Significance
Leaves display constant and complex movements. We found that the petioles of undamaged leaves of Arabidopsis thaliana undergo minute deformations when insects fed on other leaves. These deformations reported in real time the arrival and architecture of damage-triggered electrical signals called slow wave potentials. Mutants affecting cell walls in xylem vessels altered these petiole deformations, suggesting that water fluxes coincide with the passage of the electrical signals through tissues. Further analyses revealed that whole leaves distal to wounds displayed slow downward motions following the arrival of electrical signals. Monitoring insect-triggered tissue deformations in different genetic backgrounds can yield mechanistic insights into electrical signalling and may increase our understanding of the basis of plant movement.
Keywords: jasmonate, slow wave potential, wound, xylem, pressure
Abstract
Slow wave potentials (SWPs) are damage-induced electrical signals which, based on experiments in which organs are burned, have been linked to rapid increases in leaf or stem thickness. The possibility that pressure surges in injured xylem underlie these events has been evoked frequently. We sought evidence for insect feeding-induced positive pressure changes in the petioles of Arabidopsis thaliana. Instead, we found that petiole surfaces of leaves distal to insect-feeding sites subsided. We also found that insect damage induced longer-duration downward leaf movements in undamaged leaves. The transient petiole deformations were contemporary with and dependent on the SWP. We then investigated if mutants that affect the xylem, which has been implicated in SWP transmission, might modify SWP architecture. irregular xylem mutants strongly affected SWP velocity and kinetics and, in parallel, restructured insect damage-induced petiole deformations. Together, with force change measurements on the primary vein, the results suggest that extravascular water fluxes accompany the SWP. Moreover, petiole deformations in Arabidopsis mimic parts of the spectacular distal leaf collapse phase seen in wounded Mimosa pudica. We genetically link electrical signals to organ movement and deformation and suggest an evolutionary origin of the large leaf movements seen in wounded Mimosa.
Over a century ago, J. C. Bose elegantly highlighted the fact that many plant movements “escape our scrutiny” (1). Bose’s text refers in part to slow, tropic movements now known to depend on hormone-controlled differential tissue growth (2). However, a large part of Bose’s 1906 book concerned more rapid movements of a different nature. Bose focused much attention on the leaf motions of the sensitive plant Mimosa pudica and on their relationships to electrical activities in leaves. M. pudica displays a variety of different movements when touched or wounded. When touched gently, leaflets on the stimulated leaf fold upwards. These movements and those of the traps of certain carnivorous plants are associated with action potentials (3, 4) and are generally restricted to the stimulated organ. However, wounding M. pudica can trigger spectacular, turgor-driven movements in leaves distal to wounds, whereby this plant is thought to become less visible to large herbivores (5). These latter interorgan movements correlate with an electrical signal that is distinct from the action potential and which was termed the slow wave potential (SWP, otherwise termed the variation potential). First recognized as distinct signals in M. pudica (6), SWPs are widespread in plants that appear not to display obvious leaf movements when damaged (7). These species include Arabidopsis thaliana (8, 9).
Interested in generalities across the plant kingdom, Bose compared M. pudica with plants that apparently lacked rapid movements. Using strong thermal stimuli, Bose indeed detected heat-induced movement in such plants (1). The use of heating and, in particular, burning has been perhaps the most common way of triggering SWPs. Using heat as a stimulus, increases in the thickness of leaves or stems or increases in xylem pressure have been detected repeatedly in numerous plants (10–13). These burn-induced increases in organ thickness have been attributed to the rapid release of xylem tension distal to damage sites, and it has been proposed that increases in xylem pressure might activate SWP production (11, 13). Alternatively, chemical elicitors of membrane depolarization might be sucked into and along the xylem to activate electrical signaling in response to wounding (e.g., refs. 14 and 15). Here we ask whether wound-response tissue deformation occurs in Arabidopsis and, if so, whether this is likely to reflect rapid increases in xylem pressure that might trigger the SWP.
In Arabidopsis, the propagation of SWPs depends on several clade 3 GLUTAMATE RECEPTOR-LIKE (GLR) proteins (8). Leaf-to-leaf electrical signals are abolished in the glr3.3 glr3.6 double-mutant, and recent analyses showed that GLR3.3 localized to phloem sieve elements, whereas GLR3.6 localized to xylem contact cells. Therefore, 2 nonadjacent cell populations, xylem and phloem, are necessary for leaf-to-leaf SWP transmission (9). Motivated by the possibility that xylem-associated tissue pressure changes might be detectable in wounded Arabidopsis and might provide insights into SWP mechanisms, we monitored the response of this plant to burning and then, in similar experiments, to insect feeding. To do this, and to obtain quantitative outputs, we employed sensitive force probes applied to the adaxial epidermis of leaf petioles.
The experimental designs used herein are based on the fact that wounding individual leaves can initiate electrical signaling in distal leaves that share direct vascular connections with the wounded leaf. For experiments, leaves were labeled in terms of their age, with leaf 1 being the first true leaf produced. When, for example, leaf 8 on a 5-wk-old rosette is wounded, electrical signals are detected on leaves 11, 13, and 16, but not on leaf 9 (8). In most experiments conducted herein, leaf 8 was wounded, and events (electrical signals and petiole surface force changes) on leaf 13 were recorded. Based on our initial results we then extended the work to studies of mutants in SWP signaling and xylem integrity. Finally, we used video microscopy of rosettes to investigate overall leaf movement in response to feeding insects.
Results and Discussion
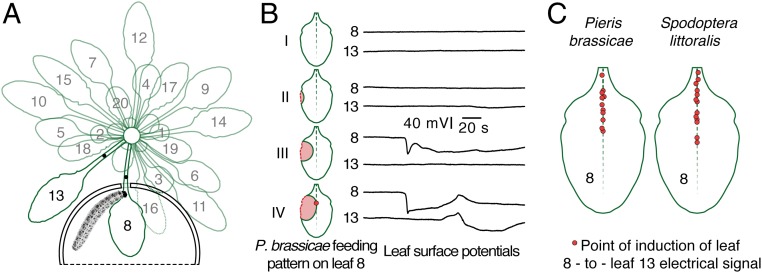
The areas of Arabidopsis leaves that need to be damaged by feeding insects in order to trigger SWPs in distal leaves were mapped. Pieris brassicae larvae were constrained to leaf 8, surface electrodes were placed on leaves 8 and 13 (Fig. 1A), and feeding behavior and electrical activity were monitored simultaneously (Movie S1). SWPs in leaf 13 were observed when insects severed the midvein (Fig. 1B). This occurred rapidly within a single feeding sweep, suggesting that both the xylem and phloem are severed during the activation of SWP propagation. The primary vein in the region of the leaf base is the region in which signals causing leaf-to-leaf SWPs are generated by feeding P. brassicae. Similar behavior was seen with an unrelated insect, Spodoptera littoralis (Fig. 1C).
Fig. 1.
Insect damage to the basipetal leaf midvein triggers leaf-to-leaf electrical signals. (A) Leaves are numbered from oldest (1) to youngest. P. brassicae larva feed in a chamber surrounding leaf 8. A surface electrode (black dot) on leaf 8 is placed 2–3 mm from the edge of the chamber. A second electrode is placed on the petiole of leaf 13. (B) Extent of P. brassicae damage on leaf 8 (red-shaded area on the scheme of leaf 8) and corresponding electrical signals in leaves 8 and 13. One representative set of traces is presented of 4 plants tested for each feeding pattern. (C) Leaf 8, indicating points of induction of surface potentials (SPs; red circles) in leaf 13 during feeding of P. brassicae and S. littoralis (see Movie S1 as an example).
Quantitative force probes (SI Appendix, Fig. S1 A and B) were used to investigate possible tissue deformations in Arabidopsis due to leaf damage. Leaf 13 was first immobilized, and a force sensor was placed on the petiole near a surface electrode. In the absence of any stimulus, force on the probe increased at 20 ± 27 µN per minute (SI Appendix, Fig. S1C). These force increases were expected due to growth and diel hyponasty (16). Smaller force changes of ∼5 μN were also detectable on individual force traces (SI Appendix, Fig. S1C) but not on averaged traces (SI Appendix, Fig. S1D). Earlier work using flame wounding reported either leaf deformation (14) or increases in the thickness of leaves (10). Previous studies showed that when caterpillars damaged tomato leaves they failed to elicit changes in leaf thickness unless the plants were almost completely defoliated prior to experiments (17). In our case, we decided against partial defoliation of Arabidopsis and kept the plants intact prior to wounding. We first used a laser to damage leaf 8 within the region that we had identified as necessary for insect-induced SWPs (SI Appendix, Fig. S2A). After initiation of a 10-s laser pulse on leaf 8, the force monitored on leaf 13 decreased abruptly during the first 3–9 s of stimulation and thereafter increased, reaching a maximum about 20 s later. The depolarization phase of the SWP occurred during the rapid force increase on the probe. After reaching a maximum about 10 s after lasing, a decrease in force on the probe was apparent (SI Appendix, Fig. S2 B and C). Our findings were therefore broadly comparable to previous data from burning leaves (7). We expected similar results when plants were damaged by insects.
Insect Feeding-Induced Petiole Surface Deformation
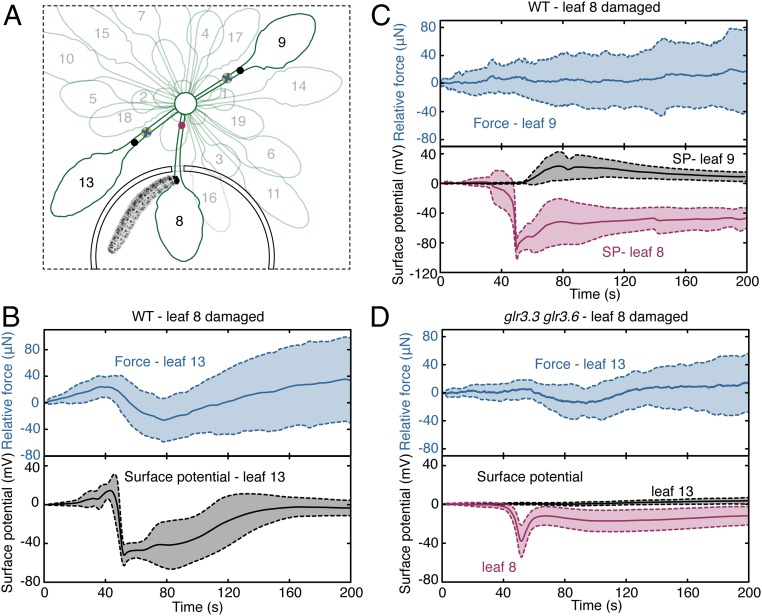
To test whether P. brassicae feeding on leaf 8 caused deformations on distal leaf 13, a force sensor and an electrode were placed on this leaf on WT plants (Fig. 2A). When P. brassicae severed the leaf 8 petiole, force decreases and SWPs appeared at leaf 13 (Movie S2). That is, while insect-induced SWPs on leaf 13 had similar structures to those elicited by the laser, lasing elicits tissue motions of an opposite vector to those generated in response to feeding herbivores. We speculate that this difference may be due to heat-driven water expansion or phase change during lasing. Averaging both outputs after insect damage revealed that the decrease of force monitored on leaf 13 started coincidently with the electrical signal (Fig. 2B). Regression analysis indicated that depolarization duration and latency to the electrical depolarization minimum correlated with force changes (SI Appendix, Fig. S3). When leaf 8 was wounded we detected no significant force changes on leaf 9, a leaf that does not display SWPs when leaf 8 was wounded (8) (Fig. 2C).
Fig. 2.
Insect-induced electrical signals correlate with petiole deformations. (A) P. brassicae larva in chamber containing leaf 8. A surface electrode was placed on leaf 8 (red dot) petiole. The microforce-sensing probe (blue cross) and surface electrode (black dot) on leaf 13 or leaf 9 were placed at an interval of 3 mm. Force measurements and surface-potential recordings were made at 33 Hz, i.e., 6,600 measurements in the 200-s recording period. (B) P. brassicae-induced mean force changes and associated SPs (solid lines) from leaf 13 of the WT. (C) Mean force traces from leaf 9 (blue solid line) of the WT during P. brassicae feeding on leaf 8. Mean SPs from leaf 8 (red solid line) and hyperpolarizations from leaf 9 (black solid lines). (D) P. brassicae-induced force changes on leaf 13 (blue solid line) of the glr3.3 glr3.6 double-mutant. Surface-potential traces from leaf 8 (red solid line) and leaf 13 (black solid lines) in the double-mutant. For glr3.3 glr3.6, SP depolarization minima on leaf 8 were used for point-to-point averaging of force changes in leaf 13. SD envelopes (shaded area delimited by dashed lines) were from 9 independent pairs of measurements.
To further examine the relationship of P. brassicae-induced force decreases and electrical activity, measurements were made on the glutamate receptor-like (glr) glr3.3 glr3.6 double-mutant that cannot propagate SWPs to distal leaf 13 (8). When electrical activity and force changes in response to insect damage to leaf 8 were monitored on leaf 13 (Fig. 2D and SI Appendix, Fig. S4) the averaged amplitude of force changes in leaf 13 of glr3.3 glr3.6 mutants was 40 ± 20 μN compared to 70 ± 31 in the WT. That is, the major force changes detectable on the surface of leaves distal to insect damage sites were GLR-dependent. Quantitative time-lapse microscopy was used to verify these results. The precision of the method provided reliable detection of height changes greater than 2 μm (SI Appendix, Fig. S5 A and B). Using the experimental design in SI Appendix, Fig. S5C a petiole surface area of 2.1 mm2 was monitored during distal insect feeding (SI Appendix, Fig. S5D). In the undamaged WT, changes in leaf 13 petiole surface elevation were below the precision of the method (SI Appendix, Fig. S6A). When insects fed on leaf 8 of the WT, decreases in leaf 13 petiole elevation were detected (SI Appendix, Fig. S6B). Importantly, similar deformations were undetectable in both undamaged or insect-damaged glr3.3 glr3.6 (SI Appendix, Fig. S6 C and D). Further analyses confirmed that the rate of elevation decrease on petiole 13 was greater in the insect-damaged WT than in glr3.3 glr3.6 (SI Appendix, Fig. S6 E and F). SI Appendix, Fig. S6G shows that the relative petiole surface elevation on leaf 13 decreased by an average of 2.4 µm as a consequence of insect feeding on leaf 8 in the WT.
Force Measurements on Exposed Veins
Surgically exposed midveins can conduct leaf-to-leaf SWPs (9). Microgripper force sensors were used to investigate possible distal vein deformation in response to insect feeding (SI Appendix, Fig. S7A). These experiments necessitated working on midveins thinner than those in leaf 8. Therefore, we wounded leaf 11 and chose leaf 6 as the distal target leaf (SI Appendix, Fig. S7B). No vein deformations were detected in the unwounded WT plant (SI Appendix, Fig. S7C). Insect damage to leaf 11 caused positive force changes in the WT (SI Appendix, Fig. S7D) but not in glr3.3 glr3.6 (SI Appendix, Fig. S7E). Data averages from leaf 6 of undamaged plants (SI Appendix, Fig. S8A) and P. brassicae-damaged plants (SI Appendix, Fig. S8B) revealed force change rates of ∼0.14 µN per min in the exposed midvein of insect-damaged plants. These rates were reduced nearly 3-fold in glr3.3 glr3.6 (SI Appendix, Fig. S8C). The vein therefore acts in an opposing manner to extravascular tissues, and GLR-dependent positive pressure in the WT vein would be expected to be exerted on extravascular tissues in response to wounding. Petiole surface deformations occurred in synchrony with SWPs. We assume that these deformations depend on water fluxes associated with membrane depolarization and repolarization during the SWP. If so, the largest of these water fluxes might occur outside the vasculature.
Xylem Integrity Mutants Restructure SWPs and Alter Tissue Deformations
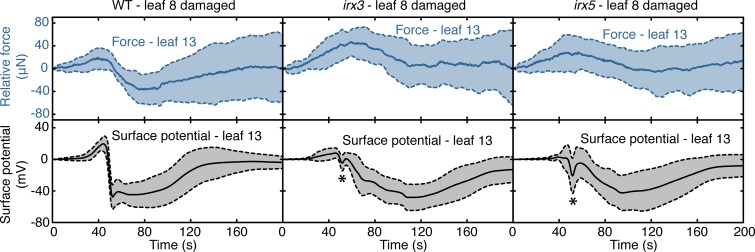
Pressure changes in the xylem have been implicated consistently in SWP initiation (10–13). Two irregular xylem (irx) mutants (18–20) were used to test whether vessel wall modification might affect electrical signaling, thereby affecting damage-stimulated leaf deformations. The mutants used, irx3 and irx5 (SI Appendix, Fig. S9A), affect secondary cell wall properties through mutations in cellulose synthase subunits CesA7 and CesA4, respectively (21). Transmission electron microscopy of midvein sections revealed that while the WT and glr3.3 had similar vessel structures, those in irx5 and irx5 glr3.3 were deformed (SI Appendix, Fig. S9 B and C). In the WT, vessel-apposed walls of the contact cell bulged slightly into the vessel. This did not appear to be the case in irx5 (SI Appendix, Fig. S9C). Monitoring of wound-stimulated electrical activity and surface force changes in the irx mutants revealed that SWP architecture was strongly altered relative to the WT. For example, depolarization spikes (marked with asterisks in Fig. 3 and SI Appendix, Fig. S10A) were detected prior to the main depolarization phase in irx3 and irx5. Similar SWP-associated spike signals have been reported in other species (3, 22). In irx3 and irx5, the slope of the principal depolarization was reduced to ∼5 mV per second relative to ∼22 mV per second in the WT. In parallel, the rates of force changes monitored on the surface of leaf 13 were attenuated in the irx mutants relative to the WT (Fig. 3). Summarizing, petiole tissue deformations correlated with electrical signals and quantitative analyses revealed that both were affected by extracellular matrix properties that were detectable in the xylem (SI Appendix, Fig. S10 B and C). We did not investigate whether the irx mutants also affected phloem cell wall properties, and this remains a possibility.
Fig. 3.
Xylem morphology mutants affect electrical signals and deformation kinetics in leaf 13. Comparison of force changes and associated SPs (solid lines) for WT, irx3, and irx5 mutants. Experimental setup as in Fig. 2A. P. brassicae larvae were allowed to feed on leaf 8, and force changes and surface potential were measured on leaf 13. Both parameters were measured at 33 Hz. Individual traces were averaged according to the first SP depolarization minimum. For irx mutants, mean (solid lines) and SD envelopes (shaded area limited by dashed lines) were from at least 11 independent pairs of measurements. Asterisks indicate depolarization spikes in irx mutants.
Epistasis studies were then performed with irx and glr mutants (SI Appendix, Fig. S11A). In irx5, irx5 glr3.3, and irx5 glr3.6 the SWP apparent velocity was reduced relative to the WT and to the glr single-mutants. Strikingly, the ∼7 cm per minute apparent velocity of the SWP in the WT was reduced to 1 cm per minute in irx5 (SI Appendix, Fig. S11B). Both irx5 glr double-mutants reduced the electrical signal amplitude to a greater extent than did the single-mutants (SI Appendix, Fig. S11C), whereas combining irx5 with glr3.3 or glr3.6 did not alter signal duration relative to that seen in the single-mutants (SI Appendix, Fig. S11D). A feature of plants harboring the irx5 allele was a reduced slope of the SWP depolarization phase relative to rapid depolarization in the WT (SI Appendix, Fig. S11E).
A primary role of the SWP in Arabidopsis is to activate the jasmonate pathway in leaves distal to wounds (8, 9). Although clade 3 glr double-mutants eliminate the SWP, it is not known whether less severe restructuring of the SWP using alternative mutational approaches would affect jasmonate pathway activation. To test this, the JAZ10 marker gene (23) was used to monitor jasmonate signaling activity in leaf 13 after insect feeding on leaf 8. All mutants affected insect-induced JAZ10 levels (SI Appendix, Fig. S11F) but not JAZ10 levels in undamaged plants (SI Appendix, Fig. S11G). Even though the irx5 glr3.3 and irx5 glr3.6 strongly attenuated distal SWPs they did not completely suppress JAZ10 induction following wounding. Together with previous work, these results reveal features of the SWP that might determine JA pathway activation. The irx mutants used herein reduced the initial membrane depolarization rate as well as the overall velocity of the SWP, but relatively high JAZ10 transcript inductions were still observed. Therefore, depolarization slope and SWP velocity do not appear to control jasmonate pathway induction. Recent work on proton pump mutants (24) suggests that the duration of the repolarization phase is one of the factors determining the extent of SWP-activated jasmonate signaling.
Mechanism of SWP Propagation
Two predominant hypotheses have emerged for the role of the xylem in the SWP. First, fast, injury-associated increases in intravessel fluid pressures might trigger membrane depolarization (e.g., refs. 10–13). Although we cannot exclude the possibility that intrinsic vessel deformability (25, 26) might attenuate xylem pressure changes in the WT, we did not detect rapid (millisecond) wound-response force changes in the exposed primary vasculature of these plants. Therefore, our results do not fit models in which rapid, wound-response changes in xylem water pressure would propagate at high speeds to activate electrical events in Arabidopsis (10, 11, 13). A second hypothesis for SWP propagation is that xylem vessels act as conduits to allow chemical elicitors produced at wound sites to be carried rapidly to distal sites (e.g., ref. 15). Our results are compatible with this hypothesis, since a loss of vessel integrity as seen in irx mutants might reduce leaf-to-leaf elicitor transport. However, we do not rule out additional roles of the xylem, for example, in water potential or cell/tissue pressure maintenance which might affect electrical signaling. We note that the primary pulvini of M. pudica contain a highly specialized xylem that is thought to have high hydraulic conductance and which is flexible, allowing pulvinar bending during the SWP (27).
Parallels with Mimosa pudica
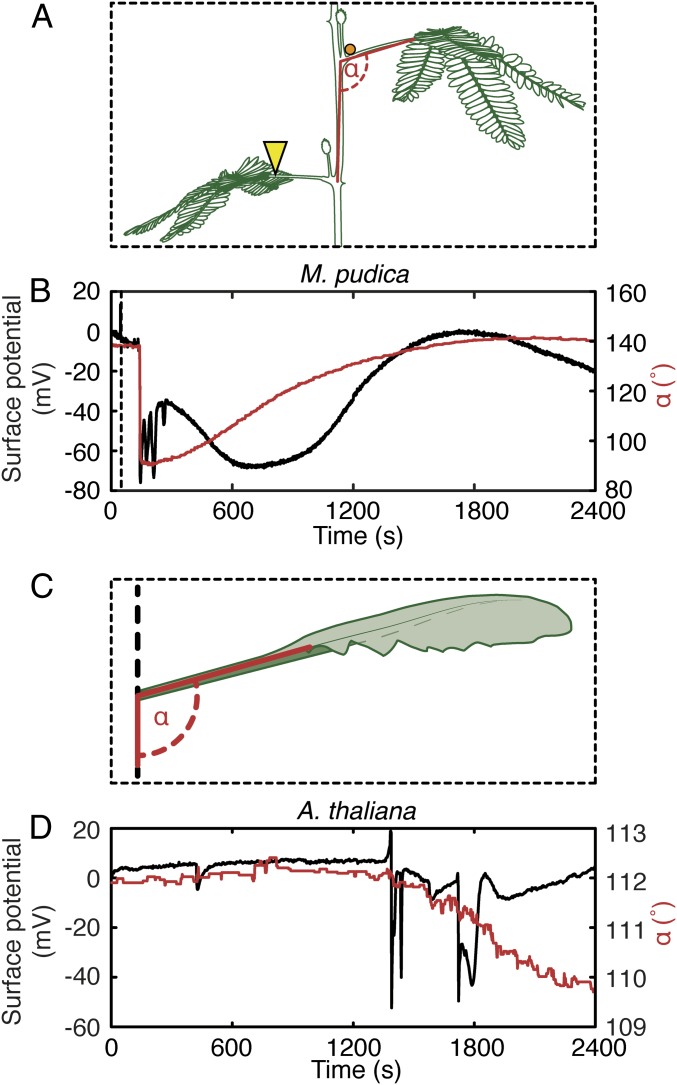
One cannot fail to notice an overall similarity between the architecture of SWPs in A. thaliana and damage-induced distal petiole movements in M. pudica. In wounded M. pudica, both the collapse of membrane potential and the collapse of distal primary pulvini occur rapidly, while membrane potentials and the restoration of petiole angle relative to the stem recover slowly (28). We monitored damage-induced M. pudica leaf movement and SWPs simultaneously (Fig. 4 A and B and Movie S3), noting that collapsed leaves began to regain their original elevations in less than 10 min after wounding a distal leaf. We next looked for free-leaf motions in insect-damaged wild-type A. thaliana. To do this, leaves distal to wounds were video-monitored. Unlike in experiments with force probes in which a leaf distal to a wound was immobilized, this allowed whole-leaf movement. Slow, downward motions lasting at least 15 min were detected in Arabidopsis leaves distal to feeding insects (Fig. 4 C and D, SI Appendix, Fig. S12 A and B, and Movie S4). These movements were observed in the WT but appeared to be reduced in glr3.3 glr3.6 (SI Appendix, Fig. S12C). Distal leaves of the WT that share direct vascular connections with leaves on which insects are feeding accurately report this damage. Indeed, inspection of Movie S4 shows that each electrical signal reaching leaf 13 causes movement in this leaf: the longer the membrane depolarization event, the clearer the downward leaf movement. However, the Arabidopsis leaves did not begin to regain their original elevations 15 min after beginning to collapse. That is, we find a stronger parallel between petiole tissue deformation in Arabidopsis (Fig. 2B) and whole-leaf movement in Mimosa (Fig. 4B) than in whole-leaf movements in the 2 plants (Fig. 4 B and D).
Fig. 4.
Similarity of wound-induced surface-potential changes and leaf motions between M. pudica and A. thaliana. (A) Setup for experiments with M. pudica. A surface electrode was placed on the upper surface of distal-leaf primary pulvinus (orange dot). An ∼1-cm section of petiole was crush-wounded (yellow arrowhead). Leaf movement was quantified as the angle between lower stem segment and the petiole of the distal leaf (indicated in red). (B) A pair of individual surface potential (black) and leaf movement (red) traces obtained from M. pudica from a leaf distal to a wound. Dotted line indicates the moment of wounding. (C) Movement of A. thaliana leaf 13 was quantified as an angle (in red) between the vertical and the petiole angle. Other details of experimental design were as in Fig. 2A. (D) Insect (P. brassicae) feeding induced surface-potential changes (black) and leaf movement (red) from leaf 13 of A. thaliana.
Herbivory Alters Daily Leaf Motions
Plants are in motion as they grow and compete for light, water, or pollinators in an ever-changing environment. These daily movements include nutations, nasties, and tropisms. Tropic movements rely on differential growth (2), and the mechanisms underlying certain nastic movements (29–31) and those of some nutations (32) are emerging. As exemplified in Arabidopsis, the movements of bilateral organs such as leaves can be particularly complex. The leaves of 2- to 3-wk-old plants show diel nastic movements at petiole bases and at lamina/petiole junctions as well as nutations during periods of upward leaf movement (16). In addition, there are overlapping diel leaf growth phases that depend on the length of the photoperiod (16). Adding further complexity, when Arabidopsis leaves encounter obstacles (such as other leaves), auxin-driven hyponastic movements are accentuated (30, 31). We show that these daily leaf movements can be interrupted abruptly when feeding insects trigger slow wave potentials. As the electrical signal passes through the plant, the petiole deforms transiently over several minutes reflecting the duration of the SWP. Then, a slow downward motion of the whole leaf is detectable, possibly reflecting a cessation of growth and a commitment to defense. The petiole deformation phase (and possibly the more sustained whole-leaf movements) is likely to be driven by transmembrane water fluxes that are linked to ion currents during electrical signaling. We assume that SWP-associated water fluxes occur in extravascular tissues, since, unlike petiole surface deformation, primary vein deformation was found to be slow and did not reflect the SWP architecture.
Herbivores cause leaf vibrations as they feed. Previous work using Pieris rapae (which is closely related to P. brassicae used in our study) employed Doppler vibrometry to detect feeding-induced vibrations in Arabidopsis (33). Using this technique, the authors were able to capture rapid bursts of vibration in the attacked leaf. These vibration bursts occurred ∼4 times per second and probably correlate with rapid mandible movements (chewing). In the same study, similar, albeit highly attenuated movements were detected on a distal leaf positioned roughly opposite to the leaf under attack. As expected due to their rapid propagation, these vibrations occurred almost simultaneously with those on the attacked leaf (33). The methods we used in the present study did not detect these rapid volleys of feeding-associated vibration. Instead, we picked up petiole deformations that correspond with the SWP and whole-leaf movements that occurred after arrival of the electrical signal. We therefore detected processes that are both different to and much slower than those detected by Appel and Cocroft (33).
In summary, 2 wound-associated motions were discovered in Arabidopsis leaves. We conclude that it is the cryptic petiole deformation and not the whole-leaf movement that most closely resembles wound-induced leaf movement in the sensitive plant M. pudica. While the physical basis of plant tissue deformation is increasingly well known (34), the genetic bases of these phenomena in leaves are less understood. In this respect, it is notable that that the deformations were attenuated in glr3.3 glr3.6 mutants. Studying insect-triggered deformations and movements in plants like Arabidopsis has the potential to yield mechanistic insights into electrical signaling. The further study of electrical signal-associated tissue deformations in different mutant backgrounds (for example, cell wall integrity mutants that affect various classes of polysaccharides rather than lignification) will be informative. We note that local heat damage and insect feeding cause different tissue deformations. To trigger slow wave potentials, it will be important to use stimuli similar to those that might be encountered naturally during the life of the plant in question.
Materials and Methods
Arabidopsis used were in the Col background. Methodology for plant culture, insect assays, deformation and surface potential measurement, video recording, and electron microscopy are presented in SI Appendix, Materials and Methods.
Data and Materials Availability.
All data underlying the study are available in the paper or SI Appendix.
Supplementary Material
Acknowledgments
We thank J. Daraspe (Electron Microscopy Facility, University of Lausanne) for micrographs. E. Pesquet (Stockholm) kindly provided irx mutants. C. Fankhauser and N. Geldner (University of Lausanne) provided critical comments on the manuscript, and N. Salamin gave useful advice on statistics. This work was funded by Swiss National Science Foundation Grants 31003A-175566 and CRSII3 154438 (to E.E.F.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. G.A.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1912386116/-/DCSupplemental.
References
- 1.Bose J. C., Plant Response as a Means of Physiological Investigation (Longmans, Green and Co., London, 1906). [Google Scholar]
- 2.Harmer S. L., Brooks C. J., Growth-mediated plant movements: Hidden in plain sight. Curr. Opin. Plant Biol. 41, 89–94 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roblin G., Mimosa pudica: A model for the study of the excitability in plants. Biol. Rev. Camb. Philos. Soc. 54, 135–153 (1979). [Google Scholar]
- 4.Simons P. J., The role of electricity in plant movements. New Phytol. 87, 11–37 (1981). [Google Scholar]
- 5.Bose J. C., Nervous Mechanism of Plants (Longmans, Green and Co., London, 1926). [Google Scholar]
- 6.Houwink A. L., The conduction of excitation in Mimosa pudica. Recl. Trav. Bot. Neerl. 32, 51–91 (1935). [Google Scholar]
- 7.Stahlberg R., et al. , “Slow wave potentials—a propagating electrical signal unique to higher plants” in Communication in Plants: Neuronal Aspects of Plant Life, Baluška F., Mancuso S., Volkmann D., Eds. (Springer, Heidelberg, Germany, 2006), pp. 291–309. [Google Scholar]
- 8.Mousavi S. A., Chauvin A., Pascaud F., Kellenberger S., Farmer E. E., GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 500, 422–426 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Nguyen C. T., Kurenda A., Stolz S., Chételat A., Farmer E. E., Identification of cell populations necessary for leaf-to-leaf electrical signaling in a wounded plant. Proc. Natl. Acad. Sci. U.S.A. 115, 10178–10183 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boari F., Malone M., Wound-induced hydraulic signals: Survey of occurrence in a range of species. J. Exp. Bot. 44, 741–746 (1993). [Google Scholar]
- 11.Malone M., Stanković B., Surface potentials and hydraulic signals in wheat leaves following localized wounding by heat. Plant Cell Environ. 14, 431–436 (1991). [Google Scholar]
- 12.Stahlberg R., Cosgrove D. J., Induction and ionic basis of slow wave potentials in seedlings of Pisum sativum L. Planta 200, 416–425 (1996). [DOI] [PubMed] [Google Scholar]
- 13.Stanković B., Zawadzki T., Davies E., Characterization of the variation potential in sunflower. Plant Physiol. 115, 1083–1088 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vodeneev V., et al. , The mechanism of propagation of variation potentials in wheat leaves. J. Plant Physiol. 169, 949–954 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Evans M. J., Morris R. J., Chemical agents transported by xylem mass flow propagate variation potentials. Plant J. 91, 1029–1037 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dornbusch T., Michaud O., Xenarios I., Fankhauser C., Differentially phased leaf growth and movements in Arabidopsis depend on coordinated circadian and light regulation. Plant Cell 26, 3911–3921 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alarcon J. J., Malone M., Substantial hydraulic signals are triggered by leaf-biting insects in tomato. J. Exp. Bot. 45, 953–957 (1994). [Google Scholar]
- 18.Turner S. R., Somerville C. R., Collapsed xylem phenotype of Arabidopsis identifies mutants deficient in cellulose deposition in the secondary cell wall. Plant Cell 9, 689–701 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown D. M., Zeef L. A., Ellis J., Goodacre R., Turner S. R., Identification of novel genes in Arabidopsis involved in secondary cell wall formation using expression profiling and reverse genetics. Plant Cell 17, 2281–2295 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar M., Turner S., Protocol: A medium-throughput method for determination of cellulose content from single stem pieces of Arabidopsis thaliana. Plant Methods 11, 46 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao Z., Mohnen D., A review of xylan and lignin biosynthesis: Foundation for studying Arabidopsis irregular xylem mutants with pleiotropic phenotypes. Crit. Rev. Biochem. Mol. Biol. 49, 212–241 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Davies E., Stanković B., “Electrical signals, the cytoskeleton, and gene expression: A hypothesis on the coherence of the cellular responses to environmental insult” in Communication in Plants: Neuronal Aspects of Plant Life, Baluška F., Mancuso S., Volkmann D., Eds. (Springer, Heidelberg, Germany, 2006), pp. 309–320. [Google Scholar]
- 23.Acosta I., Farmer E. E., “Jasmonates” in The Arabidopsis Book, (American Society of Plant Biologists, 2010), p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumari A., Chételat A., Nguyen C. T., Farmer E. E., Arabidopsis H+-ATPase AHA1 controls slow wave potential duration and wound-response jasmonate pathway activation. Proc. Natl. Acad. Sci. U.S.A. 116, 20226–20231 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cochard H., Froux F., Mayr S., Coutand C., Xylem wall collapse in water-stressed pine needles. Plant Physiol. 134, 401–408 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brodribb T. J., Holbrook N. M., Water stress deforms tracheids peripheral to the leaf vein of a tropical conifer. Plant Physiol. 137, 1139–1146 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song K., Yeom E., Lee S. J., Real-time imaging of pulvinus bending in Mimosa pudica. Sci. Rep. 4, 6466 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bose J. C., Plant Autographs and Their Revelations (Longmans, Green and Co., London, 1927). [Google Scholar]
- 29.Van Zanten M., Pons T. L., Janssen J. A. M., Voesenek L. A. C. J., Peeters A. J. M., On the relevance and control of leaf angle. Crit. Rev. Plant Sci. 29, 300–316 (2010). [Google Scholar]
- 30.Michaud O., Fiorucci A. S., Xenarios I., Fankhauser C., Local auxin production underlies a spatially restricted neighbor-detection response in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 114, 7444–7449 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pantazopoulou C. K., et al. , Neighbor detection at the leaf tip adaptively regulates upward leaf movement through spatial auxin dynamics. Proc. Natl. Acad. Sci. U.S.A. 114, 7450–7455 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitazawa D., et al. , Shoot circumnutation and winding movements require gravisensing cells. Proc. Natl. Acad. Sci. U.S.A. 102, 18742–18747 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Appel H. M., Cocroft R. B., Plants respond to leaf vibrations caused by insect herbivore chewing. Oecologia 175, 1257–1266 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forterre Y., Slow, fast and furious: Understanding the physics of plant movements. J. Exp. Bot. 64, 4745–4760 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.