Significance
Reduced expression of the serotonin transporter (5-HTT) is associated with susceptibility to stress-related psychopathology, but the underlying mechanisms remain elusive. We investigated whether an aberrant physiological and neural response to threat underlies this increased vulnerability. In a cross-species approach, we investigated the association between genetically encoded differences in 5-HTT expression and the neural correlates of fear bradycardia, a defensive response linked to vigilance. In both humans and rats, reduced 5-HTT expression was associated with exaggerated bradycardia or bradycardia-associated freezing, reduced activity of the medial prefrontal cortex, and increased threat-induced amygdala-periaqueductal grey connectivity and central amygdala somatostatin neuron activity. We have delineated a previously unknown neurogenetic mechanism underlying individual differences in the expression of anticipatory threat responses, contributing to stress sensitivity.
Keywords: serotonin transporter, bradycardia, freezing, threat
Abstract
Susceptibility to stress-related psychopathology is associated with reduced expression of the serotonin transporter (5-HTT), particularly in combination with stress exposure. Aberrant physiological and neuronal responses to threat may underlie this increased vulnerability. Here, implementing a cross-species approach, we investigated the association between 5-HTT expression and the neural correlates of fear bradycardia, a defensive response linked to vigilance and action preparation. We tested this during threat anticipation induced by a well-established fear conditioning paradigm applied in both humans and rodents. In humans, we studied the effect of the common 5-HTT-linked polymorphic region (5-HTTLPR) on bradycardia and neural responses to anticipatory threat during functional magnetic resonance imaging scanning in healthy volunteers (n = 104). Compared with homozygous long-allele carriers, the 5-HTTLPR short-allele carriers displayed an exaggerated bradycardic response to threat, overall reduced activation of the medial prefrontal cortex (mPFC), and increased threat-induced connectivity between the amygdala and periaqueductal gray (PAG), which statistically mediated the effect of the 5-HTTLPR genotype on bradycardia. In parallel, 5-HTT knockout (KO) rats also showed exaggerated threat-related bradycardia and behavioral freezing. Immunohistochemistry indicated overall reduced activity of glutamatergic neurons in the mPFC of KO rats and increased activity of central amygdala somatostatin-positive neurons, putatively projecting to the PAG, which—similarly to the human population—mediated the 5-HTT genotype’s effect on freezing. Moreover, the ventrolateral PAG of KO rats displayed elevated overall activity and increased relative activation of CaMKII-expressing projection neurons. Our results provide a mechanistic explanation for previously reported associations between 5-HTT gene variance and a stress-sensitive phenotype.
Activation of the autonomic stress system is vital to ensure an organism’s fast and accurate response to threatening situations. Threat imminence modulates the sympathetic-parasympathetic balance; more immediate threats induce a stronger sympathetic response, resulting in hypertension and tachycardia, associated fight-or-flight behavior, while more distant threats typically produce parasympathetically mediated bradycardia and behavioral freezing (1–3). Freezing functions as a preparatory reflex to avoid detection by a predator (4, 5) and is well-conserved among species, including humans (4, 6). However, the attentional focus on threat-related information accompanying this response may contribute to an attentional bias for threat-related stimuli (7–9), which may ultimately result in the development of stress-related psychopathology. The link between inappropriate autonomic stress responding and mental disease is further supported by the abnormal autonomic status and responsivity seen in patients with stress-related mental disorders (10, 11). Presumably, dysfunctions of neural circuits regulating parasympathetic responses to threats, resulting from environmental adversity and/or genetic factors, underlie maladaptive stress reactions that can culminate in stress-related disorders.
The periaqueductal gray (PAG), a midbrain region involved in homeostatic processes including threat reactions and pain (12), is instrumental in regulating the autonomic stress response (13). The PAG is organized in longitudinally structured functional columns (14) that show inhibitory interconnections and are differentially targeted by distinct fear circuits, allowing tight regulation and expression of a wide array of innate defensive or offensive responses (15). Whereas activation of the dorsolateral PAG is generally associated with flight-or-fight behavior (16) and associated sympathetic dominance (17, 18), activation of the ventrolateral PAG (vlPAG) typically induces cardiac parasympathetic activation (19) and behavioral freezing (20, 21). Other studies, however, suggest that the function of PAG subregions in modulating fear responses may depend on the exact fear or learning phase (22, 23) and the type of fear stimulus (24). Input from the central amygdala (CeA) is critical to selecting the appropriate response. Stimulation of the CeA produces freezing and bradycardia (25), whereas lesions block both autonomic and behavioral manifestations of fear (26). The activation of specifically CeA somatostatin-positive (SOM+) neurons contributes to freezing behavior (27). Fear conditioning potentiates synaptic transmission onto these CeA SOM+ neurons (28, 29), thereby converting active defensive behavior to freezing (30). SOM+ CeA neurons project directly to the PAG (28), where they inhibit vlPAG GABAergic interneurons (21, 28), ultimately disinhibiting the glutamatergic projection neurons that mediate freezing. Accordingly, human neuroimaging work (3, 31–33) has shown an association between PAG blood oxygen level-dependent (BOLD) responses and bradycardic reactions to aversive visual stimuli and threat of shock, and effective amygdala-PAG coupling during the processing of these inputs. However, it is currently unclear which factors affect the functioning of these neural circuits regulating the parasympathetic response to threat, and the associated risk for developing stress-related psychopathology.
One key vulnerability factor is the serotonin transporter (5-HTT) gene (34). A variable repeat sequence in the promotor of 5-HTT, the serotonin transporter-linked polymorphic region (5-HTTLPR) (35), determines its expression and function. The short (S) allelic variant of the 5-HTTLPR gene variant is associated with reduced transcription and function of the 5-HTT gene compared with the long (L) allele (36). S allele carriers are characterized by increased anxiety- and neuroticism-related traits and an elevated risk for stress-related disorders (35, 37). Moreover, altered freezing in S allele–carrying infants is predictive of internalizing symptom development across adolescence (38). Aberrant autonomic stress responses in S allele carriers may contribute to these associations. Various expressions of exaggerated sympathetic responding to threat have been observed in S allele carriers (39, 40), but little is known about modulation of the parasympathetic response to threat by 5-HTT gene variance, let alone its neural mechanisms.
Whereas human studies into the neural circuits regulating parasympathetic response to threat offer limited anatomic specificity, animal studies offer more fine-grained understanding of these neural mechanisms. Here we performed a cross-species study comparing the effects of the 5-HTTLPR genotype in humans to the effects of 5-HTT knockout in rodents, which mimic the increased stress sensitivity observed in human S allele carriers (37). We investigated 5-HTT–mediated modulation of the parasympathetic response to threat using a fear conditioning paradigm in (i) healthy human S allele carriers vs. LL allele carriers, by combining heart rate (HR) measurements with functional magnetic resonance imaging (fMRI), and (ii) 5-HTT knockout (KO) vs. wild-type (WT) rats, by combining behavioral measures of freezing, telemetry, and immunohistochemistry.
Results
Experiment 1.
With a total of 65 5-HTTLPR S carriers (11 SS, 54 SL), and 39 LL carriers, genotype distribution was in accordance with previous studies and in Hardy–Weinberg equilibrium (P = 0.223). S and LL carriers did not significantly differ in age, trait anxiety, or neuroticism (P > 0.39 for all).
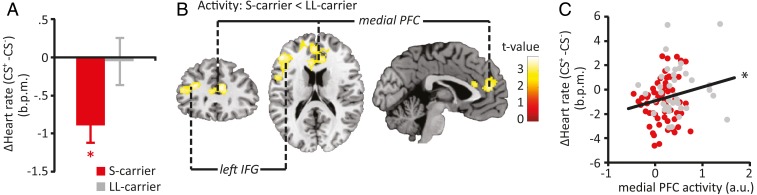
Participants showed significant fear bradycardia; a stronger reduction in HR was observed during the CS+ (mean ± SD, −5.19 ± 2.39 bpm) relative to the CS− (−4.61 ± 2.27 bpm; F(1,102) = 6.429, P = 0.013). Moreover, fear bradycardia was modulated by the 5-HTTLPR genotype, with S allele carriers displaying significantly stronger fear bradycardia than LL carriers (genotype × cue interaction: F(1,102) = 5.093, P = 0.026) (Fig. 1A). S carriers displayed differential HR response to the CS+ vs. CS− (t(64) = 4.054, P < 0.001), unlike LL carriers (P = 0.868). Baseline HR, defined as the average HR during the prestimulus period, was not different between genotypes (S carriers: 66.08 ± 12.75 bpm; LL carriers: 67.68 ± 12.55 bpm; P = 0.963).
Fig. 1.
Effects of the 5-HTTLPR genotype on fear bradycardia and neuronal activity. S allele carriers displayed increased fear bradycardia compared with LL carriers (mean ± SEM) (A), and overall reduced activity in the left IFG and medial PFC (B), which were significantly correlated (C) (medial PFC shown). *P < 0.05.
Threat anticipation was associated with the expected increase in neural activity of regions encompassing the salience network (SI Appendix, Results, Fig. S1, and Table S1). Neural activity during task execution (irrespective of threat) in two activation clusters in the frontal cortex, the left inferior frontal gyrus (IFG) and medial prefrontal cortex (mPFC)/rostral anterior cingulate cortex, was reduced in S allele carriers compared with LL allele carriers (Table 1 and Fig. 1B). Moreover, overall activity of these regions correlated significantly with fear bradycardia (left IFG: r(103) = 0.198, P = 0.045; mPFC: r(103) = 0.199, P = 0.044) (Fig. 1C), with reduced activity associated with stronger bradycardia, linking both observed phenomena in S carriers. However, this reduced frontal activity did not statistically mediate the relationship between 5-HTT genotype and fear bradycardia (P = 0.166 and 0.1733, respectively).
Table 1.
Peak voxels and corresponding t values of significant clusters in the main effect of genotype and genotype × condition interaction
| Brain region | Cluster size, cm3 | MNI coordinates, x, y, z | Peak t value |
| 5-HTTLPR S carrier < LL carrier | |||
| Medial prefrontal cortex/rostral anterior cingulate cortex | 45.28* | 8, 26, 24 | 3.84 |
| Inferior frontal gyrus, left | 32.24† | −48, 30, 20 | 3.78 |
| 5-HTTLPR S carrier > LL carrier | / | ||
| 5-HTTLPR S carrier (CS+ > CS−) < 5-HTTLPR LL carrier (CS+ > CS−) | / | ||
| 5-HTTLPR S carrier (CS+ > CS−) > 5-HTTLPR LL carrier (CS+ > CS−) | |||
| Dorsomedial prefrontal cortex | 20.75‡ | 4, 4, 50 | 4.08 |
All effects are analyzed using cluster-level statistics, implementing a height threshold at P < 0.005 uncorrected at the voxel level.
P < 0.001.
P < 0.05.
P = 0.077.
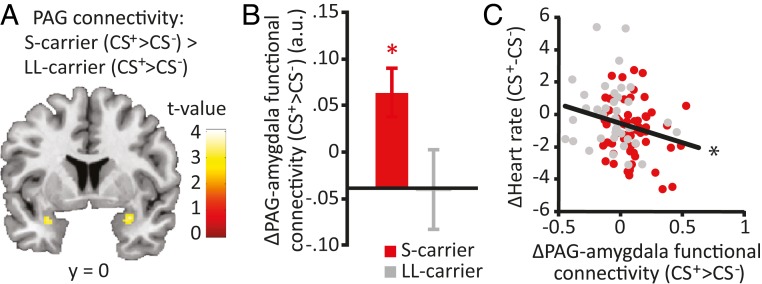
PAG activity increased during the processing of threat (F(1,101) = 36.447, P < 0.001), without displaying a main effect of genotype or threat × genotype interaction. A psychophysiological interaction (PPI) analysis investigating PAG functional connectivity during threat processing revealed a significantly greater increase in PAG connectivity to other regions within the midbrain and the right amygdala in S allele carriers compared with LL carriers (Fig. 2A and Table 2). To further investigate this genotype difference in PAG-amygdala connectivity, we extracted the mean values for the threat-induced increase in PAG coupling to the anatomically defined amygdala. These data confirmed significantly increased strengthening of PAG-amygdala coupling under threat in S carriers compared with LL carriers (t(100) = 2.212, P = 0.029) (Fig. 2B and SI Appendix, Table S2). Moreover, increased threat-induced PAG-amygdala coupling correlated with the fear bradycardia response (r(102) = −0.219, P = 0.027) (Fig. 2C). Mediation analyses confirmed that S carriers showed increased threat-induced PAG-amygdala (anatomically defined) connectivity (path a: z = −2.11, P = 0.035). Furthermore, the increased threat-induced PAG-amygdala connectivity predicted fear bradycardia (path b: z = −1.96, P = 0.0499) and significantly mediated the 5-HTT genotype’s effect on fear bradycardia (path ab: z = 2.01, P = 0.044) (see Fig. 5A). Thus, the increased threat-induced PAG-amygdala connectivity partially mediated (path c′: z = 2.17, P = 0.030) the relationship between the 5-HTT genotype and fear bradycardia (path c: z = 2.61, P = 0.009).
Fig. 2.
Effects of the 5-HTTLPR genotype on PAG-amygdala connectivity. S allele carriers displayed an enhanced threat-induced increase in connectivity between the PAG and the bilateral amygdala (A). Extracted data of the anatomically defined amygdala confirmed the association between 5-HTTLPR genotype and threat-induced recruitment of PAG-amygdala connectivity (mean ± SEM) (B), which correlated with fear bradycardia (C). *P < 0.05.
Table 2.
Peak voxels and corresponding t values of significant clusters in the PPI analysis seeding the PAG
| Brain region | Cluster size, cm3 | MNI coordinates, x, y, z | Peak t value |
| CS+ > CS− | |||
| Brainstem | 27.70* | −10, −22, −18 | 4.29 |
| CS+ < CS− | / | ||
| 5-HTTLPR S carrier (CS+ > CS−) > 5-HTT LL carrier (CS+ > CS−) | |||
| Amygdala, right | 1.93 | 30, −2, −18 | 4.06‡ |
| Amygdala, left | 0.77 | −28, 4, −18 | 3.97‡ |
| 5-HTTLPR S carrier (CS+ < CS−) > 5-HTTLPR LL carrier (CS+ < CS−) | |||
| Superior temporal/supramarginal gyrus, right | 17.66† | 32, −40, 22 | 3.90 |
All effects were analyzed using a height threshold at P < 0.005 uncorrected at the voxel level.
P < 0.01.
P < 0.05.
P < 0.01 small volume-corrected at the voxel level.
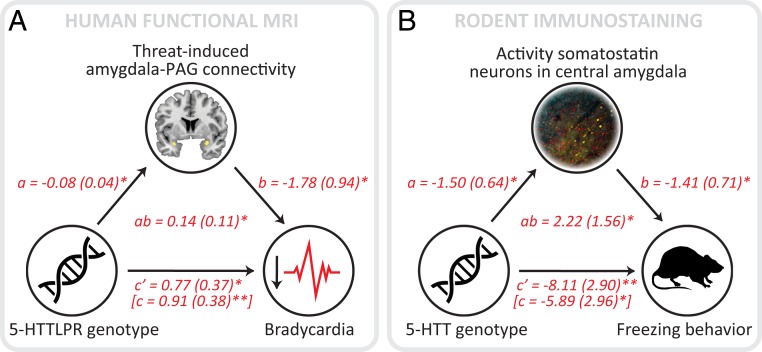
Fig. 5.
PAG-amygdala connectivity and CeA somatostatin neuron activity mediates the relationship between serotonin transporter availability and fear bradycardia (A) and behavioral freezing (B), respectively. Lines are labeled with path coefficients, and SEMs are shown in parentheses. *P < 0.05; **P < 0.01.
Experiment 2.
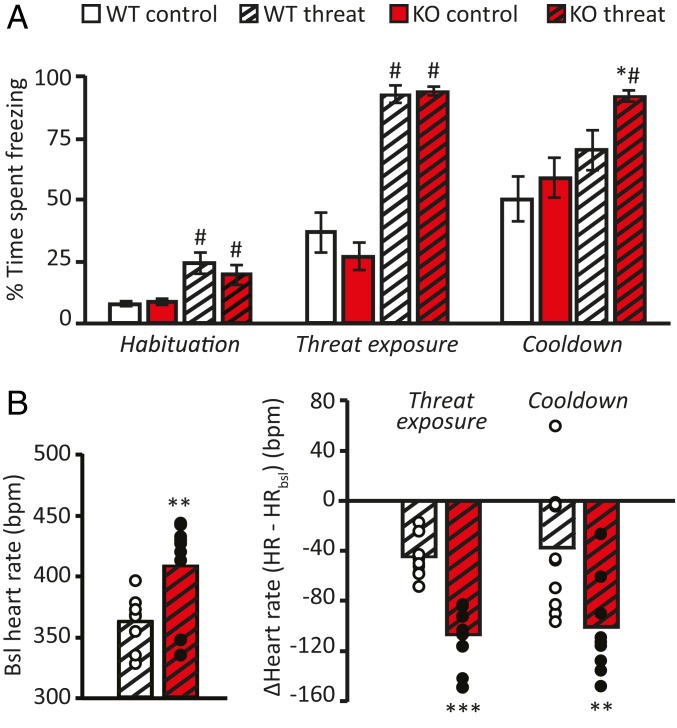
To replicate human physiological and fMRI findings and investigate the neuronal subpopulations in the PAG subnuclei as well as amygdala-PAG projection neurons mediating the 5-HTT effects, we continued with an animal model for reduced 5-HTT availability, the 5-HTT KO rats. At 1 d after conditioning, fear-conditioned animals displayed higher levels of freezing during the habituation period (F(1,51) = 9.730, P = 0.003), threat cue (F(1,55) = 178.802, P < 0.001), and intertrial interval (F(1,55) = 111.818, P < 0.001) compared with control animals, with no difference between genotypes (P > 0.075 for all) (Fig. 3A). However, after the cessation of threat (the cooldown period), fear-conditioned 5-HTT KO rats showed prolonged excessive freezing compared with WT animals (t(24.36) = 2.351, P = 0.027), which showed decreased freezing after threat cessation (t(19) = 3.397, P = 0.003) (Fig. 3A).
Fig. 3.
Effects of serotonin transporter (5-HTT) genotype on fear bradycardia and threat-induced freezing in rats. Conditioned 5-HTT KO rats displayed increased freezing compared with WT animals during the cooldown period (A), an increased baseline heart rate, and exaggerated bradycardic responses on threat reexposure that continued following threat cessation (B). Data are mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001, effect of genotype; #P < 0.05, effect of condition.
Baseline HR levels during habituation were significantly higher in threat-exposed KO animals compared with WTs (t(16) = 3.055, P = 0.008) (Fig. 3B). This was likely the result of prior fear conditioning, as baseline HR measurements in an independent batch of naïve animals (nKO = 9; nWT = 8) did not reveal any differences in basal HR (P = 0.280) (SI Appendix, Fig. S2). Mean HR measured during CS+ presentation was significantly lower than baseline in both KO (t(8) = 6.691, P < 0.001) and WT (t(8) = 5.877, P < 0.001) animals. However, this bradycardic response was significantly greater in KO animals (t(15) = 6.236, P < 0.001) (Fig. 3B) and was sustained after threat cessation (t(16) = 2.952, P = 0.009). Interestingly, bradycardic responses correlated with the sustained freezing responses during the cooldown period (r(19) = −0.509, P = 0.026), supporting the association between the two threat response measures. Moreover, total freezing behavior following threat onset correlated with bradycardia during the cooldown period (r(19) = −0.586, P = 0.008). There was no significant association between freezing behavior and bradycardia during threat exposure itself (CS+ presentation; P = 0.129), most likely due to ceiling levels of freezing during this period. In WT rats (reducing freezing behavior during the cooldown period), bradycardia during threat exposure predicted freezing behavior during cooldown (r(10) = −0.632, P = 0.050), further supporting the link between threat-induced HR deceleration and the behavioral expression of fear.
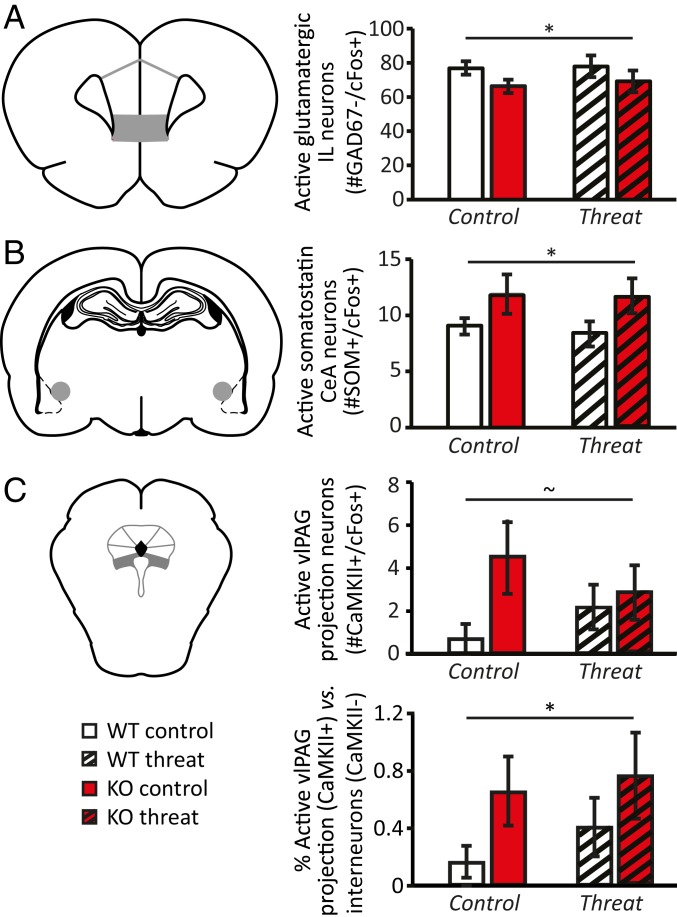
Neuronal activity as assessed by c-Fos expression was lower in the infralimbic (IL) cortex in 5-HTT KO rats compared with WT rats (F(1,33) = 8.744, P = 0.006), independent of previous conditioning (F < 1 for all). Moreover, 5-HTT KO rats had slightly fewer inhibitory (GAD67+) neurons in the IL cortex compared with WTs (F(1,34) = 4.867, P = 0.034, SI Appendix, Table S3). Both the number of activated inhibitory neurons (F(1,35) = 4.616, P = 0.039) and activated putative excitatory neurons (GAD67− and c-Fos+) (F(1,33) = 7.992, P = 0.008) were reduced in KO rats (Fig. 4A). However, IL excitatory activation did not mediate the relationship between 5-HTT genotype and the fear response (P = 0.866).
Fig. 4.
Effects of serotonin transporter (5-HTT) genotype on neuronal activity in rat brain regions involved in mediating threat response and/or revealing 5-HTT modulation in humans. 5-HTT KO rats displayed reduced activity of glutamatergic neurons (approximated by the number of GAD67−/cFos+ cells) in the IL cortex compared with WT animals (A). Activity of somatostatin neurons in the CeA was higher in 5-HTT KO rats (B). Absolute and relative activity of CaMKII-expressing projection neurons in the vlPAG tended to be higher in 5-HTT KOs (C). Data are mean ± SEM. ∼P < 0.1; *P = <0.05 effect of genotype.
As a proxy for the threat-related recruitment of CeA neurons projecting to the vlPAG, we analyzed c-Fos expression in SOM+ CeA neurons, which are known to project to the vlPAG (21, 28) and to modulate freezing behavior (27, 30) (SI Appendix, Fig. S3). The total number of CeA SOM+ neurons was marginally, but not significantly, higher in 5-HTT KO rats compared to WT rats (F(1,34) = 3.234, P = 0.081) (SI Appendix, Table S3). SOM+ neuronal activity was significantly modulated by genotype (F(1,32) = 4.722, P = 0.037), but not by condition or a condition × genotype interaction (F < 1 for all). 5-HTT KO rats displayed greater activity of CeA SOM+ neurons compared with WTs (Fig. 4B). In contrast, the activity of SOM− neurons within the CeA was significantly lower in KO rats (F(1,31) = 8.964, P = 0.005). Mediation analyses confirmed that 5-HTT KO rats showed increased CeA SOM+ neuronal activity (path a: z = −2.34, P = 0.019), and that more CeA SOM+ neuronal activity predicted exaggerated freezing during cooldown (path b: z = −2.07, P = 0.038). Most critically, and in line with the mediation analysis reported for amygdala-PAG connections in humans, CeA SOM+ neuronal activity partially mediated (path c′: z = −2.87, P = 0.004) the 5-HTT effect on this fear response (path ab: z = 2.01, P = 0.045; path c: z = −1.96, P = 0.049) (Fig. 5B).
In the vlPAG, overall c-Fos expression was higher in 5-HTT KO rats compared with WT rats (F(1,29) = 4.398, P = 0.045), without displaying effects of condition or a genotype × condition interaction (F < 1 for all). This increased activity was reflected in a significantly elevated number of activated non–CaMKII-expressing neurons in 5-HTT KO rats compared with WTs (F(1,29) = 4.347, P = 0.046) (SI Appendix, Table S3), whereas the increase in activated CaMKII-expressing projection neurons in 5-HTT KOs reached trend level significance only (F(1,27) = 2.979, P = 0.096) (Fig. 4C). Regardless, the vlPAG of 5-HTT KO rats was characterized by an increased relative contribution of CaMKII-expressing (putative glutamatergic) projection neurons to vlPAG activity compared with WTs (t(25.681) = 2.019, P = 0.05) (Fig. 4C), which might fit their behavioral phenotype. Unlike the findings for CeA SOM+ neuron activity, these PAG-related effects did not mediate the effect of genotype on freezing levels (P > 0.40 for all).
Discussion
In this study, we investigated the association between 5-HTT availability and parasympathetic domination during threat anticipation. Human 5-HTTLPR S allele carriers and 5-HTT KO rats displayed an exaggerated bradycardic threat response, reduced mPFC activation, and increased threat-induced amygdala-PAG connectivity (in humans) and activity of amygdala SOM+ neurons putatively projecting to the vlPAG (in rats). In addition, KO rats displayed heightened freezing and increased vlPAG activity, characterized by increased relative activation of CaMKII-expressing projection neurons. Mediation analysis revealed that, specifically, the altered amygdala-PAG connectivity (in humans) and amygdala SOM+ neuron activity (in rats) mediated the effect of 5-HTT availability on bradycardia and freezing.
5-HTTLPR S carriers displayed exaggerated bradycardic responses during threat anticipation in the absence of differences in basal HR. In parallel, increased bradycardic responses were observed in 5-HTT KO rats. However, fear-conditioned KO rats also displayed increased basal HR in a novel environment, indicating an association between reduced 5-HTT availability and both exaggerated bradycardia during threat anticipation and elevated novelty stress-induced tachycardia. This could explain earlier reports on 5-HTTLPR modulation of autonomic function describing both enhanced (41) and suppressed (42) HR responses to threats.
In KO rats, the increased bradycardic response was associated with exaggerated freezing (43) after threat cessation. Previous work supports an association between freezing behavior and increased fear bradycardia in rodents (19, 44). These responses likely indicate exaggerated threat responses by 5-HTT KO rodents, as fear learning is not affected (43). The increase in freezing levels observed over the course of the experiment in the control animals (F(2,17) = 28.193, P < 0.001) is likely caused by an overall reduction in exploratory movement due to familiarity with the context, rather than to increased freezing. Although we were not able to measure the behavioral freezing response in our human participants (due to incompatibility of body sway measurements and fMRI), previous work has linked HR decelerations to reduced body sway as measured on a stabilometric force platform in humans (4, 9, 45, 46). Excessive freezing may reflect increased attention to threat (47) and has been associated with altered perception (48) and heightened anxiety (9), in line with 5-HTTLPR S allele carriers’ heightened ambiguous threat perception (41). Thus, our observations of exaggerated freezing and bradycardia in individuals with inherited 5-HTT down-regulation suggest enhanced threat anticipation, which may predispose them to stress-related mental disorders (49).
Whereas the 5-HTTLPR genotype did not affect BOLD threat responses in the human PAG, KO rats displayed significantly increased vlPAG activity and increased relative activation of CaMKII-expressing (putatively glutamatergic) projection neurons. However, vlPAG activity did not relate to the genotype effect on freezing. Moreover, the increased vlPAG GABAergic (non-CaMKII) neuron activity in these animals is contrary to the reduction expected based on the increased recruitment of SOM+ CeA neurons in 5-HTT KO rats (28). Developmental compensation due to life-long dysregulation of the 5-HT system may be a potential cause of this (50, 51). Alternatively, the current subdivision of activated PAG neuronal subclasses is still too coarse, and freezing/bradycardic output might rather depend on the exact PAG neural circuitry recruited. This could also explain the absence of any condition effects on PAG activity.
Interestingly, differential recruitment of the PAG-amygdala circuit mediated the effects of 5-HTT genotype on threat response and bradycardia in both rats and humans. Animal work has indicated that stimulation of the CeA-vlPAG connection induces both freezing (25) and cardiovascular changes (52). Similar amygdala-vlPAG connectivity has been confirmed in the human brain, both structurally (53) and functionally (54). The vlPAG is targeted primarily by SOM+ neurons, which can directly inhibit vlPAG interneurons (28). Here we show that these SOM+ neurons are more active in 5-HTT KOs, and that threat processing induces stronger PAG-amygdala circuit activation in 5-HTTLPR S allele carriers, mediating 5-HTT modulatory effects on freezing and bradycardia. As raphe serotonergic projections densely innervate the amygdala (55), altered serotonergic signaling may cause this effect. Reduced expression or binding of the 5-HT1a receptor, as seen in individuals characterized by compromised 5-HTT availability (56, 57), may increase amygdala activation.
Compromised 5-HTT availability also causes aberrant prefrontal cortex function. Both 5-HTTLPR carriers and 5-HTT KO rats displayed reduced activation of the mPFC, independent of threat exposure, whereas the rats were also characterized by a reduction in inhibitory neurons. The ventromedial (IL) cortex mediates fear extinction (58, 59) and inhibits CeA output neurons (60). The mPFC sends direct connections to the PAG as well, thereby modulating the amygdala and PAG threat response (61, 62). S allele carriers are characterized by attenuated prefrontal-amygdala coupling (63). Here we show that 5-HTT down-regulation is associated with reduced mPFC activity, which correlates significantly with threat-related freezing or fear bradycardia but did not significantly mediate the exaggerated fear bradycardia.
Some limitations of this study need to be mentioned. First, HR recordings and neuronal activity were not assessed in the same animals, preventing direct comparisons. Therefore, we used behavioral freezing responses, obtained in all rats, as a proxy for bradycardia. The validity of this approach is supported by previous findings of an association between bradycardic responses to threat and freezing behavior (9, 45, 46), which we confirmed here through correlation. Furthermore, our human neuroimaging data set lacked the spatial specificity to reliably dissect the distinct PAG columns and test for associations of fear bradycardia with specific PAG subregions. This requires dedicated MRI settings and comes at the cost of a restricted field of view (54, 64), prohibiting whole brain coverage; nonetheless, this should definitely be explored in future studies. Finally, we did not directly measure amygdala-PAG connectivity in the rats, but assessed the activity of CeA SOM+ neurons. Although there is clear evidence that vlPAG neurons mediating freezing behavior are modulated by SOM+ CeA neurons (26), and these SOM+ neurons most potently affect vlPAG activity, these neurons can project to other areas as well (28). Tracing experiments could ascertain the connectivity of activated neurons, but their inherent variability prohibits quantitative assessments (65). Future dedicated studies could potentially provide unequivocal evidence for the role of SOM+ neuron CeA-vlPAG projections in mediating threat responses.
In conclusion, we have delineated and validated a previously unknown neurogenetic mechanism underlying individual differences in the expression of bradycardic and freezing anticipatory threat responses. We have demonstrated that 5-HTT availability reduces mPFC activity and affects the intensity of aversive anticipatory states through altered neural PAG-amygdala connectivity, presumably by increased CeA somatostatin neuronal activity. This provides a mechanistic explanation for previously reported associations between genetic variability in serotonin function and a stress-sensitive phenotype.
Methods
Experiment 1.
All participants provided written informed consent. The study was approved by the local ethical reviewing committee (Commissie Mensgebonden Onderzoek region Arnhem-Nijmegen, The Netherlands) and was conducted under the national legislation in accordance with basic international ethical principles (i.e., the Declaration of Helsinki). A total of 104 male participants were subjected to a fear conditioning paradigm, consisting of 18 presentations of two types of visual stimuli, one of which coterminated with a mild electric shock on one-third of the trials. During the experiment, participants’ cardiac rhythm was measured during the acquisition of fMRI data on a 1.5-T Avanto MR scanner (Siemens, Erlangen, Germany). Using recommended preprocessing procedures, a general linear model was composed in SPM8 to relate BOLD signal variation in each voxel to the task conditions: threat (conditioned stimulus [CS+]), safe (CS−), and shock (unconditioned stimulus [US]). Reactions to CS+ and CS− were contrasted in each subject to index threat-related responses, and single subject contrast maps were subjected to random-effects analyses. Mean beta weights of the anatomically defined PAG (61) were extracted for statistical analyses, as this region was considered a priori a region of interest (19). A PPI analysis (66) was used to examine PAG threat-related functional connectivity as a function of 5-HTTLPR genotype. The amygdala was considered a priori a region of interest (28, 31). Results were considered significant at P < 0.05, family-wise error-corrected.
Experiment 2.
Eighteen adult male rats (nKO = 9; nWT = 9) were implanted with a radiotelemetry device to record HR. Following recovery, the animals underwent a cued fear conditioning trial (using a tone as CS+ and footshock as US), followed by CS+ reexposure 24 h later. Freezing behavior and HR during the threat cue reexposure were recorded. A separate batch of animals (nKO = 10; nWT = 10) was subjected to the exact same behavioral procedure and transcardially perfused at 90 min after reexposure to the CS+. Nonshocked animals (nKO = 10; nWT = 10) served as controls. Using immunofluorescence, the immediate early gene c-Fos and the GABAergic neuron marker GAD67 were visualized in the mPFC to quantify threat-related non-GABAergic (c-Fos+, GAD67−) neuronal activity, which was used as a derived measure of glutamatergic activity (67). The CeA was stained for c-Fos, SOM, and tyrosine hydroxylase. PAG slices were stained for c-Fos and the projection neuron marker CaMKII, to determine the activation of CaMKII-expressing (putative glutamatergic) projection neurons.
Statistical Analyses.
Behavioral and physiological data were analyzed in SPSS version 23.0 (IBM) using independent-samples and paired-samples t tests. For correlational analyses, Pearson correlations were used. To test whether genotype-dependent neural connectivity mediated an impact of genotype on psychophysiological responses, we performed a mediation analysis with accelerated bias-corrected bootstrap significance testing (10,000 bootstrap samples), as implemented in the M3 toolbox (https://github.com/canlab) (68). The α value was set at 0.05 throughout.
Data Availability.
The MRI, heart rate, microscopy, and behavioral data reported in this paper have been deposited in the Donders Data Sharing Collection (http://hdl.handle.net/11633/aacyoplh).
Supplementary Material
Acknowledgments
We thank Anthonieke Middelman for her devoted assistance with animal breeding and genotyping and Luca Casanova and Jesse Stoop for their assistance with data collection. M.J.A.G.H. is the recipient of Veni Grant 863.15.008, J.R.H. is the recipient of Vidi Grant 864.10.003, and K.R. is the recipient of Vici Grant 453.12.001 awarded by the Netherlands Organization for Scientific Research. K.R. and E.J.H. were supported by consolidator grants from the European Research Council (2018_772337 and ERC-2015-CoG 682591).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The MRI, heart rate, microscopy, and behavioral data reported in this paper have been deposited in the Donders Data Sharing Collection (http://hdl.handle.net/11633/aacyoplh).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1904843116/-/DCSupplemental.
References
- 1.Fanselow M. S., Neural organization of the defensive behavior system responsible for fear. Psychon. Bull. Rev. 1, 429–438 (1994). [DOI] [PubMed] [Google Scholar]
- 2.Kroes M. C., Henckens M. J., Homberg J. R., How serotonin transporter gene variance affects defensive behaviours along the threat imminence continuum. Curr. Opin. Behav. Sci. 26, 25–31 (2019). [Google Scholar]
- 3.McNaughton N., Corr P. J., A two-dimensional neuropsychology of defense: Fear/anxiety and defensive distance. Neurosci. Biobehav. Rev. 28, 285–305 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Lang P. J., Davis M., Emotion, motivation, and the brain: Reflex foundations in animal and human research. Prog. Brain Res. 156, 3–29 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Wiens S., Ohman A., Unawareness is more than a chance event: Comment on Lovibond and Shanks (2002). J. Exp. Psychol. Anim. Behav. Process. 28, 27–31 (2002). [PubMed] [Google Scholar]
- 6.Hagenaars M. A., Oitzl M., Roelofs K., Updating freeze: Aligning animal and human research. Neurosci. Biobehav. Rev. 47, 165–176 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Fox E., Russo R., Bowles R., Dutton K., Do threatening stimuli draw or hold visual attention in subclinical anxiety? J. Exp. Psychol. Gen. 130, 681–700 (2001). [PMC free article] [PubMed] [Google Scholar]
- 8.Holmes E. A., Brewin C. R., Hennessy R. G., Trauma films, information processing, and intrusive memory development. J. Exp. Psychol. Gen. 133, 3–22 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Roelofs K., Hagenaars M. A., Stins J., Facing freeze: Social threat induces bodily freeze in humans. Psychol. Sci. 21, 1575–1581 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Grippo A. J., Johnson A. K., Stress, depression and cardiovascular dysregulation: A review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress 12, 1–21 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah A. J., et al. , Posttraumatic stress disorder and impaired autonomic modulation in male twins. Biol. Psychiatry 73, 1103–1110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basbaum A. I., Fields H. L., Endogenous pain control mechanisms: Review and hypothesis. Ann. Neurol. 4, 451–462 (1978). [DOI] [PubMed] [Google Scholar]
- 13.LeDoux J. E., Iwata J., Cicchetti P., Reis D. J., Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci. 8, 2517–2529 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandler R., Shipley M. T., Columnar organization in the midbrain periaqueductal gray: Modules for emotional expression? Trends Neurosci. 17, 379–389 (1994). [DOI] [PubMed] [Google Scholar]
- 15.Gross C. T., Canteras N. S., The many paths to fear. Nat. Rev. Neurosci. 13, 651–658 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Deakin J. F., Graeff F. G., 5-HT and mechanisms of defence. J. Psychopharmacol. 5, 305–315 (1991). [DOI] [PubMed] [Google Scholar]
- 17.Yardley C. P., Hilton S. M., The hypothalamic and brainstem areas from which the cardiovascular and behavioural components of the defence reaction are elicited in the rat. J. Auton. Nerv. Syst. 15, 227–244 (1986). [DOI] [PubMed] [Google Scholar]
- 18.Lovick T. A., Ventrolateral medullary lesions block the antinociceptive and cardiovascular responses elicited by stimulating the dorsal periaqueductal grey matter in rats. Pain 21, 241–252 (1985). [DOI] [PubMed] [Google Scholar]
- 19.Koba S., Inoue R., Watanabe T., Role played by periaqueductal gray neurons in parasympathetically mediated fear bradycardia in conscious rats. Physiol. Rep. 4, e12831 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyon M., The role of central midbrain structures in conditioned responding to aversive noise in the rat. J. Comp. Neurol. 122, 407–429 (1964). [DOI] [PubMed] [Google Scholar]
- 21.Tovote P., et al. , Midbrain circuits for defensive behaviour. Nature 534, 206–212 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Watson T. C., Cerminara N. L., Lumb B. M., Apps R., Neural correlates of fear in the periaqueductal gray. J. Neurosci. 36, 12707–12719 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Andrade Rufino R., Mota-Ortiz S. R., De Lima M. A. X., Baldo M. V. C., Canteras N. S., The rostrodorsal periaqueductal gray influences both innate fear responses and acquisition of fear memory in animals exposed to a live predator. Brain Struct. Funct. 224, 1537–1551 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Brandão M. L., Zanoveli J. M., Ruiz-Martinez R. C., Oliveira L. C., Landeira-Fernandez J., Different patterns of freezing behavior organized in the periaqueductal gray of rats: Association with different types of anxiety. Behav. Brain Res. 188, 1–13 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Applegate C. D., Kapp B. S., Underwood M. D., McNall C. L., Autonomic and somatomotor effects of amygdala central N. stimulation in awake rabbits. Physiol. Behav. 31, 353–360 (1983). [DOI] [PubMed] [Google Scholar]
- 26.Fendt M., Fanselow M. S., The neuroanatomical and neurochemical basis of conditioned fear. Neurosci. Biobehav. Rev. 23, 743–760 (1999). [DOI] [PubMed] [Google Scholar]
- 27.Fadok J. P., et al. , A competitive inhibitory circuit for selection of active and passive fear responses. Nature 542, 96–100 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Penzo M. A., Robert V., Li B., Fear conditioning potentiates synaptic transmission onto long-range projection neurons in the lateral subdivision of central amygdala. J. Neurosci. 34, 2432–2437 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H., et al. , Experience-dependent modification of a central amygdala fear circuit. Nat. Neurosci. 16, 332–339 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu K., Garcia da Silva P., Albeanu D. F., Li B., Central amygdala somatostatin neurons gate passive and active defensive behaviors. J. Neurosci. 36, 6488–6496 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hermans E. J., Henckens M. J., Roelofs K., Fernández G., Fear bradycardia and activation of the human periaqueductal grey. Neuroimage 66, 278–287 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Lojowska M., Ling S., Roelofs K., Hermans E. J., Visuocortical changes during a freezing-like state in humans. Neuroimage 179, 313–325 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mobbs D., et al. , When fear is near: Threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science 317, 1079–1083 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith G. S., et al. , Effects of serotonin transporter promoter polymorphisms on serotonin function. Neuropsychopharmacology 29, 2226–2234 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Lesch K. P., et al. , Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274, 1527–1531 (1996). [DOI] [PubMed] [Google Scholar]
- 36.Greenberg B. D., et al. , Genetic variation in the serotonin transporter promoter region affects serotonin uptake in human blood platelets. Am. J. Med. Genet. 88, 83–87 (1999). [PubMed] [Google Scholar]
- 37.Homberg J. R., van den Hove D. L., The serotonin transporter gene and functional and pathological adaptation to environmental variation across the life span. Prog. Neurobiol. 99, 117–127 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Niermann H. C. M., et al. , The relation between infant freezing and the development of internalizing symptoms in adolescence: A prospective longitudinal study. Dev. Sci. 22, e12763 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Klumpers F., et al. , Dorsomedial prefrontal cortex mediates the impact of serotonin transporter linked polymorphic region genotype on anticipatory threat reactions. Biol. Psychiatry 78, 582–589 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Otte C., McCaffery J., Ali S., Whooley M. A., Association of a serotonin transporter polymorphism (5-HTTLPR) with depression, perceived stress, and norepinephrine in patients with coronary disease: The Heart and Soul Study. Am. J. Psychiatry 164, 1379–1384 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crişan L. G., et al. , Genetic contributions of the serotonin transporter to social learning of fear and economic decision making. Soc. Cogn. Affect. Neurosci. 4, 399–408 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams R. B., et al. , Central nervous system serotonin function and cardiovascular responses to stress. Psychosom. Med. 63, 300–305 (2001). [DOI] [PubMed] [Google Scholar]
- 43.Shan L., Guo H. Y., van den Heuvel C. N. A. M., van Heerikhuize J., Homberg J. R., Impaired fear extinction in serotonin transporter knockout rats is associated with increased 5-hydroxymethylcytosine in the amygdala. CNS Neurosci. Ther. 24, 810–819 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshimoto M., Nagata K., Miki K., Differential control of renal and lumbar sympathetic nerve activity during freezing behavior in conscious rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R1114–R1120 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Azevedo T. M., et al. , A freezing-like posture to pictures of mutilation. Psychophysiology 42, 255–260 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Hagenaars M. A., Stins J. F., Roelofs K., Aversive life events enhance human freezing responses. J. Exp. Psychol. Gen. 141, 98–105 (2012). [DOI] [PubMed] [Google Scholar]
- 47.Blanchard D. C., Griebel G., Pobbe R., Blanchard R. J., Risk assessment as an evolved threat detection and analysis process. Neurosci. Biobehav. Rev. 35, 991–998 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Lojowska M., Gladwin T. E., Hermans E. J., Roelofs K., Freezing promotes perception of coarse visual features. J. Exp. Psychol. Gen. 144, 1080–1088 (2015). [DOI] [PubMed] [Google Scholar]
- 49.Kozlowska K., Walker P., McLean L., Carrive P., Fear and the defense cascade: Clinical implications and management. Harv. Rev. Psychiatry 23, 263–287 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaspar P., Cases O., Maroteaux L., The developmental role of serotonin: News from mouse molecular genetics. Nat. Rev. Neurosci. 4, 1002–1012 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Teissier A., Soiza-Reilly M., Gaspar P., Refining the role of 5-HT in postnatal development of brain circuits. Front. Cell. Neurosci. 11, 139 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anand B. K., Dua S., Circulatory and respiratory changes induced by electrical stimulation of limbic system (visceral brain). J. Neurophysiol. 19, 393–400 (1956). [DOI] [PubMed] [Google Scholar]
- 53.Ezra M., Faull O. K., Jbabdi S., Pattinson K. T., Connectivity-based segmentation of the periaqueductal gray matter in human with brainstem optimized diffusion MRI. Hum. Brain Mapp. 36, 3459–3471 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Faull O. K., Pattinson K. T., The cortical connectivity of the periaqueductal gray and the conditioned response to the threat of breathlessness. eLife 6, e21749 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Rourke H., Fudge J. L., Distribution of serotonin transporter labeled fibers in amygdaloid subregions: Implications for mood disorders. Biol. Psychiatry 60, 479–490 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.David S. P., et al. , A functional genetic variation of the serotonin (5-HT) transporter affects 5-HT1A receptor binding in humans. J. Neurosci. 25, 2586–2590 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Q., Wichems C., Heils A., Lesch K. P., Murphy D. L., Reduction in the density and expression, but not G-protein coupling, of serotonin receptors (5-HT1A) in 5-HT transporter knock-out mice: Gender and brain region differences. J. Neurosci. 20, 7888–7895 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morgan M. A., LeDoux J. E., Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav. Neurosci. 109, 681–688 (1995). [DOI] [PubMed] [Google Scholar]
- 59.Phelps E. A., Delgado M. R., Nearing K. I., LeDoux J. E., Extinction learning in humans: Role of the amygdala and vmPFC. Neuron 43, 897–905 (2004). [DOI] [PubMed] [Google Scholar]
- 60.Quirk G. J., Likhtik E., Pelletier J. G., Paré D., Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J. Neurosci. 23, 8800–8807 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coulombe M. A., Erpelding N., Kucyi A., Davis K. D., Intrinsic functional connectivity of periaqueductal gray subregions in humans. Hum. Brain Mapp. 37, 1514–1530 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bandler R., Keay K. A., Floyd N., Price J., Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res. Bull. 53, 95–104 (2000). [DOI] [PubMed] [Google Scholar]
- 63.Pezawas L., et al. , 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nat. Neurosci. 8, 828–834 (2005). [DOI] [PubMed] [Google Scholar]
- 64.Faull O. K., Jenkinson M., Ezra M., Pattinson K. Ts., Conditioned respiratory threat in the subdivisions of the human periaqueductal gray. eLife 5, e12047 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soiza-Reilly M., et al. , SSRIs target prefrontal to raphe circuits during development modulating synaptic connectivity and emotional behavior. Mol. Psychiatry 24, 726–745 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friston K. J., et al. , Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6, 218–229 (1997). [DOI] [PubMed] [Google Scholar]
- 67.Ritov G., Boltyansky B., Richter-Levin G., A novel approach to PTSD modeling in rats reveals alternating patterns of limbic activity in different types of stress reaction. Mol. Psychiatry 21, 630–641 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wager T. D., Davidson M. L., Hughes B. L., Lindquist M. A., Ochsner K. N., Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 59, 1037–1050 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The MRI, heart rate, microscopy, and behavioral data reported in this paper have been deposited in the Donders Data Sharing Collection (http://hdl.handle.net/11633/aacyoplh).