Significance
In mammals, the germline is set aside early in development for the later production of the gametes, either eggs or sperm. It remains unknown when, and how, the precursor cells (termed primordial germ cells [PGCs]) become committed to produce only gametes, and no other cell type. We identify an embryonic transition occurring late in development, after PGCs colonize the nascent gonad, that serves to commit these cells to produce only gametes. Our findings have broad implications for the origin of germ cell tumors in humans, and for the stepwise commitment of the germline in mammals and other vertebrates.
Keywords: germ cell, commitment, teratoma, pluripotency, Dazl
Abstract
Mammalian primordial germ cells (PGCs) are induced in the embryonic epiblast, before migrating to the nascent gonads. In fish, frogs, and birds, the germline segregates even earlier, through the action of maternally inherited germ plasm. Across vertebrates, migrating PGCs retain a broad developmental potential, regardless of whether they were induced or maternally segregated. In mammals, this potential is indicated by expression of pluripotency factors, and the ability to generate teratomas and pluripotent cell lines. How the germline loses this developmental potential remains unknown. Our genome-wide analyses of embryonic human and mouse germlines reveal a conserved transcriptional program, initiated in PGCs after gonadal colonization, that differentiates germ cells from their germline precursors and from somatic lineages. Through genetic studies in mice and pigs, we demonstrate that one such gonad-induced factor, the RNA-binding protein DAZL, is necessary in vivo to restrict the developmental potential of the germline; DAZL’s absence prolongs expression of a Nanog pluripotency reporter, facilitates derivation of pluripotent cell lines, and causes spontaneous gonadal teratomas. Based on these observations in humans, mice, and pigs, we propose that germ cells are determined after gonadal colonization in mammals. We suggest that germ cell determination was induced late in embryogenesis—after organogenesis has begun—in the common ancestor of all vertebrates, as in modern mammals, where this transition is induced by somatic cells of the gonad. We suggest that failure of this process of germ cell determination likely accounts for the origin of human testis cancer.
During embryogenesis, cells segregate into germline and somatic lineages. In mammals, this split is first evident around the time of gastrulation, when intercellular signaling induces the formation of primordial germ cells (PGCs) (1, 2). Comparative studies reveal that an inductive method of germline segregation likely existed in the common ancestor of all vertebrates (3). However, some vertebrates, such as fish, frogs, and birds, have acquired a different approach to germline segregation. It occurs much earlier in these species—during the first cell divisions of the zygote—through the action of maternally supplied RNAs known as germ plasm (4).
Despite these different strategies for PGC formation, emerging evidence suggests that migratory PGCs of nonmammalian vertebrates remain developmentally uncommitted to gametogenesis, retaining the capacity for somatic differentiation. In frogs, PGCs arising via germ plasm readily differentiate into somatic cells when transplanted into host embryos (5). Similarly, in fish, mismigrated PGCs readily adopt somatic fates if depleted of Dnd1 (6). In salamanders, where PGCs arise through inductive processes, irreversible commitment of the germline occurs late in development, long after gastrulation is complete and somatic lineages are established (7). In mammals, migratory PGCs can form teratomas if transplanted to ectopic sites (8) and give rise to pluripotent cell lines in culture (9–11). It has also been suggested that presumptive PGCs (labeled genetically by Prdm1-Cre) in the posterior region of the embryo during allantoic elongation may contribute to nongametogenic lineages (12, 13). Taken together, these observations suggest that migratory PGCs of vertebrates retain a broad developmental potential, regardless of their mode of segregation. That is, migratory PGCs, while clearly cells of the germline (the entire lineage from zygote to gamete), may not yet be germ cells, which, by definition, are committed to producing gametes and no other cell types (14).
To better understand germline commitment in mammals, we examine the transition that occurs as PGCs invade the nascent gonads. We find a transcriptional program, initiated in human and mouse PGCs after colonization of the gonad, that distinguishes germ cells from their migratory germline precursors, and from soma. Through genetic studies, we demonstrate that this program is necessary for germ cell commitment in mammals. In embryonic mice deficient in one factor induced at PGC colonization—the RNA-binding protein Dazl—the germline remains developmentally uncommitted, retaining expression of a network of pluripotency factors, the capacity for pluripotent cell line derivation, and the potential to form gonadal teratomas in mice and pigs.
Results
Germ Cell Determinants of Nonmammalian Vertebrates Are Expressed in Mice and Humans upon PGC Colonization of the Nascent Gonads.
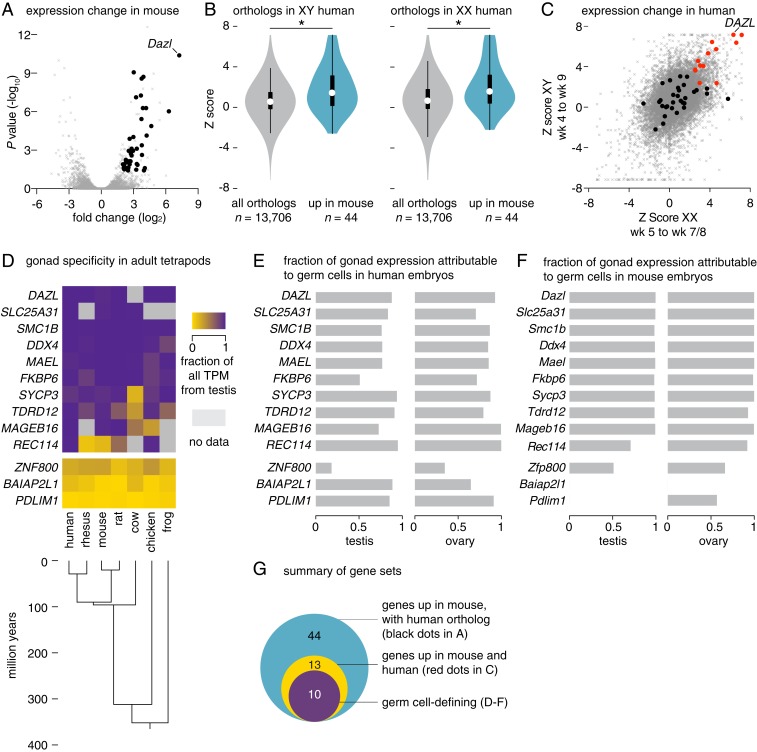
We searched for changes in gene expression that accompany PGC colonization of gonads in mammals. To this end, we reanalyzed published transcriptomes of migratory and gonadal germline cells from mouse (15) and human (16) embryos. Our analyses in mice identified 74 genes whose expression increased robustly after PGCs colonized the gonads (fold change of >4 [presented as log2 transformed] from E9.5 to E11.5, false discovery rate (FDR) < 0.05; Fig. 1A and Datasets S1 and S2). To determine whether this program of gene expression is similarly induced in human embryos, we reanalyzed the transcriptomes of single cells within a comparable developmental window (XY: weeks 4 to 9; XX: weeks 5 to 7 and 8; Dataset S3 and SI Appendix, Fig. S1). Of the 74 genes induced in mice, 44 have one-to-one orthologs in humans. As a set, these 44 genes are up-regulated in both XY and XX human germline cells after gonadal colonization, indicating that the program induced in the mouse germline at colonization is conserved in humans (Fig. 1B). Of particular importance, 13 genes up-regulated in mice were also significantly up-regulated in both the XY and XX human germlines (Fig. 1C).
Fig. 1.
A conserved program of germ cell transcription is induced upon PGC colonization of nascent gonads in mice and humans. (A) Gene expression changes in mouse germline between E9.5 and E11.5, as measured by RNA-seq. Black dots denote 44 genes that are up-regulated and have single human orthologs (fold change > 4, FDR value < 0.05); gray dots denote all other expressed genes (n = 11,282). (B and C) Gene expression changes in XY and XX human embryonic germlines between weeks 4 and 9, as measured by single cell RNA-seq. (B) Violin plots; as a set, genes induced in mouse germline (from A, n = 44) show greater expression increases in XY (Left) and XX (Right) human germline after PGCs colonize the gonads than do the set of all expressed orthologs (n = 13,706; *P value < 0.0007 by Wilcoxon rank sum test; black bar, interquartile range; circle, median value). (C) Scatter plot; black and red dots denote genes robustly up-regulated in mice, and possessing a single human ortholog (from A, n = 44); red dot genes are also significantly up-regulated in both XY and XX human germlines (n = 13). Gray dots denote all other expressed genes. (D) Heatmap; summary of gonad specificity of commonly up-regulated genes (red dots in C, n = 13), by RNA-seq, in 9 adult tissues from 7 tetrapods. Specificity fraction is determined by dividing testis expression (in TPM, transcripts per million) by sum of expression in all analyzed adult tissues, for each species. Genes with no annotated ortholog are shown in gray. (E and F) Germ cell expression of commonly up-regulated factors (red dots in C, n = 13) in (E) human embryonic testis and ovary and (F) mouse E14.5 testis and ovary by RNA-seq (SI Appendix, SI Materials and Methods). Ratio of 1 indicates germ cell-specific expression; 0 indicates somatic cell expression. (G) Euler diagram of gene sets identified through analyses in A–F.
Might this conserved program of gene expression, initiated after PGCs colonize the nascent gonads, serve to distinguish germ cells from other cell types in vivo? To address this, we examined, across diverse tetrapods, the expression breadth of these 13 genes. By reanalyzing RNA sequencing (RNA-seq) datasets of nine tissues from five mammals, as well as chicken and frog, we found that 10 of the 13 genes were predominantly or exclusively expressed in the adult testis, regardless of whether the germline is segregated by induction (as occurs in the mammalian epiblast) or via germ plasm (as occurs in the frog and chicken; Fig. 1D). In both mouse and human, each of these 10 gonad-specific genes is expressed predominantly in germ cells of embryonic gonads (Fig. 1 E and F). Notably, several factors activated on PGC colonization are components of germ plasm in nonmammalian animals, including DAZL, DDX4 (the mammalian ortholog of Vasa), MAEL, and TDRD12.
By comparison, the set of genes expressed in migratory PGCs of mouse and human embryos, immediately prior to gonadal colonization, does not display such gonad-specific expression in tetrapods; instead, these genes are expressed across adult tissues (SI Appendix, Fig. S2 A–D). The same is true for a curated set of PGC-defining factors gleaned from the literature, and also for a set of genes activated on PGC-like cell derivation (SI Appendix, Fig. S2 A–D). Further, analysis of 500,000 random-sampled gene sets found none that displayed gonad specificity comparable to that of the 13 genes up-regulated as PGCs colonize the gonads (SI Appendix, Fig. S2E).
In sum, a set of genes whose expression defines germ cells of vertebrates is first activated in the mouse and human germlines after embryonic PGCs colonize the gonads. These genes include orthologs of germ plasm components critical to germline commitment in diverse nonmammalian metazoa (Dataset S2), raising the possibility that one or more of these genes direct germ cell commitment in mammals, and that this occurs following gonadal colonization.
If this is true, then the transcriptional profiles of the germline should provide evidence of its uncommitted nature prior to gonadal entry. Indeed, we find that, in both humans and mice, migratory and newly colonized germline cells express naïve and general pluripotency factors, which identify developmentally uncommitted cells in vivo (in the inner cell mass) and in vitro (in embryonic stem [ES] cells) (17–19) (SI Appendix, Fig. S3). [Pluripotency factors are similarly expressed in migratory PGCs of fish and birds (20, 21).] Further, in humans and mice of both sexes, these pluripotency factors are markedly down-regulated after PGC colonization and induction of the germ cell-defining program of gene expression (SI Appendix, Fig. S3). Might activation of this program be necessary to down-regulate pluripotency factors and restrict the developmental potential of the germline?
Expression of Pluripotency Factors, and the Capacity for Deriving Pluripotent Cell Lines, Are Prolonged in Dazl-Deficient Mice.
We considered whether the program of gene expression induced after PGCs colonize the gonads functions in germ cell commitment. Evidence from a range of vertebrates suggests that DAZL, encoding an RNA-binding protein, might contribute to this function. For example, DAZL orthologs function in the germ plasm of fish (22, 23), frogs (24, 25), and birds (26). In C57BL/6 mice (B6), Dazl is necessary for licensing—the acquisition of meiotic and gametogenic competence—after PGCs colonize the gonads (27), and studies of mouse and human ES cells have suggested that DAZL limits the expression of pluripotency factors in vitro (28, 29). An opposing view—that Dazl serves to maintain germline pluripotency—emerges from reports that pluripotent embryonic germ (EG) cell lines could not be derived from Dazl-deficient embryos (30), and that the Dazl-deficient germline is unable to form gonadal teratomas (28), which arise when pluripotent cells differentiate to generate tissues of all three germ layers.
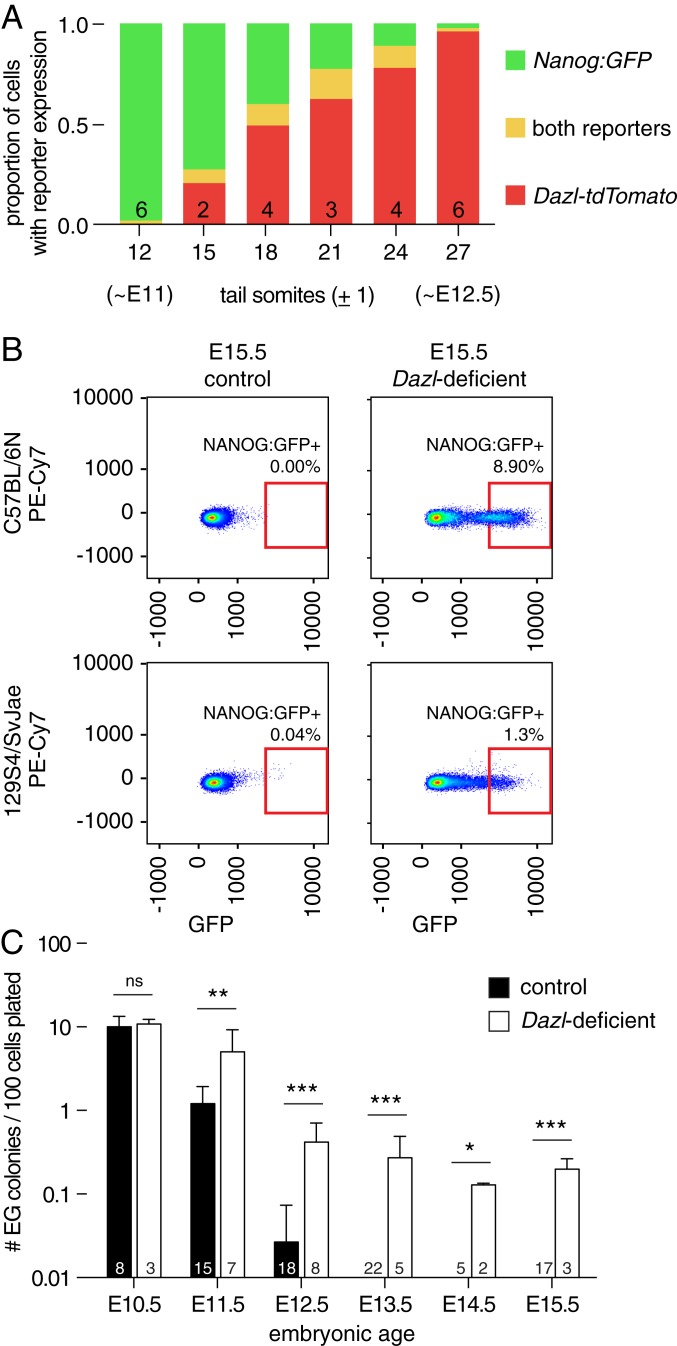
To reexamine the relationship between DAZL expression and germline commitment, we generated a reporter allele of DAZL expression (where both Dazl and tdTomato are translated from a single nascent RNA, referred to as Dazl-tdTomato; SI Appendix, Fig. S4A), and intercrossed this with a second fluorescent reporter allele, Nanog:GFP (a reporter of uncommitted cells). Flow cytometry revealed an abundance of Nanog:GFP-positive cells in E11.0 embryos (∼12 tail somites [ts]) carrying both reporters, with few if any of these cells also expressing the DAZL reporter (Fig. 2A and SI Appendix, Fig. S4B). By 15 ts, however, we began to detect DAZL reporter expression in a small population of Nanog:GFP-positive cells. With increasing embryonic age, we continued to find a small group of cells expressing both reporters, while an increasing proportion expressed the DAZL reporter alone (no longer positive for Nanog:GFP). By 27 ts (∼E12.5), very few cells were Nanog:GFP-positive, irrespective of the chromosomal sex of the embryo. These findings demonstrate, at cellular resolution, that the onset of DAZL expression is tightly correlated with the subsequent restriction of Nanog:GFP expression, across the entire population, within 36 h.
Fig. 2.
Dazl is required for restricting developmental potential of the germline in diverse strains of mice. (A) Developmental time course of germline using Dazl-tdTomato and Nanog:GFP reporters, detected by flow cytometry. At ∼12 ts (∼E11), most cells expressed only the Nanog:GFP reporter (green). As development proceeds, the proportions of cells expressing both fluorescent reporters (orange), or only the Dazl-tdTomato reporter (red), change. Numbers of embryos tested are listed in each column, and the fraction of cells expressing each reporter is shown as an average. (B) Flow cytometry for Nanog:GFP-positive cells of E15.5 control and Dazl-deficient ovaries of indicated strains. Autofluorescence in PE-Cy7 channel is shown on y axis. Red box indicates area in which Nanog:GFP-positive cells were counted. (C) Derivation of EG cell lines from control and B6.Dazl-deficient embryos. Cells were collected by fluorescence-activated cell sorting (FACS) at embryonic age indicated on x axis, and cultured under defined conditions. After 10 d, EG cell colonies were counted, and rate of EG cell derivation (per 100 EGFP-positive cells plated) was calculated. Number of embryos tested is listed in each column, mean + SD, *P value < 0.05, ** <0.01, *** <0.001, ns = not significant, using t test or Fisher’s exact test as appropriate.
Might Dazl be necessary for this restriction? To test this, we crossed the Nanog:GFP reporter to both B6 and 129S4/SvJae (129S4) backgrounds, and monitored expression in each strain (SI Appendix, SI Discussion and Fig. S5). At E15.5, germline expression of the Nanog:GFP reporter was absent from the gonads of control embryos, but maintained in Dazl-deficient gonads, regardless of the embryo’s genetic background or sex (Fig. 2B and SI Appendix, Fig. S4C). In support, reanalysis of published RNA-seq data from Dazl-deficient mouse ovaries (31) shows that a set of “general” and “naïve” pluripotency factors (19) remain expressed at E14.5 (SI Appendix, Fig. S4D). These observations indicate that Dazl is necessary, in vivo, to extinguish the expression of key markers of uncommitted cells.
To test whether Dazl is necessary to restrict the developmental potential of the germline, we attempted to derive pluripotent cell lines from control and Dazl-deficient B6 embryos. Germline cells isolated from control E10.5 embryos readily gave rise to EG cell colonies, with a mean derivation efficiency of 10.0 ± 3.4 colonies per 100 EGFP-positive cells plated (mean ± SD, n = 8 embryos; Fig. 2C and SI Appendix, Table S1). Colony formation declined precipitously with increasing embryonic age, irrespective of the donor embryo’s sex (E11.5: 1.2 ± 0.72 colonies, n = 15 embryos). At E12.5, only 5 of 18 control embryos gave rise to any EG colonies (0.03 ± 0.05 colonies), and no EG colonies were derived from E13.5 onward, irrespective of sex (n = 43 embryos). These observations were as expected, given prior reports that EG colony formation declines sharply after PGCs arrive at the gonads in mice (32), and the similarly transient capacity to derive pluripotent-like cells from human gonads (33).
Our experimental observations with Dazl-deficient embryos differed strikingly from littermate controls. At E10.5 (before Dazl is expressed), Dazl-deficient embryos gave rise to EG colonies at a frequency comparable to controls (10.8 ± 1.5 colonies per 100 EGFP-positive cells, n = 3 embryos; Fig. 2C). However, all Dazl-deficient embryos retained the capacity to generate EG colonies beyond E12.5 (n = 10 embryos, combined from E13.5 to E15.5), regardless of the embryo’s sex. Importantly, we first observed a significant difference between control and Dazl-deficient embryos at E11.5 (5.0 ± 4.2 colonies per 100 EGFP-positive cells, n = 7 embryos), concomitant with initiation of Dazl expression in the germline (Fig. 2A). Likewise, Dazl-deficient embryos isolated from an F1 cross between 129S4 and B6 mice retained the capacity to give rise to EG cell colonies until at least E15.5; the pluripotency of these cell lines was confirmed by injection into recipient blastocysts and resultant chimerism (SI Appendix, Fig. S4 E–H).
Taken together, these data establish that the Dazl-deficient germline continues to express pluripotency factors, and retains a PGC-like capacity for the derivation of pluripotent cell lines, even several days after colonization of the genital ridges in mice. We conclude that Dazl is necessary to restrict the developmental potential of the mouse germline after colonization of the gonads, regardless of genetic background, and prior to sexual differentiation of the germline.
Are other germ cell-defining factors (Fig. 1D) also required for this restriction? To address this question, we collected germline cells immediately prior to and shortly after gonadal entry, from control and Dazl-deficient embryos (34). RNA-seq analysis of these cells revealed that, apart from Dazl, expression of each of the other germ cell-defining factors is initiated upon gonadal entry in both control and Dazl-deficient embryos, indicating that DAZL is not required for their expression (SI Appendix, Fig. S4I). Further, this demonstrates that their expression is not sufficient to restrict Nanog:GFP expression in the Dazl-deficient germline—nor does their expression preclude derivation of EG cell lines (Fig. 2 B and C).
Spontaneous Gonadal Teratomas in Dazl-Deficient Mice of both Sexes.
We next considered the fate of the Dazl-deficient germline in mice. In wild-type 129 males (but not females), teratomas arise spontaneously, at low but significant frequency, from germline cells (35). We hypothesized that these tumors arise from PGCs that enter the gonads but nonetheless remain uncommitted, retaining their teratoma-forming potential.
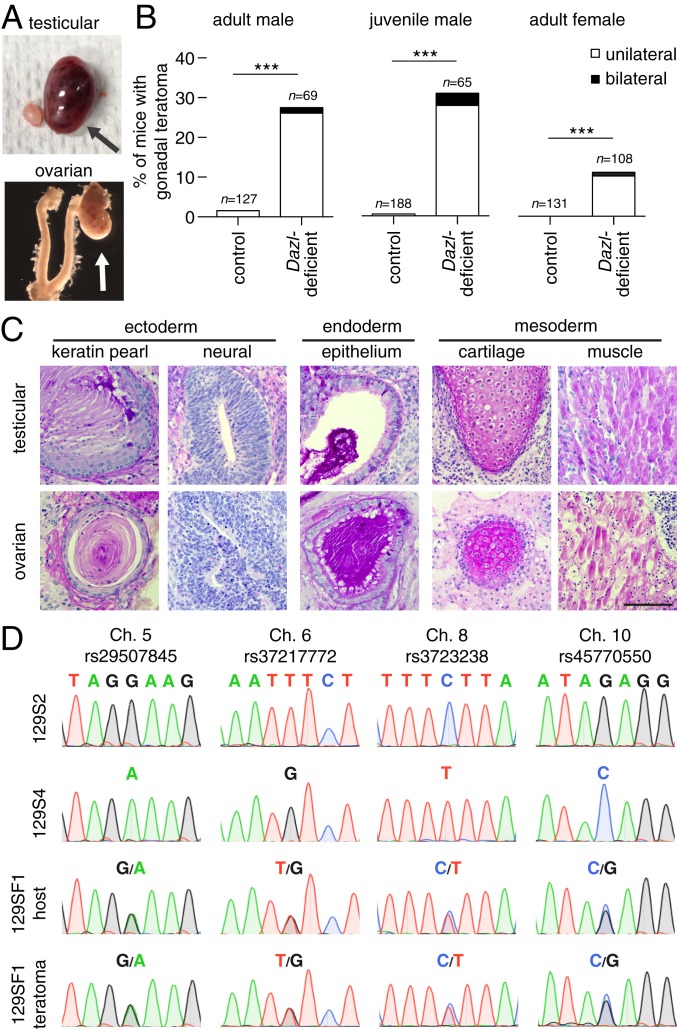
We collected 129S4.Dazl-deficient males and examined the testes for spontaneous teratomas. In control 129S4 mice at 4 mo of age, we found two teratomas among 127 males (Fig. 3 A–C), consistent with the low incidence reported in this strain (35). By contrast, among Dazl-deficient males, 19 of 69 (28%) exhibited testicular teratomas by 4 mo of age. To determine whether teratomas were present even earlier in postnatal life, we collected 129S4 mice at 4 wk of age. In controls, we found one teratoma among 188 males (Fig. 3B). By contrast, among Dazl-deficient males, 20 of 65 (31%) displayed testicular teratomas. Clearly, teratomas form at a markedly elevated rate in 129S4.Dazl-deficient males early in postnatal life, if not before.
Fig. 3.
Spontaneous gonadal teratomas occur in Dazl-deficient mice, and arise from mitotic cells. (A) Appearance of unilateral gonadal teratomas (arrows) in 129S4.Dazl-deficient mice. (B) Incidence of gonadal teratomas in mice. Males were dissected by 4 mo (adult male) or at 4 wk of age (juvenile male), and adult females were dissected at 2 mo of age; n = number of animals examined, ***P value ≤ 0.0001 using Fisher’s exact test. (C) Representative histology of teratomas from testis (Upper) and ovary (Lower) stained with periodic acid-Schiff reagent (PAS). (Scale bar, 100 µm.) (D) Representative Sanger sequencing at 4 SNP loci from 129S2 and 129S4 mice (Upper), and from 129SF1 host and teratoma (Lower). Teratomas are heterozygous at each SNP locus.
Our earlier studies of EG cell derivation and Nanog reporter expression indicated that germline cells become restricted in their developmental potential independent of and prior to their sexual differentiation. Accordingly, we asked whether the germline in 129S4.Dazl-deficient ovaries can give rise to spontaneous teratomas. At 2 mo of age, we found no ovarian teratomas in 131 control females (Fig. 3B). By contrast, 11 of 108 (10%) Dazl-deficient females displayed teratomas (Fig. 3 A–C).
Do these tumors arise from pluripotent mitotic cells (such as PGCs), or from cells that have completed meiosis I and have undertaken parthenogenetic activation of pluripotency? In humans, ovarian teratomas may arise from either mitotic or meiotic cells. In mice, there are no published reports of ovarian teratomas arising from mitotic cells; ovarian teratomas have been observed in LTXBJ mice, but these arise from meiotic germ cells, via parthenogenesis (36). To ascertain the cellular origin of teratomas in Dazl-deficient mice, we tested tumors for loss of heterozygosity (LOH)—a hallmark of cells that have completed meiosis I, and of parthenogenesis. To assay LOH, we first crossed the 129S4.Dazl mice with a genetically distinguishable substrain—129S2/SvPasCrl (129S2)—in which Dazl deficiency produces teratomas (SI Appendix, Fig. S6A). Importantly, the 129S4 and 129S2 substrains differ at 11 single-nucleotide polymorphisms (SNPs), each on a different chromosome (Dataset S4 and SI Appendix, Table S2; ref. 37). By intercrossing these mice, we generated offspring that are heterozygous at each SNP. We genotyped the resultant gonadal teratomas, along with normal host tissue, and detected no LOH in 10 ovarian and 10 testicular teratomas (Fig. 3D and SI Appendix, Fig. S6B), implying that these teratomas arose from mitotic cells.
Collectively, these studies demonstrate that Dazl is necessary for the commitment of germ cells, in the gonads, from their uncommitted precursors, independent of sex. Dazl-deficient gonadal teratomas do not reflect the parthenogenetic reactivation of pluripotency in meiotic cells, but instead arise from mitotic germline cells that have retained broad developmental potential.
Are other germ cell-defining factors also required for this commitment? Like Dazl, Ddx4 and Gcna are germ plasm constituents, first expressed after PGC colonization of the gonads in humans and mice (Fig. 1 and refs. 38 and 39). To determine whether Ddx4 or Gcna is similarly necessary for germ cell commitment, we crossed null alleles for Ddx4 [Ddx4-Cre (40)] and Gcna (38) to a 129S4 background and measured teratoma incidence. We found no gonadal teratomas in 129S4.Ddx4-deficient mice, and a single tumor in a 129S4.Gcna-deficient male (Ddx4: n = 25 males, 23 females; Gcna: n = 50 males; SI Appendix, Fig. S6 C and D), indicating that, while necessary for the completion of spermatogenesis (38, 41), both Ddx4 and Gcna are dispensable for germ cell commitment.
Sex Reversal Shows That the Testis Is a Favorable Site for Teratoma Formation.
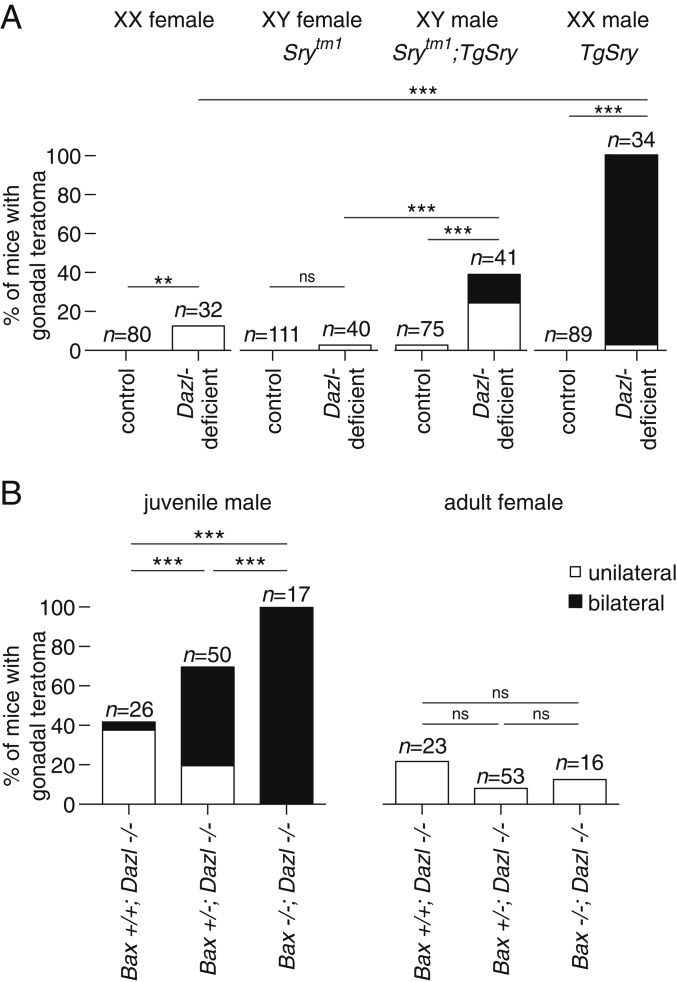
Despite the common developmental origin of teratomas in Dazl-deficient males and females, their incidence is higher in males (Fig. 3B). To test whether this male bias reflected an intrinsic difference in the developmental potency of XY germline cells, or the testicular environment to which XY PGCs migrate, we reversed the gonadal sex of both XX and XY animals. In mammals, gonadal sex is determined by expression of a Y-linked gene, Sry. An Sry transgene (TgSry) can induce male development in XX embryos (42), and disruption of Sry results in female development of XY embryos.
We generated sex-reversed Dazl-deficient mice and assessed teratoma incidence. Teratomas were observed much less frequently in Dazl-deficient 129S4 females (either XX or XY) than in Dazl-deficient XY males (Fig. 4A). Strikingly, we observed testicular teratomas in all XX male mice (XX TgSry, n = 34 animals, 33 with bilateral teratomas). Thus, the mouse testis is a more favorable environment than the ovary for teratomas to arise from either the XX or XY Dazl-deficient germline. We conclude that XX germline cells are as susceptible to teratoma formation as their XY counterparts, if not more so, given an equivalent gonadal environment. Our findings establish that, in the absence of Dazl, both XX and XY germline cells can form spontaneous teratomas, overturning the view that a Y-linked gene is essential for teratoma formation in male mice (43).
Fig. 4.
Teratoma formation in Dazl-deficient mice is affected by sex reversal, and by ablation of Bax-mediated cell death. (A) Incidence of gonadal teratomas in sex-reversed mice. Mutation of Sry (Srytm1) causes XY embryos to develop as anatomic females. Expression of Sry transgene (TgSry) causes XX embryos to develop as anatomic males. (B) Incidence of testicular teratomas (Left) and ovarian teratomas (Right) in Dazl-deficient mice (−/−) who were either homozygous wild-type (+/+), heterozygous (+/−), or deficient (−/−) for Bax; n = number of animals examined, **P value < 0.01, *** <0.0001, ns = not significant using Fisher’s exact test.
Ablating Bax-Mediated Cell Death Increases Teratoma Formation in Dazl-Deficient Male Mice.
Our findings led us to hypothesize that many (or most) gonadal germline cells either die or form spontaneous teratomas in the absence of Dazl function. Indeed, these two fates—cell death or teratoma—might represent alternative outcomes, in vivo, of germline cells whose developmental potential has not been restricted. If this were the case, then curtailing cell death pathways in Dazl-deficient mice should increase the incidence of spontaneous gonadal teratomas. To attenuate apoptotic cell death, we intercrossed mice carrying the Dazl null allele with mice carrying a Bax null allele (44, 45). Among Dazl-deficient;Bax-heterozygous males, we observed a dramatically increased incidence of teratomas (68%; including 23 with bilateral teratomas, from 50 mice; Fig. 4B and SI Appendix, Fig. S6E) compared with Dazl-deficient;Bax wild-type male littermates (42%; including one with bilateral teratomas, from 26 males). Strikingly, we observed bilateral teratomas in all 17 double-knockout males examined (compared with none in 51 Bax-deficient males, where at least one copy of Dazl remained intact). We conclude that, in the testes of 129S4.Dazl-deficient males, the failure to restrict the developmental potential of the germline usually leads to Bax-mediated cell death. By genetically attenuating or eliminating Bax-mediated cell death, the Dazl-deficient germline’s broad developmental potential—and, thereby, its capacity for teratoma formation—is revealed more fully in vivo. In females, however, we observed no significant effect of Bax deficiency on the incidence of ovarian teratomas in Dazl-deficient mice (Fig. 4B and SI Appendix, Fig. S6F). This finding parallels our observations in sex-reversed mice, demonstrating again that the sexual identity of the somatic gonad strongly influences the likelihood of teratoma formation (Fig. 4A).
DAZL-Deficient Pigs Develop Spontaneous Teratomas.
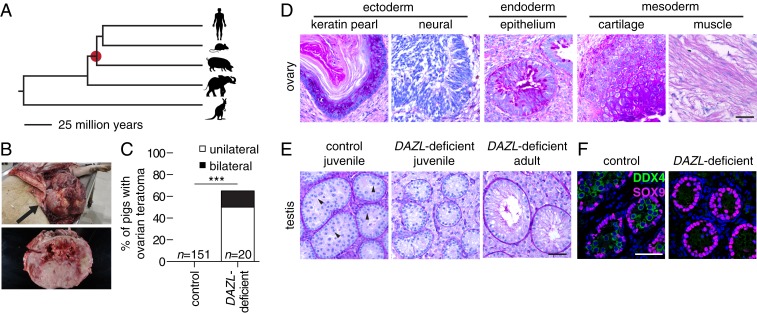
Given that Dazl is necessary for germ cell commitment in mice, we assessed whether this holds true in other mammals. We tested this in pigs, an outgroup to primates and rodents (Fig. 5A). Using a transcription activator-like effector nucleases (TALEN)-mediated gene editing strategy, we generated female pigs with targeted disruptions of DAZL (SI Appendix, Table S3). We examined the ovaries of 20 DAZL-deficient females that were at least 6 mo old: 13 had large ovarian tumors, and 3 of the 13 had bilateral tumors (Fig. 5 B and C). Histological examination revealed that these tumors were teratomas, containing disorganized mixtures of tissues derived from all three germ layers (Fig. 5D), similar to our findings in Dazl-deficient mice. We did not observe evidence of ovarian teratomas in 151 female controls of similar age [nor did we observe testicular teratomas in any of three previously generated (46) DAZL-deficient male pigs analyzed at 11 wk or 9 mo of age].
Fig. 5.
Spontaneous ovarian teratomas in DAZL-deficient pigs. (A) The pig is an outgroup to rodents and primates, sharing a common ancestor 95 million years ago (red dot). (B) Ovarian teratoma (arrow) in DAZL-deficient pig. The tumor measured 46 × 23 × 28 cm and weighed 15.4 kg. (C) Incidence of ovarian teratomas in control and DAZL-deficient pigs. ***P value < 0.0001 using Fisher’s exact test. (D) Representative histology of teratoma from ovary, stained with PAS. (E) Histology of control (Left, 18 wk, spermatocytes marked with arrow head) and DAZL-deficient testis at 11 wk (Center) and 9 mo of age (Right), stained with PAS. (F) Immunofluorescence of control (Left, 18 wk) and DAZL-deficient pig testis (Right, 11 wk). Germ cells were stained with DDX4 (green), and somatic cells were stained with SOX9 (magenta). DNA was stained with DAPI (blue). (Scale bars, 50 µm.)
To assay whether DAZL is necessary for survival of germline cells in the testes of pigs, as it is in diverse strains of mice (SI Appendix, Fig. S5), we examined testes of DAZL-deficient pigs at 11 wk and at 9 mo of age. In all gonads analyzed, we found no evidence of germ cells in either ovaries or testes (Fig. 5 D and E). Consistent with this, immunohistological analysis revealed that all cells within the seminiferous epithelium expressed the Sertoli cell factor SOX9 (Fig. 5F). These data indicate that DAZL is required for the survival of germline cells in the swine testis.
Combined with our data in humans and mice, these observations in pigs demonstrate, across eutherian mammals, that DAZL is necessary to restrict the developmental potential of PGCs after their arrival at the gonads.
Embryonic Dazl Expression Is Sufficient for Germline Survival and Oogenesis.
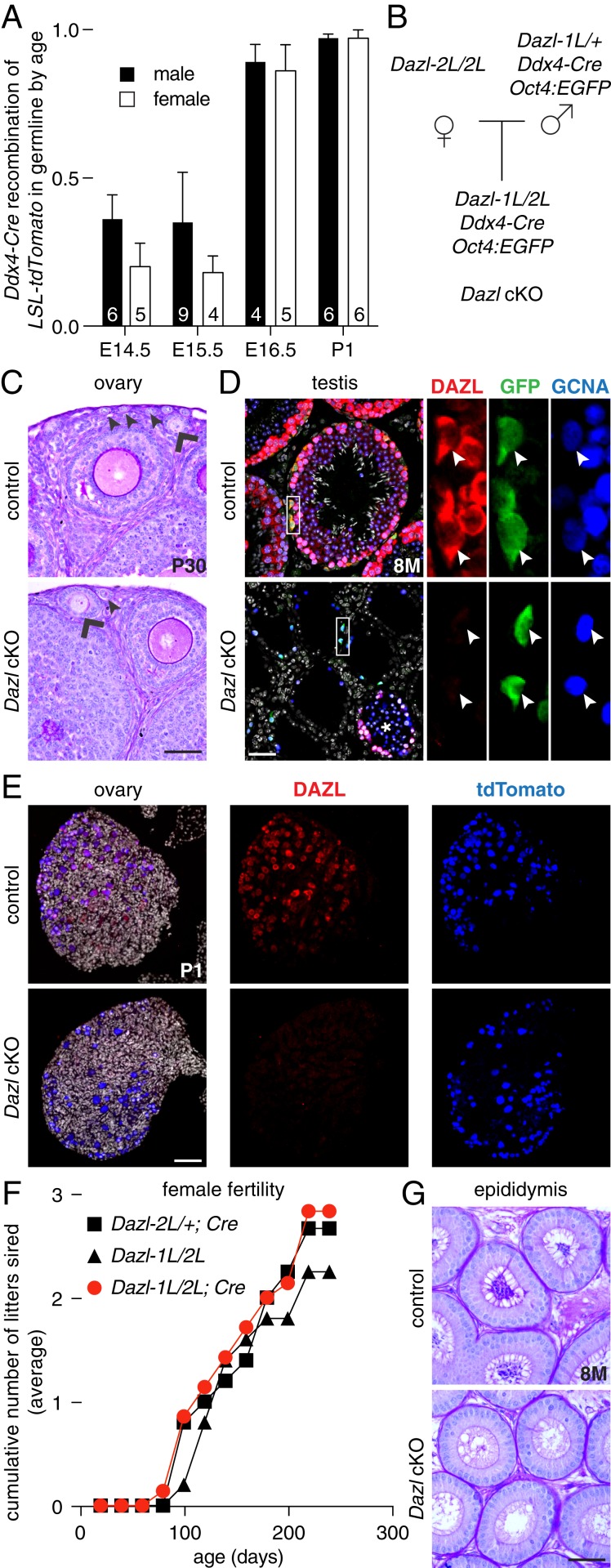
Finally, we assessed whether a brief period of Dazl expression is sufficient to initiate germ cell commitment, after which Dazl might be dispensable for gametogenesis in mice. We constructed a conditional Dazl allele on a B6 background (referred to as B6.Dazl-2L), and then generated a null allele (Dazl-1L) using a germline-specific Cre recombinase (40) (Ddx4-Cre; SI Appendix, Fig. S7 A and B). Next, we temporally ablated Dazl from ∼E14.5 onward, using Ddx4-Cre (Fig. 6A). This ablation occurred after PGCs extinguished Nanog:GFP and their potential to give rise to EG cells (Fig. 2 A and C), and after sexual differentiation had commenced in both sexes and meiosis had been initiated in females. In conditional knockout mice (B6.Dazl-1L/2L;Cre, referred to as Dazl cKO), we observed germ cells in ovaries and testes at all ages tested, through 8 mo of age (Fig. 6 B–D). In Dazl cKO ovaries, we confirmed DAZL’s absence in germ cells at birth (marked by the LSL-tdTomato reporter, recombined in germ cells by Ddx4-Cre; Fig. 6E). In Dazl cKO testes, we confirmed the loss of DAZL expression in spermatogonia (marked by the Oct4:EGFP transgene and the germ cell marker GCNA; some tubules retained DAZL expression and exhibited variable spermatogenesis). These data suggest that, once germ cell commitment has occurred, Dazl is no longer necessary for germ cell viability in mice on a B6 background (SI Appendix, SI Discussion).
Fig. 6.
A brief period of Dazl expression is sufficient for germ cell commitment, and for completion of oogenesis. (A) Time course of Ddx4-Cre (MvhCre-mOrange) recombinase activity in embryonic germline using a fluorescent Cre reporter mouse line, LSL-tdTomato. Cre-mediated recombination (resulting in tdTomato expression) in Oct4:EGFP-positive cells is assayed by flow cytometry. Numbers of embryos tested are listed in each column, mean + SD. (B) Breeding scheme for Dazl conditional knockout (Dazl cKO) mice. (C) Histology of control (Upper) and B6.Dazl cKO ovary (Lower) stained with PAS at 20 d of age, with primary (arrow head) and secondary (chevron) follicles marked. (D) Immunofluorescence of control (Upper) and B6.Dazl cKO testis (Lower) at 8 mo of age. Germ cells are immunostained by GCNA (blue), and undifferentiated spermatogonia are immunostained by GFP (green) expressed from the Oct4:EGFP reporter. Cre recombination is confirmed by immunostaining for DAZL (red). DNA is stained with DAPI (gray). Insets show each marker in Oct4:EGFP-positive spermatogonia (arrow head); * denotes tubule with incomplete Dazl recombination in cKO testis. (E) Immunofluorescence of control (Upper) and B6.Dazl cKO ovary (Lower) at postnatal day 1. Germ cells are immunostained by tdTomato protein (expressed following recombination of tdTomato-LSL allele by Ddx4-Cre; blue). Cre recombination is confirmed by immunostaining for DAZL (red). DNA is stained with DAPI (gray). (F) B6.Dazl cKO females remained fertile for at least 8 mo. (G) B6.Dazl cKO males were sterile, with no spermatozoa in epididymal ducts. All data are mean + SD. (Scale bars, 50 µm.)
To test whether Dazl is dispensable during the remainder of gametogenesis, we assessed the fertility of Dazl cKO mice. Dazl cKO females were fertile through at least 8 mo of age (n = 5 females, with the recombined allele transmitted to all progeny; Fig. 6F). In contrast, Dazl cKO males were sterile, with no spermatozoa observed in the epididymis of adults (n = 5 males; Fig. 6G). Thus, Dazl has additional functions in postnatal spermatogenesis, consistent with previous descriptions on a mixed genetic background (47–49).
We conclude that a brief period of Dazl expression, after PGC colonization of the gonads, is sufficient for germline commitment and the initiation of gametogenesis in mice, as shown by the survival of germ cells in the gonads of both sexes of Dazl cKO mice, and by the completion of oogenesis and fertility in females.
Discussion
Our studies sought to answer a fundamental question: When, where, and how does the mammalian germline restrict its developmental potential and irreversibly commit to gametogenesis? One view has been that mammalian PGCs are unipotent germ cells—only capable of giving rise to gametes (50–54). This view is challenged by several lines of evidence that reveal the broad developmental potential of migratory PGCs, including the expression of a network of pluripotency factors, the ability to produce pluripotent cell lines in culture, the occurrence of spontaneous gonadal teratomas, and, most recently, evidence that presumptive PGCs may contribute to the allantois (12, 13). Comparable observations in fish (6), frogs (5), and salamanders (7) corroborate the view that, among vertebrates, migratory-stage PGCs are not yet irreversibly committed, but instead retain the capacity for somatic differentiation, regardless of whether germline segregation occurs via induction, or by germ plasm.
Our present studies in mammals provide definitive genetic evidence that the germline’s broad developmental potential is restricted after PGC colonization of the gonads, and that Dazl is necessary for this to occur. We find that, in mouse embryos lacking Dazl function, PGCs migrate to the gonads but maintain expression of a network of pluripotency factors, and retain the ability to give rise to pluripotent cell lines until at least E15.5 in both sexes (Fig. 2 and SI Appendix, Fig. S4). We further corroborated this by following the fate of the Dazl-deficient germline in adult 129S mice, which formed gonadal teratomas at a remarkable frequency. We found testicular teratomas in 87 of 324 Dazl-deficient males, compared to 6 of 747 control mice (SI Appendix, Table S4). The incidence was even higher in certain circumstances. For example, 33 of 34 Dazl-deficient, sex-reversed (XX) males developed bilateral gonadal teratomas, as did 17 of 17 Dazl-deficient;Bax-deficient XY males (Fig. 4). Strikingly, we also discovered ovarian teratomas in 35 of 300 Dazl-deficient females, compared to none in 426 control mice, demonstrating that Dazl is necessary for germ cell commitment in both sexes.
Taken together with published reports, our findings suggest a sequence of commitment steps during mammalian germline development. In developmental biology, a cell is “specified” when it will develop autonomously after isolation from the embryo; specified cells are not yet irreversibly committed, and may adopt other fates if transplanted to a new position (55). For example, the fate of specified trophectoderm or of the inner cell mass may be altered upon relocation within the preimplantation embryo (56). Similarly, mammalian PGCs appear to be specified shortly after their induction (1, 2), without being irreversibly committed to gametogenesis.
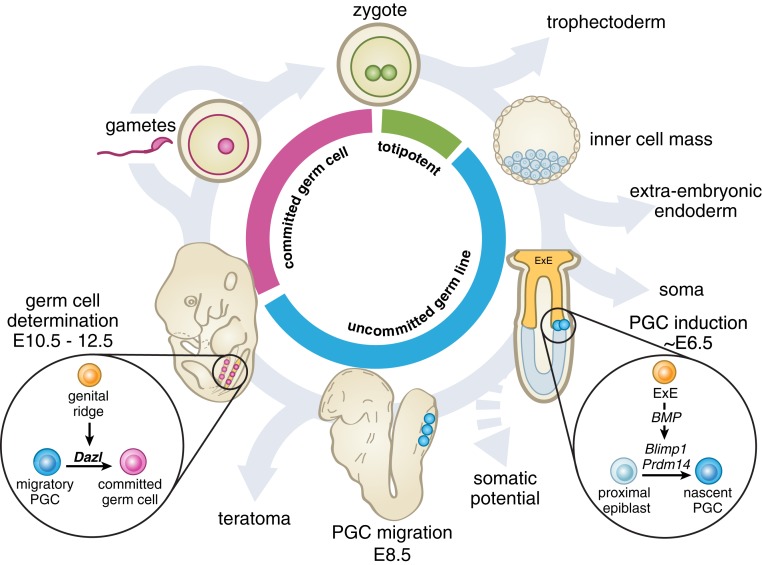
In contrast, a cell is “determined” (fully committed) when its fate cannot be reversed by grafting (55). By these definitions, cells are determined when their potential is restricted, regardless of environment. It is informative here to revisit the work of Leroy Stevens, who discovered that PGCs can give rise to teratomas in mice (35, 57). [Likewise, migratory-stage PGCs from amphibians can give rise to somatic cell lineages if transplanted (5) or in cell culture (7).] Stevens demonstrated, by grafting PGCs, that they lose the capacity to form teratomas after colonizing the gonads (8, 58). Combining Stevens’ observations with our own, we conclude that germ cells are determined in mice after PGCs colonize the gonads, and that the induction of Dazl is necessary for this commitment (Fig. 7). Utilizing a conditional allele, we show that a brief period of Dazl expression is sufficient for this commitment to gametogenesis.
Fig. 7.
A proposed model for germ cell commitment in mammals. The germline comprises all cells whose descendants include gametes. At fertilization, the totipotent zygote has the capacity to give rise to all cell lineages. As development proceeds, extraembryonic and somatic lineages differentiate away from the germline. PGCs (blue cells) are induced from the epiblast, preserving the germline, but also maintaining a broad developmental potential. At PGC colonization of the gonads, expression of a germ cell program—marked by DAZL—is induced in germline cells by the genital ridge. Our studies show that DAZL is necessary for the restriction of developmental potential in the germline, resulting in the determination of germ cells (pink cells). Determined germ cells then undertake gametogenesis and must cycle through fertilization to reestablish totipotency and continue the germline cycle in a new diploid individual. ExE, extraembryonic ectoderm.
This model is further corroborated by our findings in DAZL-deficient pigs, where the germline similarly retains the capacity for teratoma formation after gonadal colonization. These findings—in two species whose most recent common ancestor lived about 95 million years ago—strongly suggest that DAZL-dependent commitment, occurring after gonadal colonization, operated in the last common ancestor of all eutherian mammals. Moreover, in fish (22, 23), frogs (24, 25), and birds (26), orthologs of DAZL are essential constituents of germ plasm, which functions in segregating germline from soma. Thus, DAZL is a key factor in germline commitment, regardless of whether germline segregation occurs by induction or germ plasm.
Distinguishing Gonadal Germ Cells from Their Migratory Germline Precursors.
If the germline’s broad developmental potential is extinguished only after PGC colonization of the gonads, then the roles of key regulators expressed in PGCs prior to gonadal entry must also be considered. Here we will incorporate findings from both mammals and nonmammalian vertebrates to suggest that these critical regulators of migratory PGCs repress the cells’ capacity for somatic differentiation, insulating them from inductive cues.
Consider Nanos and Dnd1, which are expressed in migratory PGCs of many vertebrates and encode repressors of cellular differentiation. When dnd1 is knocked down in fish, PGCs adopt somatic cell fates in response to morphogenic signals that they would normally resist (6). Similarly, in frogs, nanos1-deficient PGCs migrating through the endoderm inappropriately activate a set of endoderm-defining factors (59). When either of these factors, Dnd1 (60, 61) or Nanos3 (62), is deleted in rodents, PGCs give rise to teratomas at high frequency. These comparative observations support the view that both Nanos and Dnd1 repress the expression of somatic factors, thereby insulating PGCs from inductive cues encountered during migration.
Similarly, nascent mammalian PGCs express a repertoire of factors (including Prdm1 [Blimp1] and Tfap2c, and also SOX17 in primates and pigs) that serve to repress cell differentiation in other contexts (63–66). These factors have also been observed to repress somatic gene expression during the derivation and culture of human and mouse PGC-like cells (67–70). Taken together, these observations suggest that PGC-expressed regulators function to insulate the migratory germline from ectopic gene expression, thereby preventing uncommitted PGCs from adopting somatic cell fates. Expression of these factors, however, is insufficient to irreversibly restrict germline potential; PGCs express a network of pluripotency factors, and will produce teratomas when transplanted (8). By contrast, once mammalian PGCs colonize the gonads, a definitive program of germ cells is induced—a program marked by the expression of deeply conserved, germ cell-exclusive factors (and a reciprocal down-regulation of pluripotency factors), and, functionally, by extinguishing the capacity for pluripotent cell line derivation and teratoma formation (Fig. 7).
Unlike other genes whose ablation yields teratomas, Dazl is expressed only in the germline (71, 72), and only after PGC colonization of the gonad (60, 61). Thus, Dazl deficiency provides unambiguous evidence of germ cell commitment occurring on PGC arrival at the nascent gonad.
That mechanisms exist to insulate PGCs from somatic inductive cues could also help explain why mouse PGCs do not contribute to somatic lineages when injected into blastocysts (73). PGC-expressed factors may prevent these cells from contributing to chimerism upon injection into blastocysts, without necessarily being instructive for germ cell determination. Thus, PGCs have been said to exhibit a “latent” pluripotency (74) whose unmasking results in teratomas and EG cell lines. The failure of mouse PGCs to contribute to chimerism could also reflect a developmental incompatibility between mouse PGCs and the environment of the blastocyst, as observed elsewhere when donor cells are not developmentally matched to their host (75). In any case, the negative results in mice contrast with a study in pigs, where freshly isolated PGCs, following injection into blastocysts, were reported to contribute to somatic lineages, as assayed by transgene expression (76).
Implications for Gametogenesis In Vitro.
Since our model states that migratory PGCs remain uncommitted, in vitro gametogenesis from these immature cells should not be possible. In seeming contradiction, complete gametogenesis from mouse ES and induced pluripotent stem cells has been reported; however, this occurred only after PGC-like cells were combined with somatic gonadal tissue, which led to Dazl expression (77, 78). We suggest that this soma-directed induction of Dazl, together with DNA demethylation to facilitate Dazl induction (79, 80), likely accounts for the ability of PGC-like cells to differentiate into functional gametes. Identifying the gonad-derived factors that induce Dazl expression in vivo will help investigators to recapitulate the entirety of gametogenesis in vitro (81, 82).
Implications for the Pathogenesis of Germ Cell Tumors.
Our insights into germ cell commitment in the embryo have ramifications for our understanding of germ cell tumors (GCTs), the most common cancer in young men (83). Specifically, our present studies in embryos converge in striking fashion with recent epidemiological and genome-wide association (GWA) studies of GCTs. For example, GWA studies implicate pluripotency factors (e.g., PRDM14, SALL4, TFCP2L1, and ZFP42), as well as genomic sites of binding for transcription factors of pluripotency (e.g., KLF4, NANOG, POU5F1, and SOX2), in the pathogenesis of testis cancer (84, 85). Most importantly, DAZL has been identified as a susceptibility locus, implicating DAZL as a key factor in both the pathogenesis of GCTs in humans (86) and germ cell commitment. Consistent with a critical role for apoptosis, GWAS has implicated BAK1 in the heritability of GCTs (87), akin to our observations in Bax-deficient mice. Along similar lines, the receptor:ligand pair KIT and KIT ligand (KITLG) function in several cellular contexts to protect cells from apoptosis (88). Like BAK1, polymorphism at KITLG is implicated in the heritability of human GCTs, and teratoma incidence in mice (87, 89, 90). Human GWA studies also implicate GATA4 (84), which is required in mice for gonad development and induction of Dazl (81). Reinforcing the view that somatic gonadal development is central to germ cell determination, young children with disorders of gonadal development are at markedly increased risk of germline neoplasia (91). These many connections lead us to suggest that germline neoplasms arise from embryonic cells that, having failed to complete germ cell determination on their arrival at the gonad, remain uncommitted and susceptible to tumor formation. Accordingly, our revised understanding of germ cell commitment will help clarify the developmental origin of GCTs, and inform efforts to account for their dramatically increased incidence in recent decades (92).
In conclusion, we demonstrate that germ cell determination in mammals occurs late in embryonic development—after the body plan has been established, and organogenesis begun—through an ancient germ cell program induced as PGCs colonize the nascent gonads (Fig. 7). This model has deep implications for the genesis of germline neoplasms in humans, and for the stepwise commitment and determination of germ cells in mammals and across the vertebrata.
Materials and Methods
Further details can be found in SI Appendix, SI Materials and Methods.
Animals.
All experiments involving mice or pigs conformed to principles and guidelines approved by the Committee on Animal Care at the Massachusetts Institute of Technology, Cincinnati Children's Hospital Medical Center, or by International Center for Biotechnology, respectively. Further details can be found in SI Appendix, SI Materials and Methods.
Cell Isolation.
Embryos carrying fluorescent reporter alleles were dissected, and gonadal cells were subjected to flow cytometry, followed by pluripotent cell line derivation, or to RNA isolation for transcriptional analysis, as described in SI Appendix, SI Materials and Methods.
Histology.
Gonads were removed and fixed in 4% paraformaldehyde, or Bouins’ solution, embedded in paraffin, sectioned, and stained for immunohistology, or stained with hematoxylin and periodic acid-Schiff. Teratoma formation was confirmed by the presence of cells from each somatic germ layer. Full details are available in SI Appendix, SI Materials and Methods.
Transcriptional Analyses.
Sequence data were aligned to the appropriate reference genome, and differential expression was calculated as outlined in SI Appendix, SI Materials and Methods.
Data Availability.
Data generated by array and RNA-seq have been deposited at Gene Expression Omnibus (accession no. GSE87771) and Sequence Read Archive (accession no. PRJNA434733), respectively.
Supplementary Material
Acknowledgments
We thank M. Goodheart and K. Igarashi for technical support; Styliani Markoulaki and the Genetically Engineered Models core facility at Whitehead Institute (WI) for CRISPR/Cas9 injections for the conditional allele; the Transgenic Animal and Genome Editing Core at Cincinnati Children’s Hospital Medical Center for generating Dazl-tdTomato mice; Howard Cooke for the Dazltm1Hjc allele; Rudolf Jaenisch for Nanog:GFP mice; the National Institute of Genetics (Japan) for the MSM/Ms strain of mice; Dirk de Rooij for advice on analysis of spermatogenesis; Martin Taylor for helpful advice on teratoma pathology; George Enders for the GCNA antibody; and R. Jaenisch, G. Matthews, M. Mikedis, H. Skaletsky, Y. Stelzer, and Z. Swartz for comments on the manuscript. We acknowledge the technical expertise of the WI FACS, Keck imaging, and Genome Technology Core facilities; the Albert Einstein College of Medicine Genomics Facility for SNP genotyping; George Bell of WI Bioinformatics and Research Computing for assistance with SNP analysis; and Meredith Fedorovsky for illustration. This work was supported by the Howard Hughes Medical Institute, where D.C.P. is an Investigator. P.K.N. is a Hope Funds for Cancer Research Fellow (Grant HFCR-15-06-06) and recipient of an Early Career Fellowship (Grant GNT1053776, National Health and Medical Research Council, Australia). H.S. was supported by Deutsche Forschungsgemeinschaft Grant Scho 503 13-1. S.N. was supported under a research grant from Biogen, Inc. Y.F. was supported by the National Natural Science Foundation of China (Grant 81471507). Swine work was funded, in part, by NIH Small Business Innovation Research Award GM108150 to I.D., D.F.C., and S.C.F. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Competing interest statement: A.L.W., D.F.C., and S.C.F. are employees and shareholders of Recombinetics Inc. I.D. is a member of the scientific advisory board of Recombinetics Inc. The remaining authors have declared that no competing interests exist.
Data deposition: Data generated using the Mouse Diversity Genotyping Array (Thermo Fisher Scientific) for the SNP genotyping of 129S2 and 129S4 substrains have been deposited at the Gene Expression Omnibus under accession no. GSE87771. Data generated from control and Dazl-deficient germ line cells at E10.3 and E11.5 have been deposited under the Sequence Read Archive BioProject accession no. PRJNA434733.
See Commentary on page 25374.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1910733116/-/DCSupplemental.
References
- 1.Lawson K. A., et al. , Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 13, 424–436 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohinata Y., et al. , A signaling principle for the specification of the germ cell lineage in mice. Cell 137, 571–584 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Extavour C. G., Akam M., Mechanisms of germ cell specification across the metazoans: Epigenesis and preformation. Development 130, 5869–5884 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Strome S., Updike D., Specifying and protecting germ cell fate. Nat. Rev. Mol. Cell Biol. 16, 406–416 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wylie C. C., Heasman J., Snape A., O’Driscoll M., Holwill S., Primordial germ cells of Xenopus laevis are not irreversibly determined early in development. Dev. Biol. 112, 66–72 (1985). [Google Scholar]
- 6.Gross-Thebing T., et al. , The vertebrate protein Dead end maintains primordial germ cell fate by inhibiting somatic differentiation. Dev. Cell 43, 704–715.e5 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Chatfield J., et al. , Stochastic specification of primordial germ cells from mesoderm precursors in axolotl embryos. Development 141, 2429–2440 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens L. C., Experimental production of testicular teratomas in mice. Proc. Natl. Acad. Sci. U.S.A. 52, 654–661 (1964). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsui Y., Zsebo K., Hogan B. L., Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell 70, 841–847 (1992). [DOI] [PubMed] [Google Scholar]
- 10.Resnick J. L., Bixler L. S., Cheng L., Donovan P. J., Long-term proliferation of mouse primordial germ cells in culture. Nature 359, 550–551 (1992). [DOI] [PubMed] [Google Scholar]
- 11.Shamblott M. J., et al. , Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc. Natl. Acad. Sci. U.S.A. 95, 13726–13731 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikedis M. M., Downs K. M., STELLA-positive subregions of the primitive streak contribute to posterior tissues of the mouse gastrula. Dev. Biol. 363, 201–218 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikedis M. M., Downs K. M., PRDM1/BLIMP1 is widely distributed to the nascent fetal-placental interface in the mouse gastrula. Dev. Dyn. 246, 50–71 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLaren A., Primordial germ cells in the mouse. Dev. Biol. 262, 1–15 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi S., et al. , Dynamics of 5-methylcytosine and 5-hydroxymethylcytosine during germ cell reprogramming. Cell Res. 23, 329–339 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L., et al. , Single-cell RNA-seq analysis maps development of human germline cells and gonadal niche interactions. Cell Stem Cell 20, 585–873.e4 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Theunissen T. W., et al. , Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell 15, 471–487 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo G., et al. , Epigenetic resetting of human pluripotency. Development 144, 2748–2763 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalkan T., et al. , Tracking the embryonic stem cell transition from ground state pluripotency. Development 144, 1221–1234 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sánchez-Sánchez A. V., et al. , Nanog regulates primordial germ cell migration through Cxcr4b. Stem Cells 28, 1457–1464 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Jean C., et al. , Transcriptome analysis of chicken ES, blastodermal and germ cells reveals that chick ES cells are equivalent to mouse ES cells rather than EpiSC. Stem Cell Res. 14, 54–67 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashimoto Y., et al. , Localized maternal factors are required for zebrafish germ cell formation. Dev. Biol. 268, 152–161 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Li M., Zhu F., Li Z., Hong N., Hong Y., Dazl is a critical player for primordial germ cell formation in medaka. Sci. Rep. 6, 28317 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houston D. W., Zhang J., Maines J. Z., Wasserman S. A., King M. L., A Xenopus DAZ-like gene encodes an RNA component of germ plasm and is a functional homologue of Drosophila boule. Development 125, 171–180 (1998). [DOI] [PubMed] [Google Scholar]
- 25.Houston D. W., King M. L., A critical role for Xdazl, a germ plasm-localized RNA, in the differentiation of primordial germ cells in Xenopus. Development 127, 447–456 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Lee H. C., et al. , DAZL expression explains origin and central formation of primordial germ cells in chickens. Stem Cells Dev. 25, 68–79 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Gill M. E., Hu Y.-C., Lin Y., Page D. C., Licensing of gametogenesis, dependent on RNA binding protein DAZL, as a gateway to sexual differentiation of fetal germ cells. Proc. Natl. Acad. Sci. U.S.A. 108, 7443–7448 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen H.-H., et al. , DAZL limits pluripotency, differentiation, and apoptosis in developing primordial germ cells. Stem Cell Reports 3, 892–904 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung D., et al. , In vitro differentiation of human embryonic stem cells into ovarian follicle-like cells. Nat. Commun. 8, 15680 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haston K. M., Tung J. Y., Reijo Pera R. A., Dazl functions in maintenance of pluripotency and genetic and epigenetic programs of differentiation in mouse primordial germ cells in vivo and in vitro. PLoS One 4, e5654 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soh Y. Q. S., et al. , A gene regulatory program for meiotic prophase in the fetal ovary. PLoS Genet. 11, e1005531 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsui Y., Tokitake Y., Primordial germ cells contain subpopulations that have greater ability to develop into pluripotential stem cells. Dev. Growth Differ. 51, 657–667 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Kerr C. L., Hill C. M., Blumenthal P. D., Gearhart J. D., Expression of pluripotent stem cell markers in the human fetal ovary. Hum. Reprod. 23, 589–599 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Nicholls P. K., Page D. C., Germ line from control and Dazl-deficient embryos. Sequence Read Archive. https://trace.ncbi.nlm.nih.gov/Traces/sra/?study=SRP133168. 20 February 2018.
- 35.Stevens L. C., Little C. C., Spontaneous testicular teratomas in an inbred strain of mice. Proc. Natl. Acad. Sci. U.S.A. 40, 1080–1087 (1954). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eppig J. J., Kozak L. P., Eicher E. M., Stevens L. C., Ovarian teratomas in mice are derived from oocytes that have completed the first meiotic division. Nature 269, 517–518 (1977). [DOI] [PubMed] [Google Scholar]
- 37.Nicholls P. K., Page D. C., Affymetrix mouse diversity genotyping array for 129S2/SvPasCrl and 129S4/SvJae. Gene Expression Omnibus. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE87771. Deposited 8 October 2016.
- 38.Carmell M. A., et al. , A widely employed germ cell marker is an ancient disordered protein with reproductive functions in diverse eukaryotes. eLife 5, e19993 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toyooka Y., et al. , Expression and intracellular localization of mouse Vasa-homologue protein during germ cell development. Mech. Dev. 93, 139–149 (2000). [DOI] [PubMed] [Google Scholar]
- 40.Hu Y.-C., de Rooij D. G., Page D. C., Tumor suppressor gene Rb is required for self-renewal of spermatogonial stem cells in mice. Proc. Natl. Acad. Sci. U.S.A. 110, 12685–12690 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka S. S., et al. , The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes Dev. 14, 841–853 (2000). [PMC free article] [PubMed] [Google Scholar]
- 42.Koopman P., Gubbay J., Vivian N., Goodfellow P., Lovell-Badge R., Male development of chromosomally female mice transgenic for Sry. Nature 351, 117–121 (1991). [DOI] [PubMed] [Google Scholar]
- 43.Anderson P. D., Lam M.-Y., Poirier C., Bishop C. E., Nadeau J. H., The role of the mouse Y chromosome on susceptibility to testicular germ cell tumors. Cancer Res. 69, 3614–3618 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stallock J., Molyneaux K., Schaible K., Knudson C. M., Wylie C., The pro-apoptotic gene Bax is required for the death of ectopic primordial germ cells during their migration in the mouse embryo. Development 130, 6589–6597 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Cook M. S., Coveney D., Batchvarov I., Nadeau J. H., Capel B., BAX-mediated cell death affects early germ cell loss and incidence of testicular teratomas in Dnd1(Ter/Ter) mice. Dev. Biol. 328, 377–383 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan W., et al. , Efficient nonmeiotic allele introgression in livestock using custom endonucleases. Proc. Natl. Acad. Sci. U.S.A. 110, 16526–16531 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruggiu M., et al. , The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature 389, 73–77 (1997). [DOI] [PubMed] [Google Scholar]
- 48.Schrans-Stassen B. H., Saunders P. T., Cooke H. J., de Rooij D. G., Nature of the spermatogenic arrest in Dazl -/- mice. Biol. Reprod. 65, 771–776 (2001). [DOI] [PubMed] [Google Scholar]
- 49.Saunders P. T. K., et al. , Absence of mDazl produces a final block on germ cell development at meiosis. Reproduction 126, 589–597 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Durcova-Hills G., Tang F., Doody G., Tooze R., Surani M. A., Reprogramming primordial germ cells into pluripotent stem cells. PLoS One 3, e3531 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murakami K., et al. , NANOG alone induces germ cells in primed epiblast in vitro by activation of enhancers. Nature 529, 403–407 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kobayashi T., et al. , Principles of early human development and germ cell program from conserved model systems. Nature 546, 416–420 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reik W., Surani M. A., Germline and pluripotent stem cells. Cold Spring Harb. Perspect. Biol. 7, a019422 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saitou M., Yamaji M., Primordial germ cells in mice. Cold Spring Harb. Perspect. Biol. 4, a008375 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slack J. M. W., From Egg to Embryo (Cambridge University Press, Cambridge, United Kingdom, ed. 2, 1991). [Google Scholar]
- 56.Tarkowski A. K., Suwińska A., Czołowska R., Ożdżeński W., Individual blastomeres of 16- and 32-cell mouse embryos are able to develop into foetuses and mice. Dev. Biol. 348, 190–198 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Stevens L. C., Origin of testicular teratomas from primordial germ cells in mice. J. Natl. Cancer Inst. 38, 549–552 (1967). [PubMed] [Google Scholar]
- 58.Stevens L. C., Development of resistance to teratocarcinogenesis by primordial germ cells in mice. J. Natl. Cancer Inst. 37, 859–867 (1966). [PubMed] [Google Scholar]
- 59.Lai F., Singh A., King M. L., Xenopus Nanos1 is required to prevent endoderm gene expression and apoptosis in primordial germ cells. Development 139, 1476–1486 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Youngren K. K., et al. , The Ter mutation in the dead end gene causes germ cell loss and testicular germ cell tumours. Nature 435, 360–364 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Northrup E., et al. , The ter mutation in the rat Dnd1 gene initiates gonadal teratomas and infertility in both genders. PLoS One 7, e38001 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schemmer J., et al. , Transcription factor TFAP2C regulates major programs required for murine fetal germ cell maintenance and haploinsufficiency predisposes to teratomas in male mice. PLoS One 8, e71113 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vincent S. D., et al. , The zinc finger transcriptional repressor Blimp1/Prdm1 is dispensable for early axis formation but is required for specification of primordial germ cells in the mouse. Development 132, 1315–1325 (2005). [DOI] [PubMed] [Google Scholar]
- 64.Auman H. J., et al. , Transcription factor AP-2gamma is essential in the extra-embryonic lineages for early postimplantation development. Development 129, 2733–2747 (2002). [DOI] [PubMed] [Google Scholar]
- 65.Werling U., Schorle H., Transcription factor gene AP-2 gamma essential for early murine development. Mol. Cell. Biol. 22, 3149–3156 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kanai-Azuma M., et al. , Depletion of definitive gut endoderm in Sox17-null mutant mice. Development 129, 2367–2379 (2002). [DOI] [PubMed] [Google Scholar]
- 67.Magnúsdóttir E., et al. , A tripartite transcription factor network regulates primordial germ cell specification in mice. Nat. Cell Biol. 15, 905–915 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakaki F., et al. , Induction of mouse germ-cell fate by transcription factors in vitro. Nature 501, 222–226 (2013). [DOI] [PubMed] [Google Scholar]
- 69.Irie N., et al. , SOX17 is a critical specifier of human primordial germ cell fate. Cell 160, 253–268 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kojima Y., et al. , Evolutionarily distinctive transcriptional and signaling programs drive human germ cell lineage specification from pluripotent stem cells. Cell Stem Cell 21, 517–532.e5 (2017). [DOI] [PubMed] [Google Scholar]
- 71.Matson C. K., et al. , DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 476, 101–104 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krentz A. D., et al. , The DM domain protein DMRT1 is a dose-sensitive regulator of fetal germ cell proliferation and pluripotency. Proc. Natl. Acad. Sci. U.S.A. 106, 22323–22328 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leitch H. G., et al. , On the fate of primordial germ cells injected into early mouse embryos. Dev. Biol. 385, 155–159 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leitch H. G., Smith A., The mammalian germline as a pluripotency cycle. Development 140, 2495–2501 (2013). [DOI] [PubMed] [Google Scholar]
- 75.Cohen M. A., Markoulaki S., Jaenisch R., Matched developmental timing of donor cells with the host is crucial for chimera formation. Stem Cell Reports 10, 1445–1452 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mueller S., et al. , Chimeric pigs following blastocyst injection of transgenic porcine primordial germ cells. Mol. Reprod. Dev. 54, 244–254 (1999). [DOI] [PubMed] [Google Scholar]
- 77.Hayashi K., Ohta H., Kurimoto K., Aramaki S., Saitou M., Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 146, 519–532 (2011). [DOI] [PubMed] [Google Scholar]
- 78.Hayashi K., et al. , Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science 338, 971–975 (2012). [DOI] [PubMed] [Google Scholar]
- 79.Maatouk D. M., et al. , DNA methylation is a primary mechanism for silencing postmigratory primordial germ cell genes in both germ cell and somatic cell lineages. Development 133, 3411–3418 (2006). [DOI] [PubMed] [Google Scholar]
- 80.Ohta H., et al. , In vitro expansion of mouse primordial germ cell-like cells recapitulates an epigenetic blank slate. EMBO J. 36, 1888–1907 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hu Y.-C., et al. , Licensing of primordial germ cells for gametogenesis depends on genital ridge signaling. PLoS Genet. 11, e1005019 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miyauchi H., et al. , Bone morphogenetic protein and retinoic acid synergistically specify female germ-cell fate in mice. EMBO J. 36, 3100–3119 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oosterhuis J. W., Looijenga L. H. J., Testicular germ-cell tumours in a broader perspective. Nat. Rev. Cancer 5, 210–222 (2005). [DOI] [PubMed] [Google Scholar]
- 84.Litchfield K., et al. ; UK Testicular Cancer Collaboration ; PRACTICAL Consortium , Identification of 19 new risk loci and potential regulatory mechanisms influencing susceptibility to testicular germ cell tumor. Nat. Genet. 49, 1133–1140 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Z., et al. ; Testicular Cancer Consortium , Meta-analysis of five genome-wide association studies identifies multiple new loci associated with testicular germ cell tumor. Nat. Genet. 49, 1141–1147 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ruark E., et al. ; UK Testicular Cancer Collaboration (UKTCC) , Identification of nine new susceptibility loci for testicular cancer, including variants near DAZL and PRDM14. Nat. Genet. 45, 686–689 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rapley E. A., et al. ; UK Testicular Cancer Collaboration , A genome-wide association study of testicular germ cell tumor. Nat. Genet. 41, 807–810 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Runyan C., et al. , Steel factor controls midline cell death of primordial germ cells and is essential for their normal proliferation and migration. Development 133, 4861–4869 (2006). [DOI] [PubMed] [Google Scholar]
- 89.Heaney J. D., Lam M.-Y. J., Michelson M. V., Nadeau J. H., Loss of the transmembrane but not the soluble kit ligand isoform increases testicular germ cell tumor susceptibility in mice. Cancer Res. 68, 5193–5197 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kanetsky P. A., et al. , Common variation in KITLG and at 5q31.3 predisposes to testicular germ cell cancer. Nat. Genet. 41, 811–815 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Looijenga L. H. J., et al. , Tumor risk in disorders of sex development (DSD). Best Pract. Res. Clin. Endocrinol. Metab. 21, 480–495 (2007). [DOI] [PubMed] [Google Scholar]
- 92.Znaor A., Lortet-Tieulent J., Laversanne M., Jemal A., Bray F., International testicular cancer incidence trends: Generational transitions in 38 countries 1900-1990. Cancer Causes Control 26, 151–158 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated by array and RNA-seq have been deposited at Gene Expression Omnibus (accession no. GSE87771) and Sequence Read Archive (accession no. PRJNA434733), respectively.