Significance
While alcohol is widely known for its proinflammatory effects, there is also evidence that it may improve outcomes in several autoimmune conditions. Alcohol’s mechanism of potential beneficial effects is unclear, and there are no clinical guidelines on the acceptable level of patient consumption. Using a model of autoimmune neuroinflammation, experimental autoimmune encephalomyelitis, we present data that moderate alcohol consumption ameliorates disease symptoms, decreases central nervous system (CNS) microglia, and enriches protective gut microbial networks in a sex-specific pattern. Our results implicate the gut–CNS axis in alcohol’s effects in autoimmunity and raise important broader implications about sex-specific diet effects.
Keywords: multiple sclerosis, EAE, alcohol, gut–CNS axis, diet
Abstract
Alcohol is a widely consumed dietary component by patients with autoimmune neuroinflammatory diseases, but current evidence on the effects of alcohol in these conditions is confounding. Epidemiological studies suggest moderate consumption of alcohol may be protective in some autoimmune diseases; however, this correlation has not been directly investigated. Here, we characterize the effects of moderate-dose alcohol in a model system of autoimmune neuroinflammation, experimental autoimmune encephalomyelitis (EAE). Male and female C57BL/6J mice were fed a 2.6% alcohol or isocaloric diet for 3 wk prior to MOG35–55 EAE induction. Surprisingly, alcohol-fed males experienced significantly greater disease remission compared to alcohol-fed females and control-fed counterparts. We observed a male-specific decrease in microglial density in alcohol-consuming animals in cervical and thoracic spinal cord in late-stage disease. In the gut, alcohol diet resulted in several sex-specific alterations in key microbiota known for their regulatory immune roles, including Turicibacter, Akkermansia, Prevotella, and Clostridium. Using a correlation network modeling approach, we identified unique bacterial modules that are significantly enriched in response to treatment and sex, composed of Clostridial taxa and several Firmicutes known to be protective in EAE. Together, these data demonstrate the potential of alcohol to significantly alter the course of autoimmunity differentially in males and females via effects on gut bacterial networks and support further need to evaluate dose and sex-specific alcohol effects in multiple sclerosis (MS) and other autoimmune neuroinflammatory conditions.
There is a growing consensus that environmental factors play a critical role in the rising incidence of neuroinflammatory autoimmune disorders worldwide (1). In particular, diet has been implicated as a major risk factor in autoimmune disease susceptibility (2, 3). Different dietary components have been shown to alter the gut microbiome (4), which is composed of trillions of symbiotic microorganisms in the intestinal tract. The gut microbiota are thought to impact immune homeostasis, inflammation (5), and autoimmunity (6) via metabolite production and interactions with gut immune cells (7). Microbial immune influence is of particular interest for neuroinflammatory disorders, as gut microbes can have a direct effect on central nervous system (CNS) inflammation through the gut–CNS axis (2, 8), a bidirectional communication system between intestinal microbiota and CNS microglia. Thus, as a directly modifiable environmental risk factor, dietary intervention presents an attractive treatment option that is generally well tolerated, cost effective, and may be used in conjunction with existing medical therapies.
While the influence of many dietary factors such as salt, fat, and carbohydrates (9) have been widely studied, relatively little attention has been devoted to the role of alcohol in neuroinflammatory conditions despite its widespread consumption by patients (10). Alcohol is known to activate microglia in CNS inflammation (11) and induce gut dysbiosis (12). However, in several studies of autoimmune conditions, including lupus, hypothyroidism, and rheumatoid arthritis (13–15), alcohol has been shown to improve disease outcomes. Thus, alcohol’s precise role in autoimmunity is largely controversial, contributing toward the challenge of offering evidence-based recommendations on alcohol consumption to patients.
In this study, we used experimental autoimmune encephalomyelitis (EAE) as a model system to directly evaluate the effects of alcohol on autoimmune neuroinflammation. EAE is a widely used animal model of multiple sclerosis (MS) (16, 17), an autoimmune disease resulting in CNS demyelination and progressive neurodegeneration. Both gut microbiome (18) and microglial activation (19) are known to influence the course of EAE, and thus EAE is an optimal model to study alcohol’s effects on CNS autoimmunity. We hypothesized that exposure to daily moderate doses of alcohol would result in reduced EAE severity. We also predicted that alcohol would differentially affect male and female gut microbiome and CNS microglia, since 1) there is a known sex bias in autoimmune disease pathogenesis (20), 2) alcohol has differential effects on male and female physiology (21) and immune system activation (22), as well as 3) there are sex differences in gut microbiota (23), which can be exacerbated by alcohol.
Results
Moderate Alcohol Consumption Significantly Reduces Clinical EAE Severity in a Sex-Specific Pattern.
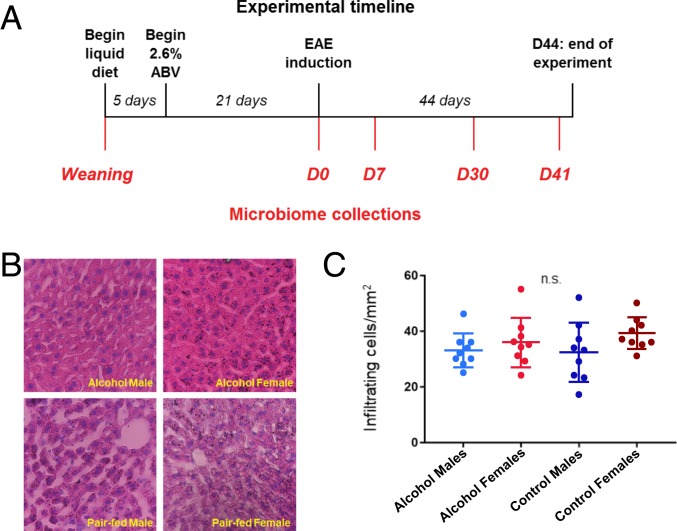
To evaluate the potential beneficial effects of moderate alcohol consumption in an MS model, mice were fed either a 2.6% ABV Lieber–DeCarli diet or an isocaloric pair-fed control diet starting at 3 wk prior to EAE induction and continued for 44 d postinduction (D44), as described in Materials and Methods (Fig. 1A). Diets were well tolerated as evidenced by lack of motor impairment, ataxia, or weight loss. Animals maintained expected weight gain, and there was no notable difference in body weight between the treatment groups within sex (SI Appendix, Fig. S1). As expected, animals across experimental groups lost weight coinciding with peak disease (D14) followed by a recovery of the weight along with their motor function (Fig. 2). Alcohol-fed groups consumed 7 to 9 g of alcohol per kg of body weight per d, with no significant difference in consumption between males and females (SI Appendix, Fig. S1). There were also no significant differences noted in liver histology between experimental groups, suggesting that 2.6% Lieber–DeCarli diet did not contribute toward end-organ damage (Fig. 1 B and C).
Fig. 1.
Alcohol treatment ameliorates EAE course in a sex-specific pattern. (A) Animals were provided a liquid diet as their sole food source beginning 4 wk prior to EAE induction. Alcohol-fed mice received an up-taper of alcohol diet until they reached 2.6% ABV and continued for the duration of the experiment. Control mice received an isocaloric alcohol-free liquid diet. Microbiome samples were collected at 5 time points, as shown. (B) Liver samples were collected and stained with hematoxylin and eosin to quantify mononuclear cell infiltration. (C) Cellular infiltration did not significantly differ between groups (n = 3 per group, 3 sequential slices per subject; P = 0.21).
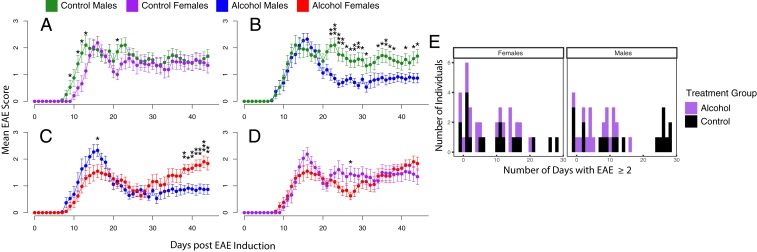
Fig. 2.
Alcohol treatment ameliorates EAE course in a sex-specific pattern. (A) Post MOG 35–55 EAE induction, control-fed males experience a slightly earlier disease onset compared to control-fed females but overall no significant sex difference at later disease stages. (B) Males on alcohol diet show a slightly greater EAE peak disease followed by significant and sustained amelioration post peak disease, while females show significant disease progression after day 39. (C) Males on alcohol diet experience a sustained symptomatic improvement post peak disease compared with control males. (D) Overall, no differences were noted between females on alcohol diet and controls. n = 19 to 20 per group, combined data for 3 individual experiments (Student’s t test, *P < 0.05,**P < 0.01, and ***P < 0.001). (E) Poisson regression model of the frequency of days with EAE score ≥ 2 demonstrates a significant treatment effect (P < 0.01), sex effect (P < 0.01), and treatment:sex interaction (P < 0.01).
Following EAE induction, mice were scored daily for symptomatic onset and disease progression per standard EAE scoring criteria, reflecting the degree of motor impairment (16). Mice began to display motor deficits by approximately D9 and reached peak disease scores by D15. Overall, there was no difference in peak disease scores (D12 to D18) between treatment groups within sex (Fig. 2). As expected in C57BL/6J EAE, we did not observe a difference in scores between males and females on pair-fed control diet (Fig. 2A).
Interestingly, there was a significant sex difference in EAE scores within the alcohol-fed groups during the recovery phase (Fig. 2). Specifically, alcohol-fed males experienced a greater reduction in disease severity postpeak sustained to D44 compared to alcohol-fed females and control males (Fig. 2 B and C). Alcohol-fed females initially experienced a decrease in disease scores D20 to D30, followed by relapse in higher disease scores post D30, reaching significance by D38 (Fig. 2C). There was no sustained difference in disease scores between control males and females throughout the experimental course (Fig. 2A). Linear mixed-effect modeling of the clinical scores run on aligned EAE scores to day of EAE onset, confirmed an interaction between day, treatment group, and sex (P = 1.12e-6) (SI Appendix, Fig. S2). A Poisson regression model of the frequency of days with EAE score ≥ 2 also demonstrated a treatment and sex interaction (P = 4.86e-3) (Fig. 2E). Thus, our data suggest that alcohol can have divergent effects in males and females throughout the EAE disease course, with greatest potential to impart a protective phenotype in alcohol-consuming males.
Moderate Alcohol Consumption Reduces Neuroinflammation.
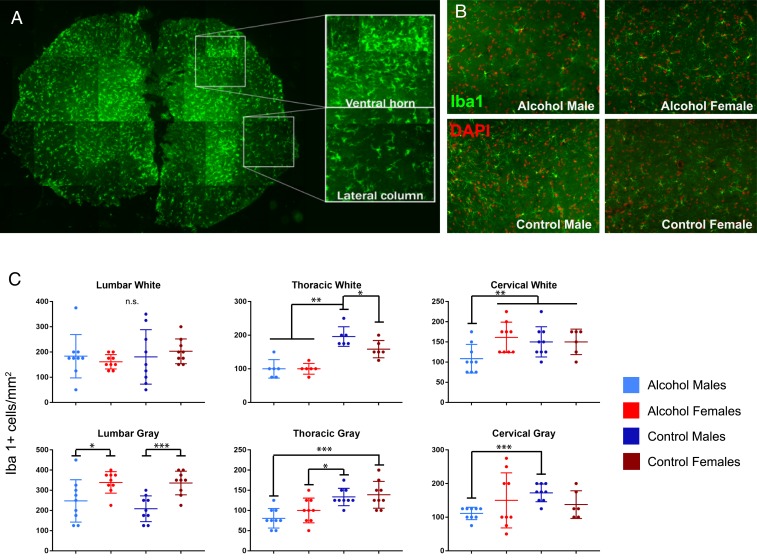
Alcohol is known to influence microglia in the CNS (11, 24). To investigate whether the effects of moderate alcohol diet may be acting on microglial activation during EAE, we collected spinal cords from alcohol-consuming and control animals on D44. Iba1+ microglial cells were quantified within white matter (lateral white matter column) and gray matter (ventral horn efferent motor area) of the lumbar, thoracic, and cervical regions of the spinal cord. Microglial density per square millimeter was significantly reduced in both the lateral white matter columns and ventral horn efferent motor area in thoracic regions of alcohol-consuming males and females. In cervical spinal cord, microglial reduction was observed specifically in alcohol-consuming males. While no significant differences were found between groups in the lumbar lateral columns, there was a marked sex difference in the lumbar ventral horn (Fig. 3C). The male-specific decrease in microglial density in cervical and thoracic spinal cord suggests that alcohol may be acting in a sex-specific pattern to alter CNS inflammation and disease progression.
Fig. 3.
Alcohol consumption reduces spinal cord microglial density in males. (A) Spinal cords collected 44 d postinduction were stained for the microglial marker Iba1 to quantify microglial density in the ventral horn and lateral columns. (B) Representative images of ventral horn staining from cervical spinal cord. (C) Microglial density was significantly lower in the cervical spinal cord of alcohol-consuming males compared to other groups in both the ventral horn and lateral column. In thoracic regions, alcohol-consuming males and females had reduced microglial density compared to control counterparts. Additionally, alcohol-consuming females had a lower density of Iba1+ cells compared to control females in thoracic lateral columns. In the lumbar spinal cord, there were no significant differences between microglial density in the lateral columns. Density in the lumbar ventral horn was significantly lower in males compared to females regardless of treatment (n = 3 per group, 3 slices per subject; Student’s t test, *P < 0.05, **P < 0.01, and ***P < 0.001).
Moderate Alcohol Treatment Alters Gut Microbiome in a Sex-Specific Pattern.
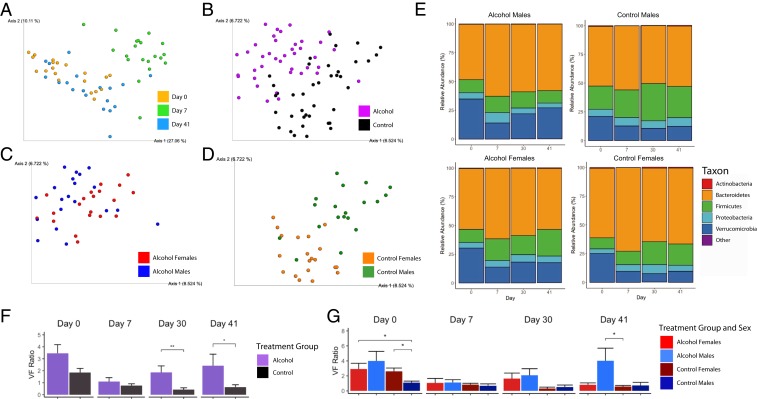
We next asked whether moderate alcohol consumption could lead to alterations within the gut microbiome in the EAE model. Bacterial DNA was extracted from fecal samples from male and female mice at weaning, pre-EAE (D0), on day 7 postinduction (D7), and during EAE-recovery stage (D30 and D41), and analyzed by 16S rRNA sequencing. Principal-coordinate analysis (PCoA) of beta diversity using Bray–Curtis dissimilarity index showed clustering of groups clearly distinguishing D7 time point samples from D0 and D41 time points (Fig. 4A). Analysis of the Jaccard’s beta diversity index demonstrated separation by treatment group as well as by sex within treatment group at D41 (Fig. 4 B–D). The weaning time point was used to confirm there was no significance difference between the groups’ microbiomes, and to serve as a baseline for each individual mouse. Alpha diversity remained similar throughout the course of the experiment (SI Appendix, Fig. S3).
Fig. 4.
Alcohol treatment and EAE induction shifts gut microbiome beta diversity and induces phylum level gut microbiome changes. (A) Principal-coordinate analysis (PCoA) of Bray–Curtis dissimilarity index illustrates a shift of the D7 microbiome compared to D0 and D41 prior to clinical symptom onset. (B) PCoA of Jaccard’s index reveals a treatment effect that can further be divided by sex as shown in C and D, emphasizing that both the alcohol-fed and control groups’ microbiomes respond differentially between the sexes. (E) Mosaic plots illustrating the average composition of the gut microbiome at the phylum taxonomic level in the 4 treatment groups. (F) Verrucomicrobia-to-Firmicutes ratio (VF) of the treatment groups reveals a stronger restoration of the gut microbiome to pre-EAE levels in the alcohol-fed mice. (G) VF ratio of the sexes and their respective treatment groups demonstrate a trend of a higher VF ratio in the alcohol-fed males post-EAE induction (n = 4 to 5 per group; Wilcoxon rank sum tests and Benjamini–Hochberg corrections, *P < 0.05, **P < 0.01, and ***P < 0.001).
At the phylum taxonomic level, relative abundance changes were analyzed over the course of EAE. We observed a marked reduction in the relative abundance of Verrucomicrobia and an increase in Firmicutes on D7 compared to D0 in all groups irrespective of treatment (Fig. 4E). As the shift in these phyla appeared to be a characteristic of EAE onset, we investigated the Verrucomicrobia-to-Firmicutes ratio (VF ratio) as a potential marker of the disease state. The VF ratio was significantly higher in alcohol-fed groups on D30 and D41 compared to controls, and was particularly higher in alcohol-fed males on D41 (Fig. 4 F and G).
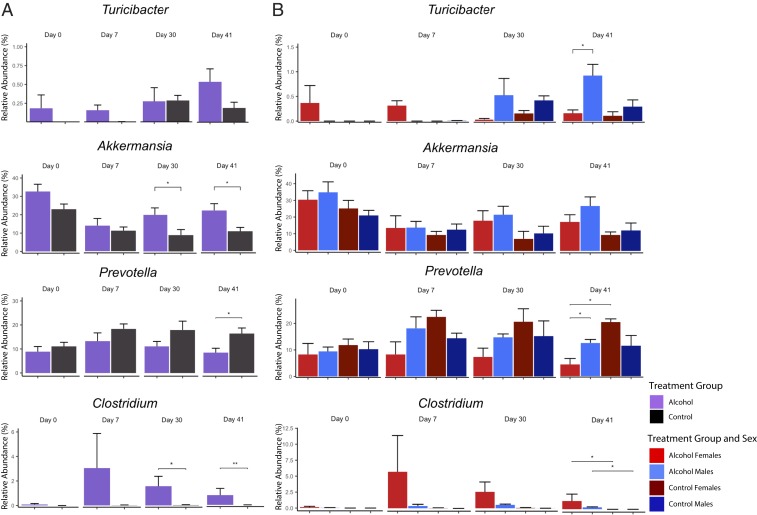
Analysis at the genus taxonomic level revealed several genera differentially expressed across treatment timepoints (Fig. 5). The genus Turicibacter began to increase in alcohol-fed males compared to the alcohol-fed females on D30 and reached significance on D41 (P = 0.037). The genus Akkermansia was significantly elevated in alcohol-fed male and female groups on D30 (P = 0.023) and D41 (P = 0.027) compared to pair-fed controls. In addition, the genus Prevotella was noted to be reduced in alcohol-consuming females on D7 and D30 and reached significance on D41 (P = 0.012) compared to pair-fed females. Finally, the genus Clostridium was increased in all alcohol-fed groups on D30 (P = 0.011), and D41 (P = 0.004). (SI Appendix, Tables S1–S3).
Fig. 5.
Alcohol consumption induces genus-level gut microbiome changes in a sex-specific pattern. (A) Effects of alcohol treatment on specific genera’s relative abundance in the gut microbiome. (B) Sex-specific effects of the taxa depicted in A. (Error bars calculated with SE for n = 4 to 5 per group; Wilcoxon rank-sum test and Benjamini–Hochberg corrections: *P < 0.05 and **P < 0.01.)
Moderate Alcohol Treatment Alters Microbiota Networks in the Gut.
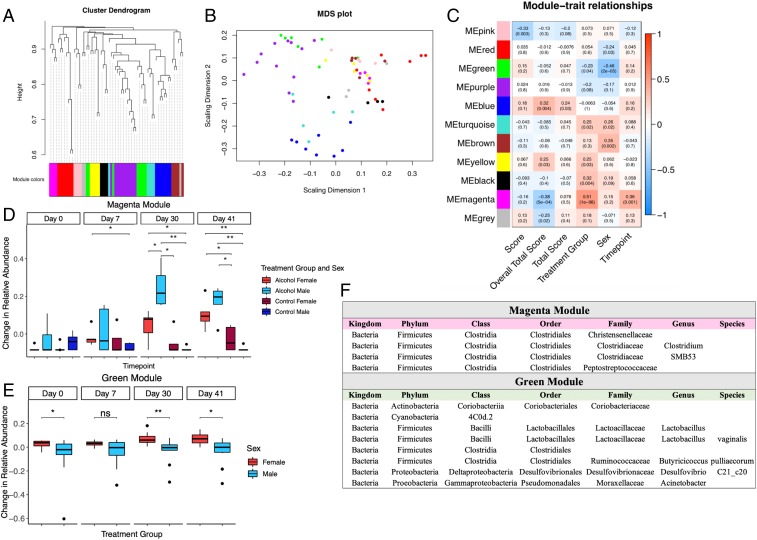
To better characterize the biological relevance of alcohol-induced taxonomic shifts, we developed an approach to evaluate gut microbiome network interactions through modified use of unbiased weighted gene coexpression network analysis (WGCNA). This technique, originally developed by Horvath and colleague (25), allows to cluster correlated changes in gene expression and has been used widely for transcriptomics studies. Just like genes acting in networks, it is likely that gut bacterial taxa also function in biological networks within an organism. We thus applied WGCNA to identify clusters of bacterial taxa whose differential representation was correlated across treatment groups, time points, or both. Our analysis resulted in a network clustered into 11 distinct modules of highly correlated bacteria (Fig. 6A), of which the largest module contained 11 bacterial species and the smallest module contained 3 bacterial species.
Fig. 6.
Alcohol consumption induces genus-level gut microbiome changes in a sex-specific pattern. (A) Hierarchical clustering of taxa and assignment to modules based on the log relative abundance ratios. (B) Multidimensional scaling of the taxa demonstrates Euclidean separation of modules. (C) Correlative heat map of traits with their respective P values (score = score day of collection; overall total score = cumulative score of mouse for the entire experiment; total score = cumulative score to day of collection.) (D) Box-and-whisker plot of the magenta module demonstrates increased eigentaxa representation in the alcohol-fed mice, particularly in the males. (E) Box-and-whisker plot of the green module reveals an inherent sex effect with the females displaying enriched eigentaxa compared to the males in both treatment groups. (F) Constituent taxa of the green and magenta modules (n = 4 to 5 per group; Wilcoxon rank sum test and Benjamini–Hochberg corrections, *P < 0.05, **P < 0.01, and ***P < 0.001).
Each sample was then assigned a relative score similar to WGCNA’s eigengenes, which we have respectively termed “eigentaxa.” These eigentaxa scores were then tested to determine how modules correlated with differing traits (Fig. 6C). Of the identified modules, 2 modules presented intriguing results. The magenta module had an eigentaxa that was positively correlated with treatment group, and the green module had an eigentaxa that was differentially affected by sex independent of treatment.
Notably, the magenta module (Fig. 6D) was significantly enriched in the alcohol-fed mice at D30 and D41, with the alcohol-fed males presenting the strongest relative increase in constituents of this module. Interestingly, the taxa of the magenta module are all in the order of Clostridiales, and within this order, the families of Christensenellaceae, Clostridiaceae, and Peptostreptococcaceae were specifically identified in our data. Clostridiaceae had 2 specific genera identified within it, SMB53 and Clostridium.
The green module, consisting of a large array of bacteria, stood out for a sexually dimorphic relative abundance change at D0 (P = 0.045), D30 (P = 0.007), and D41 (P = 0.009) regardless of treatment groups (Fig. 6E). The absence of a sex difference in this module at D7 could be explained by the dramatic shift at this time point mediated by EAE induction and disease onset. Interestingly, the majority of the taxa in this module are from the Firmicutes phylum, which has been previously noted to contribute to a latent sex difference in the microbiome (23, 26).
In conclusion, weighted coexpression network analysis identified a clear difference in bacterial populations between alcohol-consuming and control diet animals, which correlates with disease progression. Thus, weighted coexpression network analysis is suitable for adaptation to longitudinal microbiome studies.
Discussion
Here, we present evidence that an environmental factor in the form of moderate alcohol can alter the course of a neuroinflammatory disease. Combining a clinical EAE study, CNS histology, gut microbiome sequencing, and analysis of weighted coexpression gut bacterial networks, our findings implicate the gut–CNS axis in mediating moderate alcohol’s beneficial effects in a sex-specific pattern.
Specifically, we observed that males on a moderate alcohol diet experienced significantly greater disease remission compared to alcohol-fed females and control males and females. Females on the alcohol diet initially trended toward disease amelioration, followed by disease progression in the late stages of EAE. Our results suggest that moderate daily alcohol dosing may have differential effects on the sexes, and potentially could have a protective effect in males. These findings are unexpected, as C57BL/6J mice are not known to display a sex difference in EAE (27). Traditionally, sexually dimorphic phenotypes are ascribed to primary hormonal or sex chromosomal effects (28). Here, we demonstrate that a dietary factor can directly uncover and effectuate disease pathogenesis differentially in males and females. The known influence of alcohol consumption on estrogen and testosterone production (22, 29) and direct action of alcohol on sex hormone receptors in the CNS (30) necessitates further investigation to elucidate this potential mechanism of action in the EAE model.
Microglial activation has previously been linked to both EAE severity and alcohol-related CNS inflammation (31). However, activated microglia also present in diverse phenotypes and have been shown to drive remyelination and mitigate peripheral lymphocyte infiltration (32). Evaluation of microglial density in our study in late-stage EAE revealed a significant reduction in microglia in the cervical and thoracic spinal cord of alcohol-fed males correlating with observed clinical amelioration in EAE scores. This sex-specific reduction in microglial density suggests that either alcohol is acting on the CNS to down-regulate proinflammatory microglia or to up-regulate neuroprotective microglia in alcohol-fed males. It is also possible that the microglial density is influenced by alcohol-induced changes on the peripheral immune system, which drive CNS lymphocyte infiltration. In future studies, it will be important to define the effect of alcohol on microglial signatures in the EAE model and to correlate microglial phenotypes with the inflammatory milieu and infiltration of peripheral immune cells.
Our microbiome analysis revealed several intriguing results. The combined use of the Bray–Curtis and Jaccard’s beta diversity indices allowed for a more powerful method of visualizing true variance in the data. Jaccard’s index illustrated clear treatment effects and sex differences between groups. In turn, Bray–Curtis dissimilarity showed a clear separation of bacterial populations on D7 compared to the other experimental time points in both treatment groups. Taken together, these findings indicate that alcohol affects microbial constituents over the course of time in EAE differentially in males and females. In particular, the microbiome composition was most distinct on D7 post-EAE induction, insinuating that changes to the microbiota precede the development of clinical symptoms and likely stage the immune system for subsequent course of the disease. The restoration of the gut microbiome to predisease condition by D41 suggests that, as the clinical disease reaches a steady state physiologically, a similar “settling effect” can be observed within the gut microbiome.
Focusing on specific microbiota taxa in the gut, we identified phylum- and genus-level signature microbiomic shifts in response to alcohol treatment over the course of EAE. For example, there was a higher abundance of Turicibacter (phylum Firmicutes) in alcohol-fed males on D30 and D41, at time of disease quiescence. Turicibacter is known to be correlated with lower disease scores in murine EAE models (33). Interestingly, we observed a female-specific reduction in Prevotella (phylum Bacteroidetes) at later disease stages. This taxon is known to be decreased in untreated MS patients and increases with MS treatment (34). Another significant genera increased in alcohol-fed animals, with a trend for higher abundance in males, was Akkermansia (phylum Verrucomicrobia), which has been shown in different studies to regulate immune system function through short-chain fatty acid (SCFA) production (35) and is found to be decreased in alcohol binge studies correlating with higher inflammatory states (36). Of note, Akkermansia has been found to be increased in untreated MS patients (37, 38), although this species is thought to be protective in other autoimmune diseases such as type 1 diabetes (39). Furthermore, Akkermansia has been shown to improve metabolic profiles and reduce systemic inflammation in several animal models (40, 41). Last, we also observed an increase in the genus Clostridium (phylum Firmicutes) in alcohol-fed animals, which is composed of ∼100 species within the gut, with some species having potent antiinflammatory activity via butyrate production (42).
We observed that the D7 microbiome profile, known as the time of highest peripheral inflammation prior to clinical EAE onset, was primarily characterized by reduction in Verrucomicrobia relative to Firmicutes regardless of treatment or sex. Of note, Firmicutes are known to be increased in MS patients during times of higher disease activity and decreased at disease quiescence (43). Conversely, Verrucomicrobia, solely composed of Akkermansia in the gut (44), is a keystone of a healthy microbiome and known for an immunoregulatory role via SCFA production (35). Interestingly, VF ratios were increased in alcohol-consuming mice prior to disease induction and were significantly elevated in alcohol-treated males during late-stage disease, suggesting that this ratio may be associated with disease amelioration. Thus, we propose the VF ratio as a potentially useful marker indicative of EAE disease state akin to the Bacteroidetes-to-Firmicutes ratio, previously described as a biomarker of inflammatory microbiome shifts in models of inflammatory bowel disease, obesity, and MS (43, 45, 46). However, due to novelty of our proposed VF ratio, it requires further replication and validation in future studies in EAE and MS.
While individual species shifts within the microbiome reveal interesting biological associations, this type of analysis ignores interactions between networks of bacteria. A common approach to quantifying bacterial networks is using simple correlation-based construction or a dissimilarity measure, methods that rely primarily on adjacency correlation between neighbors but do not account for more complex interactions between nonadjacent neighbors. A more realistic approach to biological networks is gene network analysis, which utilizes a scale-free network with a topological overlap matrix (TOM) (47). The use of the TOM allows for better module detection within the networks by weighing direct adjacency in the network in combination with connections through neighboring nodes. From the perspective of systems biology, the bacteria in the same module are more likely to have similar biological function. Applying this approach to gut bacterial network analysis allowed us to identify groups of bacteria in modules that increase or decrease in unison over the course of the experiment.
Although WGCNA is not prominent in microbiome studies, its fundamental techniques, including hierarchical clustering, principal-component analysis, and correlation analysis are commonly applied separately in microbiome studies. The combined use of these individual techniques offers a powerful approach to study longitudinal microbial correlations within the microbiome. Future studies with large-scale microbiome data will help to further validate this method of data transformation in application to WGCNA.
One of the most notable modules in our study, magenta, was significantly enriched in the alcohol-fed males and was composed of taxa within the order Clostridiales. Specific taxa within this order are known for immune-modulating activity and previously found to be reduced in MS patients (48). For example, the family of Christensenellaceae appears to be higher in mice who either fail to develop EAE or present a mild disease phenotype (33), and Clostridium is known to have antiinflammatory properties via butyrate production (42). The genus Peptostreptococcaceae is correlated with low levels of vitamin D in MS patients and, like the mucin degrading genus, Akkermansia municiphilia, has an immunoregulatory role through SCFA production (35).
Several recent studies have highlighted sex-specific differences in the gut microbiome constituents (23, 26). We identified a sex-specific module, green, which consisted of multiple bacteria, including taxa from the class of Bacillus and the family Ruminococceae. This finding implies that postweaning, males and females may be developing sex-specific differences within the gut microbiome. Given that a sex difference in MS emerges postpuberty (49), our findings implicate the gut microbiome in potentially contributing toward the known age-specific sex differences in MS.
In summary, we present data that moderate alcohol diet is well tolerated in the EAE model and can drive CNS changes differentially in males and females. The identification of a dietary factor that results in EAE amelioration in a sex-specific pattern has several important implications. First, our results suggest that diet independent of other factors could influence onset and progression of MS autoimmunity and as such could be contributing toward the known global rise of MS in females (50). Our findings highlight the need for further mechanistic diet studies in both sexes to best guide personalized recommendations on dietary habits in the treatment of patients with MS and other autoimmune diseases. In addition, our observation that gut microbiota shift early in the disease course in response to alcohol suggests that dietary factors influencing the gut microbiome composition during early development and perhaps during adolescence could ultimately influence the future course of autoimmunity.
The existing human epidemiological studies in MS are controversial with respect to potential beneficial versus detrimental effects of alcohol (51–53) and there are no current guidelines on alcohol for MS patients (54). Thus, clinical advice on continuing versus stopping alcohol consumption in MS remains largely speculative. Our finding that moderate alcohol is well tolerated in mice and exerts beneficial effects suggests that low-dose alcohol consumption may be a consideration in patients with MS. A limitation of our study is that we did not directly evaluate alcohol in human patients. Thus, further long-term follow-up mechanistic studies in humans are critical to ascertain the relationship between exact dosing of alcohol and disease activity, as well as potential side effects associated with alcohol consumption. Additional studies will also be necessary to differentiate the direct CNS effects of alcohol from the physiological effects caused by alcohol-induced microbiome changes. Since alcohol can have differential effects on male and female physiology (21, 29), it is important that future studies are carried out in both sexes. Inevitably, a more comprehensive understanding of alcohol’s effects in MS could unveil molecular pathways and therapeutics that could be targeted to recapitulate the protective effects of alcohol without direct alcohol consumption. Ultimately, a better understanding of the mechanisms by which moderate alcohol exerts a beneficial effect in neuroinflammation will also shed light unto beneficial effects of other dietary factors in MS and other neuroinflammatory conditions.
Materials and Methods
Animals.
Male and female C57BL/6J mice were housed under specific-pathogen–free conditions in microisolator cages (n = 4 to 5 per cage) under a 12-h light/dark cycle. Mice received a liquid Lieber–DeCarli diet as their sole food source from weaning (postnatal day 21). Alcohol groups had ad libitum access to a 2.6% alcohol by volume formula (BioServ; F1258SP). Control groups were given an alcohol-free control diet (BioServ; F1259SP) and were isocalorically pair-fed as previously described (55). Water was provided ad libitum. All animal experiments were approved by the University of Texas at Austin Institutional Animal Care and Use Committee.
Experimental Autoimmune Encephalomyelitis.
EAE was induced in 7-wk-old mice by subcutaneous injection of 100 µg of MOG35–55 peptide (Genemed Synthesis Inc.) dissolved in Complete Freund’s Adjuvant at 1 mg/mL. Intraperitoneal injections of 250 ng pertussis toxin (List Biological Laboratories Inc.) were administered immediately following MOG35–55 and 48 h after. EAE severity was scored on a 0 to 5 scale reflecting degree of limb paralysis and motor dysfunction per Hooke Laboratories protocol (“Appendix A: EAE scoring guide,” Hooke catalog no. EK-2110).
Histology and Immunohistochemistry.
Mice were intracardially perfused with 10 mL of cold PBS and 10 mL of cold 4% paraformaldehyde (PFA). Whole spinal cords and livers were extracted and fixed in 4% PFA for 24 h, and then cryoprotected in 30% sucrose prior to sectioning. Spinal cords were sliced at 20 µm and stained with rabbit anti-Iba1 (WAKO; 1:1,000 dilution), FITC-conjugated donkey anti-rabbit (Abcam; 1:200), and DAPI (1 µg/mL). Livers were sectioned at 10 µm and stained with 0.1% hematoxylin and 0.1% eosin. Image analysis was performed using FIJI ImageJ software. Figures were generated in GraphPad Prism 7 and R.
Microbiome Analysis.
Fecal samples were immediately frozen on dry ice and stored at −80 °C before processing. Bacterial genomic DNA was extracted using QIAGEN DNeasy PowerSoil Kit following the manufacturer’s protocol. 16S rRNA sequencing was performed at the Genomic Sequencing and Analysis Facility. Sequencing data were processed in Qiime2 (2018.11) using DADA2 to denoise, and the GreenGene classifier to assign taxonomy. The read counts were fractionated over the total read counts of each sample to get a representative percent relative abundance.
Statistical Analysis.
Significance for the score curves, weights, food consumption, and microglial quantification was determined with Student’s t test with error bars calculated from SEM. For the Poisson distribution, the count of the number of days with “high” EAE scores (defined as EAE ≥ 2) was taken as the dependent variable. Significance for the microbiome analysis was determined using Wilcoxon rank-sum tests and Benjamini and Hochberg corrections in R:
The WGCNA package in R was used to analyze and group taxa into modules. A log-transformed ratio of the experimental time points (ε) over the weaning time point (α) of each mouse including a pseudocount of 5 reads over the total reads (θ) to create a ratio of change (φ) that was used for the WGCNA clustering analysis. The scale-free topological overlay matrix selection criteria were used to select β = 2. Visualization utilized the ggplot2 package for R.
Data and Materials Availability.
The data reported in this paper have been deposited to the Sequence Read Archive of the National Center for Biotechnology Information of the National Library of Medicine (PRJNA564343) (56).
Supplementary Material
Acknowledgments
We are grateful to K. Helmsdoerfer, J. Podnar and Genomic Sequencing and Analysis Facility, and R. Von Leden for excellent technical support; and to G. Otto and R. Messing for thoughtful discussions. We also thank W. Schwartz, A. Harris, H. Hofmann, and M. Han for critical reading of the manuscript. This work was supported by Dell Medical School start-up funding (to E.M.), Texas Institute For Discovery Education In Science (TIDES) Advance Summer Research Fellowship (to C.M.), and NIH Grant T32AA007471 (to B.C.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited to the Sequence Read Archive of the National Center for Biotechnology Information of the National Library of Medicine (PRJNA564343).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1912359116/-/DCSupplemental.
References
- 1.Jörg S., et al. , Environmental factors in autoimmune diseases and their role in multiple sclerosis. Cell. Mol. Life Sci. 73, 4611–4622 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haghikia A., et al. , Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 43, 817–829 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Vieira S. M., Pagovich O. E., Kriegel M. A., Diet, microbiota and autoimmune diseases. Lupus 23, 518–526 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turnbaugh P. J., et al. , The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 1, 6ra14 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maranduba C. M., et al. , Intestinal microbiota as modulators of the immune system and neuroimmune system: Impact on the host health and homeostasis. J. Immunol. Res. 2015, 931574 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colpitts S. L., Kasper L. H., Influence of the gut microbiome on autoimmunity in the central nervous system. J. Immunol. 198, 596–604 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Mizuno M., Noto D., Kaga N., Chiba A., Miyake S., The dual role of short fatty acid chains in the pathogenesis of autoimmune disease models. PLoS One 12, e0173032 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ochoa-Repáraz J., Mielcarz D. W., Begum-Haque S., Kasper L. H., Gut, bugs, and brain: Role of commensal bacteria in the control of central nervous system disease. Ann. Neurol. 69, 240–247 (2011). [DOI] [PubMed] [Google Scholar]
- 9.van den Hoogen W. J., Laman J. D., ’t Hart B. A., Modulation of multiple sclerosis and its animal model experimental autoimmune encephalomyelitis by food and gut microbiota. Front. Immunol. 8, 1081 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bombardier C. H., et al. , Alcohol and drug abuse among persons with multiple sclerosis. Mult. Scler. 10, 35–40 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Alfonso-Loeches S., Pascual-Lucas M., Blanco A. M., Sanchez-Vera I., Guerri C., Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J. Neurosci. 30, 8285–8295 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leclercq S., et al. , Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc. Natl. Acad. Sci. U.S.A. 111, E4485–E4493 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan H.-F., Leng R.-X., Wang J., Li X.-P., Ye D.-Q., Protective role of moderate alcohol drinking in systemic lupus erythematosus. Clin. Rheumatol. 29, 337 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Di Giuseppe D., Alfredsson L., Bottai M., Askling J., Wolk A., Long term alcohol intake and risk of rheumatoid arthritis in women: A population based cohort study. BMJ 345, e4230 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlé A., et al. , Moderate alcohol consumption may protect against overt autoimmune hypothyroidism: A population-based case-control study. Eur. J. Endocrinol. 167, 483–490 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Constantinescu C. S., Farooqi N., O’Brien K., Gran B., Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 164, 1079–1106 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinman L., Zamvil S. S., How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Ann. Neurol. 60, 12–21 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Berer K., et al. , Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. U.S.A. 114, 10719–10724 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almolda B., Gonzalez B., Castellano B., Antigen presentation in EAE: Role of microglia, macrophages and dendritic cells. Front. Biosci. 16, 1157–1171 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Voskuhl R., Sex differences in autoimmune diseases. Biol. Sex Differ. 2, 1 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomasson H. R., Gender differences in alcohol metabolism. Physiological responses to ethanol. Recent Dev. Alcohol. 12, 163–179 (1995). [DOI] [PubMed] [Google Scholar]
- 22.Grossman C. J., et al. , Sex differences and the effects of alcohol on immune response in male and female rats. Alcohol. Clin. Exp. Res. 17, 832–840 (1993). [DOI] [PubMed] [Google Scholar]
- 23.Markle J. G., et al. , Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339, 1084–1088 (2013). [DOI] [PubMed] [Google Scholar]
- 24.McCarthy G. M., Farris S. P., Blednov Y. A., Harris R. A., Mayfield R. D., Microglial-specific transcriptome changes following chronic alcohol consumption. Neuropharmacology 128, 416–424 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langfelder P., Horvath S., WGCNA: An R package for weighted correlation network analysis. BMC Bioinformatics 9, 559 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Org E., et al. , Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 7, 313–322 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papenfuss T. L., et al. , Sex differences in experimental autoimmune encephalomyelitis in multiple murine strains. J. Neuroimmunol. 150, 59–69 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Voskuhl R. R., Gold S. M., Sex-related factors in multiple sclerosis: Genetic, hormonal and environmental contributions. Nat. Rev. Neurol. 8, 255–263(2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eriksson C. J., Fukunaga T., Lindman R., Sex hormone response to alcohol. Nature 369, 711 (1994). [DOI] [PubMed] [Google Scholar]
- 30.Chung K. W., Effect of ethanol on androgen receptors in the anterior pituitary, hypothalamus and brain cortex in rats. Life Sci. 44, 273–280 (1989). [DOI] [PubMed] [Google Scholar]
- 31.Chu F., et al. , The roles of macrophages and microglia in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Neuroimmunol. 318, 1–7 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Dubbelaar M. L., Kracht L., Eggen B. J. L., Boddeke E. W. G. M., The kaleidoscope of microglial phenotypes. Front. Immunol. 9, 1753 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colpitts S. L., et al. , A bidirectional association between the gut microbiota and CNS disease in a biphasic murine model of multiple sclerosis. Gut Microbes 8, 561–573 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J., et al. , Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 6, 28484 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belzer C., de Vos W. M., Microbes inside—from diversity to function: The case of Akkermansia. ISME J. 6, 1449–1458 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grander C., et al. , Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 67, 891–901 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Jangi S., et al. , Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 7, 12015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cekanaviciute E., et al. , Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. U.S.A. 114, 10713–10718 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hansen C. H., et al. , Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia 55, 2285–2294 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Schneeberger M., et al. , Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 5, 16643 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao S., et al. , Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice. J. Mol. Endocrinol. 58, 1–14 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Van den Abbeele P., et al. , Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 7, 949–961 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cosorich I., et al. , High frequency of intestinal TH17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci. Adv. 3, e1700492 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujio-Vejar S., et al. , The gut microbiota of healthy Chilean subjects reveals a high abundance of the phylum Verrucomicrobia. Front. Microbiol. 8, 1221 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walters W. A., Xu Z., Knight R., Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 588, 4223–4233 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ley R. E., Turnbaugh P. J., Klein S., Gordon J. I., Microbial ecology: Human gut microbes associated with obesity. Nature 444, 1022–1023 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Li A., Horvath S., Network neighborhood analysis with the multi-node topological overlap measure. Bioinformatics 23, 222–231 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Calvo-Barreiro L., Eixarch H., Montalban X., Espejo C., Combined therapies to treat complex diseases: The role of the gut microbiota in multiple sclerosis. Autoimmun. Rev. 17, 165–174 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Tintoré M., Arrambide G., Early onset multiple sclerosis: The role of gender. J. Neurol. Sci. 286, 31–34 (2009). [DOI] [PubMed] [Google Scholar]
- 50.Rotstein D. L., et al. , Temporal trends in multiple sclerosis prevalence and incidence in a large population. Neurology 90, e1435–e1441 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Foster M., et al. , Associations of moderate alcohol consumption with clinical and MRI measures in multiple sclerosis. J. Neuroimmunol. 243, 61–68 (2012). [DOI] [PubMed] [Google Scholar]
- 52.D’hooghe M. B., Haentjens P., Nagels G., De Keyser J., Alcohol, coffee, fish, smoking and disease progression in multiple sclerosis. Eur. J. Neurol. 19 616–624 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Hedström A. K., Hillert J., Olsson T., Alfredsson L., Alcohol as a modifiable lifestyle factor affecting multiple sclerosis risk. JAMA Neurol. 71, 300–305 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Fragoso Y. D., Cardoso M., Is alcohol harmful for patients with multiple sclerosis? J. Mult. Scler. (Foster City) 4, 201 (2017). [Google Scholar]
- 55.Guo F., Zheng K., Benedé-Ubieto R., Cubero F. J., Nevzorova Y. A., The Lieber-DeCarli diet—a flagship model for experimental alcoholic liver disease. Alcohol. Clin. Exp. Res. 42, 1828–1840 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Caslin B., Maguire C., Melamed E., Longitudinal microbiome samples from alcohol mice with EAE. Sequencing Read Archive, https://www.ncbi.nlm.nih.gov/bioproject/PRJNA564343. Deposited 6 September 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.