Significance
Nicotine addiction that results from long-term smoking is a worldwide health epidemic. Here we show that nicotinic receptors containing specific subunits, located in the brain reward system, mediate the experience of both the acute rewarding and aversive effects of nicotine, depending on which neurons they are located. These results provide contradictory evidence to the popularly held belief that the brain dopaminergic system exclusively mediates the rewarding effects of abused drugs, instead providing evidence that the aversive motivational effects of nicotine are signaled through this system as well. Our results lead to a better understanding of the neurobiological substrates of nicotine reward and aversion and thus may lead to new possible targets for pharmacotherapeutic treatments of tobacco addiction.
Keywords: nicotinic receptors, place-conditioning, nicotine motivation, reward, aversion
Abstract
Evidence shows that the neurotransmitter dopamine mediates the rewarding effects of nicotine and other drugs of abuse, while nondopaminergic neural substrates mediate the negative motivational effects. β2* nicotinic acetylcholine receptors (nAChR) are necessary and sufficient for the experience of both nicotine reward and aversion in an intra-VTA (ventral tegmental area) self-administration paradigm. We selectively reexpressed β2* nAChRs in VTA dopamine or VTA γ-amino-butyric acid (GABA) neurons in β2−/− mice to double-dissociate the aversive and rewarding conditioned responses to nicotine in nondependent mice, revealing that β2* nAChRs on VTA dopamine neurons mediate nicotine’s conditioned aversive effects, while β2* nAChRs on VTA GABA neurons mediate the conditioned rewarding effects in place-conditioning paradigms. These results stand in contrast to a purely dopaminergic reward theory, leading to a better understanding of the neurobiology of nicotine motivation and possibly to improved therapeutic treatments for smoking cessation.
Drug abuse, specifically nicotine addiction, is a worldwide epidemic that claims millions of lives each year. Many reports show that the neurotransmitter dopamine is crucial for the mediation of drug reward (1) and that other nondopaminergic neural substrates are responsible for the negative effects experienced after drug use (2, 3), although there is evidence that dopaminergic neurons can also be modulated by aversive stimuli (see ref. 4 for review). Nicotine could produce its separate rewarding and aversive effects (5, 6) by activating different nicotinic acetylcholine receptor (nAChR) subtypes and/or by activating similar nAChR subtypes located on different cell populations, including the independent dopaminergic and γ-amino-butyric acid (GABA) neurons in the ventral tegmental area (VTA) of the brain reward system (7–10). An acute systemic injection of nicotine activates nAChRs on VTA neurons, increasing both dopaminergic (11–13) and GABAergic activity (13). nAChRs are pentameric, ligand-gated ion channels that contain various combinations of subunits, designated α2-α10 and β2-β4, that bind acetylcholine and nicotine (11). The β2-subunit–containing (β2*) nAChRs in the VTA are necessary and sufficient for the experience of both the rewarding and aversive effects of nicotine self-administered directly into the VTA (11, 13, 14) and also have been implicated in nicotine-elicited conditioned place preference (CPP) (15), as further confirmed in a recent study using light-controllable β2* nAChRs (16). However, the role of dopamine versus GABA neurons in signaling rewarding versus aversive nicotine-induced effects in a place-conditioning paradigm, where rewarding and aversive effects can be measured separately, remains a topic of debate. There is evidence that coactivation of both populations mediates nicotine’s motivational effects (13); however, other studies have suggested that dopaminergic VTA neurons signal aversion, while GABAergic VTA projection neurons signal reward (9, 17). These different results suggest more complex roles for dopamine and GABA in nicotine’s acute motivational effects.
Here we aimed to unravel the role of VTA β2* nAChRs expressed on dopamine or GABA VTA neurons in the conditioned aversive and rewarding effects of nicotine, using a place-conditioning paradigm that pairs nicotine exposure and environmental context to assess the primary motivational value of a nicotine stimulus. We utilized lentiviral vector-mediated selective reexpression of the β2* nAChRs in VTA dopamine or GABA neurons in constitutive β2−/− mice (β2-knockout [KO]; ref. 13) using a gene transfer expression strategy combining lentiviral delivery and the Cre/loxP recombination system to double-dissociate the CPP and conditioned place aversion (CPA) for acute nicotine in nondependent mice.
Results
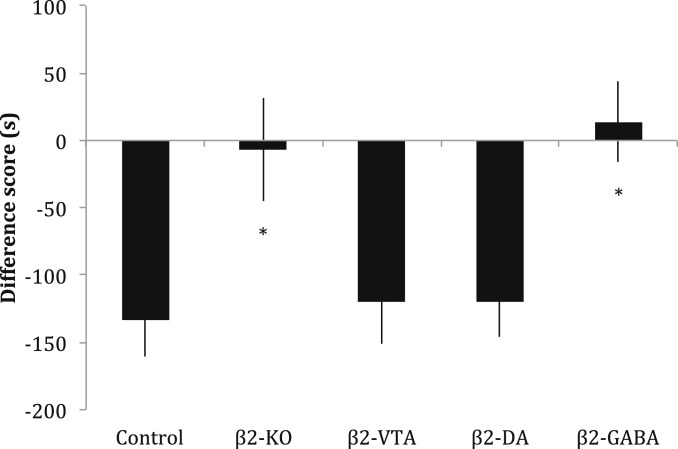
Acute nicotine injected s.c. at a dose of 1.75 mg/kg will consistently induce a CPA in an unbiased procedure (18, 19). After confirming that this dose was the minimum dose that could elicit a reliable CPA in our place-conditioning procedure (F6,66 = 49.72, P < 0.05; SI Appendix), we administered it to our 5 different groups of mice: wild-type C57BL/6J mice (controls), β2-KO mice, β2-KO mice injected with a β2-subunit–expressing lentivector into the entire VTA (β2-VTA), β2-KO × DAT-Cre mice with β2 receptors reexpressed selectively on VTA dopamine neurons (β2-DA), and β2-KO × GAD67-Cre mice with β2 receptors expressed selectively on VTA GABA neurons (β2-GABA). The reexpression of β2* receptors was confirmed by immunostaining and autoradiography on dopamine or GABA neurons in the VTA (Fig. 1). Quantification of epibatidine autoradiography binding showed lentiviral restoration of β2*-nAChRs in the VTA (SI Appendix). We observed a conditioned aversive response for the nicotine-paired environment in the control, β2-VTA and β2-DA groups that was not present in the β2-KO or β2-GABA groups of mice (F4,97 = 5.158, P < 0.05; Fig. 2). The groups of mice with β2* nAChRs reintroduced on VTA DA neurons behaved like control mice and showed a conditioned response (post hoc Bonferroni test comparing β2-DA and control groups, P > 0.05), while the groups of mice with β2* nAChRs reintroduced on VTA GABA neurons showed no conditioned effect of the aversive dose of acute nicotine, instead behaving as if they were complete KO mice (post hoc Bonferroni test comparing β2-GABA and KO, P > 0.05). These results suggest that signaling of nicotine’s conditioned aversive effects is through β2* nAChRs located on dopamine (but not GABA) neurons in the VTA. Control groups of mice injected with saline in both the black and white environments showed no preference for either environment (t11 = 0.3618, P > 0.05; SI Appendix).
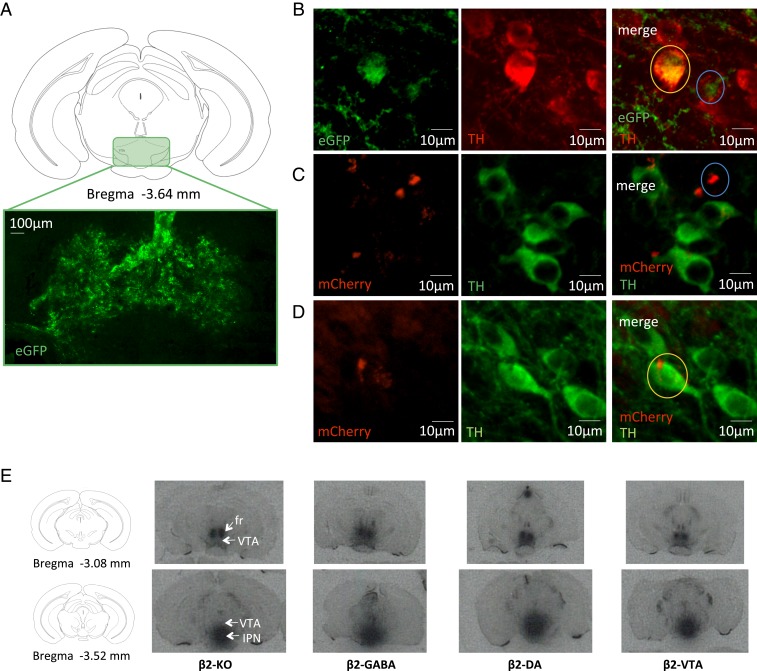
Fig. 1.
Lentivirus-induced reexpression of β2*nAChRs in β2-KO mouse brain. In the conditional lentiviral vector used in the present study, β2 transcription is prevented by a lox P-flanked mCherry-Stop DNA sequence, such that in the presence of the Cre-recombinase, both mCherry and the stop codon are excised, thereby allowing β2 expression. mCherry is thus expressed only in neurons that do not express the Cre-recombinase. (A) Coronal section drawing of a C57BL6/J mouse brain showing ventral tegmental area (VTA) location and representative immunofluorescence image (×20) of localization and spreading of lentivirus reporter gene eGFP in the VTA (example of a β2-VTA mouse). (B) Higher-magnification/higher-resolution images (spinning-disk confocal microscope, 40×) from a β2-VTA mouse showing lentivirus reporter gene eGFP expression (green) and TH staining for DA neurons (red); the orange circle shows an example of an eGFP and TH coexpressing cell (β2 expression in DA neurons), while the blue circle shows an example of eGFP expression in a TH-negative cell (β2 expression in non-DA neurons). (C) Higher-magnification/higher-resolution images (spinning-disk confocal microscope, 40×) from a β2-DA mouse showing floxed lentivirus reporter gene mCherry expression (red, indicating lack of β2* nAChRs) and TH staining for DA neurons (green); the blue circle shows an example of mCherry expression only in a TH-negative cell. (D) Higher-magnification/higher-resolution images (spinning-disk confocal microscope, 40×) from a β2-GABA mouse showing floxed lentivirus reporter gene mCherry expression (red, indicating lack of β2* nAChRs) and TH staining for DA neurons (green); the orange circle shows an example of mCherry expression only in TH-positive cells. (E) [125I]Epibatidine autoradiography on coronal brain slices in the VTA of β2-KO, β2-GABA, β2-DA, and β2-VTA mice. fr, fasciculus retroflexus; IPN, interpeduncular nucleus.
Fig. 2.
Nicotine-induced CPA is mediated by VTA dopamine neurons. A high dose of acute nicotine (1.75 mg/kg) in mice produces a conditioned aversive response in control mice. This CPA was not observed in β2-KO and β2-GABA mice, but rescued in β2-VTA and β2-DA mice. Data and error bars represent mean ± SEM. *P < 0.05.
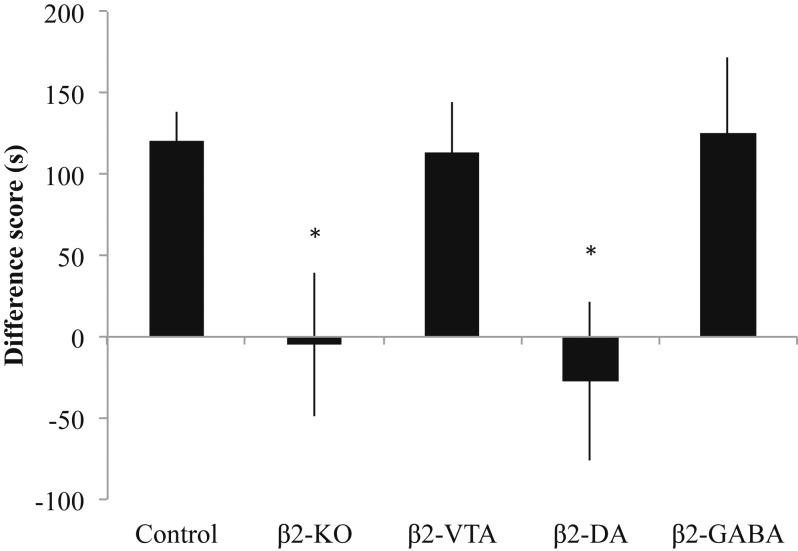
It is difficult to observe conditioned rewarding effects of nicotine in our place-conditioning paradigm; thus we next utilized a lower dose of nicotine (0.35 mg/kg) after pretreatment with the dopamine receptor antagonist α-flupenthixol to block the aversive motivational effects of nicotine (18, 19). This treatment protocol produces a reliable CPP for the nicotine-paired environment in control mice, which was also observed in groups of β2-VTA and β2-GABA mice, but not the β2-KO and β2-DA groups (F4,68 = 3.817, P < 0.05; Fig. 3). With this lower dose of nicotine and α-flupenthixol pretreatment, neuronal subpopulation-targeted β2 reexpression had opposite consequences to those that were observed with a high dose of acute nicotine: mice with β2* nAChRs reintroduced on VTA GABA neurons behaved like control mice and showed a conditioned rewarding response (post hoc Bonferroni test comparing β2-GABA and control groups, P > 0.05), while mice with β2* nAChRs reintroduced on VTA DA neurons showed neither a CPP nor CPA (post hoc Bonferroni test comparing β2-GABA and β2-DA, P > 0.05), behaving as if they were complete KO mice (post hoc Bonferroni test comparing β2-DA and KO, P > 0.05). These results suggest that nicotine’s conditioned rewarding effects after dopamine receptor blockade are signaled through β2* nAChRs on GABA (but not dopamine) neurons in the VTA. Of note, control groups of mice given α-flupenthixol (0.8 mg/kg) in 1 environment and saline in the other environment showed no preference for either environment (t14 = 0.1518, P = 0.8815; SI Appendix). This result suggests that the dose of α-flupenthixol used in this study is neither rewarding nor aversive.
Fig. 3.
Nicotine-induced CPP is mediated by VTA GABA neurons. After pretreatment with the dopamine antagonist α-flupenthixol, a low dose of acute nicotine (0.35 mg/kg) produced a conditioned rewarding response in control mice. This CPP was not observed in β2-KO and β2-DA mice, but rescued in β2-VTA and β2-GABA mice. Data and error bars represent mean ± SEM. *P < 0.05.
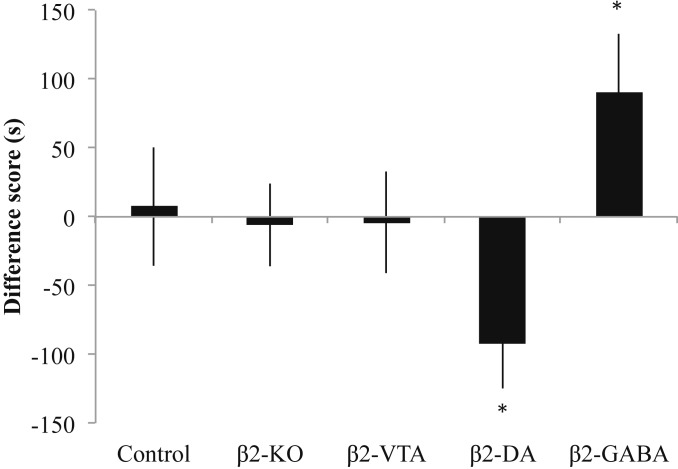
It is possible that pretreatment with α-flupenthixol prevented the expression of a conditioned response in the β2-DA group; thus we gave new groups (as well as extinguished groups) of mice the low dose of acute nicotine (0.35 mg/kg) without α-flupenthixol pretreatment (F4,76 = 4.063, P < 0.05; Fig. 4). This dose of nicotine did not cause any observable preference or aversion for the nicotine-paired environment in the control, β2-KO, and β2-VTA groups. However, this dose of nicotine produced a significant CPP for the nicotine-paired environment in the β2-GABA group (P < 0.05 compared to controls). By contrast, we observed a significant CPA in the β2-DA group (P < 0.05 compared to controls), which, similar to previous studies (9, 17), suggests that nicotine produces both aversive and rewarding conditioned effects that are mediated in the VTA. We suggest that the absence of a conditioned response observed after a low dose of nicotine in the groups with functional β2* nAChRs on both dopamine and GABA VTA neurons (the control and β2-VTA groups) is due to the competing positive and negative effects that are simultaneously occurring after administration of acute low-dose nicotine. However, the group of β2-DA mice, with functional β2* nAChRs rescued only on their VTA dopamine neurons, demonstrated a conditioned aversive response that could be observed when the mice were not pretreated with the dopamine antagonist α-flupenthixol. The β2-GABA mice, with functional β2* nAChRs on their VTA GABA neurons only, showed a conditioned reward response to this dose of nicotine. These results provide a double-dissociation of the motivational effects of acute nicotine: activation of VTA β2* nAChRs located on GABA neurons causes reward in the absence of β2* nAChRs located on dopamine neurons; however, when β2* nAChRs located on GABA neurons are absent, activation of β2* nAChRs located on dopamine neurons induces a conditioned aversive response.
Fig. 4.
Nicotine-induced CPP and CPA are revealed by separate β2-GABA and β2-DA rescue, respectively. A low dose of acute nicotine (0.35 mg/kg) produced no observable response in control mice, β2-KO mice, and β2-VTA mice. The aversive effects of this dose were revealed when β2* nAChRs were rescued in β2-DA mice, and rewarding effects were revealed with β2* nAChR rescue in β2-GABA mice. Data and error bars represent mean ± SEM. *P < 0.05.
Discussion
The conditioned effects of nicotine depend upon a process involving both rewarding and aversive components that are hypothesized to work through different neurobiological substrates. The present report utilizes lentiviral rescue of the β2* nAChRs in mice to demonstrate that nicotine-induced CPP acts through GABAergic VTA neurons, while nicotine-induced CPA is signaled by dopaminergic VTA neurons. By rescuing β2* nAChRs selectively on dopaminergic or GABAergic VTA neurons in mice that previously lacked these receptors, mice exhibited conditioned aversive and rewarding effects to acute nicotine, respectively. These results are in line with previous studies showing that the conditioned rewarding effects of acute nicotine are mediated by a VTA GABAergic connection with the tegmental pedunculopontine nucleus of the brainstem (9) and that the conditioned aversive response to acute nicotine is mediated by dopaminergic signaling (17, 19) through a specific pattern of phasic activity at dopamine D1 receptors (18). This latter study showed that both agonism and antagonism of dopamine D1 receptors prevented the CPA to acute nicotine (18), suggesting that either increasing or decreasing activity at dopamine receptors prevents the specific pattern of firing that signals the conditioned aversive response to nicotine. Recent optogenetic studies have suggested that stimulation of dopaminergic VTA neurons elicits CPP (20), and activation of GABA VTA neurons disrupts reward consumption (21) or leads to CPA (22). However, these optogenetic results might be seen as consistent with our hypothesis that a specific pattern of VTA dopaminergic (or GABA) activity signals nicotine’s conditioned rewarding and aversive effects and that optogenetic stimulation-induced modification of this pattern may obscure the signaling of motivation. Indeed, the optogenetic stimulation parameters employed (20–22) did not attempt to mimic the specific firing patterns that we suggest signal the conditioned aversive and rewarding responses to nicotine (18) and thus may have obscured such specific patterns of neuronal activity. The present data suggest that the acute rewarding and aversive effects of nicotine occur simultaneously and can be revealed by reconstituting the endogenous patterns of dopaminergic signaling (for aversion) and GABAergic signaling (for reward). Furthermore, both effects require the presence of functional β2* nAChRs in the VTA. These results are in line with previous studies using intracerebral nicotine administration in nondependent rats (9, 17) and, considered with recent optogenetic findings (16, 20–22), support the hypothesis that specific patterns of activity in VTA β2* nAChR-containing GABA and dopaminergic neurons respectively signal nicotine reward and aversion (SI Appendix). β2* nAChR-mediated modifications in the GABA/dopaminergic activity balance is also likely to switch motivational states from reward to aversion. In accordance with this hypothesis, it was previously established that lentiviral β2* nAChR rescue on VTA dopaminergic neurons, in anesthetized β2-KO mice, does not restore spontaneous and nicotine-evoked firing or bursting patterns of these neurons, that are constitutively altered in these mice (13). By contrast, lentiviral β2* nAChR rescue on VTA GABAergic neurons was found to restore spontaneous activity of dopamine neurons, but to cause decreased firing and bursting dopaminergic activity in response to nicotine (13). Future studies will now be needed to further characterize the response of both VTA dopaminergic and GABAergic neurons to cell-selective β2* nAChR rescue in awake mice and to understand how these responses translate into nicotine reward or aversion, also depending on the nicotine dose and route of administration.
Although we suggest here that β2* nAChRs on dopaminergic VTA neurons are critical for nicotine’s acute aversive effects, other studies have suggested that α5* nAChRs on medial habenular projections to serotonergic brain centers (23) and to the interpeduncular nucleus (7, 24) are important for the aversive effects of self-administered nicotine. These results suggest that many different nicotinic receptor subtypes and neurobiological substrates play a role in nicotine’s behavioral effects. Indeed, systemic nicotine normally will stimulate simultaneously many neural areas and nicotinic receptors, including both reward and aversion circuitry proximal and distal to the VTA. We suggest that this simultaneous activation of VTA GABA and dopamine neurons leads to GABAergic inhibition of the aversive dopamine signal and therefore a rewarding motivational experience.
We utilized constitutive β2-KO mice in this study. It is possible that the absence of β2* nAChRs during development could have led to differential expression of remaining nAChR subunits and potentially some developmental rewiring that might have contributed to the motivated behavior of our groups. However, the β2-VTA groups of mice that had reexpression of β2* nAChRs on both DA and GABA VTA neurons showed almost identical conditioned responses to nicotine as C57BL/6J wild-type mice at every dose tested, suggesting that constitutive knockout of β2* nAChRs (followed by reexpression) did not lead to significant developmental effects.
The present results differ from those in the study conducted by Tolu and colleagues (13) that used the same lentiviral vector rescue paradigm, but with more extensive nicotine exposure in mice that were trained to self-administer nicotine into the VTA over a period of 7 d. These authors observed the optimal reinstatement of a form of nicotine self-administration when β2* nAChRs were reexpressed on both VTA DA and GABA neurons (13). Several key differences between the procedures used across the studies might account for these apparently contradictory results. Intra-VTA self-administration leads to multiple doses of nicotine in 1 session (which likely leads to different amounts of nicotine in the VTA than the systemic dose used in the present study) that is repeated over many sessions, which may lead to tolerance effects after repeated activation of nAChRs expressed only within the VTA (25), while place-conditioning follows a single systemic, experimenter-administered drug administration, to a maximum of 4 times. Thus, it is likely that 1) the state of the nAChRs (such as activated, desensitized, or up-regulated), 2) the afferent modulations of VTA GABA and dopamine neurons, and 3) the amount of nicotine exposure and route of administration differ between the 2 procedures. Indeed, we suggest that this substantial difference in the overall amount of nicotine and frequency of administration leads to switching between naive and nonnaive motivational states and that the neurobiological substrates mediating nicotine reward and aversion in these distinct naive versus nonnaive motivational states differ, even within the same brain region (19). In nicotine-dependent (nonnaive) mice, the motivational effects of nicotine appear to depend primarily on dopaminergic function (18, 19). Further, the VTA is composed of anatomically and functionally heterogeneous dopaminergic subpopulations with different axonal projections (26) and GABA neurons that provide local inhibition of dopamine signaling as well as long-range inhibition of projection regions (27). Indeed, a recent study showed that nicotine concurrently excites and inhibits VTA dopamine neurons (28), and another study showed that only a subset of VTA dopaminergic neurons will express the stress neurotransmitter corticotropin-releasing factor after chronic nicotine administration and that such expression is necessary for the aversive effects of chronic nicotine withdrawal (29). Taken together, these studies suggest that there are distinct subpopulations of dopamine and GABA neurons in the VTA that may serve different functions for nicotine motivation (SI Appendix), contributing distinctly to the conditioned rewarding and aversive responses observed after the administration of nicotine. Indeed, optogenetic activation or silencing of VTA dopamine neurons leads to rewarding or aversive responses, respectively (30), suggesting that silencing of “rewarding” VTA DA neurons may lead to aversive responses. Further, nAChRs have been shown to influence the response of the reward system to other drugs and rewards than nicotine, including cocaine, alcohol, and food (31–33), and the motivational effects of nicotine, opiates, and food can be doubly dissociated by inactivating VTA dopaminergic versus GABAergic neurons (34, 35). Thus, VTA β2* nAChRs located on dopaminergic or GABAergic neurons also may differentially influence aversive and rewarding effects of stimuli other than nicotine.
The identification of the exact processes that mediate reward versus aversion in response to nicotine administration is an important step in the development of new treatments for nicotine addiction and smoking cessation. We suggest here that the VTA mediates both reward and aversion to nicotine through a specific pattern of signaling by different neuronal types: dopamine for aversion and GABA for reward. These results imply that selective modulation of dopamine or GABA systems may provide novel therapeutic targets for smoking cessation pharmacotherapies.
Materials and Methods
The behavioral studies were conducted at the University of Toronto. All animal use procedures were approved by the University of Toronto Animal Care Committee, in accordance with the Canadian Council on Animal Care guidelines.
Mice.
Male C57BL/6 mice (Charles River, Canada, or Institut Pasteur, France), β2* nAChR KO mice (Institut Pasteur, France), and β2-DAT-Cre and β2-GAD67-Cre mice (Institut Pasteur, France) ranging in age from 3 to 6 mo were housed in a temperature-controlled room with lights on from 7 AM to 7 PM. The β2-KO mice used in the experiments had been backcrossed with wild-type C57BL/6J mice for at least 19 generations.
Drugs.
Nicotine hydrogen tartrate salt (Sigma-Aldrich) was dissolved in saline at pH 7.0 ± 0.3 and administered via s.c. injection at a variety of doses (SI Appendix). The dopamine receptor antagonist α-flupenthixol (0.8 mg/kg) was purchased from Sigma-Aldrich, dissolved in saline, and administered i.p. 60 min prior to conditioning (18, 19, 36). All doses of drugs are expressed as milligrams of free base/kg of body weight. Doses and time of injections were selected based on the results from our dose–response curve (SI Appendix) and our previous studies (18, 19, 36).
Lentiviral Vectors.
The nonselective reexpression lentivector [phosphoglycerate kinase (PGK)-β2-Ires2–enhanced green fluorescent protein (eGFP)] is a bicistronic β2-IRES2-eGFP construct, previously described (11, 13, 37). Briefly, the mouse PGK promoter was amplified from an expression vector and ligated into pTRIPΔU3. To generate the β2-IRES2-eGFP construct a cloning site was created in the pIRES2-eGFP expression plasmid to introduce the wild-type mouse β2-subunit cDNA. Then the entire cassette coding the β2-IRES2-eGFP was transferred into pTRIPΔU3. To create the conditional lentivectors [PDGF-Floxed-mCherryStop-β2], where β2 transcription is prevented by a lox P-flanked mCherry-Stop DNA sequence, a previously described subcloning strategy was used (13, 38). The PGK-eGFP control lentivector was identical to the bicistronic version, but lacked the β2-IRES2 portion.
Lentiviral Vector Injections.
Lentiviral vector injection occurred at the Institut Pasteur in an identical manner to previous reports (13, 37). Mice aged 10 to 12 wk were anesthetized using 250 μL of ketamine 1.5% (Merial)/xylazine 0.05% (Bayer Healthcare) in PBS. Mice were placed into a stereotaxic frame adapted for mice. Lentivirus (2 μL at 100 ng p24 protein per microliter for β2 lentiviral vectors and 2 μL at 37.5 ng p24 protein per microliter for eGFP vectors) was injected bilaterally at: anteroposterior −3.4 mm, lateral ±0.5 mm from Bregma, and −4.4 mm from the surface for VTA injection. In Cre-mice, after lentiviral transduction and integration into the host genome, the dormant gene is activated by Cre-mediated excision of the floxed cassette in Cre-containing cells only. For this study, 5 different experimental groups of male mice were generated: wild-type C57BL/6J mice injected with eGFP-expressing lentivector in the VTA (controls), β2 constitutive KO mice (β2-KO) injected with eGFP-expressing lentivector into the VTA, β2 KO mice injected with a β2-subunit–expressing lentivector into the VTA (β2-VTA), β2 KO × DAT-Cre mice injected with a Cre recombinase-activated (floxed) β2-expressing lentivector into the VTA (12) for reexpression selectively on VTA dopamine neurons (β2-DA), and β2 KO × GAD67-Cre mice injected with a floxed β2-expressing lentivector into the VTA for reexpression selectively on VTA GABA neurons (β2-GABA). In the β2-DA and β2-GABA groups, the β2 gene is expressed after lentiviral transduction by Cre-mediated excision of the floxed cassette in Cre-containing cells only. In the presence of the Cre-recombinase, both mCherry and the stop codon are excised, thereby allowing β2 expression, such that mCherry is expressed only in cells that do not express the Cre-recombinase, while β2 is expressed only in cells that express the Cre-recombinase. The efficacy and specificity of our system has been demonstrated previously by confocal analysis of immunostained mouse VTA and its dopamine and GABA-containing neurons (13, 37). Such reexpressed β2* nAChRs are functional and are activated by nicotine (11, 13, 37). All procedures were carried out in accordance with European Commission directives 219/1990 and 220/1990 and approved by Animalerie centrale and Médecine du travail, Institut Pasteur. The mice were tested after 10–12 wk of viral expression.
Autoradiography and Quantification.
To highlight the presence of high-affinity nicotinic β2*nAChR sites after vectorization, brains from a group of mice were dissected, frozen in dry ice, and stored at −80 °C until use. Two series of consecutive coronal sections, 20 μm thick, were cut at −20 °C and thaw-mounted on Menzel Glasser SuperFrost Plus microscope slides. Slides were then incubated at room temperature, with 220 pM [125I]-epibatidine (NEN Perkin-Elmer, specific activity 2,200 Ci/mmol) in 50 mM Tris (pH 7.4) for 1 h in presence or not of 10 µM of nicotine (Sigma-Aldrich) to identify nonspecific binding sites. After incubation, sections were rinsed twice 5 min in the same buffer and briefly in distilled water. Sections were then dried and exposed for 48 to 72 h to Carestream Kodak BioMax MR films (Sigma-Aldrich). The quantification of lentiviral restoration of β2*-nAChRs in the VTA was carried out by ImageJ (National Institutes of Health) on 5 to 8 coronal sections for each brain, for both without- and with-nicotine (for nonspecific binding) conditions. An internal background was measured for each section from the superior colliculus. The relative intensity for each individual was calculated as the mean value, from all quantified sections, of: (1/luminosity minus internal background, without nicotine) – (1/luminosity minus internal background, with nicotine).
Immunostaining.
To analyze eGFP and mCherry transduction, sections from a group of vectorized mice were stained for GFP (Life Technologies SAS), Tyrosine Hydroxylase (TH) (Sigma-Aldrich) for dopamine cell visualization, and RFP (Millipore Bioscience) for mCherry visualization. Fluorescence immunohistochemistry was performed as follows: free-floating 50-µm VTA brain sections were incubated 1 h at 4 °C in a fixative solution of PBS containing 10% normal goat serum (Sigma) and 0.2% Triton X-100 and then overnight at 4 °C in PBS containing the primary antibody (rabbit anti-GFP at 1:2,000 dilution, mouse anti-TH at 1:400 dilution or mouse anti-RFP at 1:400 dilution), 2% normal goat serum and 0.2% Triton X-100. The next day, sections were rinsed with PBS and then incubated for 3 h at room temperature with secondary antibody (AlexaFluor 488-conjugated anti-rabbit and DyLight 594-conjugated anti-mouse, Jackson Immunoresearch) at 1:200 dilution in a solution of 2% normal goat serum in PBS. After 3 rinses in PBS, slices were wet-mounted using Mowiol 4-88 (Calbiochem Corporation).
Place-Conditioning.
The place-conditioning apparatus was obtained from Med Associates, Inc. (SOF-700RA-25 two-chamber place preference apparatus). One environment was black with a metal rod floor, and the other was white with a wire mesh floor. An intermediate gray area housed a removable partition. Each cage was cleaned between animals, and each group was fully counterbalanced. During preference testing, the dividing partition was removed, and mice were given free access to both environments. All place-conditioning and testing was performed between 10 AM and 6 PM (18, 19, 36).
For conditioning sessions, mice were pretreated i.p. with saline or α-flupenthixol (0.8 mg/kg) 1 h prior to conditioning and given an s.c. injection of nicotine (0.35 or 1.75 mg/kg) or saline (18, 19, 36). Each group underwent 8 conditioning trials over 8 consecutive days (4 alternating drug and vehicle pairings) in 1 of the conditioning environments for 15 min (18, 19, 36). All conditioning was unbiased and fully counterbalanced for treatment compartment and order of drug presentation. A single 10-min preference-testing session was performed 2–5 d after the last conditioning day, when subjects were drug-free (18, 19, 36). The divider was removed from the apparatus, and the mouse was placed in the central gray compartment. The location of the mouse was recorded automatically by beam breaks in the environments. The difference score for each animal was calculated by subtracting the time spent in the saline-paired environment from the time spent in the nicotine-paired environment (18, 19, 36).
Twenty-one of the 98 mice shown in Fig. 2, and 23 of the 69 mice shown in Fig. 3, were extinguished for their conditioned place preferences by exposing them to all conditioning environments for 10 min per day for as many sessions as necessary to reduce their preference to less than 100 s (between 2 and 10 sessions). They were then conditioned in a new, fully counterbalanced treatment schedule, where half of the mice that had initially received the large aversive dose of nicotine (1.75 mg/kg) were conditioned with the lower dose (0.35 mg/kg), and vice versa. Most important, the groups that were conditioned only once did not differ from the groups that were extinguished and conditioned a second time, as measured by Student’s t tests: Controls (t11 = 0.8969, P = 0.3890), β2-KO (t13 = 0.7311, P = 0.4777), β2-VTA (t13 = 0.3819, P = 0.7087), β2-DA (t12 = 0.4653, P = 0.6501), and β2-GABA (t10 = 0.3654, P = 0.7224).
Statistical Analysis.
Results were analyzed similar to our previous studies (18, 19, 36) using a 1-way analysis of variance (ANOVA) or Student’s t tests with a significance level of 0.05. In all cases a normality test and equal variance test were performed before an ANOVA to ensure its validity. Post hoc Bonferroni tests were used where appropriate. Data are shown as mean ± SEM.
Data Availability.
All data are available in the manuscript and SI Appendix. Raw individual data are available to readers upon request.
Supplementary Material
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research, the Institut Pasteur, Paris, and the Agence Nationale Pour la Recherche, Paris. We thank Brenda Coles and the University of Toronto Division of Comparative Medicine (DCM) staff for technical assistance.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1908724116/-/DCSupplemental.
References
- 1.Kalivas P. W., Volkow N. D., The neural basis of addiction: A pathology of motivation and choice. Am. J. Psychiatry 162, 1403–1413 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Koob G. F., Le Moal M., Neurobiology of Addiction (Elsevier, Inc, San Diego, 2006). [Google Scholar]
- 3.George O., et al. , CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc. Natl. Acad. Sci. U.S.A. 104, 17198–17203 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thibeault K. C., Kutlu M. G., Sanders C., Calipari E. S., Cell-type and projection-specific dopaminergic encoding of aversive stimuli in addiction. Brain Res. 1713, 1–15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grunberg N. E., Overview: Biological processes relevant to drugs of dependence. Addiction 89, 1443–1446 (1994). [DOI] [PubMed] [Google Scholar]
- 6.Laviolette S. R., van der Kooy D., The neurobiology of nicotine addiction: Bridging the gap from molecules to behaviour. Nat. Rev. Neurosci. 5, 55–65 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Fowler C. D., Lu Q., Johnson P. M., Marks M. J., Kenny P. J., Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature 471, 597–601 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grace A. A., The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction 95 (suppl. 2), S119–S128 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Laviolette S. R., Alexson T. O., van der Kooy D., Lesions of the tegmental pedunculopontine nucleus block the rewarding effects and reveal the aversive effects of nicotine in the ventral tegmental area. J. Neurosci. 22, 8653–8660 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nestler E. J., Is there a common molecular pathway for addiction? Nat. Neurosci. 8, 1445–1449 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Maskos U., et al. , Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436, 103–107 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Mameli-Engvall M., et al. , Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron 50, 911–921 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Tolu S., et al. , Co-activation of VTA DA and GABA neurons mediates nicotine reinforcement. Mol. Psychiatry 18, 382–393 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Shoaib M., et al. , The role of nicotinic receptor beta-2 subunits in nicotine discrimination and conditioned taste aversion. Neuropharmacology 42, 530–539 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Walters C. L., Brown S., Changeux J. P., Martin B., Damaj M. I., The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology (Berl.) 184, 339–344 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Durand-de Cuttoli R., et al. , Manipulating midbrain dopamine neurons and reward-related behaviors with light-controllable nicotinic acetylcholine receptors. eLife 7, e37487 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laviolette S. R., van der Kooy D., Blockade of mesolimbic dopamine transmission dramatically increases sensitivity to the rewarding effects of nicotine in the ventral tegmental area. Mol. Psychiatry 8, 50–59 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Grieder T. E., et al. , Phasic D1 and tonic D2 dopamine receptor signaling double dissociate the motivational effects of acute nicotine and chronic nicotine withdrawal. Proc. Natl. Acad. Sci. U.S.A. 109, 3101–3106 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grieder T. E., et al. , Dopaminergic signaling mediates the motivational response underlying the opponent process to chronic but not acute nicotine. Neuropsychopharmacology 35, 943–954 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai H. C., et al. , Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324, 1080–1084 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Zessen R., Phillips J. L., Budygin E. A., Stuber G. D., Activation of VTA GABA neurons disrupts reward consumption. Neuron 73, 1184–1194 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan K. R., et al. , GABA neurons of the VTA drive conditioned place aversion. Neuron 73, 1173–1183 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu Y. W., et al. , Medial habenula output circuit mediated by α5 nicotinic receptor-expressing GABAergic neurons in the interpeduncular nucleus. J. Neurosci. 33, 18022–18035 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fowler C. D., Tuesta L., Kenny P. J., Role of α5* nicotinic acetylcholine receptors in the effects of acute and chronic nicotine treatment on brain reward function in mice. Psychopharmacology (Berl.) 229, 503–513 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matta S. G., et al. , Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl.) 190, 269–319 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Lammel S., Lim B. K., Malenka R. C., Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 76, 351–359 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Creed M. C., Ntamati N. R., Tan K. R., VTA GABA neurons modulate specific learning behaviors through the control of dopamine and cholinergic systems. Front. Behav. Neurosci. 8, 1–7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eddine R., et al. , A concurrent excitation and inhibition of dopaminergic subpopulations in response to nicotine. Sci. Rep. 5, 8184 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grieder T. E., et al. , VTA CRF neurons mediate the aversive effects of nicotine withdrawal and promote intake escalation. Nat. Neurosci. 17, 1751–1758 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilango A., et al. , Similar roles of substantia nigra and ventral tegmental dopamine neurons in reward and aversion. J. Neurosci. 34, 817–822 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuesta L. M., Fowler C. D., Kenny P. J., Recent advances in understanding nicotinic receptor signaling mechanisms that regulate drug self-administration behavior. Biochem. Pharmacol. 82, 984–995 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahman S., Engleman E. A., Bell R. L., Recent advances in nicotinic receptor signaling in alcohol abuse and alcoholism. Prog. Mol. Biol. Transl. Sci. 137, 183–201 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Besson M., Forget B., Correia C., Blanco R., Maskos U., Profound alteration in reward processing due to a human polymorphism in CHRNA5: A role in alcohol dependence and feeding behavior. Neuropsychopharmacology 44, 1906–1916 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laviolette S. R., Gallegos R. A., Henriksen S. J., van der Kooy D., Opiate state controls bi-directional reward signaling via GABAA receptors in the ventral tegmental area. Nat. Neurosci. 7, 160–169 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Bechara A., van der Kooy D., A single brain stem substrate mediates the motivational effects of both opiates and food in nondeprived rats but not in deprived rats. Behav. Neurosci. 106, 351–363 (1992). [DOI] [PubMed] [Google Scholar]
- 36.Grieder T. E., et al. , Deletion of α5 nicotine receptor subunits abolishes nicotinic aversive motivational effects in a manner that phenocopies dopamine receptor antagonism. Eur. J. Neurosci. 46, 1673–1681 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tolu S., et al. , A versatile system for the neuronal subtype specific expression of lentiviral vectors. FASEB J. 24, 723–730 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Morel C., et al. , Nicotine consumption is regulated by a human polymorphism in dopamine neurons. Mol. Psychiatry 19, 930–936 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the manuscript and SI Appendix. Raw individual data are available to readers upon request.