Significance
Bacterial toxins belonging to the family of superantigens are potent immunostimulatory antigens capable of activating T cells in a nonconventional manner. This results in an overzealous activation of immune cells and release of pathologic levels of pro-inflammatory cytokines, which underlies severe disease manifestations such as streptococcal toxic shock syndrome. Here, we provide evidence that mucosal-associated invariant T (MAIT) cells are major contributors to the overall cytokine response elicited by group A streptococci. Both streptococcal superantigens and surface-attached bacterial factors activate MAIT cells, but through different mechanisms. Furthermore, activated MAIT cells could be detected in patients during the acute phase of streptococcal toxic shock syndrome. Thus, this study identifies an actor and potential target for intervention in streptococcal toxic shock syndrome.
Keywords: superantigens, MAIT cells, cytokine, Streptococcus pyogenes
Abstract
Streptococcal toxic shock syndrome (STSS) is a rapidly progressing, life-threatening, systemic reaction to invasive infection caused by group A streptococci (GAS). GAS superantigens are key mediators of STSS through their potent activation of T cells leading to a cytokine storm and consequently vascular leakage, shock, and multiorgan failure. Mucosal-associated invariant T (MAIT) cells recognize MR1-presented antigens derived from microbial riboflavin biosynthesis and mount protective innate-like immune responses against the microbes producing such metabolites. GAS lack de novo riboflavin synthesis, and the role of MAIT cells in STSS has therefore so far been overlooked. Here we have conducted a comprehensive analysis of human MAIT cell responses to GAS, aiming to understand the contribution of MAIT cells to the pathogenesis of STSS. We show that MAIT cells are strongly activated and represent the major T cell source of IFNγ and TNF in the early stages of response to GAS. MAIT cell activation is biphasic with a rapid TCR Vβ2-specific, TNF-dominated response to superantigens and a later IL-12- and IL-18-dependent, IFNγ-dominated response to both bacterial cells and secreted factors. Depletion of MAIT cells from PBMC resulted in decreased total production of IFNγ, IL-1β, IL-2, and TNFβ. Peripheral blood MAIT cells in patients with STSS expressed elevated levels of the activation markers CD69, CD25, CD38, and HLA-DR during the acute compared with the convalescent phase. Our data demonstrate that MAIT cells are major contributors to the early cytokine response to GAS, and are therefore likely to contribute to the pathological cytokine storm underlying STSS.
Streptococcus pyogenes, also known as group A streptococci (GAS) are Gram-positive, beta-hemolytic cocci, which can cause a wide range of diseases in humans. Although most commonly causing superficial infections, such as impetigo and pharyngitis, GAS are also able to cause severe invasive conditions, such as necrotizing fasciitis and streptococcal toxic shock syndrome (STSS) (1). STSS is a state characterized by rapidly progressing multiorgan failure associated with high morbidity and mortality (2). Streptococcal exotoxins belonging to the family of superantigens have been implicated as key players in the pathogenesis of STSS (3). Superantigens are toxins mainly produced by GAS and Staphylococcus aureus, but also by some other bacteria and viruses. They are potent immune stimulators that activate T cells without prior cellular processing through binding to MHC class II molecules on antigen-presenting cells and the Vβ region of the T cell receptor (TCR), thereby bypassing conventional antigen processing and presentation (4, 5). Each superantigen is specific for 1 or a few Vβ regions and triggers polyclonal activation and expansion of these Vβ-specific T cell populations. This can result in rapid and excessive activation of up to 20% of the T cell pool. This activation results in a massive release of cytokines by the T cells and consequent downstream activation of other cell types, leading to a proinflammatory cytokine cascade often referred to as a cytokine storm. The cytokine storm underlies the shock, vascular leakage, and multiorgan failure associated with septic shock.
Superantigen involvement in patients with STSS was proposed by studies on peripheral blood demonstrating a skewed Vβ repertoire in T cells during the acute phase (6–8). Only limited data are available on the cytokine responses in patients, but an initial report by Sriskandan et al. (9) demonstrated elevated levels of TNFβ (lymphotoxin-α). Also, assessment of frequencies of cytokine-producing cells in peripheral blood mononuclear cells (PBMCs) from patients during the acute phase of infection revealed significantly higher frequencies of IL-2, IL-6, and TNF-producing cells in patients with STSS and/or necrotizing fasciitis compared with milder invasive GAS infections (10). Superantigen involvement was further demonstrated at the local tissue site of infection in patients with necrotizing fasciitis, and the severity of disease was associated with elevated levels of TNFβ and IFNγ, indicating a predominant Th1 response (11). Utilization of HLA-DR1 transgenic mice demonstrated that superantigens trigger an early response dominated by IL-2, IL-6, and TNF, which is followed by an IL-12-driven IFNγ response (12). Taken together with in vitro stimulation experiments, it has been proposed that superantigens are typically associated with a strong Th1 cytokine response characterized by high TNFβ and IFNγ, as well as proinflammatory cytokines such as TNF and IL-1 (reviewed in ref. 13).
Mucosal-associated invariant T (MAIT) cells are unconventional T cells characterized by the expression of a semi-invariant TCR with invariant usage of the Vα7.2 segment, together with expression of CD161 (14). This α chain is paired with a limited array of β chains, with predominant usage of certain Vβ including Vβ2 (15, 16). In contrast, murine MAIT cells express a Vα19-Jα33 TCRα chain predominantly paired with Vβ6- or Vβ8-containing β chains (17). MAIT cells are present in high frequencies at mucosal sites, and are most abundant in the liver (14, 18). In peripheral blood, the frequency of circulating MAIT cells in adults varies widely among healthy individuals, ranging from around 0.2 to more than 20% of T cells (19) MAIT cells are rapid responders to bacterial infections, recognizing metabolites originating from the riboflavin biosynthesis pathway and presented by the MHC class I-like MR1 molecule (20–23). In addition to the TCR-mediated activation, MAIT cells can be activated independent of MR1 by the cytokines IL-12 and IL-18 in an innate-like manner (24, 25). Upon activation, MAIT cells produce large quantities of cytokines including TNF, IFNγ, IL-17A, and IL-2 (26). Recent findings indicate that MAIT cells play an important role in antimicrobial immunity at mucosal sites, and in particular the lung (27–29). Le Bourhis et al. (21) reported that GAS did not activate MAIT cells, consistent with their lack of de novo riboflavin synthesis (20, 22). However, GAS was demonstrated to induce a MR1-dependent activation of a small MAIT cell-like subset of T cells expressing a distinct Vα2.1-carrying TCR (30). A recent report by Shaler et al. (31) demonstrated that MAIT cells were activated by the superantigen staphylococcal enterotoxin B (SEB), resulting in a substantial cytokine response. Although this activation could occur in a Vβ-specific manner, it was primarily cytokine-driven and largely IL-12 and IL-18 dependent.
As many of the streptococcal superantigens are Vβ2 specific, we hypothesized that MAIT cells might be involved in the cytokine storm associated with STSS despite GAS lacking de novo riboflavin synthesis. To explore this, we conducted a comprehensive analysis of human MAIT cell activation and cytokine responses to GAS strains and streptococcal superantigens. The results demonstrated strong MAIT cell activation and cytokine release in response to GAS superantigens, as well as whole bacteria.
Results
Both Secreted and Surface Factors of GAS Trigger MAIT Cell Activation and Cytokine Production.
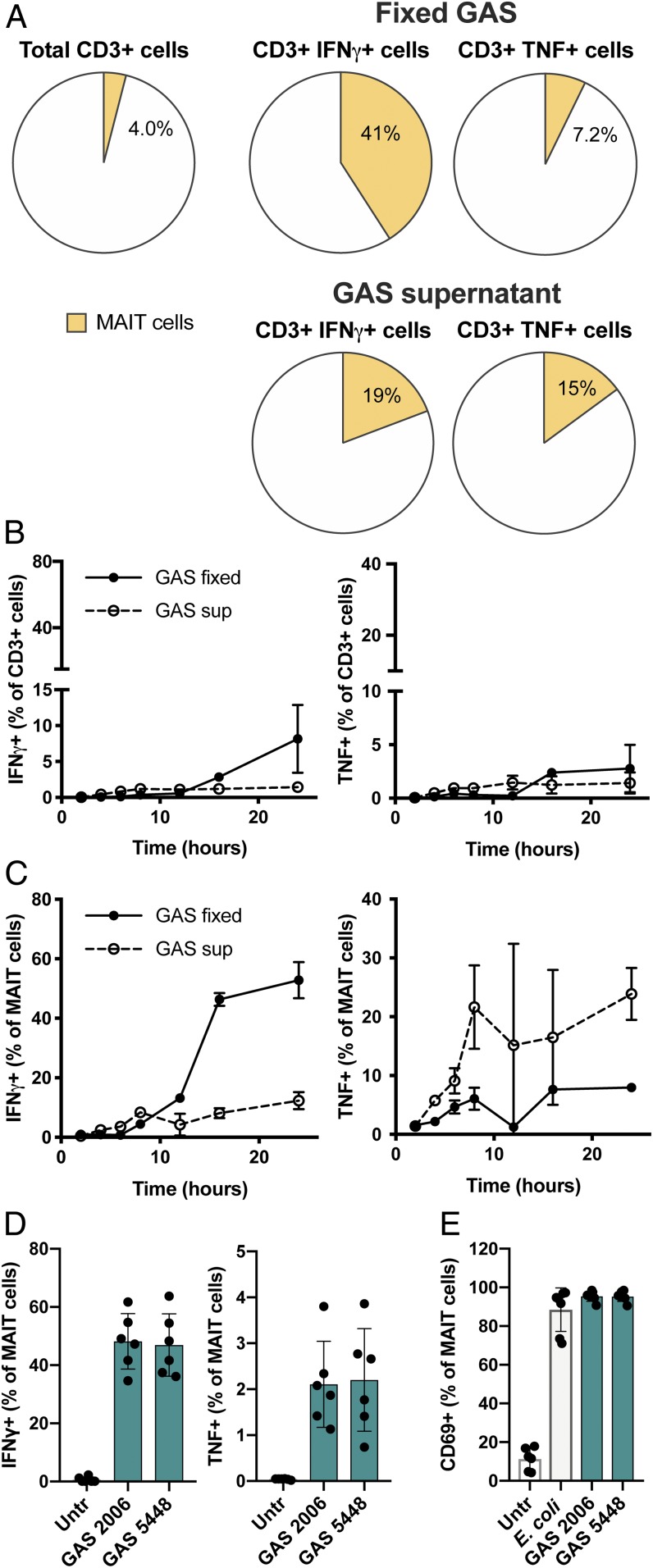
Both surface-attached and secreted virulence factors are known to contribute to cytokine responses in GAS infections (reviewed in ref. 13). We therefore investigated the response to both fixed bacteria and exotoxin-containing supernatant prepared from cultures of a clinical GAS isolate (strain 2006; emm1 type; superantigen genes: speA, speG, speJ) from a case with STSS and necrotizing fasciitis (32). PBMCs from healthy donors were stimulated with fixed GAS or GAS supernatants for 24 h, followed by analysis of TNF and IFNγ production in subsets of the CD3+ T cell pool. Although MAIT cells, defined as Vα7.2+ CD161+ T cells, represented 1 to 10% of the total T cell population, they constituted an average 41% (range, 28.4 to 59.1%) of the total IFNγ-producing CD3+ T cell populations after stimulation with fixed GAS (Fig. 1A and SI Appendix, Fig. S1). Similarly, in response to GAS supernatant, MAIT cells were also a major contributor to IFNγ and TNF production, representing an average 19% (range, 7.9 to 32.5%) and 15% (range, 7.3 to 21.1%), respectively, of the responding CD3+ T cells (Fig. 1A and and SI Appendix, Fig. S1). In contrast, the MAIT cell contribution to TNF production was modest in response to fixed bacteria.
Fig. 1.
MAIT cells are major contributors to cytokine production in response to fixed GAS or GAS supernatant. (A) Frequency of Vα7.2+CD161+ MAIT cells among total CD3+ cells and among CD3+ IFNγ+ or CD3+ TNF+ cells after 24 h stimulation of PBMCs with fixed GAS 2006 or GAS 2006 supernatant assessed by flow cytometry. (B and C) Kinetics of cytokine production after stimulation of PBMCs with GAS, presented as frequency of IFNγ+ or TNF+ cells among (B) total CD3+ cells or (C) MAIT cells assessed by flow cytometry. Media control yielded less than 1.5% IFNγ+ and 3% TNF+ cells at any time. (D) Frequencies IFNγ+ or TNF+ MAIT cells after 24 h stimulation with fixed GAS strains 2006 and 5448. (E) Frequency of CD69+ MAIT cells after stimulation with E. coli, GAS 2006, and GAS 5448. Data are presented as mean frequencies from (A and D) 6, (E) 7, or (B and C) 4 donors ± SD.
Next, we stimulated PBMCs with fixed GAS, as well as supernatants, and determined the kinetics of the cytokine response in total CD3+ cells (Fig. 1B) and in the MAIT cell population (Fig. 1C) during the first 24 h. The GAS supernatants induced a rapid cytokine response, evident already after 6 h, which was dominated by TNF with low levels of IFNγ (Fig. 1 B and C). In contrast, the response to fixed bacteria occurred later, after 12 to 16 h, and was dominated by high levels of IFNγ and low levels of TNF. To ensure that the noted activation was not exclusive for strain 2006, we included the clinical STSS isolate GAS strain 5448, widely used in studies of invasive GAS infections (33, 34). Fixed GAS 5448 triggered an equally strong and almost identical MAIT cell response as that of GAS strain 2006, in terms of both IFNγ and TNF production (Fig. 1D). To further study MAIT cell activation in response to fixed GAS, CD69 expression was assessed in cocultures of purified MAIT cells and monocytes stimulated with fixed bacterial strains, including clinical GAS strains 2006 and 5448, as well as the riboflavin-synthesizing bacterium Escherichia coli as a positive control. GAS stimulated CD69 up-regulation on MAIT cells to the same extent as E. coli (Fig. 1E).
MAIT Cell Activation by Whole GAS Is Independent of MR1, but Requires IL-12 and IL-18.
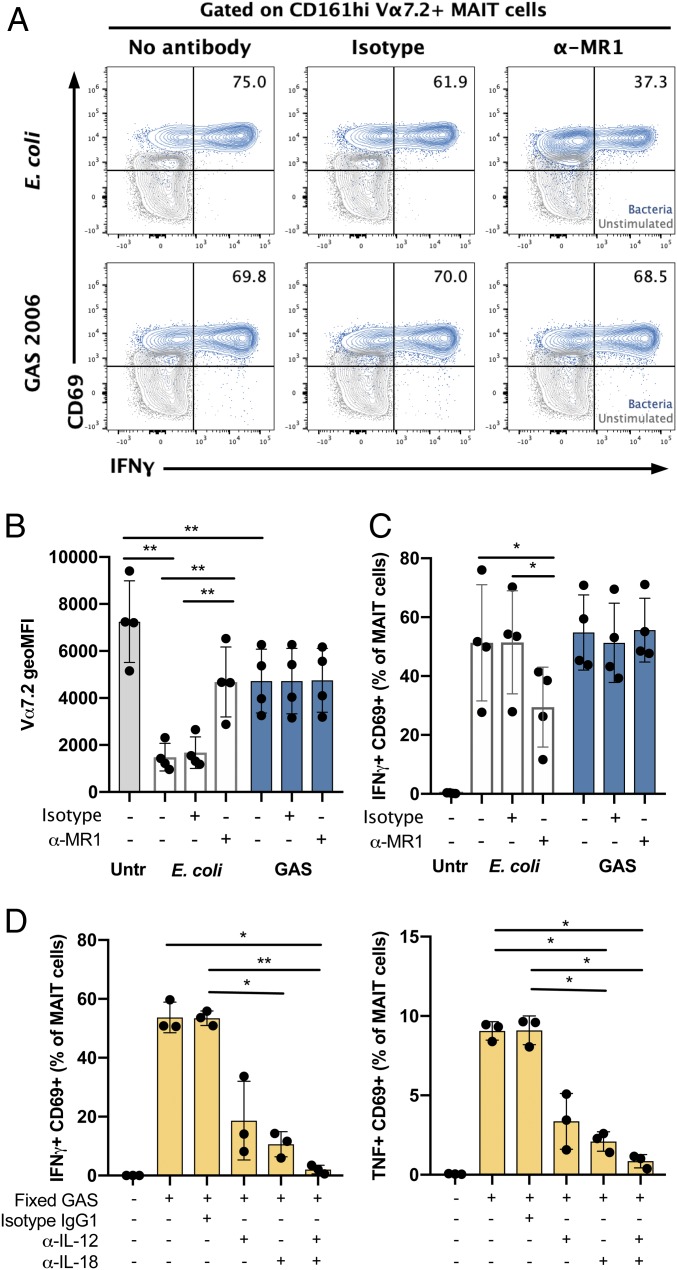
To test whether the activation of MAIT cells by fixed GAS was MR1-dependent, an MR1 blocking antibody was used in the stimulation experiment. Blocking of MR1 reduced the cytokine expression in E. coli-stimulated MAIT cells, but had no effect on the IFNγ production by GAS-stimulated MAIT cells (Fig. 2 A and C). Also, the surface Vα7.2 staining density decreased on stimulation with both GAS and E. coli, indicative of TCR triggering and concomitant down-regulation, but the down-regulation was significantly more pronounced after E. coli stimulation (Fig. 2B). Notably, blocking of MR1 could partially prevent the Vα7.2 down-regulation in response to E. coli, but not after stimulation with GAS. This suggests that GAS activate MAIT cells independent of TCR-MR1 interactions, consistent with GAS lacking de novo riboflavin synthesis.
Fig. 2.
MAIT cell activation by fixed GAS is dependent on IL-12 and IL-18, but not on MR1. MAIT cells were incubated with monocytes stimulated with fixed bacteria for 24 h, and frequency of activated cells was assessed by flow cytometry. (A–C) Stimulation with fixed E. coli or GAS 2006 in the presence of anti-MR1 antibody or IgG2A isotype control. (A and C) IFNγ+CD69+ MAIT cells in (A) 1 representative donor and (C) mean ± SD of 4 donors. (B) Vα7.2 expression presented as geoMFI. Mean ± SD of 4 donors. (D) Frequencies of IFNγ+ CD69+ or TNF+ CD69+ MAIT cells after stimulation with fixed GAS 2006 in the presence of anti-IL-12 and/or anti-IL-18 antibody or IgG1 isotype control. Mean ± SD of 3 donors. (B–D) One-way ANOVA was used to detect significant differences between paired samples. **P < 0.01; *P < 0.05.
To investigate whether MAIT cell activation by whole fixed GAS occurs through the IL-12 and IL-18 produced by the antigen-presenting cells, blocking antibodies against IL-12 and IL-18 were added to the PBMC 1 h after stimulation with bacteria. Neutralization of either IL-12 or IL-18 caused a significant decrease in the IFNγ and TNF production in response to fixed GAS after 24 h of stimulation (Fig. 2D). Combined blocking of both IL-12 and IL-18 almost completely abolished cytokine production by MAIT-cells (96% and 91% reduction for IFNγ and TNF respectively), thus confirming a cytokine-dependent activation.
Secreted Streptococcal Superantigens Trigger Early MAIT Cell Activation and Cytokine Production in a Vβ2-Dependent Manner.
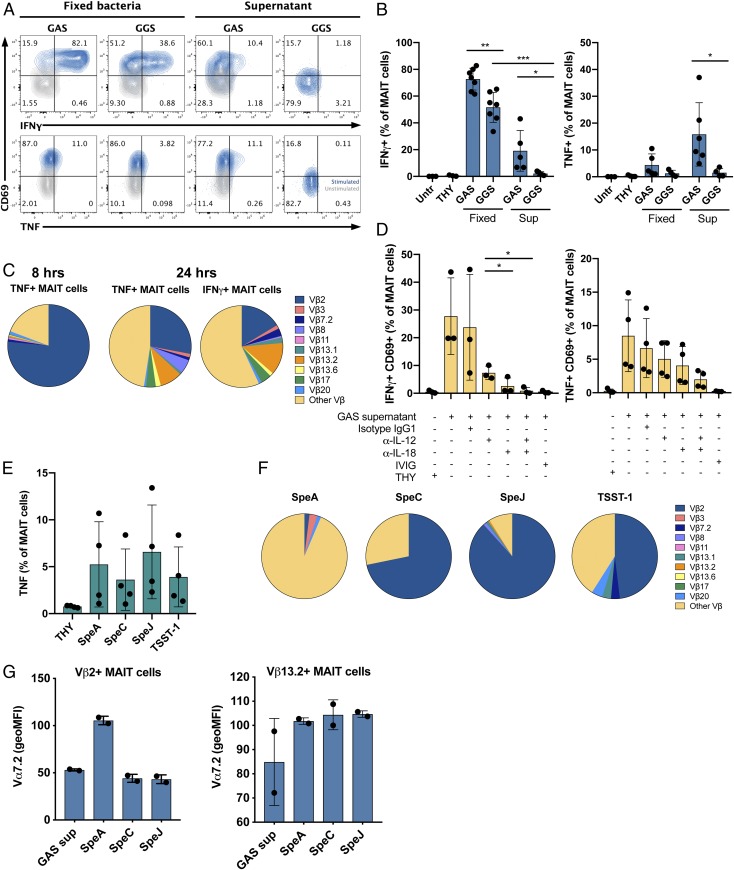
To explore whether the noted MAIT cell activation by secreted streptococcal factors is due to superantigens, we included a clinical group G Streptococcus (GGS), the Streptococcus dysgalactiae strain 6017, lacking superantigen genes. Indeed, GGS 6017 did not show superantigenic activity toward human PBMCs when tested in standard mitogenicity assays, in contrast to GAS 2006 and 5448 supernatants (SI Appendix, Fig. S2A). PBMCs were stimulated for 24 h with GAS and GGS. Similar to GAS strains, fixed GGS activated MAIT cells and induced a strong IFNγ but weaker TNF response (Fig. 3 A and B). However, supernatants of GGS cultures failed to induce CD69 up-regulation or cytokine production in MAIT cells. This suggests that the activation of MAIT cell by GAS supernatants is likely mediated by superantigens.
Fig. 3.
MAIT cells are activated by streptococcal superantigens in a Vβ2-dependent manner. (A and B) Cytokine production by MAIT cells after stimulation of PBMC with fixed bacteria and supernatants of GAS 2006 and GGS 6017 for 24 h assessed by flow cytometry. (A) FACS plot of IFNγ+ or TNF+ MAIT cells from 1 representative donor. (B) Mean frequencies± SD of IFNγ+ or TNF+ MAIT cells of 4 to 7 donors. (C) Stimulation of PBMCs for 8 or 24 h with GAS 2006 supernatant. Vβx expression among cytokine+ MAIT cells was assessed by flow cytometry. (D) Frequencies of IFNγ+ CD69+, or TNF+ CD69+ MAIT cells after stimulation with GAS 2006 supernatant in the presence of anti-IL-12 and/or anti-IL-18 antibody, IgG1 isotype control, or IVIG. Mean ± SD of 3 to 4 donors. (E–G) PBMCs were stimulated with recombinant superantigens and analyzed by flow cytometry. (E) Frequencies of TNF+ MAIT cells after stimulation for 8 h. Mean ± SD of 4 donors. (F) Vβx expression among TNF+ MAIT cells after 8 h of stimulation. Mean ± SD of 2 to 5 donors. (G) Vα7.2 expression (geoMFI normalized to unstimulated control) among Vβ2 or Vβ13.2 MAIT cells after 8 h of stimulation with superantigens of GAS supernatant. Mean ± SD of 2 donors. (B and D) One-way ANOVA was used to detect significant differences between paired samples. ***P < 0.001; **P < 0.01; *P < 0.05.
As superantigens are known to activate T cells in a Vβ-dependent manner, the Vβ profile of GAS supernatant activated MAIT cells were determined for the 10 Vβ chains most commonly expressed by MAIT cells (15, 16). Guided by the cytokine kinetics data (Fig. 1C), Vβ expression was assessed in TNF-producing MAIT cells after 8 h of stimulation, and in both TNF- and IFNγ-producing MAIT cells at 24 h (Fig. 3C). MAIT cells isolated from different donors were used, and although a great interdonor variation in the Vβ repertoire of unstimulated cells was noted, Vβ2 and Vβ13.2 were most frequent, amounting to 17.3% and 4.6%, on average (SI Appendix, Fig. S2B). In contrast, early MAIT cell activation determined by TNF production after 8 h stimulation with GAS supernatant was predominantly Vβ2-specific, with 62 to 91% (mean, 76.5%) of the TNF-producing cells expressing Vβ2 (Fig. 3C and SI Appendix, Fig. S2C). Notably, after 24 h of stimulation, the dominant Vβ2-specificity was lost and other Vβ subsets of MAIT cells also became activated (Fig. 3C and SI Appendix, Fig. S2D). The production of TNF was still partially Vβ2-biased, but some other subsets had started to produce TNF as well. The IFNγ-producing cells displayed a broader Vβ repertoire with Vβ2, Vβ7.2, Vβ13.1, and Vβ13.2. These data indicate that the early activation of MAIT cells is Vβ-specific, and thereby TCR-mediated. However, the broader Vβ-repertoire of activated cells observed at later points indicates that other mechanisms also contribute to the activation. We therefore assessed whether the activation of MAIT cells by GAS supernatants was dependent on IL-12 and IL-18 production. Indeed, neutralization of IL-12 and IL-18 inhibited the production of IFNγ and TNF after 24 h of incubation, but did not abolish it completely (Fig. 3D). Both blocking antibodies could decrease the cytokine production when used alone, but they were most effective when used in combination (97% and 77% reduction for IFNγ and TNF, respectively). In addition, polyspecific i.v. Ig (IVIG) was added as a blocking agent, as it has been shown to contain neutralizing antibodies to a broad spectrum of superantigens (35). The IVIG preparation used completely (100%) inhibited proliferative response elicited by GAS supernatants (SI Appendix, Fig. S2A). In addition, addition of IVIG resulted in a 98% reduction of the cytokine production (Fig. 3D).
The early activation of Vβ2+ MAIT cells suggests that Vβ2-specific superantigens mediated the activation, such as SpeJ, which targets Vβ2 (36, 37), and is relevant here, as the GAS strain 2006 harbors the speJ gene (32). To further explore the action of Vβ2-specific superantigens, MAIT cells were stimulated with a panel of recombinant superantigens targeting Vβ2; that is, SpeC, SpeJ, and TSST-1, and as control, SpeA, which does not bind Vβ2 nor any other Vβ reported to be present on MAIT cells. All superantigens tested activated MAIT cells, as assessed by TNF production after 8 h stimulation (Fig. 3E). The data reflect a great variation between donors, which is in line with varying HLA class II and Vβ repertoires influencing superantigen responses. As expected, SpeC, SpeJ, and TSST-1 activated MAIT cells in a Vβ2-specific manner, whereas SpeA activation of MAIT cells was not specific to any of the analyzed Vβ chains (Fig. 3F). Similarly, Vα7.2 was selectively down-regulated in Vβ2+ subsets after SpeC and SpeJ, but not SpeA, stimulation (Fig. 3G and SI Appendix, Fig. S2E). These data are all from 8 h stimulations due to the substantial loss of MAIT cells at later points, particularly the loss of the Vβ2+ subsets in SpeC-, SpeJ-, and TSST-1-stimulated cultures (SI Appendix, Fig. S3 A and B). The depletion of cells was preceded by a significant increase in caspase-3 activity specifically in Vβ2-specific SpeC- and SpeJ-activated cells, but not in SpeA-activated cells (SI Appendix, Fig. S3 C and D). In Vβ13.2+ MAIT cells, no increase in caspase-3 activity was detected after superantigen stimulation. The findings demonstrate that the Vβ2-specific superantigens SpeC and SpeJ are potent MAIT cell activators. Notably, MAIT cells stimulated with GAS supernatant did not display a significant depletion, nor caspase-3 activation in the Vβ2+ MAIT cell population (SI Appendix, Fig. S3 A–D), despite eliciting a Vβ2-specific cytokine response. Further studies are needed to understand this observation, which is likely linked to the more complex content of the GAS supernatant. Taken together, these data are consistent with Vβ2 being the most commonly used Vβ segment in MAIT cells and provides an explanation as to why such a large fraction of MAIT cells is activated by GAS supernatants.
MAIT Cells Are Key Contributors to the Proinflammatory Cytokine Profile Elicited by Streptococcal Factors.
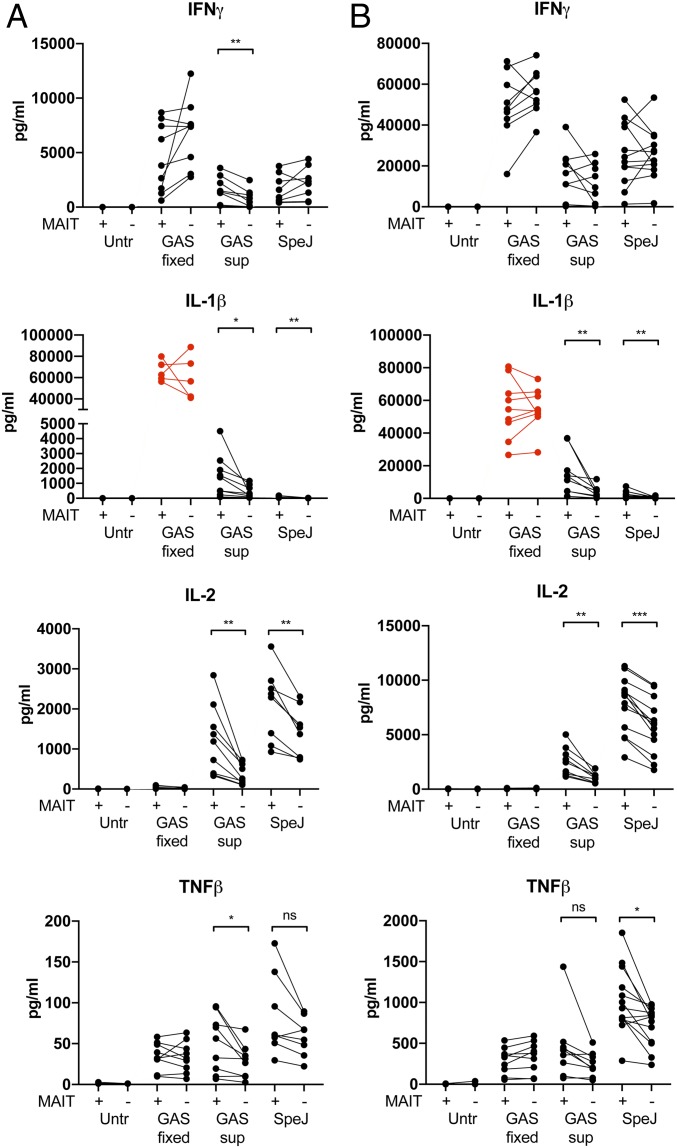
Next, we evaluated the cytokine response in PBMC supernatants after 8 and 24 h of stimulation with fixed GAS, GAS supernatants, and SpeJ, using a multiplex assay. Also, the relative contribution of MAIT cells was tested by inclusion of PBMCs depleted of MAIT cells. The cytokines IL-1β, IL-2, TNFβ, and IFNγ were selected because of their association with the STSS-associated cytokine storm. IL-2 and IFNγ are cytokines reported to be produced by MAIT cells, whereas IL-1β and TNFβ are not. All cytokines were produced in response to all tested stimuli except IL-2, which was only induced by GAS supernatant and SpeJ (Fig. 4 A and B). MAIT cell depletion did not significantly influence the cytokine response elicited by fixed GAS. In contrast, levels of IL-1β, IL-2, and TNFβ were reduced in the MAIT cell-depleted cultures stimulated with GAS supernatant and SpeJ. IFNγ levels were significantly reduced in the 8-h MAIT cell-depleted cultures stimulated with GAS supernatant, but not with SpeJ. Overall, the data indicate that MAIT cells contribute both directly and indirectly to the cytokine response elicited by GAS supernatants and superantigens. Hence, the data support a role for MAIT cells in the superantigen-triggered cytokine storm mediating STSS.
Fig. 4.
MAIT cells are major contributor to the total cytokine production in response to GAS. MAIT cells were depleted from PBMC and the PBMC with (+) or without (−) MAIT cells were stimulated with fixed GAS 2006, GAS 2006 supernatant, or recombinant SpeJ. Total cytokine levels in the cell culture supernatants were assessed using a multiplex assay. (A and B) Concentration (pg/mL) of cytokines in the culture supernatants after (A) 8 h or (B) 24 h of stimulation. Lines in the graph represent individual donors (n = 8–9). IL-1β levels were indicated as out of range after stimulation with fixed bacteria, and are therefore marked in red. The paired t test was used to detect significant differences between paired samples. ***P < 0.001; **P < 0.01; *P < 0.05; ns, nonsignificant.
MAIT Cell Activation in Peripheral Blood of Patients with STSS during the Acute Phase.
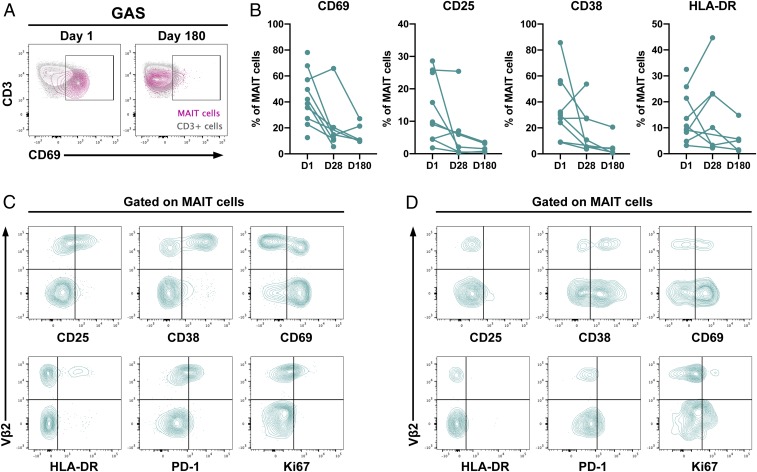
To seek in vivo evidence for MAIT cell activation in patients, frozen PBMCs from patients with GAS STSS collected during acute and convalescent phases were analyzed. The cryopreserved samples were available from the study of Darenberg et al. (35). Consistent with the in vitro results, MAIT cells from patients with STSS expressed the activation marker CD69 at day 1 after diagnosis. Eight patients had both acute and convalescent samples available, and in all cases, the frequency of CD69+ MAIT cells declined in the convalescent phase (Fig. 5 A and B). In addition, MAIT cells positive for the activation markers CD25, CD38, and HLA-DR were detected at day 1, and the expression decreased at day 28 and 180 for all except HLA-DR (Fig. 5B). In 3 of the patients, a Vβ2-specific activation profile was noted (Fig. 5C), whereas in the other patients, the activation was seen in both the Vβ2+ and Vβ2− subsets (Fig. 5D). The variation in Vβ2 specificity between patients could be due to differences in the Vβ specificity of superantigens expressed during infection, or it could be timing, as our in vitro data showed that activation of MAIT cells was Vβ2 specific primarily at early times. Nonetheless, these results support the hypothesis that MAIT cells are activated by GAS superantigens during the acute phase of STSS.
Fig. 5.
MAIT cells are activated during the acute phase of STSS. (A) CD69 expression in MAIT cells (pink) and CD3+ cells (gray) at day 1 and day 180 in 1 representative STSS patient (GAS) (B) Expression of activation markers in acute (D1) and convalescent (D28 and D180) phases of STSS. Lines in the graphs represent individual patients. (C) Representative plots from 1 of 3 donors showing Vβ2-specific expression of CD25, CD38, CD69, HLA-DR, PD-1, and Ki67 on MAIT cells. (D) Representative plots from 1 of 7 donors showing Vβ2-independent expression of CD25, CD38, CD69, HLA-DR, PD-1, and Ki67 on MAIT cells.
Discussion
The MAIT cell–MR1 axis is an evolutionarily conserved system to protect the host against microbial infections (38). However, the contribution of MAIT cells in severe invasive GAS infections such as sepsis and STSS has so far been unclear. Initial reports proposed that GAS did not activate MAIT cells due to lack of riboflavin synthesis (21), consistent with the reported unaltered frequencies in streptococcal sepsis, a pattern different from sepsis caused by riboflavin-synthesizing bacteria, such as S. aureus (39). However, Shaler et al. (31, 39) reported that select superantigens could activate both human and mouse MAIT cells. In this study, we have conducted a comprehensive analysis of human MAIT cell responses to GAS factors, both surface-attached and secreted. We demonstrate that both fixed GAS and streptococcal superantigens are potent activators of MAIT cells. In relation to the overall cytokine response, MAIT cells were found to have a marked role in the production of STSS-associated cytokines, such as IFNγ, IL-1β, IL-2, and TNFβ, in response to GAS. An involvement of MAIT cells during the immunopathogenesis of GAS infections was further supported by the finding of up-regulation of activation markers on MAIT cells in PBMCs of patients with STSS.
The finding that fixed GAS activated both CD69 up-regulation and cytokine production in MAIT cells contradicts previous reports in which no up-regulation of CD69 was noted (21). This discrepancy could be caused by differences in the experimental design, including human versus murine MAIT cells and use of different bacterial culture media and fixation procedure, as well as different bacterial GAS strains. In the present study, 2 well-characterized clinical GAS strains isolated from patients with STSS with or without necrotizing fasciitis infections were used; both belong to the highly virulent emm1 type (33, 34, 40). The 2 strains triggered almost identical activation profiles, with MAIT cells accounting for up to 60% (on average, 40%) of the total IFNγ-producing CD3+ pool. Our data indicate that the activation is MR1-independent, thus concurring with the lack of de novo riboflavin synthesis. Rather, blocking experiments showed that the activation is primarily cytokine-driven. Further experiments are required to decipher which GAS factors expressed on the bacterial surface contribute to this cytokine-driven, MR1-independent, MAIT cell activation. In addition, fixed GGS was a potent MAIT cell activator, suggesting that the streptococcal surface factor responsible for MAIT cell activation is not a superantigen. Meermeier et al. (30) described the existence of an MR1-presented GAS ligand that activates a particular MAIT-like MR1-restricted subset characterized by Vα2.1 expression. It should be noted that this cell subset is not included in our analyses.
Comparison of fixed GAS with GAS supernatants revealed that while both were potent activators, the cytokine response and receptors involved varied. The MAIT cell activation showed a biphasic response with an early, TCR-specific TNF response to GAS supernatant, and a later IL-12- and IL-18-dependent IFNγ-dominated response elicited by both fixed GAS and secreted factors. The early cytokine responses triggered by supernatant and Vβ2-targeting superantigens displayed a dominant Vβ2 signature. In addition, Vα7.2 was down-regulated in Vβ2+, but not Vβ13.2+, MAIT cells after activation by GAS supernatant or Vβ2-stimulating GAS superantigens, supporting a direct TCR-dependent response. In the initial report on superantigen activation of MAIT cells, it was concluded that SEB activation can occur in a TCR Vβ-specific manner, but was primarily dependent on IL-12 and IL-18 (31). This is also in line with our results on the early Vβ2-specific response, which is followed by a cytokine-driven response at later points, resulting in a broader Vβ repertoire among activated MAIT cells. In addition, we observe a strong IL-12- and IL-18-driven IFNγ response to fixed bacteria that, during infection, are likely to contribute to the cytokine storm.
Our results of a Vβ2-specific response to GAS superantigens, as well as TSST-1, is of particular importance considering that skewed Vβ profiles, involving either expansion or depletion of Vβ2-expressing CD3+ cells, have been reported in superantigen-associated toxic shock cases caused by either S. aureus or GAS (7, 8, 41). Taken together, with Vβ2 being the dominant Vβ expressed by human MAIT cells, this provides an explanation to the high frequency of superantigen-triggered cytokine production in MAIT cells compared with the total CD3+ compartment. Several superantigens target Vβ2, including the staphylococcal TSST-1 and the streptococcal SpeC and SpeJ produced by many invasive GAS strains. In contrast, the superantigen SEB, which also activates MAIT cells (31) and is associated with staphylococcal toxic shock syndrome, targets Vβ13.2, the second most common Vβ expressed by MAIT cells.
As the MAIT cells comprise around 1 to 10% of the total CD3+ compartment, it was of importance to assess their relative contribution to the overall cytokine response. To this end, we depleted MAIT cells from PBMCs and compared the cytokine response after stimulation. The data revealed a significant reduction in the 4 cytokines studied: IFNγ, IL-2, IL-1β, and TNFβ. These cytokines were chosen due to their association with the cytokine storm observed in patients with STSS (9–11). It should be noted that IFNγ and IL-2 are produced by MAIT cells, while IL-1β and TNFβ are probably not, indicating both a direct and indirect impact of MAIT cells on the cytokine response. The indirect effect is intriguing and warrants further studies to delineate the underlying mechanisms.
Combined, the findings in this study indicate that MAIT cells contribute to the cytokine response elicited by GAS, both whole bacteria and superantigens. This was further supported by analyses of PBMC from patients with STSS, where MAIT cells displayed several activation markers, including CD25, CD38, CD69, and HLA-DR, during the acute STSS episode, whereas the markers decreased during convalescent phases days 28 and 180. In addition, in the 3 patients with a distinct Vβ2-specific signature, expression of the proliferation marker Ki67 was evident. This implicates MAIT cell activation during the acute phase of GAS STSS. However, the results do not allow for conclusions as to whether MAIT cell activation is a cause or effect of the cytokine storm associated with STSS.
Considering the rapidity by which STSS develops and the severity of this condition, with mortality rates up to 50%, and 26% already within 1 d (42), it is of utmost importance to identify the early mediators of the disease that can serve as diagnostic biomarkers. This report identifies MAIT cells as prominent and rapid responders to GAS secreted and surface factors, resulting in a cytokine response including the hallmark cytokines of STSS. Taken together, the study identifies an actor and potential target for diagnosis or intervention in STSS.
Materials and Methods
Study Subjects.
Peripheral blood was collected from healthy donors recruited at the blood transfusion clinic at Karolinska Hospital, Huddinge. The study was approved by the Regional Ethics Review Board in Stockholm. Frozen PBMCs from 10 patients with STSS collected from 1999 to 2000 in the study by Darenberg et al. (35) were used. Patient age ranged between 29 and 64 y (median, 48 y). Written informed consent was given by each patient, conforming to provisions of the Declaration of Helsinki and approved by the Regional Ethics Review Board in Stockholm. The samples had been stored frozen, in liquid nitrogen, for 19 to 20 y. The long-term storage had not affected the viability of the cells, as the recovery rate exceeded 80% in all samples. The cells were washed once in RPMI-1640 supplemented with 10% FBS (Sigma-Aldrich), 2 mM l-glutamine (Thermo Fisher Scientific), and 25 mM Hepes (Thermo Fisher Scientific), and immediately stained for flow cytometry as described here.
Cell Isolation.
PBMCs were isolated from peripheral blood by Ficoll-Hypaque density gradient centrifugation (Lymphoprep, Axis-Shield). The cells were rested overnight at 4 °C in RPMI-1640 supplemented with 10% FBS (Sigma-Aldrich), 2 mM l-glutamine (Thermo Fisher Scientific), 25 mM Hepes (Thermo Fisher Scientific), 50 μg/mL gentamicin (Life Technologies), and 100 μg/mL normocin (InvivoGen). For Vα7.2+ cell isolation, the PBMCs were incubated with anti-Vα7.2 PE-conjugated antibody (Clone 3C10, BioLegend) for 10 min at 4 °C. The cells were washed, followed by incubation with anti-PE microbeads for 15 min at 4 °C. Positive selection of Vα7.2+ cells was performed using magnetic-activated cell sorting. Monocytes were isolated by negative selection, using RosetteSep human monocyte enrichment mixture (STEMCELL Technologies).
Bacterial Strains.
GAS strains included 1 strain isolated from a patient with STSS and necrotizing soft tissue infection (GAS 2006;emm1) (34, 40), and GAS strain 5448 isolated from a patient with STSS, which has been commonly used in pathogenesis studies (GAS 5448; emm1) (43). The GGS strain 6017 cultured from a patient with necrotizing soft tissue infection was also included. Genomic paired-end libraries (250 bp) were prepared according to the instructions from the TruSeq DNA LT Sample Prep Kit (Illumina). Whole-genome sequencing was then performed via the HiSEq. 2500 Ultra-High-Throughput Sequencing System (Illumina), and the sequencing output was visually checked for quality, using FastQC (44). Sequencing adapters were removed from the FASTQ files with scythe, and then low-quality sequences were trimmed with sickle (45, 46). The trimmed sequences were assembled and scaffolded using multiple k-mer sets (automatic selection based on read lengths), using SPAdes (47), and the resulting contigs were annotated with Prokka (48). The genomic sequence was submitted to the European Nucleotide Archive (ENA database) (49). The sequence was screened for all known streptococcal superantigens.
The 2006 and 6017 strains were collected in the INFECT study (ClinicalTrials.gov, number NCT01790698). All GAS and GGS strains were cultured from single colonies in Todd-Hewitt broth (Invitrogen) supplemented with 1.5% (wt/vol) yeast extract (Invitrogen) at 37 °C. E. coli strain D1 was cultured in Luria-Bertani broth at 37 °C. Overnight GAS and GGS culture supernatants were sterile-filtered and used immediately for PBMC or MAIT cell activation. Fixation of bacteria was performed as previously described (16, 50). Bacteria were washed once in PBS, fixed in 1% formaldehyde for 3 min, and washed 3 times before PBMC or MAIT cell activation. The bacteria were diluted and plated on blood agar plates before fixation, and cultured overnight at 37 °C. The CFU counts were determined the following day.
In Vitro Stimulation Assays.
PBMC (106 cells/well in a 96-well plate) were incubated at 37 °C for 2 h before the addition of fixed bacteria (E. coli MOI = 100; GAS 2006, GAS 5448, and GGS 6017 MOI = 10), supernatants (1:20 dilution), or recombinant superantigens SpeA, SpeC, SpeJ, or TSST-1 (all at 100 ng/mL), which were prepared as described (51, 52). The cells were stimulated for from 2 to 24 h. For FACS analysis experiments, monensin (GolgiStop, BD Biosciences) and brefeldin A (GolgiPlug, BD Biosciences) were added at 1:1,000 final concentration for the last 6 h of incubation or 30 min after addition of stimuli for the shorter incubations. The MAIT cell activation and MR1 blocking assay were performed as previously described (16, 49, 52). Monocytes were incubated at 37 °C for 2 h before the addition of bacteria. Three hours later, the monocytes were washed and Vα7.2+ cells were added at a Vα7.2+ cell-to-monocyte ratio of 2:1, together with anti-MR1 blocking Ab (26.5; BioLegend) or IgG2a isotype control (MOPC-173; BioLegend). After 1 h, 1.25 μg/mL anti-CD28 mAb (L293; BD Biosciences) was added. The MAIT cells were stimulated for 24 h. Monensin (GolgiStop; BD Biosciences) and brefeldin A (GolgiPlug; BD Biosciences) were added at 1:1,000 final concentration for the last 6 h of incubation. For IL-12 and IL-18 blocking experiments, isotype control (IgG1), anti-IL-12p70 mAb (Miltenyi Biotec, Clone C8.6) and/or anti-IL-18 mAb (MBL Life science, Clone 125–2H) were added at 5 μg/mL 2 h after the addition of microbes, supernatants, or superantigens in both the MAIT cell and PBMC stimulation assays. IVIG (1 mg/mL) was added 2 h after addition of stimuli, when indicated.
Flow Cytometry.
Surface and intracellular stainings were performed as previously described (50). Cells were stained for surface markers for 20 min, followed by fixation and permeabilization with CytoFix/Cytoperm (BD Bioscience) for 30 min before intracellular staining for 30 min. The antibodies used are listed in SI Appendix, Table S1. All stainings were performed at 4 °C. Samples were acquired on an LSRFortessa flow cytometer (BD Biosciences) equipped with 355-, 405-, 488-, 561-, and 639-nm lasers. Compensation was performed using single-stained polystyrene beads (BD Biosciences), and the compensation platform in the FlowJo software v. 10.
Proliferation Assay.
The supernatants were tested for superantigenic activity and neutralization by IVIG in a 3H-thymidine proliferation assay, using PBMCs isolated from buffy coats of healthy volunteers, as previously detailed (35). In brief, PBMC were stimulated with 1:100 dilution of GAS supernatants in the presence or absence of 1 mg/mL IVIG (Privigen, CSL Behring) and [3H]-thymidine (PerkinElmer) uptake assessed after 72 h.
MAIT Cell Depletion.
For MAIT cell depletion, PBMCs were incubated with anti-Vα7.2 PE-conjugated antibody (Clone 3C10, BioLegend) for 10 min at 4 °C. The cells were washed, followed by incubation with anti-PE microbeads (Miltenyi Biotec) for 15 min at 4 °C. Depletion of Vα7.2+ cells was performed by magnetic-activated cell sorting, using an LD column (Miltenyi Biotec).
Multiplex.
PBMCs with or without MAIT cells (depleted as described earlier) were stimulated with fixed GAS, GAS supernatants, or SpeJ for 8 or 24 h, as described earlier. Culture supernatants were harvested and stored at −20 °C. Before the assay, the supernatants were centrifuged for 5 min at 13,000 rpm.
IL-1β, IL-2, IFNγ, and TNFβ levels in the supernatants were determined using a customized Bioplex Cytokine kit (Bio-Rad) and analyses on the Bio-plex MAGPIX multiplex reader (Bio-Rad).
Data Availability Statement.
Additional data and experimental details are available in SI Appendix. Genomic sequences are publicly available as detailed (49).
Supplementary Material
Acknowledgments
We thank Nirupama Benis for assistance with sequence analysis. This work was supported by grants from the European Union (FP7/2007-2013) under the grant agreement 305340 (to A.N.-T. and S.S.), by the Swedish Research Council (2014-6722 to A.N.-T. and S.S.; 2018-02475 to A.N.-T.; 2016-03052 to J.K.S.), the Swedish Cancer Society (CAN 2017/777 to J.K.S.), and the Canadian Institutes of Health Research (MOP-142137 to J.K.M.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. P.M.S. is a guest editor invited by the Editorial Board.
Data deposition: The sequence of strain 6017 was deposited in the European Nucleotide Archive (ENA database) under the BioProject PRJNA524111, accession no. SJLQ00000000.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1910883116/-/DCSupplemental.
References
- 1.Walker M. J., et al. , Disease manifestations and pathogenic mechanisms of Group A Streptococcus. Clin. Microbiol. Rev. 27, 264–301 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous; The Working Group on Severe Streptococcal Infections , Defining the group A streptococcal toxic shock syndrome. Rationale and consensus definition. JAMA 269, 390–391 (1993). [PubMed] [Google Scholar]
- 3.Lappin E., Ferguson A. J., Gram-positive toxic shock syndromes. Lancet Infect. Dis. 9, 281–290 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Kappler J., et al. , V beta-specific stimulation of human T cells by staphylococcal toxins. Science 244, 811–813 (1989). [DOI] [PubMed] [Google Scholar]
- 5.Dellabona P., et al. , Superantigens interact with MHC class II molecules outside of the antigen groove. Cell 62, 1115–1121 (1990). [DOI] [PubMed] [Google Scholar]
- 6.Watanabe-Ohnishi R., et al. ; Ontario Streptococcal Study Project , Selective depletion of V beta-bearing T cells in patients with severe invasive group A streptococcal infections and streptococcal toxic shock syndrome. J. Infect. Dis. 171, 74–84 (1995). [DOI] [PubMed] [Google Scholar]
- 7.Javouhey E., et al. , Similarities and differences between staphylococcal and streptococcal toxic shock syndromes in children: Results from a 30-case cohort. Front Pediatr. 6, 360 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michie C., Scott A., Cheesbrough J., Beverley P., Pasvol G., Streptococcal toxic shock-like syndrome: Evidence of superantigen activity and its effects on T lymphocyte subsets in vivo. Clin. Exp. Immunol. 98, 140–144 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sriskandan S., Moyes D., Cohen J., Detection of circulating bacterial superantigen and lymphotoxin-alpha in patients with streptococcal toxic-shock syndrome. Lancet 348, 1315–1316 (1996). [DOI] [PubMed] [Google Scholar]
- 10.Norrby-Teglund A., et al. , Host variation in cytokine responses to superantigens determine the severity of invasive group A streptococcal infection. Eur. J. Immunol. 30, 3247–3255 (2000). [DOI] [PubMed] [Google Scholar]
- 11.Norrby-Teglund A., et al. , Evidence for superantigen involvement in severe group a streptococcal tissue infections. J. Infect. Dis. 184, 853–860 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Faulkner L., Cooper A., Fantino C., Altmann D. M., Sriskandan S., The mechanism of superantigen-mediated toxic shock: Not a simple Th1 cytokine storm. J. Immunol. 175, 6870–6877 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Johansson L., Thulin P., Low D. E., Norrby-Teglund A., Getting under the skin: The immunopathogenesis of Streptococcus pyogenes deep tissue infections. Clin. Infect. Dis. 51, 58–65 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Treiner E., et al. , Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422, 164–169 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Lepore M., et al. , Parallel T-cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRβ repertoire. Nat. Commun. 5, 3866 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Dias J., Leeansyah E., Sandberg J. K., Multiple layers of heterogeneity and subset diversity in human MAIT cell responses to distinct microorganisms and to innate cytokines. Proc. Natl. Acad. Sci. U.S.A. 114, E5434–E5443 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahimpour A., et al. , Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J. Exp. Med. 212, 1095–1108 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dusseaux M., et al. , Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 117, 1250–1259 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Lee O. J., et al. , Circulating mucosal-associated invariant T cell levels and their cytokine levels in healthy adults. Exp. Gerontol. 49, 47–54 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Kjer-Nielsen L., et al. , MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 491, 717–723 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Le Bourhis L., et al. , Antimicrobial activity of mucosal-associated invariant T cells. Nat. Immunol. 11, 701–708 (2010). Corrected in: Nat. Immunol.11, 969 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Patel O., et al. , Recognition of vitamin B metabolites by mucosal-associated invariant T cells. Nat. Commun. 4, 2142 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Corbett A. J., et al. , T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature 509, 361–365 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Ussher J. E., et al. , CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur. J. Immunol. 44, 195–203 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slichter C. K., et al. , Distinct activation thresholds of human conventional and innate-like memory T cells. JCI Insight 1, e86292 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howson L. J., Salio M., Cerundolo V., MR1-Restricted mucosal-associated invariant T cells and their activation during infectious diseases. Front. Immunol. 6, 303 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meierovics A., Yankelevich W. J., Cowley S. C., MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection. Proc. Natl. Acad. Sci. U.S.A. 110, E3119–E3128 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H., et al. , MAIT cells protect against pulmonary Legionella longbeachae infection. Nat. Commun. 9, 3350 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pincikova T., et al. , Severely impaired control of bacterial infections in a patient with cystic fibrosis defective in mucosal-associated invariant T cells. Chest 153, e93–e96 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Meermeier E. W., et al. , Human TRAV1-2-negative MR1-restricted T cells detect S. pyogenes and alternatives to MAIT riboflavin-based antigens. Nat. Commun. 7, 12506 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaler C. R., et al. , MAIT cells launch a rapid, robust and distinct hyperinflammatory response to bacterial superantigens and quickly acquire an anergic phenotype that impedes their cognate antimicrobial function: Defining a novel mechanism of superantigen-induced immunopathology and immunosuppression. PLoS Biol. 15, e2001930 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Babbar A., et al. ; INFECT Study Group , Pivotal role of preexisting pathogen-specific antibodies in the development of necrotizing soft-tissue infections. J. Infect. Dis. 218, 44–52 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Walker M. J., et al. , DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat. Med. 13, 981–985 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Chella Krishnan K., et al. , Genetic architecture of group A streptococcal necrotizing soft tissue infections in the mouse. PLoS Pathog. 12, e1005732 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darenberg J., Söderquist B., Normark B. H., Norrby-Teglund A., Differences in potency of intravenous polyspecific immunoglobulin G against streptococcal and staphylococcal superantigens: Implications for therapy of toxic shock syndrome. Clin. Infect. Dis. 38, 836–842 (2004). [DOI] [PubMed] [Google Scholar]
- 36.McCormick J. K., Pragman A. A., Stolpa J. C., Leung D. Y., Schlievert P. M., Functional characterization of streptococcal pyrogenic exotoxin J, a novel superantigen. Infect. Immun. 69, 1381–1388 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Proft T., Arcus V. L., Handley V., Baker E. N., Fraser J. D., Immunological and biochemical characterization of streptococcal pyrogenic exotoxins I and J (SPE-I and SPE-J) from Streptococcus pyogenes. J. Immunol. 166, 6711–6719 (2001). [DOI] [PubMed] [Google Scholar]
- 38.Lantz O., Legoux F., MAIT cells: An historical and evolutionary perspective. Immunol. Cell Biol. 96, 564–572 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Grimaldi D., et al. , Specific MAIT cell behaviour among innate-like T lymphocytes in critically ill patients with severe infections. Intensive Care Med. 40, 192–201 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Siemens N., et al. ; INFECT Study Group , Biofilm in group A streptococcal necrotizing soft tissue infections. JCI Insight 1, e87882 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi Y., et al. , Selective expansion of T cells expressing V beta 2 in toxic shock syndrome. J. Exp. Med. 172, 981–984 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamagni T. L., et al. , Predictors of death after severe Streptococcus pyogenes infection. Emerg. Infect. Dis. 15, 1304–1307 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Chatellier S., et al. , Genetic relatedness and superantigen expression in group A streptococcus serotype M1 isolates from patients with severe and nonsevere invasive diseases. Infect. Immun. 68, 3523–3534 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andrews S., Fast Q. C., A Quality Control tool for High Throughput Sequence Data (Version 0.11.7, 2010). http://www.bioinformatics.babraham.ac.uk/projects/fastqc/. Accessed 6 September 2018.
- 45.Buffalo V., Scythe (Version 0.991, 2011). https://github.com/vsbuffalo/scythe. Accessed 6 September 2018.
- 46.Joshi N., Fass J., Sickle: A sliding-window, adaptive, quality-based trimming tool for FastQ files (Version 0.991, 2011). https://github.com/najoshi/sickle. Accessed 6 September 2018.
- 47.Bankevich A., et al. , SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19, 455–477 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seemann T., Prokka: Rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069 (2014).24642063 [Google Scholar]
- 49.Barrantes I., et al. , INFECT Study Group, Novel genomic isolates of S. pyogenes and S. dysgalactiae subsp. equisimilis associated to necrotising fasciitis (NSTI) obtained from several European hospitals. European Nucleotide Archive. https://www.ebi.ac.uk/ena/data/view/PRJNA524111. Deposited 25 February 2019.
- 50.Dias J., Sandberg J. K., Leeansyah E., Extensive phenotypic analysis, transcription factor profiling, and effector cytokine production of human MAIT cells by flow cytometry. Methods Mol. Biol. 1514, 241–256 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Kasper K. J., et al. , Bacterial superantigens promote acute nasopharyngeal infection by Streptococcus pyogenes in a human MHC Class II-dependent manner. PLoS Pathog. 10, e1004155 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dias J., Sobkowiak M. J., Sandberg J. K., Leeansyah E., Human MAIT-cell responses to Escherichia coli: Activation, cytokine production, proliferation, and cytotoxicity. J. Leukoc. Biol. 100, 233–240 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional data and experimental details are available in SI Appendix. Genomic sequences are publicly available as detailed (49).