figures and Anna Schumann for the assistance in data management.
References
- [1].Casjens S. Prophages and bacterial genomics: what have we learned so far? Molecular microbiology. 2003;49:277–300. doi: 10.1046/j.1365-2958.2003.03580.x. [DOI] [PubMed] [Google Scholar]
- [2].Canchaya C, Fournous G, Brüssow H. The impact of prophages on bacterial chromosomes. Molecular microbiology. 2004;53:9–18. doi: 10.1111/j.1365-2958.2004.04113.x. [DOI] [PubMed] [Google Scholar]
- [3].Hatfull GF, Hendrix RW. Bacteriophages and their genomes. Current opinion in virology. 2011;1:298–303. doi: 10.1016/j.coviro.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bondy-Denomy J, Davidson AR. When a virus is not a parasite: the beneficial effects of prophages on bacterial fitness. Journal of microbiology. 2014;52:235–42. doi: 10.1007/s12275-014-4083-3. [DOI] [PubMed] [Google Scholar]
- [5].Pennington JM, Rosenberg SM. Spontaneous DNA breakage in single living Escherichia coli cells. Nature genetics. 2007;39:797–802. doi: 10.1038/ng2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nanda AM, Thormann K, Frunzke J. Impact of spontaneous prophage induction on the fitness of bacterial populations and host-microbe interactions. J Bacteriol. 2015;197:410–9. doi: 10.1128/JB.02230-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lwoff A. Lysogeny. Bacteriological reviews. 1953;17:269–337. doi: 10.1128/br.17.4.269-337.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nanda AM, Heyer A, Kramer C, Grunberger A, Kohlheyer D, Frunzke J. Analysis of SOS-induced spontaneous prophage induction in Corynebacterium glutamicum at the single-cell level. J Bacteriol. 2014;196:180–8. doi: 10.1128/JB.01018-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pfeifer E, Hünnefeld M, Popa O, Polen T, Kohlheyer D, Baumgart M, et al. Silencing of cryptic prophages in Corynebacterium glutamicum. Nucleic Acids Res. 2016;44:10117–31. doi: 10.1093/nar/gkw692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].de la Cruz F, Davies J. Horizontal gene transfer and the origin of species: lessons from bacteria. Trends Microbiol. 2000;8:128–33. doi: 10.1016/s0966-842x(00)01703-0. [DOI] [PubMed] [Google Scholar]
- [11].Ochman H, Lawrence JG, Groisman EA. Lateral gene transfer and the nature of bacterial innovation. Nature. 2000;405:299–304. doi: 10.1038/35012500. [DOI] [PubMed] [Google Scholar]
- [12].Darmon E, Leach DRF. Bacterial Genome Instability. Microbiol Mol Biol R. 2014;78:1–39. doi: 10.1128/MMBR.00035-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lamberte LE, Baniulyte G, Singh SS, Stringer AM, Bonocora RP, Stracy M, et al. Horizontally acquired AT-rich genes in Escherichia coli cause toxicity by sequestering RNA polymerase. Nat Microbiol. 2017;2 doi: 10.1038/nmicrobiol.2016.249. 16249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rohwer F, Youle M, Maughan H, Hisakawa N, Pantéa LL, Darby B. Life in our phage world: a centennial field guide to the Earth's most diverse inhabitants. 2015 [Google Scholar]
- [15].Labrie SJ, Samson JE, Moineau S. Bacteriophage resistance mechanisms. Nature reviews Microbiology. 2010;8:317–27. doi: 10.1038/nrmicro2315. [DOI] [PubMed] [Google Scholar]
- [16].Doron S, Melamed S, Ofir G, Leavitt A, Lopatina A, Keren M, et al. Systematic discovery of antiphage defense systems in the microbial pangenome. Science. 2018:359. doi: 10.1126/science.aar4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stern A, Sorek R. The phage-host arms race: shaping the evolution of microbes. BioEssays : news and reviews in molecular, cellular and developmental biology. 2011;33:43–51. doi: 10.1002/bies.201000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dupuis ME, Villion M, Magadan AH, Moineau S. CRISPR-Cas and restriction-modification systems are compatible and increase phage resistance. Nat Commun. 2013;4:2087. doi: 10.1038/ncomms3087. [DOI] [PubMed] [Google Scholar]
- [19].Navarre WW. The Impact of Gene Silencing on Horizontal Gene Transfer and Bacterial Evolution. Adv Microb Physiol. 2016;69:157–86. doi: 10.1016/bs.ampbs.2016.07.004. [DOI] [PubMed] [Google Scholar]
- [20].Singh K, Milstein JN, Navarre WW. Xenogeneic Silencing and Its Impact on Bacterial Genomes. Annu Rev Microbiol. 2016;70:199–213. doi: 10.1146/annurev-micro-102215-095301. [DOI] [PubMed] [Google Scholar]
- [21].Will WR, Bale DH, Reid PJ, Libby SJ, Fang FC. Evolutionary expansion of a regulatory network by counter-silencing. Nat Commun. 2014;5 doi: 10.1038/ncomms6270. 5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Navarre WW, McClelland M, Libby SJ, Fang FC. Silencing of xenogeneic DNA by H-NS-facilitation of lateral gene transfer in bacteria by a defense system that recognizes foreign DNA. Genes Dev. 2007;21:1456–71. doi: 10.1101/gad.1543107. [DOI] [PubMed] [Google Scholar]
- [23].Tendeng C, Soutourina OA, Danchin A, Bertin PN. MvaT proteins in Pseudomonas spp.: a novel class of H-NS-like proteins. Microbiology. 2003;149:3047–50. doi: 10.1099/mic.0.C0125-0. [DOI] [PubMed] [Google Scholar]
- [24].Smits WK, Grossman AD. The transcriptional regulator Rok binds A+T-rich DNA and is involved in repression of a mobile genetic element in Bacillus subtilis. PLoS Genet. 2010;6:e1001207. doi: 10.1371/journal.pgen.1001207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gordon BR, Imperial R, Wang L, Navarre WW, Liu J. Lsr2 of Mycobacterium represents a novel class of H-NS-like proteins. J Bacteriol. 2008;190:7052–9. doi: 10.1128/JB.00733-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dorman CJ. H-NS-like nucleoid-associated proteins, mobile genetic elements and horizontal gene transfer in bacteria. Plasmid. 2014;75:1–11. doi: 10.1016/j.plasmid.2014.06.004. [DOI] [PubMed] [Google Scholar]
- [27].Duan B, Ding P, Hughes TR, Navarre WW, Liu J, Xia B. How bacterial xenogeneic silencer rok distinguishes foreign from self DNA in its resident genome. Nucleic Acids Res. 2018;46:10514–29. doi: 10.1093/nar/gky836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rocha EP, Danchin A. Base composition bias might result from competition for metabolic resources. Trends Genet. 2002;18:291–4. doi: 10.1016/S0168-9525(02)02690-2. [DOI] [PubMed] [Google Scholar]
- [29].Nishida H. Comparative analyses of base compositions, DNA sizes, and dinucleotide frequency profiles in archaeal and bacterial chromosomes and plasmids. Int J Evol Biol. 2012;2012 doi: 10.1155/2012/342482. 342482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ali SS, Xia B, Liu J, Navarre WW. Silencing of foreign DNA in bacteria. Curr Opin Microbiol. 2012;15:175–81. doi: 10.1016/j.mib.2011.12.014. [DOI] [PubMed] [Google Scholar]
- [31].Ding P, McFarland KA, Jin S, Tong G, Duan B, Yang A, et al. A Novel AT-Rich DNA Recognition Mechanism for Bacterial Xenogeneic Silencer MvaT. PLoS Pathog. 2015;11:e1004967. doi: 10.1371/journal.ppat.1004967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lang B, Blot N, Bouffartigues E, Buckle M, Geertz M, Gualerzi CO, et al. High-affinity DNA binding sites for H-NS provide a molecular basis for selective silencing within proteobacterial genomes. Nucleic Acids Res. 2007;35:6330–7. doi: 10.1093/nar/gkm712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gulvady R, Gao Y, Kenney LJ, Yan J. A single molecule analysis of H-NS uncouples DNA binding affinity from DNA specificity. Nucleic Acids Res. 2018;46:10216–24. doi: 10.1093/nar/gky826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Grainger DC. Structure and function of bacterial H-NS protein. Biochem Soc Trans. 2016;44:1561–9. doi: 10.1042/BST20160190. [DOI] [PubMed] [Google Scholar]
- [35].Kotlajich MV, Hron DR, Boudreau BA, Sun Z, Lyubchenko YL, Landick R. Bridged filaments of histone-like nucleoid structuring protein pause RNA polymerase and aid termination in bacteria. Elife. 2015;4:36. doi: 10.7554/eLife.04970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Winardhi RS, Fu W, Castang S, Li Y, Dove SL, Yan J. Higher order oligomerization is required for H-NS family member MvaT to form gene-silencing nucleoprotein filament. Nucleic Acids Res. 2012;40:8942–52. doi: 10.1093/nar/gks669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Arold ST, Leonard PG, Parkinson GN, Ladbury JE. H-NS forms a superhelical protein scaffold for DNA condensation. Proc Natl Acad Sci U S A. 2010;107:15728–32. doi: 10.1073/pnas.1006966107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gordon BR, Li Y, Wang L, Sintsova A, van Bakel H, Tian S, et al. Lsr2 is a nucleoid-associated protein that targets AT-rich sequences and virulence genes in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2010;107:5154–9. doi: 10.1073/pnas.0913551107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Winardhi RS, Yan J, Kenney LJ. H-NS Regulates Gene Expression and Compacts the Nucleoid: Insights from Single-Molecule Experiments. Biophys J. 2015;109:1321–9. doi: 10.1016/j.bpj.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Will WR, Whitham PJ, Reid PJ, Fang FC. Modulation of H-NS transcriptional silencing by magnesium. Nucleic Acids Res. 2018;46:5717–25. doi: 10.1093/nar/gky387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Liu Y, Chen H, Kenney LJ, Yan J. A divalent switch drives H-NS/DNA-binding conformations between stiffening and bridging modes. Genes Dev. 2010;24:339–44. doi: 10.1101/gad.1883510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dame RT, Luijsterburg MS, Krin E, Bertin PN, Wagner R, Wuite GJ. DNA bridging: a property shared among H-NS-like proteins. J Bacteriol. 2005;187:1845–8. doi: 10.1128/JB.187.5.1845-1848.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Qu Y, Lim CJ, Whang YR, Liu J, Yan J. Mechanism of DNA organization by Mycobacterium tuberculosis protein Lsr2. Nucleic Acids Res. 2013;41:5263–72. doi: 10.1093/nar/gkt249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Paytubi S, Madrid C, Forns N, Nieto JM, Balsalobre C, Uhlin BE, et al. YdgT, the Hha paralogue in Escherichia coli, forms heteromeric complexes with H-NS and StpA. Molecular microbiology. 2004;54:251–63. doi: 10.1111/j.1365-2958.2004.04268.x. [DOI] [PubMed] [Google Scholar]
- [45].Ueda T, Takahashi H, Uyar E, Ishikawa S, Ogasawara N, Oshima T. Functions of the Hha and YdgT proteins in transcriptional silencing by the nucleoid proteins, H-NS and StpA, in Escherichia coli. DNA Res. 2013;20:263–71. doi: 10.1093/dnares/dst008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kim MS, Bae SH, Yun SH, Lee HJ, Ji SC, Lee JH, et al. Cnu, a novel oriC-binding protein of Escherichia coli. J Bacteriol. 2005;187:6998–7008. doi: 10.1128/JB.187.20.6998-7008.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Seid CA, Smith JL, Grossman AD. Genetic and biochemical interactions between the bacterial replication initiator DnaA and the nucleoid-associated protein Rok in Bacillus subtilis. Molecular microbiology. 2017;103:798–817. doi: 10.1111/mmi.13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Boudreau BA, Hron DR, Qin L, van der Valk RA, Kotlajich MV, Dame RT, et al. StpA and Hha stimulate pausing by RNA polymerase by promoting DNA-DNA bridging of H-NS filaments. Nucleic Acids Res. 2018;46:5525–46. doi: 10.1093/nar/gky265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Castang S, Dove SL. High-order oligomerization is required for the function of the H-NS family member MvaT in Pseudomonas aeruginosa. Molecular microbiology. 2010;78:916–31. doi: 10.1111/j.1365-2958.2010.07378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sun Z, Vasileva D, Suzuki-Minakuchi C, Okada K, Luo F, Igarashi Y, et al. Differential protein-protein binding affinities of H-NS family proteins encoded on the chromosome of Pseudomonas putida KT2440 and IncP-7 plasmid pCAR1. Biosci Biotechnol Biochem. 2018;82:1640–6. doi: 10.1080/09168451.2018.1484277. [DOI] [PubMed] [Google Scholar]
- [51].Yun CS, Takahashi Y, Shintani M, Takeda T, Suzuki-Minakuchi C, Okada K, et al. MvaT Family Proteins Encoded on IncP-7 Plasmid pCAR1 and the Host Chromosome Regulate the Host Transcriptome Cooperatively but Differently. Appl Environ Microbiol. 2016;82:832–42. doi: 10.1128/AEM.03071-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yun CS, Suzuki C, Naito K, Takeda T, Takahashi Y, Sai F, et al. Pmr, a histone-like protein H1 (H-NS) family protein encoded by the IncP-7 plasmid pCAR1, is a key global regulator that alters host function. J Bacteriol. 2010;192:4720–31. doi: 10.1128/JB.00591-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Dilweg IW, Dame RT. Post-translational modification of nucleoid-associated proteins: an extra layer of functional modulation in bacteria? Biochem Soc Trans. 2018;46:1381–92. doi: 10.1042/BST20180488. [DOI] [PubMed] [Google Scholar]
- [54].Castang S, Dove SL. Basis for the essentiality of H-NS family members in Pseudomonas aeruginosa. J Bacteriol. 2012;194:5101–9. doi: 10.1128/JB.00932-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Li C, Wally H, Miller SJ, Lu CD. The multifaceted proteins MvaT and MvaU, members of the H-NS family, control arginine metabolism, pyocyanin synthesis, and prophage activation in Pseudomonas aeruginosa PAO1. J Bacteriol. 2009;191:6211–8. doi: 10.1128/JB.00888-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hong SH, Wang X, Wood TK. Controlling biofilm formation, prophage excision and cell death by rewiring global regulator H-NS of Escherichia coli. Microbial biotechnology. 2010;3:344–56. doi: 10.1111/j.1751-7915.2010.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Freiherr von Boeselager R, Pfeifer E, Frunzke J. Cytometry meets next-generation sequencing - RNA-Seq of sorted subpopulations reveals regional replication and iron-triggered prophage induction in Corynebacterium glutamicum. Sci Rep. 2018;8 doi: 10.1038/s41598-018-32997-9. 14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Albano M, Smits WK, Ho LT, Kraigher B, Mandic-Mulec I, Kuipers OP, et al. The Rok protein of Bacillus subtilis represses genes for cell surface and extracellular functions. J Bacteriol. 2005;187:2010–9. doi: 10.1128/JB.187.6.2010-2019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zeng Z, Liu X, Yao J, Guo Y, Li B, Li Y, et al. Cold adaptation regulated by cryptic prophage excision in Shewanella oneidensis. The ISME journal. 2016;10:2787–800. doi: 10.1038/ismej.2016.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Caramel A, Schnetz K. Lac and lambda repressors relieve silencing of the Escherichia coli bgl promoter. Activation by alteration of a repressing nucleoprotein complex. Journal of molecular biology. 1998;284:875–883. doi: 10.1006/jmbi.1998.2191. [DOI] [PubMed] [Google Scholar]
- [61].Yu RR, DiRita VJ. Regulation of gene expression in Vibrio cholerae by ToxT involves both antirepression and RNA polymerase stimulation. Molecular microbiology. 2002;43:119–34. doi: 10.1046/j.1365-2958.2002.02721.x. [DOI] [PubMed] [Google Scholar]
- [62].De la Cruz MA, Fernandez-Mora M, Guadarrama C, Flores-Valdez MA, Bustamante VH, Vazquez A, et al. LeuO antagonizes H-NS and StpA-dependent repression in Salmonella enterica ompS1. Molecular microbiology. 2007;66:727–43. doi: 10.1111/j.1365-2958.2007.05958.x. [DOI] [PubMed] [Google Scholar]
- [63].Navarre WW, Halsey TA, Walthers D, Frye J, McClelland M, Potter JL, et al. Co-regulation of Salmonella enterica genes required for virulence and resistance to antimicrobial peptides by SlyA and PhoP/PhoQ. Molecular microbiology. 2005;56:492–508. doi: 10.1111/j.1365-2958.2005.04553.x. [DOI] [PubMed] [Google Scholar]
- [64].Heroven AK, Nagel G, Tran HJ, Parr S, Dersch P. RovA is autoregulated and antagonizes H-NS-mediated silencing of invasin and rovA expression in Yersinia pseudotuberculosis. Molecular microbiology. 2004;53:871–88. doi: 10.1111/j.1365-2958.2004.04162.x. [DOI] [PubMed] [Google Scholar]
- [65].Bustamante VH, Santana FJ, Calva E, Puente JL. Transcriptional regulation of type III secretion genes in enteropathogenic Escherichia coli: Ler antagonizes H-NS-dependent repression. Molecular microbiology. 2001;39:664–78. doi: 10.1046/j.1365-2958.2001.02209.x. [DOI] [PubMed] [Google Scholar]
- [66].Mellies JL, Benison G, McNitt W, Mavor D, Boniface C, Larabee FJ. Ler of pathogenic Escherichia coli forms toroidal protein-DNA complexes. Microbiology. 2011;157:1123–33. doi: 10.1099/mic.0.046094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Garcia J, Cordeiro TN, Prieto MJ, Pons M. Oligomerization and DNA binding of Ler, a master regulator of pathogenicity of enterohemorrhagic and enteropathogenic Escherichia coli. Nucleic Acids Res. 2012;40:10254–62. doi: 10.1093/nar/gks846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].McDaniel TK, Jarvis KG, Donnenberg MS, Kaper JB. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci U S A. 1995;92:1664–8. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Winardhi RS, Gulvady R, Mellies JL, Yan J. Locus of enterocyte effacement-encoded regulator (Ler) of pathogenic Escherichia coli competes off histone-like nucleoid-structuring protein (H-NS) through noncooperative DNA binding. J Biol Chem. 2014;289:13739–50. doi: 10.1074/jbc.M113.545954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Williamson HS, Free A. A truncated H-NS-like protein from enteropathogenic Escherichia coli acts as an H-NS antagonist. Molecular microbiology. 2005;55:808–27. doi: 10.1111/j.1365-2958.2004.04421.x. [DOI] [PubMed] [Google Scholar]
- [71].Levine JA, Hansen AM, Michalski JM, Hazen TH, Rasko DA, Kaper JB. H-NST induces LEE expression and the formation of attaching and effacing lesions in enterohemorrhagic Escherichia coli. PLoS One. 2014;9:e86618. doi: 10.1371/journal.pone.0086618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Perez-Rueda E, Ibarra JA. Distribution of putative xenogeneic silencers in prokaryote genomes. Comput Biol Chem. 2015;58:167–72. doi: 10.1016/j.compbiolchem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- [73].Liu Q, Richardson CC. Gene 5.5 protein of bacteriophage T7 inhibits the nucleoid protein H-NS of Escherichia coli. Proc Natl Acad Sci U S A. 1993;90:1761–5. doi: 10.1073/pnas.90.5.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ali SS, Beckett E, Bae SJ, Navarre WW. The 5.5 protein of phage T7 inhibits H-NS through interactions with the central oligomerization domain. J Bacteriol. 2011;193:4881–92. doi: 10.1128/JB.05198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wagemans J, Delattre AS, Uytterhoeven B, De Smet J, Cenens W, Aertsen A, et al. Antibacterial phage ORFans of Pseudomonas aeruginosa phage LUZ24 reveal a novel MvaT inhibiting protein. Front Microbiol. 2015;6:1242. doi: 10.3389/fmicb.2015.01242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Patterson-West J, Arroyo-Mendoza M, Hsieh ML, Harrison D, Walker MM, Knipling L, et al. The Bacteriophage T4 MotB Protein, a DNA-Binding Protein, Improves Phage Fitness. Viruses. 2018;10 doi: 10.3390/v10070343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ho CH, Wang HC, Ko TP, Chang YC, Wang AH. The T4 phage DNA mimic protein Arn inhibits the DNA binding activity of the bacterial histone-like protein H-NS. J Biol Chem. 2014;289:27046–54. doi: 10.1074/jbc.M114.590851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Melkina OE, Goryanin II, Zavilgelsky GB. The DNA-mimic antirestriction proteins ArdA ColIB-P9, Arn T4, and Ocr T7 as activators of H-NS-dependent gene transcription. Microbiol Res. 2016;192:283–91. doi: 10.1016/j.micres.2016.07.008. [DOI] [PubMed] [Google Scholar]
- [79].Kronheim S, Daniel-Ivad M, Duan Z, Hwang S, Wong AI, Mantel I, et al. A chemical defence against phage infection. Nature. 2018 doi: 10.1038/s41586-018-0767-x. [DOI] [PubMed] [Google Scholar]
- [80].Brucoli F, Guzman JD, Maitra A, James CH, Fox KR, Bhakta S. Synthesis, anti-mycobacterial activity and DNA sequence-selectivity of a library of biaryl-motifs containing polyamides. Bioorg Med Chem. 2015;23:3705–11. doi: 10.1016/j.bmc.2015.04.001. [DOI] [PubMed] [Google Scholar]
- [81].Pinault L, Han JS, Kang CM, Franco J, Ronning DR. Zafirlukast inhibits complexation of Lsr2 with DNA and growth of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2013;57:2134–40. doi: 10.1128/AAC.02407-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Skennerton CT, Angly FE, Breitbart M, Bragg L, He S, McMahon KD, et al. Phage encoded H-NS: a potential achilles heel in the bacterial defence system. PLoS One. 2011;6:e20095. doi: 10.1371/journal.pone.0020095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Russell DA, Hatfull GF. PhagesDB: the actinobacteriophage database. Bioinformatics. 2017;33:784–6. doi: 10.1093/bioinformatics/btw711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Mihara T, Nishimura Y, Shimizu Y, Nishiyama H, Yoshikawa G, Uehara H, et al. Linking Virus Genomes with Host Taxonomy. Viruses. 2016;8:66. doi: 10.3390/v8030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].McNair K, Bailey BA, Edwards RA. PHACTS, a computational approach to classifying the lifestyle of phages. Bioinformatics. 2012;28:614–8. doi: 10.1093/bioinformatics/bts014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Pul U, Wurm R, Arslan Z, Geissen R, Hofmann N, Wagner R. Identification and characterization of E. coli CRISPR-cas promoters and their silencing by H-NS. Molecular microbiology. 2010;75:1495–512. doi: 10.1111/j.1365-2958.2010.07073.x. [DOI] [PubMed] [Google Scholar]
- [87].Irwin NAT, Martin BJE, Young BP, Browne MJG, Flaus A, Loewen CJR, et al. Viral proteins as a potential driver of histone depletion in dinoflagellates. 2018;9:1535. doi: 10.1038/s41467-018-03993-4. Nat Commun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Gornik SG, Ford KL, Mulhern TD, Bacic A, McFadden GI, Waller RF. Loss of nucleosomal DNA condensation coincides with appearance of a novel nuclear protein in dinoflagellates. Curr Biol. 2012;22:2303–12. doi: 10.1016/j.cub.2012.10.036. [DOI] [PubMed] [Google Scholar]
- [89].Talbert PB, Henikoff S. Chromatin: packaging without nucleosomes. Curr Biol. 2012;22:R1040–3. doi: 10.1016/j.cub.2012.10.052. [DOI] [PubMed] [Google Scholar]
- [90].Carroll AC, Wong A. Plasmid persistence: costs, benefits, and the plasmid paradox. Can J Microbiol. 2018;64:293–304. doi: 10.1139/cjm-2017-0609. [DOI] [PubMed] [Google Scholar]
- [91].San Millan A, MacLean RC. Fitness Costs of Plasmids: a Limit to Plasmid Transmission. Microbiol Spectr. 2017;5 doi: 10.1128/microbiolspec.mtbp-0016-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Doyle M, Fookes M, Ivens A, Mangan MW, Wain J, Dorman CJ. An H-NS-like stealth protein aids horizontal DNA transmission in bacteria. Science. 2007;315:251–2. doi: 10.1126/science.1137550. [DOI] [PubMed] [Google Scholar]
- [93].Banos RC, Vivero A, Aznar S, Garcia J, Pons M, Madrid C, et al. Differential regulation of horizontally acquired and core genome genes by the bacterial modulator H-NS. PLoS Genet. 2009;5:e1000513. doi: 10.1371/journal.pgen.1000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Dillon SC, Cameron AD, Hokamp K, Lucchini S, Hinton JC, Dorman CJ. Genome-wide analysis of the H-NS and Sfh regulatory networks in Salmonella Typhimurium identifies a plasmid-encoded transcription silencing mechanism. Molecular microbiology. 2010;76:1250–65. doi: 10.1111/j.1365-2958.2010.07173.x. [DOI] [PubMed] [Google Scholar]
- [95].Banos RC, Aznar S, Madrid C, Juarez A. Differential functional properties of chromosomal- and plasmid-encoded H-NS proteins. Res Microbiol. 2011;162:382–5. doi: 10.1016/j.resmic.2011.02.003. [DOI] [PubMed] [Google Scholar]
- [96].Fernandez-de-Alba C, Berrow NS, Garcia-Castellanos R, Garcia J, Pons M. On the origin of the selectivity of plasmidic H-NS towards horizontally acquired DNA: linking H-NS oligomerization and cooperative DNA binding. J Mol Biol. 2013;425:2347–58. doi: 10.1016/j.jmb.2013.03.006. [DOI] [PubMed] [Google Scholar]
- [97].Niu C, Wang D, Liu X, Liu H, Liu X, Feng E, et al. An H-NS Family Protein, Sfh, Regulates Acid Resistance by Inhibition of Glutamate Decarboxylase Expression in Shigella flexneri 2457T. Front Microbiol. 2017;8:1923. doi: 10.3389/fmicb.2017.01923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Takeda T, Yun CS, Shintani M, Yamane H, Nojiri H. Distribution of genes encoding nucleoid-associated protein homologs in plasmids. Int J Evol Biol. 2011;2011 doi: 10.4061/2011/685015. 685015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Walkinshaw MD, Taylor P, Sturrock SS, Atanasiu C, Berge T, Henderson RM, et al. Structure of Ocr from bacteriophage T7, a protein that mimics B-form DNA. Mol Cell. 2002;9:187–94. doi: 10.1016/s1097-2765(02)00435-5. [DOI] [PubMed] [Google Scholar]
- [100].McMahon SA, Roberts GA, Johnson KA, Cooper LP, Liu H, White JH, et al. Extensive DNA mimicry by the ArdA anti-restriction protein and its role in the spread of antibiotic resistance. Nucleic Acids Res. 2009;37:4887–97. doi: 10.1093/nar/gkp478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Hyatt D, Chen GL, Locascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010;11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.