Significance
Vascularized composite allotransplantation (VCA) is an emerging field that is particularly beneficial for select amputees and patients with devastating soft tissue loss that is not amenable to conventional reconstructive surgeries. As with solid organ transplantation, VCA recipients are subjected to a lifelong regimen of antirejection drugs with a well-established sequela. Herein, we report a synthetic, controlled-release microparticle system (referred to as TRI-MP) that aims to locally enrich naturally occurring, suppressive lymphocytes to prevent allograft rejection and promote tolerance. While this study exclusively focuses on VCA, this technology has implications for other conditions characterized by aberrant inflammation.
Keywords: controlled release, drug delivery, transplantation, regulatory T cells, biomaterials
Abstract
For individuals who sustain devastating composite tissue loss, vascularized composite allotransplantation (VCA; e.g., hand and face transplantation) has the potential to restore appearance and function of the damaged tissues. As with solid organ transplantation, however, rejection must be controlled by multidrug systemic immunosuppression with substantial side effects. As an alternative therapeutic approach inspired by natural mechanisms the body uses to control inflammation, we developed a system to enrich regulatory T cells (Tregs) in an allograft. Microparticles were engineered to sustainably release TGF-β1, IL-2, and rapamycin, to induce Treg differentiation from naïve T cells. In a rat hindlimb VCA model, local administration of this Treg-inducing system, referred to as TRI-MP, prolonged allograft survival indefinitely without long-term systemic immunosuppression. TRI-MP treatment reduced expression of inflammatory mediators and enhanced expression of Treg-associated cytokines in allograft tissue. TRI-MP also enriched Treg and reduced inflammatory Th1 populations in allograft draining lymph nodes. This local immunotherapy imparted systemic donor-specific tolerance in otherwise immunocompetent rats, as evidenced by acceptance of secondary skin grafts from the hindlimb donor strain and rejection of skin grafts from a third-party donor strain. Ultimately, this therapeutic approach may reduce, or even eliminate, the need for systemic immunosuppression in VCA or solid organ transplantation.
Vascularized composite allotransplantation (VCA) is an emerging field, encompassing transplantation of multiple tissue types with disparate embryonic origins. More than 100 VCAs have been performed in the past decade, including primarily hand and face transplants but also penis and uterine transplants (1, 2). Although VCA is not life-saving like solid organ transplantation, it can be transformational in improving the quality of life of amputees and other individuals who have sustained massive composite tissue loss. Unfortunately, chronic immunosuppression (IS) required to sustain these highly immunogenic transplants (due to the skin component in many VCAs) has hampered broader clinical applicability (3–8). Most systemic maintenance IS protocols for VCA consist of at least 2 to 3 drugs with well-established toxicity profiles (1), yet 85% of current hand transplant recipients still experience at least 1 episode of acute rejection during the first year, compared to only 10% of solid organ recipients (9). Thus, there is a clinical need for alternative strategies to achieve graft-specific immune hyporesponsiveness while obviating concerns with life-long IS (8, 10–12).
One such strategy involves harnessing the powerful immunoregulatory properties of a subset of naturally occurring lymphocytes known as regulatory T cells (Tregs). Indeed, Tregs are known to play a critical role in the induction and maintenance of experimental allograft tolerance (13–17). Due to their relative paucity, use of Tregs as cellular therapeutics in the clinic typically involves ex vivo culture and expansion of a patient’s own Tregs, followed by systemic reinfusion (14, 18–21). However, clinical implementation of Treg therapy faces several challenges, including the need for good manufacturing practice (GMP) facilities, as well as potential instability of Tregs and their propensity to transdifferentiate into harmful proinflammatory effector cells (14, 18, 22–24).
Enriching endogenous Treg populations in situ represents an alternative to Treg therapy that obviates some of these concerns. One such approach involves recruitment of circulating Tregs to a specific site by establishing a functional gradient of the Treg-recruiting chemokine CCL22 (25–29). However, since circulating Tregs are rare, representing ∼2–3% of all lymphocytes in the human body (19), recruiting a sufficient number of functional Tregs to resolve VCA inflammation may be challenging. Therefore, we hypothesized that in vivo induction of Tregs from naïve CD4+ T cells (a much larger population of circulating lymphocytes) could represent a more effective means to enrich Tregs at a local inflammatory site (e.g., allograft). To that end, we exploited natural mechanisms by which tolerogenic dendritic cells and some malignant tumors (as a means of evading immune recognition) induce Treg differentiation from naïve CD4+ T cells via secretion of Treg-trophic factors, such as TGF-β1 and IL-2 (30–34). In addition, suppression of Th1 and Th17 effector T cell differentiation and maintenance of the Treg suppressive function are critically important (24) and can be mediated by the naturally occurring immunosuppressive drug rapamycin (35). Accordingly, we developed a biodegradable controlled-release system—referred to as Treg-inducing (TRI) microparticles (MP)—to controllably deliver extremely small quantities of factors (ng/kg/d for TGF-β1 and IL-2; μg/kg/d for rapamycin) to the local physiological milieu (36). We previously demonstrated that TRI-MP promotes differentiation of naïve CD4+ T cells to Tregs in vitro (36) and can expand Treg populations in vivo to reduce inflammation and abrogate symptoms in rodent models of dry eye disease and allergic contact dermatitis (37, 38).
Here, we report that locally administered TRI-MP promotes indefinite allograft survival (>300 d) and donor-specific tolerance in a stringent, complete major histocompatibility complex (MHC)-mismatched rat hindlimb VCA model. Notably, this allotransplantation model involves chronic alloantigen-specific inflammation, making it fundamentally different from other acute models of nonspecific inflammation or contact allergy previously studied by our group (37, 38). As such, this study demonstrates the potential of this local immunotherapy strategy for preventing transplant rejection, with broader implications for treatment of a variety of other chronic inflammatory conditions.
Results
Characterization of TRI-MP.
TGF-β1-MP, Rapa-MP, and IL-2-MP were prepared using a biodegradable poly(lactic-coglycolic acid) (PLGA) polymer. MPs were engineered to be ∼10–20 μm in diameter in order to avoid phagocytic clearance (SI Appendix, Fig. S1A), and mean diameters for TGF-β1-MP, Rapa-MP, and IL-2-MP were 16.7, 15.7, and 17.2 μm, respectively. Scanning electron micrographs (SI Appendix, Fig. S1B) revealed spherical particles and confirmed the size distributions. IL-2-MP had slightly porous exterior surfaces, as they were formulated to be porous to obtain an initial burst followed by continuous release (SI Appendix, Fig. S1C). TGF-β1-MP and Rapa-MP released continuously for at least 3 wk (SI Appendix, Fig. S1C).
Intragraft Administration of TRI-MP Prolongs Rodent Hindlimb Transplant Survival Indefinitely.
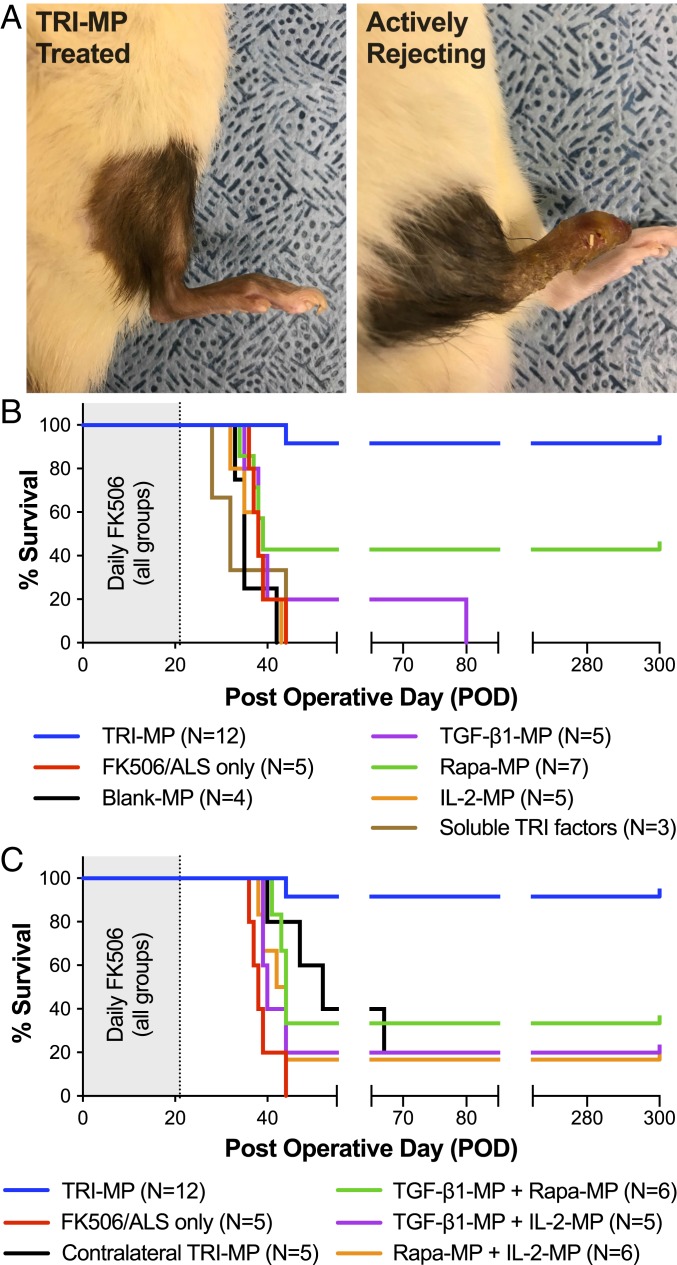
To investigate the influence of TRI-MP on VCA survival, we employed a rodent hindlimb allotransplant model. Fully MHC-mismatched hindlimbs were transplanted from Brown Norway (BN) donors to Lewis (LEW) recipients by experienced microsurgeons. All allograft recipients were given a short course of baseline IS consisting of daily FK506 (d 0–21) and rabbit antirat lymphocyte serum (ALS) (d −4 and 1). Graft recipients given this baseline IS alone consistently rejected their transplants 2–3 wk after systemic FK506 was discontinued. In contrast, 11 of 12 hindlimb allograft recipients treated with the same baseline IS plus subcutaneous (s.c.) injections of TRI-MP on d 0 and 21 survived >300 d without any treatment beyond the first 21 d (Fig. 1 A and B). To demonstrate the importance of each component of the TRI-MP system, some rats were treated with individual microparticle formulations (TGF-β1-MP, Rapa-MP, or IL-2-MP) or with combinations of 2 formulations. Notably, all 3 components were required for long-term allograft survival, as no individual microparticle formulation, or combination of 2 formulations, provided reliable long-term allograft survival (Fig. 1 B and C). To determine whether local delivery of Treg-inducing factors was necessary for therapeutic efficacy, TRI-MP was administered in the contralateral (nontransplanted) hindlimb of some rats. Delivery of Treg-inducing factors at a distal site did not affect allograft survival, suggesting that local intragraft release is required for therapeutic efficacy of TRI-MP.
Fig. 1.
TRI-MP prevents rejection and promotes long-term limb allograft survival in recipients. (A) Representative images of TRI-MP–treated hindlimb allograft (POD > 300) showing no signs of rejection and an actively rejecting untreated control graft (POD = 38). (B) TRI-MP prolongs hindlimb survival (>300 d in 11/12 animals) in the absence of continued systemic IS (P < 0.001 for TRI-MP vs. baseline IS). Individual components alone (TGF-β1-MP, Rapa-MP, IL-2-MP) did not confer reliable long-term survival (P < 0.05 for TRI-MP vs. Rapa-MP, P < 0.001 for TRI-MP vs. other individual components). (C) Neither pairwise iterations of TRI-MP nor administration of TRI-MP in the contralateral (nontransplanted) limb prolonged graft survival as well as TRI-MP (P < 0.001 for TRI-MP vs. all pairwise controls).
TRI-MP–Treated Long-Term Surviving Allografts Exhibit Normal Histology, Reduced Expression of Inflammatory Mediators, and Increased Expression of Treg-Associated Suppressive Cytokines.
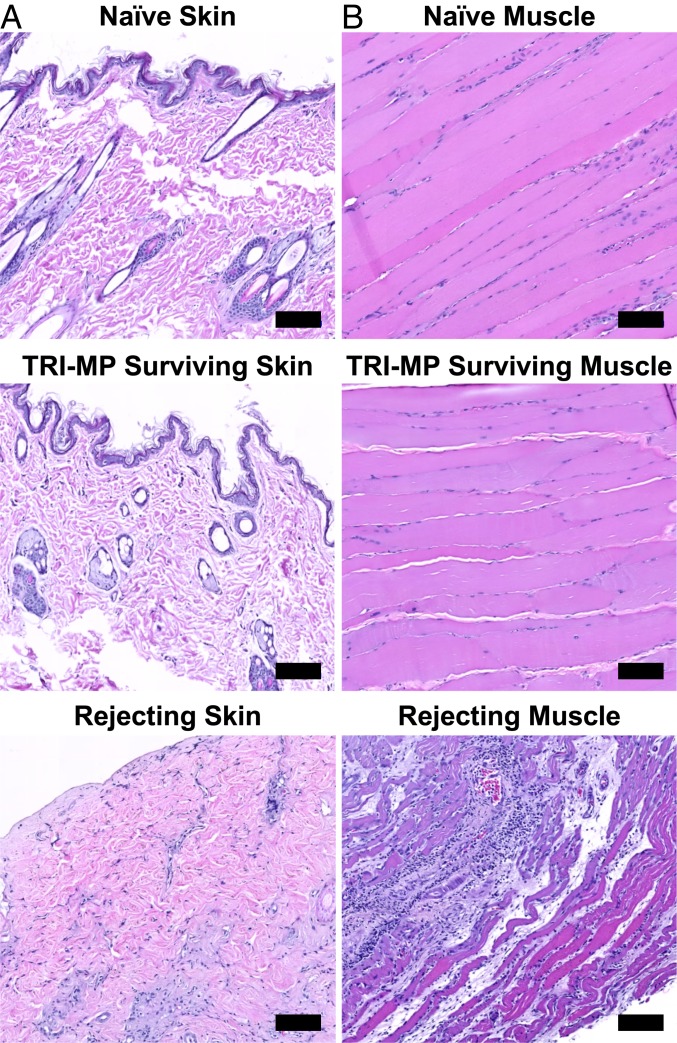
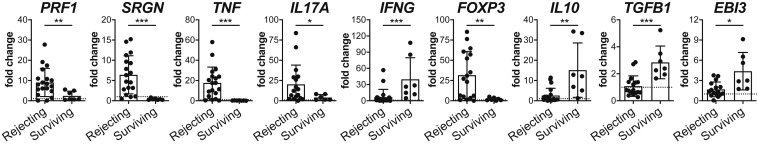
Gross observations of prolonged allograft survival in allograft recipients treated with TRI-MP (as in Fig. 1A) were corroborated by histology and qRT-PCR analyses. Histologically, skin from TRI-MP–treated hindlimb allografts exhibited intact dermis and epidermis with limited mononuclear infiltration and tissue architecture, similar to that observed in normal skin (Fig. 2A). Muscle also exhibited normal tissue architecture with limited cellular infiltration (Fig. 2B), and there was no evidence that TRI-MP injections adversely affected native tissues. In contrast, skin and muscle from actively rejecting animals showed dense mononuclear infiltration and significant destruction of tissue architecture, consistent with macroscopic observations (Fig. 2). Expression of several inflammatory mediators and Treg-associated factors in allograft skin and recipient inguinal graft draining lymph nodes (DLNs) was also quantified and compared to that of naïve controls. Skin from actively rejecting allografts had elevated levels of TNF-α, IL-17A, serglycin, and perforin-1, while expression of these inflammatory mediators in TRI-MP–treated long-term surviving allografts was comparable to that of naïve controls (Fig. 3). In contrast, IFN-γ expression was elevated in TRI-MP–treated surviving grafts compared to rejecting grafts and naïve skin (Fig. 3). While rejecting grafts had higher expression of FoxP3, 3 Treg-associated suppressive cytokines (IL-10, TGF-β1, and IL-35 [determined by expression of EBI3, a subunit of the IL-35 heterodimer]) were significantly increased in TRI-MP–treated long-term surviving allografts (Fig. 3). Similar trends in expression of inflammatory mediators and Treg-associated factors were observed in allograft DLNs except IFN-γ, IL-10, and TGF-β1, which were not elevated in lymph nodes from TRI-MP–treated rats (SI Appendix, Fig. S2).
Fig. 2.
TRI-MP administration preserves the architectural integrity of intragraft tissue components. Representative histology of (A) skin and (B) muscle from hindlimb allografts and naïve limbs (hematoxylin and eosin (H&E) staining). Allograft tissue samples taken from rejecting animals (POD = 38–44) display complete destruction of tissue architecture with dense mononuclear cell infiltration. In contrast, tissue samples taken from TRI-MP–treated grafts (POD > 300) exhibit normal/preserved tissue architecture similar to that of hindlimbs from naïve rats. (Scale bars, 100 μm.)
Fig. 3.
TRI-MP treatment yields long-term surviving allografts with reduced expression of inflammatory mediators (PRF1, SRGN, TNF, and IL17A) and increased expression of IFNG and Treg-associated cytokines (IL10, TGFB1, and EBI3 [subunit of IL-35 heterodimer]). Relative mRNA expression in skin samples from rejecting hindlimb allografts (n = 17–20, POD = 33–45) vs. surviving TRI-MP–treated hindlimb allografts (n = 7, POD > 300). Expression levels are presented as fold changes (2-ΔΔCt) relative to naïve skin (n = 11–13). Bars represent mean ± SD, and dots represent values from individual rats. Significant differences are indicated by *P < 0.05, **P < 0.01, or ***P < 0.001.
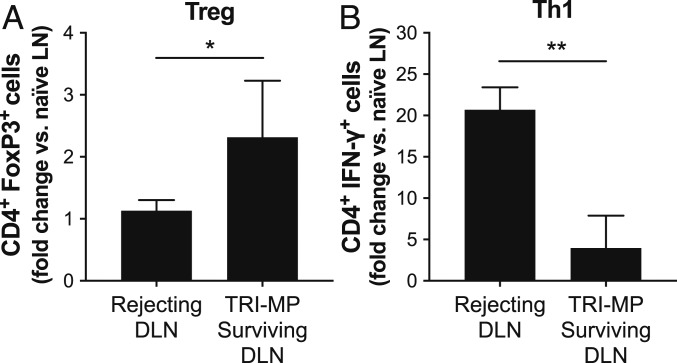
TRI-MP Increases Treg Populations and Decreases Th1 Populations in Allograft Draining Lymph Nodes.
To determine the frequencies of local regulatory and proinflammatory effector T cells in TRI-MP–treated animals and actively rejecting controls, inguinal allograft DLNs were harvested and isolated lymphocytes were stained for markers of Tregs (FoxP3) and type I effector T cells (IFN-γ). Notably, frequencies of CD4+ FoxP3+ Tregs were increased significantly in DLNs of TRI-MP–treated animals compared to those from naïve recipient limbs and actively rejecting allografts (Fig. 4). Conversely, percentages of CD4+ IFN-γ+ Th1 cells were significantly higher in DLNs of actively rejecting rats compared to TRI-MP–treated graft recipients, and Th1 frequencies in TRI-MP–treated DLNs were only slightly elevated compared to naïve lymph nodes (Fig. 4).
Fig. 4.
Phenotypic analysis of CD4+ T cells in allograft draining inguinal lymph nodes from TRI-MP–treated long-term surviving grafts (n = 4, POD > 300) and rejecting controls (n = 3, POD = 35–40). (A) CD4+ FoxP3+ Tregs and (B) CD4+ IFN-γ+ Th1 percentages in TRI-MP–treated and rejecting animals were normalized to percentages from naïve recipient strain (LEW) rats. The flow cytometry gating strategy is presented in the SI Appendix, Fig. S3. Significant differences are indicated by *P < 0.05 or **P < 0.01.
Tregs from TRI-MP–Treated Animals Exhibit Superior Suppressive Function and Donor Antigen Specificity.
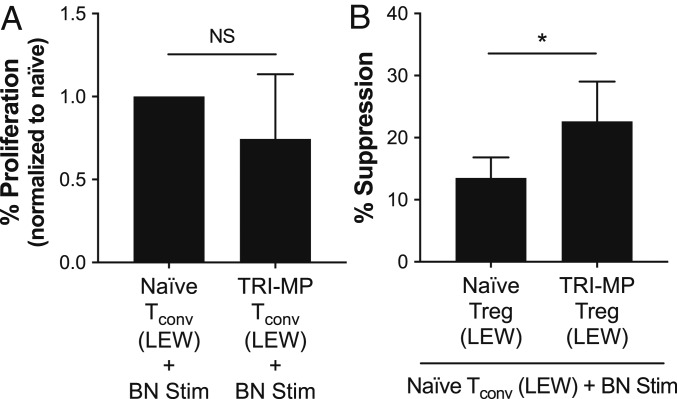
Total CD4+ T cells were isolated from splenocytes of TRI-MP–treated, long-surviving graft recipients as well as naïve LEW rats, and further sorted into CD4+ CD25hi Treg and CD4+ CD25– conventional T cell (Tconv) populations. When stimulated with irradiated BN (donor) splenocytes, Tconvs from TRI-MP–treated long-term surviving allograft recipients exhibited antidonor proliferative responses comparable to those of Tconvs from naïve animals (Fig. 5A). To assess the suppressive capacity of Tregs, isolated CD4+ CD25hi Tregs were cocultured with CD4+ CD25− Tconvs from naïve LEW rats and stimulated with irradiated BN (donor) splenocytes. Notably, Tregs from the TRI-MP–treated animals were significantly more effective at suppressing the antidonor proliferative responses of Tconvs than Tregs from naïve rats (Fig. 5B).
Fig. 5.
Functional analysis of T cells isolated from animals with TRI-MP–treated grafts. (A) Proliferative capacity of CD4+ CD25– Tconvs isolated from naïve LEW rats (n = 3) and TRI-MP–treated LEW rats with surviving BN allografts (n = 3, POD > 300). Tconvs were stimulated with BN splenocytes, and proliferation was normalized to that of naïve Tconvs. (B) CD4+ CD25hi Tregs isolated from TRI-MP–treated animals (n = 5, POD > 300) were more effective at suppressing BN-induced naïve Tconv proliferation than CD4+ CD25hi Tregs isolated from naïve rats (n = 4). Assays with cells from each rat were performed in triplicate, and statistical analyses were performed on mean results from each rat. Significant differences are indicated by *P < 0.05 (NS = not significant).
TRI-MP Promotes Systemic Donor-Specific Tolerance in Hindlimb Allograft Recipients.
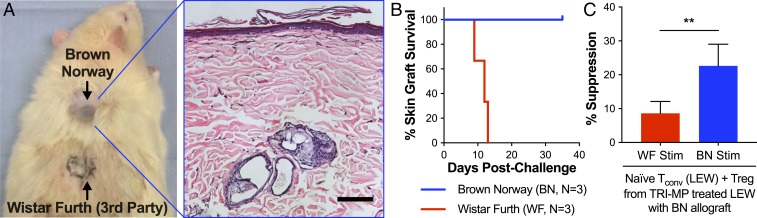
Alloantigen-specific tolerance in TRI-MP–treated rats with long-term surviving BN hindlimb allografts (>300 d) was evaluated by secondary challenge with nonvascularized skin grafts from both BN (donor) and Wistar Furth (WF; third-party) rats. Long-term survivors accepted BN grafts indefinitely, as evidenced by healed skin and hair growth (Fig. 6 A and B). In contrast, third-party skin grafts were rejected, as evidenced by extensive graft necrosis and contracture within 14 d (Fig. 6 A and B). To evaluate tolerance at the cellular level, CD4+ CD25hi Tregs from TRI-MP–treated rats were tested for antigen specificity using cocultures with purified CD4+ CD25− Tconvs from naïve LEW rats and stimulation with either irradiated BN (donor) or WF (third-party) splenocytes. In addition to superior suppressive function compared to Tregs from naïve animals, Tregs from TRI-MP–treated animals suppressed antidonor T cell responses significantly more than T cell responses directed against third-party splenocytes (Fig. 6C).
Fig. 6.
TRI-MP imparts donor-specific tolerance to rat hindlimb allograft recipients. (A and B) To test for donor antigen–specific tolerance in vivo, TRI-MP–treated animals with long-term surviving grafts were challenged with full-thickness nonvascularized skin grafts from BN and WF donors. TRI-MP–treated animals accepted BN grafts (as evidenced by wound healing and hair growth), but failed to accept WF grafts (as evidenced by extensive graft necrosis resulting in an eschar). Histological evaluation of the BN skin graft reveals an intact epidermal layer with normal tissue architecture. (Scale bar, 100 μm.) (C) CD4+ CD25hi Tregs isolated from TRI-MP–treated animals (n = 4–5, POD > 300) were cocultured with CD4+ CD25– Tconvs purified from naïve LEW rats and then stimulated with either donor BN or third-party WF splenocytes. Tregs isolated from TRI-MP–treated animals were more effective at suppressing BN-induced proliferation than WF-induced proliferation. Significant difference is indicated by **P < 0.01.
Discussion
The pinnacle achievement for transplantation continues to be induction of robust allograft tolerance in the absence of systemic IS (39). Complications associated with IS drug therapy are further magnified in the emerging field of VCA, as these grafts are not viewed as life-saving, thereby presenting significant ethical dilemmas in offering these treatments to eligible subjects (2, 9). Development of therapeutic strategies that reduce or eliminate the burden of IS is imperative, and wider clinical application of VCA will not be realized until such tolerogenic strategies are implemented in the clinic.
In this study, we explored the potential of local Treg induction as a means to establish allograft tolerance in a stringent, well-established, and clinically relevant rat hindlimb VCA model. Allograft recipients received a short course of IS, consisting of 3 wk of injected FK-506 and perioperative lymphocyte depletion with ALS to allow sufficient time for immunoregulatory factors to diffuse from TRI-MP and influence the local microenvironment. This approach also allowed us to demonstrate that TRI-MP could be used in tandem with commonly used antirejection agents, which is important because clinical trials for novel immunotherapies in transplantation typically involve weaning patients from a standard systemic IS regimen. Such clinical trial design also allows more flexibility for dose optimization by minimizing the consequences of failed tolerance induction.
Notably, administration of just 2 doses of TRI-MP, the first at the time of transplant and the second 21 d later, significantly prolonged hindlimb VCA survival (indefinitely in 11 of 12 rats), compared to control animals given only baseline IS for the first 21 d. As TRI-MP releases factors for ∼3–5 wk (SI Appendix, Fig. S1), allografts survived more than 34 wk without any systemic IS or delivery of immunomodulatory factors. None of the individual microparticle formulations of TGF-β1, rapamycin, or IL-2 could consistently confer long-term survival comparable to recipients treated with TRI-MP. Long-term graft survival in 3/7 recipients treated with Rapa-MP is not particularly surprising, as rapamycin is a clinically used immunosuppressive agent known to promote Tregs (40). While rapamycin is not consistently effective in preventing clinical VCA rejection as a monotherapy, our observation suggests that future rapamycin-based controlled-release systems (in tandem with FK506) could potentially be optimized for superior efficacy to inhibit rejection after transplantation. As with individual TRI-MP components, none of the pairwise iterations of TRI-MP reliably prolonged VCA survival, suggesting that all 3 factors were important for adequate Treg induction and establishing graft tolerance. These findings are consistent with results from prior studies using TRI-MP in vitro and in other animal models of inflammation (36–38).
Local activity of TRI-MP was confirmed by the fact that administration of TRI-MP to contralateral (nontransplanted) limbs failed to consistently promote long-term allograft tolerance (Fig. 1B). This finding is consistent with prior studies showing that TRI-MP does not promote allergen-specific Treg differentiation when administered at sites distal to allergen exposure (37). The lack of systemic effects also makes sense, since proteins released from a s.c. microparticle depot travel through the interstitium and can enter lymphatic vessels that lead to draining lymph nodes, but cannot enter the less permeable blood capillaries that lead to systemic circulation (41). Interestingly, contralateral administration of TRI-MP did appear to delay allograft rejection by 10–14 d relative to controls. This may be attributed to local induction of Tregs in the contralateral limb (in the absence of alloantigen), as Tregs induced distally could circulate to the allograft but would be less effective at suppressing rejection due to a lack of alloreactivity. According to the US Food and Drug Administration, doses for locally administered therapeutics, especially those with minimal subsequent systemic distribution, should be scaled based on concentrations at the site of administration. Therefore, we expect TRI-MP doses to be scaled based on allograft volume, and planned studies with TRI-MP in a porcine VCA model will help to validate this dose-scaling approach.
We hypothesized that local induction of FoxP3+ Tregs was the primary mechanism by which TRI-MP induced VCA tolerance. In support of this hypothesis, the frequency of CD4+ FoxP3+ Tregs in the DLNs of TRI-MP–treated grafts was significantly higher than in the DLNs of rejecting grafts or naïve limbs (Fig. 4A). In addition, there were significantly fewer CD4+ IFN-γ+ Th1 effector T cells in the DLNs of TRI-MP–treated graft recipients compared to those from rejecting graft recipients (Fig. 4B). TRI-MP also reduced cutaneous expression of inflammatory mediators (TNF-α, serglycin, perforin-1, and IL-17A) that were elevated in rejecting allografts to levels observed in naïve skin (Fig. 3). Traditionally regarded as proinflammatory, IFN-γ also reportedly promotes survival and function of alloantigen-specific Tregs in the context of transplantation (42, 43), so elevated IFN-γ expression in TRI-MP–treated surviving allografts (Fig. 3) may support tolerance. Furthermore, while rejecting allografts had greater FoxP3 expression (consistent with increased Treg numbers at sites of inflammation [37, 44]), TRI-MP–treated allografts had higher levels of Treg-associated suppressive cytokines, including IL-10, TGF-β1, and IL-35 (measured by EBI3 expression) (Fig. 3). Collectively, these results suggest that tolerance may be supported by fewer but more functionally suppressive Tregs present in TRI-MP–treated surviving allografts.
To explore possible mechanisms underlying allograft tolerance induced by TRI-MP, we assessed the function of Tregs and Tconvs isolated from naïve and TRI-MP–treated animals. The ability of Tconvs to respond to donor stimulation was not affected by TRI-MP treatment, as Tconvs from TRI-MP–treated allograft recipients and naïve rats proliferated comparably in response to stimulation with BN splenocytes (Fig. 5A). This suggests that the allograft tolerance in TRI-MP–treated rats was a result not of clonal deletion or anergy but rather of Treg-mediated suppression. Indeed, Tregs isolated from TRI-MP–treated recipients more than 300 d after transplants suppressed donor-induced proliferation of Tconvs to a greater extent than Tregs from naïve animals (Fig. 5B), a finding consistent with long-term functional stability. In future translational studies, stability of TRI-MP–induced Tregs may be confirmed at earlier timepoints by DNA methylation analysis (bisulfite pyrosequencing), as demethylation of the FOXP3 gene is indicative of stable FoxP3 expression and suppressive function (45). Tregs from TRI-MP–treated animals also better suppressed proliferation of Tconvs induced by donor splenocytes compared to third-party splenocytes, demonstrating Treg donor specificity. Finally, alloantigen-specific tolerance was confirmed in vivo, as TRI-MP–treated animals with long-term surviving grafts accepted nonvascularized skin grafts from BN donors but failed to accept grafts from third-party donors. Importantly, skin graft recipients had received no systemic IS for >300 d, consistent with persistent donor-specific tolerance.
In summary, this study demonstrates that a cell-sized, biodegradable, Treg-inducing microparticle formulation can prolong rodent hindlimb VCA survival indefinitely in the absence of long-term, systemic IS. Furthermore, our data suggest that such tolerance is specific to donor antigens. These results warrant further efforts to validate the efficacy of TRI-MP for resolution of inflammation and restoration of homeostasis in other chronic immune-mediated pathologies, including autoimmune disorders like rheumatoid arthritis, psoriasis, lupus, and inflammatory bowel disease.
Materials and Methods
More detailed descriptions of the experimental procedures are provided in the SI Appendix.
Hindlimb Transplantation and Skin Graft Challenge.
Lewis (LEW; RT1l), Brown Norway (BN; R71h), and Wistar Furth (WF; RT1u) rats were used for studies with Institutional Animal Care and Use Committee approval at the University of Pittsburgh. Hindlimbs from donor BN rats were transplanted to LEW recipients, and allografts were monitored daily for visible signs of progressive grade III rejection (46). Skin from donor strain (BN) or third-party strain (WF) rats was grafted onto surviving rats >300 d after hindlimb VCA. Skin grafts were monitored daily, and rejection was evidenced by necrosis and wound contracture.
TRI-MP Fabrication.
TRI-MP was prepared as previously reported (36), except with different loading. MP were fabricated using 10 μg rmIL-2, 2 μg rhTGF-β1, or 2 mg rapamycin per 200 mg PLGA (RG502H) polymer.
Study Design and Groups.
All hindlimb recipients received the same baseline IS protocol: FK506 (0.5 mg/kg/d i.p., postoperative days (POD) = 0–21) plus rabbit antirat lymphocyte serum (0.5 mL i.p., POD = −4 and 1). MP or soluble TRI factors were injected s.c. in the lateral aspect of the transplanted limb unless otherwise noted (POD = 0 and 21, 3 mg each MP formulation, 10 mg/mL in phosphate buffered saline). Some rats received single MP formulations, 2 of 3 MP, TRI-MP in contralateral (nontransplanted) limbs, or Blank-MP.
Tissue and Cellular Analyses.
Skin and muscle samples were H&E-stained. Total RNA was extracted from skin and lymph nodes for analysis by qRT-PCR. Cells from lymph nodes were stained for T cell markers and analyzed by flow cytometry. Tconvs and Tregs were sorted from spleens of rats with long-term surviving allografts or naïve LEW rats, and their function was evaluated by allogeneic mixed leukocyte reactions and Treg suppression assays.
Statistics.
Data are presented as mean ± SD. Significant differences (P < 0.05) were determined by 2-tailed Student t test, Welch’s t test, Mann–Whitney U test, 1-sample t test, or log-rank test, as appropriate.
Data Availability.
All data are available in the manuscript and the SI Appendix.
Supplementary Material
Acknowledgments
We thank Aarika MacIntyre and Dewayne Falkner for assistance with FACS. Histological services were provided by the McGowan Institute for Regenerative Medicine Histology Core. Flow cytometry and FACS were conducted at the University of Pittsburgh’s Unified Flow Core and benefited from a special BD LSRFortessa analyzer funded by the NIH (1-S10-OD011925-01). This research was funded by the NIH National Institute of Allergy and Infectious Diseases (NIAID) (R01-AI118777 and U19-AI131453 to A.W.T.; R01-HL122489 to H.R.T.), NIH National Institute of Dental and Craniofacial Research (R01-DE021058 to S.R.L.), Department of Defense Congressionally Directed Medical Research Programs (W81XWH-15-2-0027 to A.W.T.; W81XWH-15-1-0244 to S.R.L. and V.S.G.), and the Camille & Henry Dreyfus Foundation (to S.R.L.). J.D.F. is supported by a fellowship from the NIH NIAID (T32-AI074490), and S.C.B. is supported by a fellowship from the NIH National Cancer Institute (T32-CA175294).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1910701116/-/DCSupplemental.
References
- 1.Petruzzo P., et al. , The International Registry on Hand and Composite Tissue Transplantation. Transplantation 90, 1590–1594 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Tobin G. R., et al. , The history of human composite tissue allotransplantation. Transplant. Proc. 41, 466–471 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Lee W. P., et al. , Relative antigenicity of components of a vascularized limb allograft. Plast. Reconstr. Surg. 87, 401–411 (1991). [DOI] [PubMed] [Google Scholar]
- 4.Chadha R., Leonard D. A., Kurtz J. M., Cetrulo C. L. Jr, The unique immunobiology of the skin: Implications for tolerance of vascularized composite allografts. Curr. Opin. Organ Transplant. 19, 566–572 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Eun S. C., Composite tissue allotransplantation immunology. Arch. Plast. Surg. 40, 141–153 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leonard D. A., Kurtz J. M., Cetrulo C. L. Jr, Vascularized composite allotransplantation: Towards tolerance and the importance of skin-specific immunobiology. Curr. Opin. Organ Transplant. 18, 645–651 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Morelon E., Kanitakis J., Petruzzo P., Immunological issues in clinical composite tissue allotransplantation: Where do we stand today? Transplantation 93, 855–859 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Schnider J. T., et al. , Site-specific immunosuppression in vascularized composite allotransplantation: Prospects and potential. Clin. Dev. Immunol. 2013, 495212 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J. H., Eun S. C., Therapeutic application of T regulatory cells in composite tissue allotransplantation. J. Transl. Med. 15, 218 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gajanayake T., et al. , A single localized dose of enzyme-responsive hydrogel improves long-term survival of a vascularized composite allograft. Sci. Transl. Med. 6, 249ra110 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Jindal R., et al. , Spontaneous resolution of acute rejection and tolerance induction with IL-2 fusion protein in vascularized composite allotransplantation. Am. J. Transplant. 15, 1231–1240 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Unadkat J. V., et al. , Single implantable FK506 disk prevents rejection in vascularized composite allotransplantation. Plast. Reconstr. Surg. 139, 403e–414e (2017). [DOI] [PubMed] [Google Scholar]
- 13.Bilate A. M., Lafaille J. J., Induced CD4+ Foxp3+ regulatory T cells in immune tolerance. Annu. Rev. Immunol. 30, 733–758 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Riley J. L., June C. H., Blazar B. R., Human T regulatory cell therapy: Take a billion or so and call me in the morning. Immunity 30, 656–665 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wood K. J., Bushell A., Hester J., Regulatory immune cells in transplantation. Nat. Rev. Immunol. 12, 417–430 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Kingsley C. I., Karim M., Bushell A. R., Wood K. J., CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J. Immunol. 168, 1080–1086 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Raimondi G., et al. , Mammalian target of rapamycin inhibition and alloantigen-specific regulatory T cells synergize to promote long-term graft survival in immunocompetent recipients. J. Immunol. 184, 624–636 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Safinia N., Sagoo P., Lechler R., Lombardi G., Adoptive regulatory T cell therapy: Challenges in clinical transplantation. Curr. Opin. Organ Transplant. 15, 427–434 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Tang Q., Lee K., Regulatory T-cell therapy for transplantation: How many cells do we need? Curr. Opin. Organ Transplant. 17, 349–354 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Trzonkowski P., et al. , First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127− T regulatory cells. Clin. Immunol. 133, 22–26 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Brunstein C. G., et al. , Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: Safety profile and detection kinetics. Blood 117, 1061–1070 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbi J., Pardoll D., Pan F., Treg functional stability and its responsiveness to the microenvironment. Immunol. Rev. 259, 115–139 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou X., et al. , Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 10, 1000–1007 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Overacre A. E., Vignali D. A., T(reg) stability: To be or not to be. Curr. Opin. Immunol. 39, 39–43 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glowacki A. J., et al. , Prevention of inflammation-mediated bone loss in murine and canine periodontal disease via recruitment of regulatory lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 110, 18525–18530 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jhunjhunwala S., et al. , Bioinspired controlled release of CCL22 recruits regulatory T cells in vivo. Adv. Mater. 24, 4735–4738 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ratay M. L., et al. , Treg-recruiting microspheres prevent inflammation in a murine model of dry eye disease. J. Control. Release 258, 208–217 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curiel T. J., et al. , Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 10, 942–949 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Montane J., et al. , Prevention of murine autoimmune diabetes by CCL22-mediated Treg recruitment to the pancreatic islets. J. Clin. Invest. 121, 3024–3028 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Battaglia M., et al. , Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate antigen-specific transplantation tolerance. Diabetes 55, 40–49 (2006). [PubMed] [Google Scholar]
- 31.Yadav M., Stephan S., Bluestone J. A., Peripherally induced tregs—role in immune homeostasis and autoimmunity. Front. Immunol. 4, 232 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whiteside T. L., Schuler P., Schilling B., Induced and natural regulatory T cells in human cancer. Expert Opin. Biol. Ther. 12, 1383–1397 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whiteside T. L., Induced regulatory T cells in inhibitory microenvironments created by cancer. Expert Opin. Biol. Ther. 14, 1411–1425 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horwitz D. A., Zheng S. G., Wang J., Gray J. D., Critical role of IL-2 and TGF-beta in generation, function and stabilization of Foxp3+CD4+ Treg. Eur. J. Immunol. 38, 912–915 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Kopf H., de la Rosa G. M., Howard O. M., Chen X., Rapamycin inhibits differentiation of Th17 cells and promotes generation of FoxP3+ T regulatory cells. Int. Immunopharmacol. 7, 1819–1824 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jhunjhunwala S., et al. , Controlled release formulations of IL-2, TGF-β1 and rapamycin for the induction of regulatory T cells. J. Control. Release 159, 78–84 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balmert S. C., et al. , In vivo induction of regulatory T cells promotes allergen tolerance and suppresses allergic contact dermatitis. J. Control. Release 261, 223–233 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ratay M. L., et al. , TRI microspheres prevent key signs of dry eye disease in a murine, inflammatory model. Sci. Rep. 7, 17527 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sachs D. H., Tolerance: Of mice and men. J. Clin. Invest. 111, 1819–1821 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomson A. W., Turnquist H. R., Raimondi G., Immunoregulatory functions of mTOR inhibition. Nat. Rev. Immunol. 9, 324–337 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richter W. F., Jacobsen B., Subcutaneous absorption of biotherapeutics: Knowns and unknowns. Drug Metab. Dispos. 42, 1881–1889 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Nomura M., et al. , Cytokines affecting CD4+ T regulatory cells in transplant tolerance. II. Interferon gamma (IFN-γ) promotes survival of alloantigen-specific CD4+ T regulatory cells. Transpl. Immunol. 42, 24–33 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Hill M., et al. , Cell therapy with autologous tolerogenic dendritic cells induces allograft tolerance through interferon-gamma and Epstein-Barr virus-induced gene 3. Am. J. Transplant. 11, 2036–2045 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Chauhan S. K., Saban D. R., Lee H. K., Dana R., Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J. Immunol. 182, 148–153 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Floess S., et al. , Epigenetic control of the Foxp3 locus in regulatory T cells. PLoS Biol. 5, e38 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Unadkat J. V., et al. , Composite tissue vasculopathy and degeneration following multiple episodes of acute rejection in reconstructive transplantation. Am. J. Transplant. 10, 251–261 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the manuscript and the SI Appendix.