Significance
The Justinianic Plague (circa 541 to 750 CE) has recently featured prominently in scholarly and popular discussions. Current consensus accepts that it resulted in the deaths of between a quarter and half of the population of the Mediterranean, playing a key role in the fall of the Roman Empire. Our contribution argues that earlier estimates are founded on a small subset of textual evidence and are not supported by many other independent types of evidence (e.g., papyri, coins, inscriptions, and pollen archaeology). We therefore conclude that earlier analyses of the mortality and social effects of the plague are exaggerated, and that the nontextual evidence suggests plague did not play a significant role in the transformation of the Mediterranean world or Europe.
Keywords: Justinianic Plague, first plague pandemic, Late Antiquity, plague, Yersinia pestis
Abstract
Existing mortality estimates assert that the Justinianic Plague (circa 541 to 750 CE) caused tens of millions of deaths throughout the Mediterranean world and Europe, helping to end antiquity and start the Middle Ages. In this article, we argue that this paradigm does not fit the evidence. We examine a series of independent quantitative and qualitative datasets that are directly or indirectly linked to demographic and economic trends during this two-century period: Written sources, legislation, coinage, papyri, inscriptions, pollen, ancient DNA, and mortuary archaeology. Individually or together, they fail to support the maximalist paradigm: None has a clear independent link to plague outbreaks and none supports maximalist reconstructions of late antique plague. Instead of large-scale, disruptive mortality, when contextualized and examined together, the datasets suggest continuity across the plague period. Although demographic, economic, and political changes continued between the 6th and 8th centuries, the evidence does not support the now commonplace claim that the Justinianic Plague was a primary causal factor of them.
The 3 plague pandemics (caused by the bacterium Yersinia pestis) are considered among the most infamous—and most fatal—biological events in human history. Significant recent scholarship has investigated the so-called First Pandemic, which began with the Justinianic Plague of circa 541 to 544 CE (hereafter JP), and reoccurred in western Eurasia and North Africa over the next two centuries (1–8). The Second Pandemic, beginning with the Black Death, devastated late medieval and early modern Europe, southwestern Asia and North Africa, as well as other regions (9). The Third Pandemic globalized Y. pestis and killed millions in South and East Asia at the turn of the 20th century.
Since research began on the Third Pandemic, scholars have used similar criteria and models to investigate the 3 plague pandemics, frequently making assumptions about the nature of 1 pandemic by drawing upon precedents from the others. Early researchers of the Third Pandemic explicitly connected it to the earlier two (10–12). For decades now, research on prelaboratory plagues has been a multidisciplinary endeavor and scholarly cross-pollination has reinforced the consensus that the first 2 pandemics had similar outcomes (e.g., ref. 6).
Recent isolations of Y. pestis DNA in prelaboratory European human remains has validated the long-held assumption that the same pathogen was present during the 3 pandemics (4, 13–17). Based on molecular and historical research, a broad consensus (hereafter “the maximalist position”) has developed across disciplines that estimates JP mortality in the tens of millions, with numbers ranging between 15 and 100 million, or alternatively, 25 to 60% of the estimated population of the Late Roman Empire (for recent estimates, see refs. 3, 6, and 17). This conception of the plague’s impact has shaped modern histories of Eurasia. Plague mortality is alleged to have depopulated the “known world” and to have accelerated the transition to a “backward” “Dark Age” period, devastating the Late Roman Empire and extinguishing antiquity (e.g., refs. 5 and 6). Yet this narrative overlooks the fact that the political structures of the Western Empire had already collapsed in the 5th century and the Eastern Empire did not decline politically until the 7th century (18, 19).
The extraordinary claims of plague’s effects demand extraordinary evidence, but the consensus maximalist position has offered little evidential support when evaluated critically (7). The existing direct evidence consists of several historical narrative texts and 2 inscriptions (for texts, see refs. 6, 20, and 21; for inscriptions, see ref. 1). Additional evidence, such as the recent molecular identifications of Y. pestis in late antique Europeans, suffers from low temporal resolution (uncertainty intervals of 50 y or more) and a minuscule number of known cases (∼45). Moreover, the presence of plague does not in itself confirm a vast mortality in any spatial or temporal terms. A small quantity of indirect evidence that correlates temporally with the First Pandemic, but is not necessarily causally related to it—ranging from ambiguous texts to cultural and economic trends—has also been gathered (e.g., refs. 5, and 22, 23). Problematically, these data are rarely contextualized; rather, they are cherry-picked to construct maximalist interpretations of the JP while overlooking their uncertainty and low temporal and spatial resolution (24).
In this paper, we attempt to determine plague’s potential effects. We focus on quantitative measures that could reflect population size and illuminate the possible extent of the plague’s demographic toll. While methodological limitations do not allow us to establish the absolute size of late antique populations, the datasets we assess indicate there was no 6th century population collapse. By examining several higher-resolution (annual to decadal) datasets before, during, and after the onset of plague in the Mediterranean region in 541 CE, we can begin to determine whether there were significant changes in late antique population levels. In addition, we employ lower-resolution measures to support our argument that evidence for a demographically significant JP is lacking.
Textual (Historical) Evidence to Support Plague Severity Has Been Misused
To date, 3 catalogs for the JP have been assembled, using late antique texts and inscriptions to trace plague outbreaks from circa 541 to 750/767 (6, 20, 21). They remain the key reference works for JP research. Although they differ in their methodology, all 3 point to repeated occurrences of the JP (termed “waves” or “amplifications”) following the initial outbreak of 541 to 544. The earliest catalog proposed 14 occurrences and the most comprehensive one has 18; others have argued for 21 (25). Each recurrence supposedly caused mass death, with a recent estimate of 10% population mortality per recurrence, keeping population levels low for two centuries (6).
A quantitative examination of the original texts, however, casts doubts upon the purported gravity of most recurrences. The initial JP occurrence is documented at length in Procopius (26) and John of Ephesus (27). That this outbreak was a demographic watershed is based upon these authors’ writings, implying that late antique plague severity correlates with the quantity and detail of extant contemporary evidence. Following this line of thinking, one would expect that if recurrences were also catastrophic, contemporaries would have discussed them in similar detail. With a few exceptions, however, the recurrences are thinly documented and poorly understood, facts rarely acknowledged in JP research.
Moreover, late antique plague narratives are complex literary accounts with multiple layers of rhetorical meaning. Their authors had political, ideological, and personal purposes for their writing and the texts contain significant biases that must be contextualized. An account of a catastrophic plague could reflect reality or be a gross exaggeration employed to underscore a particular point (e.g., divine disapproval). One key account of the JP, for example, asserts that the initial occurrence killed 99.9% of the population (28), while another influential late antique author claims that Emperor Justinian—whom the author claims was an “evil demon”—killed 1 trillion people during his reign in various disasters (29). As these examples imply, late antique claims that plague was omnipresent, or nearly so, must be treated suspiciously. Although each passage should be examined critically, both within the context of the work in which it is encountered and within the work’s particular cultural setting, maximalists tend to read plague passages positivistically and to accept late antique claims and commentary on the JP at face value.
Although it has been argued that the JP garnered less attention over time because of the high frequency of plague recurrences, it is striking how numerous late antique texts, including several that purportedly overlook or skim lightly over later JP recurrences, treat other natural disasters, like earthquakes, in detail. Indeed, seismic events are thought to have been far more common and less lethal than outbreaks of the JP, yet they retained the attention of authors throughout late antiquity and beyond (cf. ref. 30). Contemporaries chose to remember and commemorate certain calamities, such as earthquakes and relatively minor volcanic eruptions, but did not memorialize plague (7).
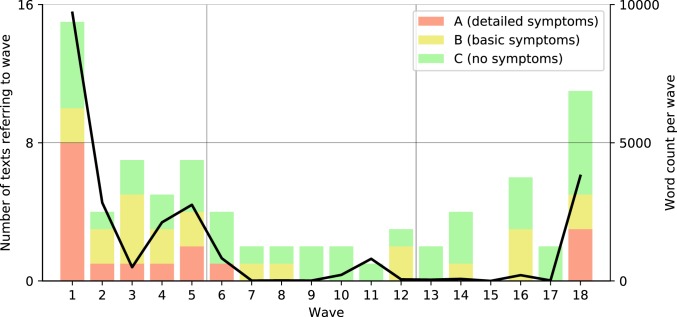
The plague catalogs group together different texts to reconstruct recurrences, implying that they were significant and that many authors wrote about them. Fig. 1 examines the 18 waves the most comprehensive catalog describes (21). The results reveal how scant the written evidence is for most of these recurrences, even after we have included sections in which the JP plays a tangential role and texts written centuries after these events. Only 2 short sentences, for example, discuss the supposed 17th wave (circa 732 to 735): “In this year there was a plague [thanatikon, literally a mortality] in Syria and many people died” (31) and “And in this year there occurred in Palestine and Egypt a severe pestilence [wabāʾ, literally an epidemic]” (32). Moreover, attempts to construct recurrences overlook the hundreds of contemporary texts in the 5 languages that do not discuss plague. As examples, 1 database has collected the works of approximately 120 authors in Greek in the 6th century alone (http://stephanus.tlg.uci.edu/); another reference work lists 107 Latin authors known in that period (33). As Fig. 1 demonstrates, only a handful of these authors refer to plague in their work. Moreover, the diagnosis of plague is far from clear; half the cataloged sources describe only a vague mortality that could refer to many diseases, and only 20% provide more than a single basic symptom (i.e., buboes) that scholars accept as sufficient for diagnosing plague (20, 21).
Fig. 1.
The number of texts referring to each plague “wave” (colored bars) and the total number of words in them (black line). For details, see explanation of the figure in SI Appendix, Materials and Methods.
Within the historical texts, the attention given to plague is minimal. Procopius, the author of the most influential passage on the JP, discusses it in less than 1% of his work. Gregory of Tours, the key western European plague reference, similarly devotes at most 1.3% of his work to plague. With 1 or 2 exceptions, similar percentages are the norm. In addition, when there are multiple references to the same outbreak, most authors refer to plague briefly to convey broader ideological or pedagogical concepts and rarely provide details (e.g., mortality, symptoms; discussion in ref. 7, pp. 9–16).
The quantitative approach to the written evidence suggested above reveals that the common maximalist JP narrative, which is based almost exclusively on the written evidence, was founded on weak premises. Although it is easy to draw a broad image of plague, portraying it as an almost unparalleled catastrophe, examining the evidence at a higher resolution demonstrates the fatal flaws in this hypothesis.
Promulgation of Laws and Issues of Coinage Not Affected by Plague
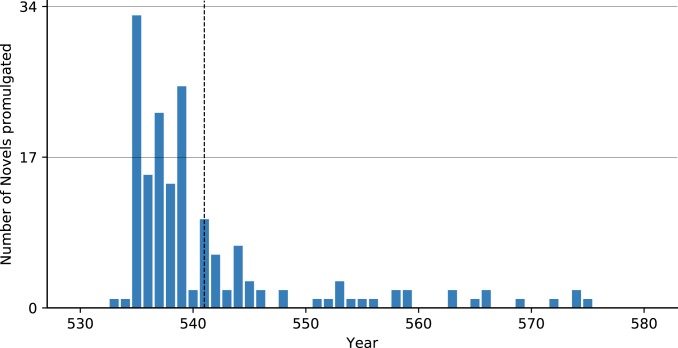
Few quantitative proxies have been used as evidence for plague given the low data resolution of late antique datasets. Fig. 2 and SI Appendix, Fig. S1 reveal how 2 measures cited as evidence for plague—the number of laws promulgated per year, and the ratio between the values of gold and bronze coins—appear unaffected by plague. Once legislation is examined at an annual resolution, rather than a chronological division into the pre/postplague reign of Justinian (527 to 565), it becomes clear that the quantity of legislation decreased before the first outbreak of plague in 541 (Fig. 2). Previously, the legal evidence was used at low data resolution (7). Moreover, the issuing of legislation dropped before the plague since Justinian had just completed a massive codification project: The Corpus Iuris Civilis (i.e., the Code of Justinian) from 529 to 534 (34). In the immediate aftermath of this codification project, Justinian issued a series of new laws (“novels” in Fig. 2) to resolve problems and issue corrections beginning in 534, hence the “burst” of legislative activity before the plague. The number of laws promulgated, therefore, fell before the plague, since the codification project and its corrections were now complete, and the commission organized to resolve legal problems was disbanded (35). Both immediately before the plague outbreak and afterward there would have been fewer pressing reasons to issue more laws (contra ref. 36).
Fig. 2.
Legislation (novels) promulgation per year (34, 79, 80). The first outbreak of plague in 541 is marked on this and the other figures below as a vertical dashed line.
Similarly, the gold to bronze ratio plummeted before the JP and increased sharply a few years after the decrease attributed to plague (SI Appendix, Fig. S1). Moreover, the connection between the plague and any broader fiscal concerns, including the fiduciary issuing of coinage, are deeply unclear (37).
As the figures show, a high-resolution analysis that considers the broader chronological context fails to identify the influence of the JP. Moreover, as the ensuing discussion notes, ignoring the broader context of legal and economic history in the years before, during, and after the plague outbreak confuses the causality involved.
Papyri Demonstrate Strong Continued Administration and Economic Vitality
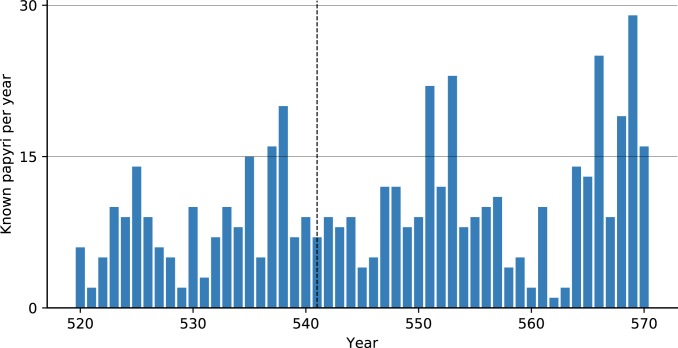
A quantitative assessment of papyrological evidence to understand local governance and economic growth has not featured in JP debates. Due to environmental conditions, papyri survive in large numbers only in Egypt, where plague entered the Mediterranean. If plague devastated the Egyptian population, as is often stressed, we should expect some discussion of it, or at least adaptation to these changes, in the best-surviving documentary evidence from the period. This, however, is not the case. A survey of the 505 papyri precisely dated to individual years from 520 to 570 reveals neither an observable reduction nor a marked increase (due to more need for documentation based on more deaths) in the total numbers of surviving papyri. There is no evidence corresponding to population decline, land abandonment, or tax revenue reductions that could be attributed to the plague in the second half of the 6th century or even to the initial outbreak of 541 in Egypt (Fig. 3). Moreover, the authors know of no papyrus document that definitely refers to the plague among the tens of thousands of surviving papyri from the period in which plague allegedly ravaged the Mediterranean (37, 38).
Fig. 3.
Known papyri per year, annual resolution (http://papyri.info/).
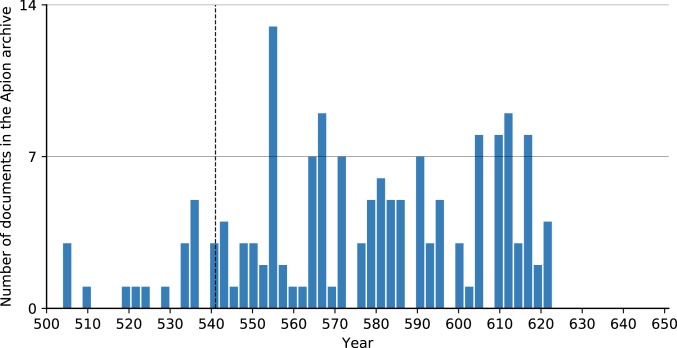
The same effect is visible in the subset of papyri from the archive of the wealthy Egyptian Apion family, the best documented economic entity in the Roman Empire between the 5th and 7th centuries. This high-resolution papyri dataset reveals no economic stress on the family’s estates, which show a substantial increase in revenue (about 30%) between the 540s and 580s, suggesting that plague had no discernible economic effects. Previous work that described JP-driven trends in shifts in Egyptian land tenure rests on a faulty reading of the source material (23; critique in ref. 7). Similarly, the mid-6th century saw an increase in the number of surviving dated papyri, which do not contain references to the plague (Fig. 4).
Fig. 4.
Dated documents of the Apion archive, up to 3-y resolution (81, 82).
Contemporary Inscriptions Reveal Continuity over the Plague Period
Only 2 inscriptions explicitly refer to the JP over two centuries. Previous scholars have posited that the plague caused a shift away from inscriptions to explain this dearth of evidence (1). Although numerous compilations of inscriptions have been published, they are not comprehensive, have varying emphases (spatial, chronological, linguistic, and so forth), and overlap at times. Moreover, the data they preserve is often messy, the ways in which they were collected are biased, and the inscriptions themselves are only rarely dated to a specific year. Despite these limitations, our quantitative examination of several compilations found no effect we could ascribe to plague.
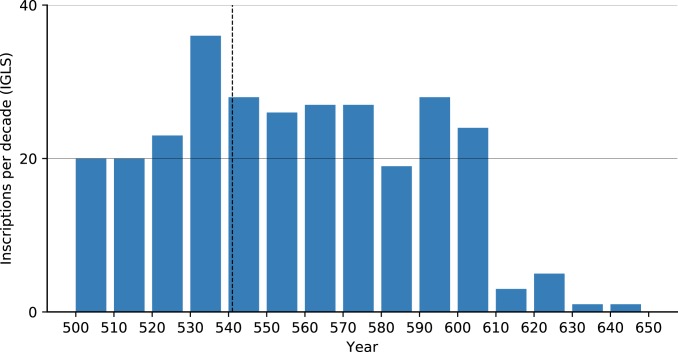
We used the Inscriptions Grecques et Latines de la Syrie (IGLS, https://inscriptions.packhum.org/) collection from Syria, a region for which we have much literary evidence for plague and a region that scholars argue the JP devastated (e.g., ref. 6). The digitized IGLS collection includes 288 inscriptions dating to specific years between 500 and 650. Examining them (Fig. 5) reveals no observable effect that could be attributed to plague: Neither a sharp decline in inscription production reflecting demographic collapse, nor a slow decrease in inscription production that could reflect economic decline. The drop in the number of inscriptions in the early 7th century, on the other hand, is the visible outcome of the Sasanian conquest of Syria during the 610s. The trend did not reverse during the brief Byzantine reconquest or the subsequent early Islamic conquests in later decades. Notably, had this dataset been examined at a lower resolution (50- to 100-y intervals) as is sometimes done with “datable” plague data, the 610s drop could easily be added to one of the first waves and misinterpreted as plague-related depopulation.
Fig. 5.
Inscriptions per decade in the IGLS collection, annual resolution (https://inscriptions.packhum.org/).
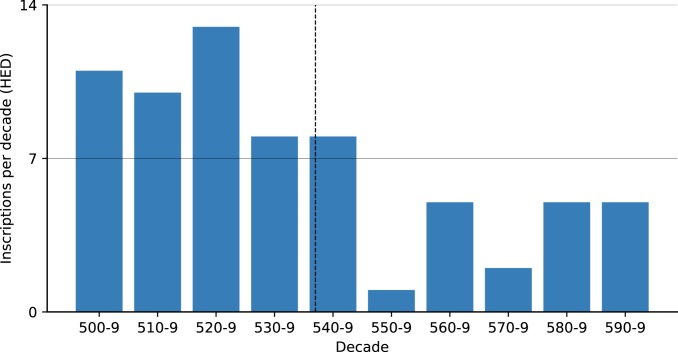
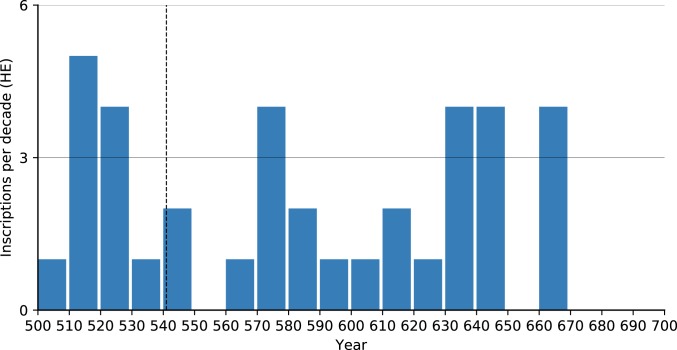
A similar search using the Heidelberg Epigraphic Database (https://edh-www.adw.uni-heidelberg.de/home), a dataset of 79,846 mostly Latin inscriptions predominantly from the central and western Mediterranean, found 68 6th century inscriptions datable to a single year (Fig. 6). Although the decadal scale of this high-resolution corpus shows a general decrease over time, no immediate plague effect was observed. The substantial drop in inscriptions occurred only a decade after the onset of plague (the 550s). Since the decrease did not persist—the next decade saw an upswing—the brief reduction during the 550s should probably be seen as a random outlier in the slower decreasing trend in inscription production in the post-Roman kingdoms.
Fig. 6.
Inscriptions per decade in the Epigraphic Database Heidelberg (HED), annual resolution (https://edh-www.adw.uni-heidelberg.de/home).
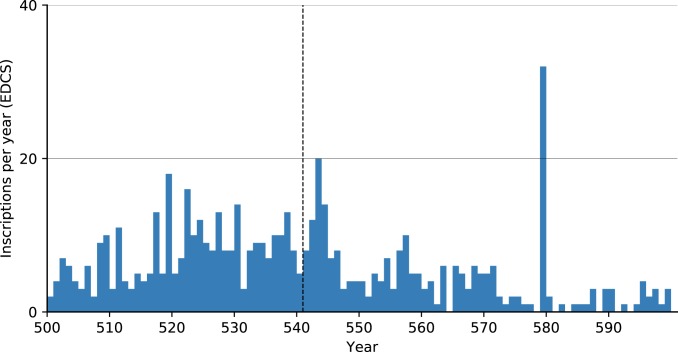
The Epigrafik-Datenbank Clauss-Slaby (EDCS, http://www.manfredclauss.de/) epigraphic database, which records 519,109 inscriptions from 36 databases, reveals similar results. The majority of inscriptions had resolutions of decades to centuries, and we included only the 570 that were dated to within an annual interval. Upon closer examination, half the inscriptions of the small spike in 543 come from Rome and Sbeitla (Fig. 7).
Fig. 7.
Inscriptions per year (annual level resolution) in the EDCS (http://www.manfredclauss.de/). Note the outlier at 579.
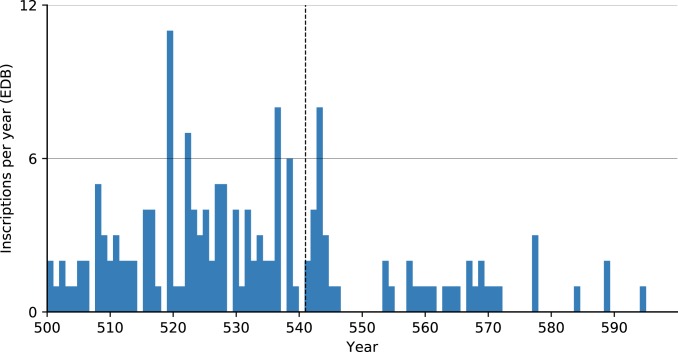
Another high-resolution subset of inscriptions (161 of over 41,000 inscriptions that are dated to a specific year in the 6th century) from late antique Rome shows a spike in inscriptions in 543 and a subsequent decline (Fig. 8). While some have seen in this evidence for plague (21), temporal correlation is insufficient to confirm a connection. The only direct evidence for plague in Rome in this period is a one-line reference to “a great pestilence [that] ravaged the land of Italy” in 543 in the continuation of the chronicler Marcellinus (39). This epidemic coincided with the Gothic Wars, which devastated Italy in the early and mid-540s. Rome was besieged several times, notably from 545 to 546, causing famine as well as deaths due to warfare. Procopius, who reports on the siege in detail, claims that only 500 local men (likely an underestimate) remained in the city after it (26). These political–military factors more readily explain the absence of inscriptions in the late 540s and early 550s. An additional dataset of ∼31,000 inscriptions from Spain includes only 40 that can be precisely dated within an annual resolution, but nonetheless does not show a substantial decline in inscriptions beginning in the 540s (Fig. 9).
Fig. 8.
Inscriptions per year (annual resolution) in the Epigraphic Database Bari (EDB) (http://www.edb.uniba.it).
Fig. 9.
Inscriptions per decade in Spain, annual resolution (http://eda-bea.es).
Contemporary Coins Show No Obvious Break during the Plague Period
Numismatic evidence for the JP’s impact on economic relations is also nonexistent. Although no comprehensive datasets for 6th century coin production have been assembled, 2 important local datasets show no visible change in coin circulation in the 540s. In SI Appendix, Figs. S2 and S3, coins can be dated to a specific year based on their mint mark, another high-resolution dataset. Like inscriptions, of the many thousands of coins recovered in the excavations in each city only a few could be dated with annual precision.
Antioch was perhaps the third largest city in the Empire and the Mediterranean, and one of the few cities that produced coins. In 540, the Sasanian emperor Khosrow sacked the city. The attack undoubtedly explains the absence of coins minted in Antioch for most of the 540s. Nonetheless, coins produced in other cities did reach Antioch, suggesting that both coin production and circulation continued as earlier. The absence of a clear signal (i.e., short drop or long-term decline) in the data serves as more evidence against demographic collapse or disruption of trade networks due to plague outbreaks. The smaller provincial capital Berytus (modern Beirut) was not attacked and similarly shows no visible drop associated with plague. Although John of Ephesus likely passed through or near both cities en route to Constantinople, the numismatic evidence appears to contradict his apocalyptic report (27).
Land Use during the Plague Period Does Not Confirm Plague Narrative
Land use, independent of the historical and archaeological evidence, can be examined through palynological data, which shows changes in the proportion of pollen of different plants from subsequent layers of lake or peat sediments. Until now, this evidence has not been brought to bear on the JP. However, given the tendency among maximalists to take the Black Death as the model for the demographic and economic impact of the initial outbreak of the JP (e.g., refs. 6, 40, and 41), comparing the impact that the initial outbreaks of the 2 plague pandemics had on the landscape may help validate the maximalist paradigm for the JP.
The initial outbreak of the Second Pandemic in Europe, the Black Death, had dramatic effects on the countryside in several European regions (42). As villages were depopulated and fields were abandoned, landscapes were transformed. Less anthropogenic pressure resulted in the “rewilding” of the landscape and a widespread process of secondary ecological succession of uncultivated species into former fields or pastures. This environmental effect of the Black Death, and the late medieval crisis more generally, has been convincingly demonstrated for several regions, such as the southern Swedish uplands (43). There a large, regional-scale analysis of multiple comparatively high-resolution cereal pollen datasets has shown a decline soon after the Black Death. Although not all European regions for which palynological data are available evidence a dramatic change of scenery following the onset of the Second Pandemic, areas of Ireland and Estonia for example (44), evidence similar to that available for southern Sweden exists for The Netherlands (44, 45), northern Greece (46, 47), central Italy (48), and perhaps the Czech lands and Brandenburg (49). In all of these cases, visible landscape change occurred within decades of the outbreak of the Black Death and has been argued to stem from high plague mortality and outward migration (despite low data resolution that ranges between 50 and 100 y).
Abundant palynological data exist also for the first millennium CE in the eastern Mediterranean (50, 51), with aggregated data available for large regions in Greece, Bulgaria, and Turkey [47, following the methods of Izdebski et al. (49), which are more precise and robust than those of Lagerås et al. (43)] (see SI Appendix, Fig. S4 for a detailed map). If the outbreak of the JP in 541 reduced anthropogenic pressure on the landscape in a manner similar to the outbreak of the Black Death in 1346 to 1353, these pollen data should reveal it in the form of significant trend changes over the 6th century record.
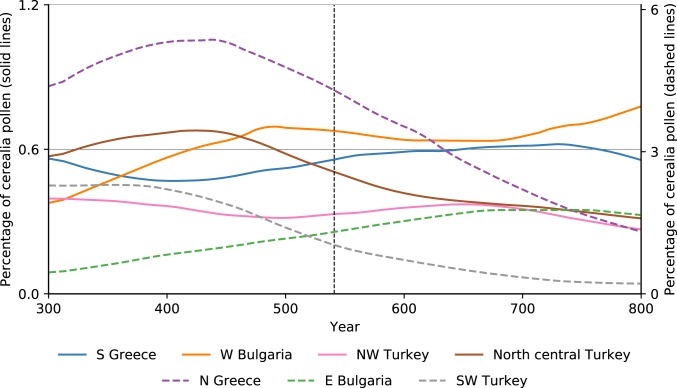
Following an earlier study of the region’s palynology (47), we considered the 2 most important indicators. Cerealia-type pollen represents the proportion of the pollen of cultivated cereals (wheat, barley, rye, and oat) in a given sample. This is a proxy for the overall spatial scale of cereal cultivation in a given region and should reflect the impact that plague-related depopulation could have on a regional landscape. The results for the northeastern Mediterranean in the first millennium CE are shown in Fig. 10. The 6th century saw no significant change in the overall trend in the proportion of cereal pollen. Regions already experiencing a decline in cereal cultivation continue the same trend (e.g., northern Greece, related to warfare and invasions in the Balkans), and regions where cereal cultivation was expanding did not experience a reversal in this trend (e.g., southern Greece, related to the increased demand for grain after the founding of Constantinople). Therefore, the most important pollen indicator for plague- and demography-related landscape change, which has been used to trace the environmental impact of the Black Death, does not show similar effects for the outbreak of the JP.
Fig. 10.
Regional percentage values of cereal pollen for selected eastern Mediterranean regions. The values for northern Greece, eastern Bulgaria, and southwest Turkey appear on the secondary (right) axis (47).
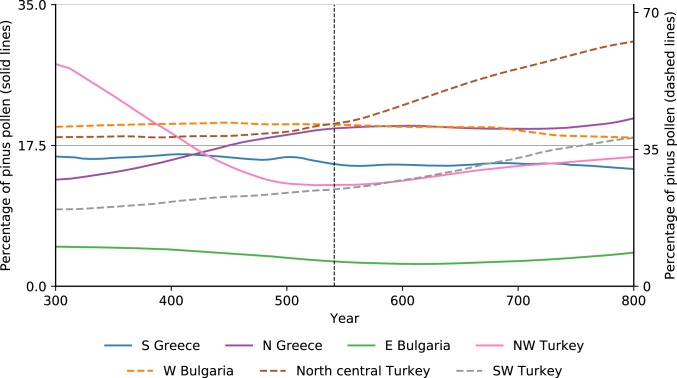
Since pine is one of the first plants to flourish on abandoned agricultural land and pastures in the northern parts of the eastern Mediterranean (true today and in late antiquity), its pollen can be used to approximate the scale of the rewilding of the landscape (52). As Fig. 11 demonstrates, there is no unexpected mid-6th century increase in pine pollen that would attest to the quick growth of pine on abandoned agricultural lands. The evidence rejects any hypothesis that the mid-6th century was a major watershed in landscape history in 6 of the 7 regions for which data are available. Although north central Turkey (inland Pontus, Amasya-Tokat area) shows a change in the proportional values of pine pollen around the 6th century, there is no written evidence to tie changes there to the JP. Northwest Turkey (lower Sakarya valley) displays the beginning of a minor trend that appears insignificant within the broader context.
Fig. 11.
Regional percentage values of pine pollen for selected eastern Mediterranean regions. Values for north-central Turkey, western Bulgaria, and southwestern Turkey appear on the secondary (right) axis (47).
Additionally, the most highly-resolved pollen record from the eastern Mediterranean covering this period shows no evidence of catastrophic agrarian decline or land abandonment during the mid-6th century. The sediments from Nar lake in Cappadocia are annually layered and pollen has been sampled at a 20-y time interval (53). Whereas the pollen of cereals and olive trees declines abruptly and catastrophically at the end of the 7th century, coincident with the early Islamic conquests, there was no such impact during the decades following the first occurrence of the plague pandemic, indicating rural landscape continuity.
In sum, palynological data from several eastern Mediterranean regions, including some close to important Roman trade networks and large urban centers, such as Constantinople, said to have been devastated by plague, not only fail to support the maximalist interpretation of the JP, but provide evidence for rejecting the 14th century Black Death as a model for the 541 outbreak of the JP. These data do not show an impact following the initial occurrence of the JP on landscapes in the eastern Mediterranean that is in any way similar to the impact that the Black Death had on landscapes in Europe.
Plague DNA, Epidemiology, and Ecology
One line of evidence that has, despite its novelty, received significant attention in histories of the JP and the First Pandemic is the isolation of Y. pestis DNA from the skeletal remains of late antique plague victims. Successful captures of the pathogen have, for most scholars, resolved a controversial debate about the plague diagnosis of the JP and the First Pandemic (e.g., refs. 21, 54, and 55). They are also employed to uphold the claim that the JP and late antique plague recurrences killed millions.
To date, Y. pestis DNA has been isolated from ∼45 individuals in central and western Europe dating to late antiquity (SI Appendix, Table S1). The identification of the plague pathogen in late antique skeletal assemblages is often construed to suggest that the JP and late antique plague recurrences killed a very large number of people (1, 6, 17, 22, 40), but this is merely a conjecture. It cannot be assumed that Y. pestis killed thousands or millions everywhere it is found. In the absence of written or serial evidence for demographic trends, such as that available for the Second Pandemic, the evidence presented above is among the most representative of population trends during the First Pandemic and it fails to support the claim that the JP significantly impacted population levels.
False-negatives are a reality for several reasons (SI Appendix), but few Y. pestis DNA finds correlate spatiotemporally (roughly or with precision) to documented outbreaks of late antique plague, such as Procopius’ account of the JP at Constantinople. Furthermore, few Y. pestis isolations are associated with mass graves (SI Appendix, Table S1). While such burials are not necessarily markers of plague outbreaks, as discussed below, they have received much attention in relation to the JP (1, 2). Roughly 40 mass graves (5 or more simultaneous inhumations) in the Mediterranean region and Europe are known for the 6th and 7th centuries, which surpasses the number known for the 5th and 8th centuries (1, 2), but this is nonetheless a minuscule tally. Several factors account for the failure to isolate Y. pestis DNA consistently or at all from mass graves and areas where large plague outbreaks are reported (SI Appendix), but the current concentration of positive detections in other contexts does not support maximalist accounts.
Some of the early attempts to isolate Y. pestis DNA remain problematic and incomplete (55, 56). For example, PCR-amplified Y. pestis DNA, identified in mass graves from Sens and Vienne (SI Appendix, Table S1), could not have been present in those remains or was mistyped, as it was identified as a branch 1 strain (Orientalis) and, likely evolved less than 250 y ago (15, 16).
The more recent single-nucleotide polymorphism (SNP)-typing and genomic work that has now identified Y. pestis in England, France, Germany, and Spain (4, 16, 17) provides several important insights on First Pandemic plague. It is now clear, for example, that the JP and the Black Death were separate emergences of a very similar yet distinct Y. pestis strain from what are likely to have been wild (but as of yet unidentified) rodent reservoirs, that plague was present in England in the mid-6th century, that Y. pestis diversified and was sustained in Europe during the 6th and 7th centuries, and that late antique plague strains interleave Y. pestis strains isolated from wild rodents and their fleas in Xinjiang, China, and neighboring Kyrgyzstan. Available data also suggest that the JP resulted from a singular emergence with what appears presently to be low genetic diversity, although this is a common characteristic of this clonal pathogen and the available sample size is small.
As valuable as these insights are, they do not lend credence to maximalist interpretations of the JP or the First Pandemic as some suppose. In fact, based on current genetic evidence, it could be argued that plague was less prevalent in the First Pandemic than the second. Y. pestis genomes isolated from late antique remains have no known descendants, meaning either that they have yet to be sampled in an existing plague reservoir or that they went extinct as many Y. pestis strains have over the bacterium’s evolutionary history (4, 16, 17, 57). This stands in current contrast with Second Pandemic strains, the most basal of which sits very close to the base of a polytomy of multiple branches on the Y. pestis tree, which are in turn very closely related (putatively directly ancestral) to Third Pandemic strains and others disseminated across the globe today (58). That currently sequenced Y. pestis strains associated with the JP and the First Pandemic are not directly ancestral to later strains could mean that late antique plague was not established in multiple, long-lived, or highly active reservoirs in any region. This includes western Eurasia and North Africa, where the First Pandemic is known to have spread, central Asia, where it may have emerged and where other plague strains have long persisted, and East Africa, from where some scholars hypothesize the JP reached the Mediterranean (4, 16, 58, 59). Notably, the fewer, shorter lived, and less active the reservoirs, the less human plague there will be.
Furthermore, the recent detection of virulence-linked gene loss in some JP-era Y. pestis genomes, one of which dates to within 80 y of the onset of the First Pandemic, may be important (16). This gene decay may have “facilitated immune evasion,” as previously suggested (16), or it may indicate that the pathogen adjusted to European reservoir species or that it went through attenuation not long after the First Pandemic commenced. This attenuation could have minimized the number of successful transmissions (i.e., virulence) and, therefore, the number of potentially infected individuals. Thus, while the isolation of Y. pestis from 7th and possibly 8th century contexts (SI Appendix, Table S1) indicates late antique Mediterranean or European plague reservoirs maintained the pathogen for decades or longer, and while plague has been isolated from late antique individuals from multiple European regions, available genomic data leave open the possibility that First Pandemic plague was less prevalent than Second Pandemic plague.
Many questions remain unanswered, and more genetic data are certain to emerge, but in the available DNA evidence itself one cannot find confirmation or support of maximalist readings of the JP or First Pandemic as previously claimed. It is also critical to emphasize that our understanding of JP-era Y. pestis is incomplete. Most reconstructed First and Second Pandemic genomes are draft genomes; they have been, as future reconstructed genomes will also be, enriched via the use of baits (small RNA molecules), designed on the basis of existing genomic diversity of Y. pestis from publicly available databases (60). Truly “novel” sequence, in late antique Y. pestis genomes, which might have enabled transmission and thus wide dissemination for instance, will not, under the current methods, be detected, unless enough Y. pestis DNA preserves in an individual from which the genome can be de novo reconstructed.
To understand the scope and probable demographic toll of the JP and the First Pandemic, an idea of how the disease spread within and between settlements would be very valuable. The debate about the mechanics of the Black Death’s dissemination within Mediterranean and European spheres, and beyond, continues, as it does for later Second Pandemic outbreaks (9, 54, 61). With far less evidence for the JP and First Pandemic plague, we are unlikely to solve this problem soon, if at all. Plague is an ecologically complex disease and a number of variables must coalesce for it to spread widely and kill many. The well-documented Third Pandemic demonstrates how, in a more recent context, mortality and prevalence were considerably lower than the Second Pandemic and highly variable on regional and local scales.
That transmission mechanics of Y. pestis during late antiquity remain unknown must further caution us against claiming that the JP or late antique plague in general was nearly omnipresent. Indeed, for over a century scholars have debated how the JP diffused within and between settlements. Current evidence and models implicate sylvatic and commensal rodents, rodent fleas, human ectoparasites (fleas and lice), and respiratory transmission (pneumonic plague). Gastrointestinal plague has hitherto received less attention. Although transmission mechanics greatly determine probable disease incidence, prevalence, and mortality, there is currently no way to discern how the JP or First Pandemic recurrences spread. Therefore, mortality estimates for settlements or regions where the disease was present remain wholly speculative. That potential for Y. pestis dispersal and transmission mechanics would have differed greatly between settlements and regions in late antiquity, as they have in other periods, complicates this matter considerably.
Archaeological Contributions Reveal Continuity Rather than Change
In recent JP literature, evidence of multiple or mass burials has been used as a proxy for high plague mortality, especially through association with positive ancient DNA tests of remains from those burials. The archaeological contexts in which Y. pestis has been identified, however, have garnered less attention (1, 2). For example, much attention has been devoted to the late antique cemetery of Aschheim, Bavaria. The first reliable isolations of JP-era Y. pestis came from individuals buried there (SI Appendix, Table S1). Although maximalists made much of these discoveries (3, 6), little effort was made to contextualize them. Significantly, reconstructed demographics for Aschheim reveal population fluctuations that are incompatible with the maximalist narrative: While a small decrease in the settlement’s population occurred in the decades around the JP’s outbreak, it increased sharply immediately afterward (SI Appendix, Fig. S5) (62). Similarly, we must rethink our approach to the relationship between burial practices and late antique plague, particularly in the light of still insufficient DNA testing of mass burials (SI Appendix, Table S1).
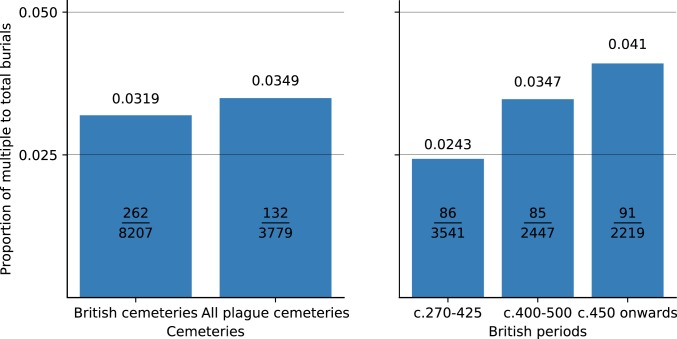
Not all mass or multiple burials are plague pits or the result of disease. Burial in the same grave may reflect cultural factors, such as social or family ties (63), rather than disease-related deaths. As Fig. 12 demonstrates (coverage in SI Appendix, Fig. S6 and Table S2), there is no obvious difference between the ratio of multiple burials to total burials found in all of the cemeteries in which Y. pestis has been identified to date, and the equivalent ratio from a near-comprehensive survey of inhumation burials in Britain between the mid-4th through the 6th and early 7th centuries. In other words, the onset of the JP did not result in an increase in the multiple burial ratio in the subsequent few centuries.
Fig. 12.
(Left) Multiple inhumation burials from all First Pandemic cemeteries in which Y. pestis was isolated compared to a near comprehensive British dataset of burials. (Right) Increasing frequency in multiple inhumation burials began in Britain at least a century before the JP (16, 64, 65).
Notably, a closer examination of the British data reveals that burials with multiple inhumations had been slowly increasing since the 4th century. In a study of 8,207 burials across the island from cemeteries used in the late Roman and early medieval periods, the data demonstrate that multiple burials (both contemporary and consecutive) were increasing (64, 65). They comprised ∼2.43% of 3,541 graves from cemeteries used in the late Roman and early post-Roman periods, 3.47% of 2,447 burials in cemeteries used from circa 400 to 500, and 4.10% of 2,219 graves begun in the second half of the 5th century. Hence, the ratio of multiple burials reached the level associated with the JP around the mid-5th century, if not earlier. Similar analysis is needed for the continent for more secure results, but the prevalence of multiple inhumations was not linked to the JP in Britain at least, despite the DNA evidence for plague there in the 6th century and the emphasis of Bede and others on “pestilence” and “mortality” (16, 41).
Many individuals were interred in multiple burials in late Roman and early-medieval Britain for reasons having nothing to do with their cause of death. Social identity(-ies) were likely more important factors than cause of death in the choice to include the individuals in multiple burials. Stoodley (65) has demonstrated that there is a gendered patterning to multiple burials of individuals using Anglo-Saxon material culture: Infants and young children were more likely to be buried with adult females, while older children were more likely to be buried with adult males. Children in particular may have been included in multiple inhumations because their communities ascribed to them different social identities than adults (67). Both interpretations, gendered patterning and social identity, are convincing because children, given their less-robust immune systems, are rarely buried together, suggesting that multiple burials had multiple meanings, and often had nothing to do with the simultaneous death of the individuals from disease (66, 67).
Even in the event of contemporary death and inhumation of more than 1 individual within a community, Y. pestis need not be the cause. Poor health and inadequate diets in late antiquity compromised immune systems and made death from disease more likely, especially for younger individuals in urban environments (68, 69). Physical and nutritional stress contributed to the impact of epidemics, including those of Y. pestis (70). Furthermore, one must not ascribe all multiple fatalities to the JP. Other epidemics, perhaps pandemics and subsistence crises, negatively impacted late antique populations (21, 71, 72). Their victims filled mass graves too. Diseases such as malaria could have also resulted in atypical morbidity patterns (73, 74). Importantly, the pathogenic remnants of some of these diseases (notably RNA viruses) are difficult to detect today (60).
Upon closer examination, the claim that trends in multiple burials evidence plague mortality in late antiquity is dubious. Late antique burial customs—in the form of the social identities created for the deceased by the living—are a more likely explanation for the practice of multiple burials than the JP. Even in the case of simultaneous or near-simultaneous death within the same community, non-JP epidemics and food shortages could be the cause. Further genetic research into late antique Y. pestis, therefore, warrants random sampling of single burials.
Conclusion
The consensus maximalist position asserts that the JP reduced the population of the late antique Mediterranean and Europe by more than a third, killing tens of millions and ending antiquity. Such extraordinary claims demand extraordinary evidence. We find little evidential support for the claim that the JP was a watershed event. In this paper, we have introduced recent evidence and contextualized old evidence relevant to the discussion of the first recorded plague pandemic. We have built upon work in distinct but complementary fields of study, integrating multiple independent high-resolution lines of data: Papyri, inscriptions, and coins, alongside more comprehensive surveys of lower-resolution data, such as pollen and burials. We also critically assessed late antique Y. pestis finds.
Our survey of this evidence reveals that a massive plague mortality is all but invisible in contemporary quantitative datasets. We contend that this is sufficient evidence to reject the current scientific and humanistic consensus of the JP as a major driver of demographic change in the 6th century Mediterranean region.
Although precise estimates are beyond the currently available evidence, the multiple quantitative and qualitative datasets collected here indicate no plague-related empire-wide demographic contraction. We have no more basis for the current maximalist estimate of a 50% demographic loss than we would have for a minimalist estimate of 0.1%. Late antique plague mortality was spatially uneven, as plague ecology and epidemiology dictates. Some regions may have suffered higher mortality at certain times—such as Constantinople during the first outbreak—but it cannot be assumed all did. On the basis of the available data, sudden and dramatic population loss was almost certainly the exception, not the rule. All high-resolution and most low-resolution quantitative measures the authors know of show no observable effect of plague on late antique demographics.
We suggest that further research should analyze plague events at the local level in regions endowed with multiple lines of evidence instead of constructing grand narratives of “Roman decline” and demographic collapse. For each site, the available direct and indirect evidence for plague should be employed to understand if and how disease transformed local communities. Such microstudies would serve as a foundation for a more nuanced interpretation of late antique plague.
Based on the evidence presented above, we believe that the JP and the so-called “First Pandemic” bear comparatively little resemblance to the Second Pandemic and the Black Death, which significantly affected the demography, economy, and landscape of western Eurasia and North Africa (75–77). In light of the paucity of supporting evidence, the “First Pandemic” label is problematic. More broadly, the scholarly treatment of the JP demonstrates the dangers of uncritical multidisciplinarity (78). Although interdisciplinary approaches to the study of the past frequently advance research, they can also inadvertently employ circular reasoning to produce and reinforce erroneous narratives that are often difficult to detect.
Supplementary Material
Acknowledgments
We thank Princeton University’s History Department for supporting this project; David Gyllenhaal (Princeton University), who contributed to the Syriac sources in SI Appendix, Table S1; John Haldon (Princeton University) and Neil Roberts (University of Plymouth and Oxford University) for commenting on drafts of this paper; and Bernard Palme (University of Vienna) for commenting on the papyri section. The work of L.M., M.E., T.P.N., and A.I. was supported by Princeton’s Climate Change and History Research Initiative. L.M. and M.E.’s work was also supported by the National Socio-Environmental Synthesis Center funded by the National Science Foundation (DBI-1639145). T.P.N. acknowledges support from the Georgetown Environmental Initiative. A.I. recognizes funding received from the Max Planck Society and from Poland’s Ministry of Science and Higher Education (2012–2014, 2016–2019). J.E.K. recognizes support from an American Council of Learned Societies/Mellon Dissertation Fellowship. J.E.K. and M.E. acknowledge support from Princeton University’s Center for Digital Humanities. H.P. recognizes support from a Social Sciences and Humanities Research Council of Canada Insight Grant, the Canadian Research Council Program, Canadian Institute for Advances Research, and McMaster University.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. N.L. is a guest editor invited by the Editorial Board.
Data deposition: The data aggregated in this study have been deposited in GitHub, https://github.com/leemordechai/PNAS-JP.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1903797116/-/DCSupplemental.
References
- 1.McCormick M., Tracking mass death during the fall of Rome’s empire (I). J. Roman Archaeol. 28, 325–357 (2015). [Google Scholar]
- 2.McCormick M., Tracking mass death during the fall of Rome’s empire (II): A first inventory of mass graves. J. Roman Archaeol. 29, 1004–1046 (2016). [Google Scholar]
- 3.Mitchell S., A History of the Later Roman Empire, AD 284-641 (Wiley Blackwell, Chichester, ed. 2, 2015). [Google Scholar]
- 4.Feldman M., et al. , A high-coverage Yersinia pestis genome from a sixth-century Justinianic Plague victim. Mol. Biol. Evol. 33, 2911–2923 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meier M., The ‘Justinianic Plague’: The economic consequences of the pandemic in the eastern Roman empire and its cultural and religious effects. Early Mediev. Eur. 24, 267–292 (2016). [Google Scholar]
- 6.Harper K., The Fate of Rome: Climate, Disease, and the End of an Empire (Princeton University Press, Princeton, 2017). [Google Scholar]
- 7.Mordechai L., Eisenberg M., Rejecting catastrophe: The case of the Justinianic Plague. Past Present 244, 3–50 (2019). [Google Scholar]
- 8.Eisenberg M., Mordechai L., The Justinianic Plague: An interdisciplinary review. Byzantine Mod. Greek Stud. 43, 156–180 (2019). [Google Scholar]
- 9.Green M., Taking “pandemic” seriously: Making the Black Death global. Medieval Globe 1, 27–61 (2014). [Google Scholar]
- 10.Nutton V., “Introduction” in Pestilential Complexities: Understanding Medieval Plague, Nutton V., Ed. (Wellcome Trust Centre for the History of Medicine at UCL, London, 2008), pp. 1–16. [PMC free article] [PubMed] [Google Scholar]
- 11.Royer K., “The blind men and the elephant: Imperial medicine, medieval historians and the role of rats in the historiography of plague” in Medicine and Colonialism, Bala P., Ed. (Pickering & Chatto, London, 2015), pp. 113–124. [Google Scholar]
- 12.Simpson W. J., A Treatise on Plague: Dealing with the Historical, Epidemiological, Clinical, Therapeutic and Preventive Aspects of the Disease (Cambridge University Press, Cambridge, 1905). [Google Scholar]
- 13.Bos K. I., et al. , A draft genome of Yersinia pestis from victims of the Black Death. Nature 478, 506–510 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haensch S., et al. , Distinct clones of Yersinia pestis caused the black death. PLoS Pathog. 6, e1001134 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harbeck M., et al. , Yersinia pestis DNA from skeletal remains from the 6th century AD reveals insights into Justinianic Plague. PLoS Pathog. 9, e1003349 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller M., et al. , Ancient Yersinia pestis genomes from across western Europe reveal early diversification during the first pandemic (541-750). Proc. Natl. Acad. Sci. U.S.A. 116, 12363–12372 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner D. M., et al. , Yersinia pestis and the plague of Justinian 541-543 AD: A genomic analysis. Lancet Infect. Dis. 14, 319–326 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Heather P., The Fall of the Roman Empire: A New History of Rome and the Barbarians (Oxford University Press, Oxford, UK, 2005). [Google Scholar]
- 19.Haldon J., The Empire that Would Not Die (Harvard University Press, Cambridge, MA, 2016). [Google Scholar]
- 20.Biraben J. N., Le Goff J., “The plague in the early Middle Ages” in Biology of Man in History, Forster R., Ranum O., Eds. (The Johns Hopkins University Press, Baltimore, 1975), pp. 48–80. [Google Scholar]
- 21.Stathakopoulos D. C., Famine and Pestilence in the Late Roman and Early Byzantine Empire: A Systematic Survey of Subsistence Crises and Epidemics (Ashgate Publishing Ltd., Aldershot, UK, 2004). [Google Scholar]
- 22.Gruber H., Indirect evidence for the social impact of the Justinianic Pandemic: Episcopal burial and conciliar legislation in Visigothic Hispania. J. Late Antiq. 11, 193–215 (2018). [Google Scholar]
- 23.Sarris P., The Justinianic plague: Origins and effects. Contin. Chang. 17, 169–182 (2002). [Google Scholar]
- 24.Baillie M. G. L., Suck-in and smear: Two related chronological problems for the 90s. J Theor. Archaeol. 2, 12–16 (1991). [Google Scholar]
- 25.Tsiamis C., Epidemic waves during Justinian’s plague in the Byzantine Empire (6th-8th c. AD). Vesalius 17, 36–41 (2011). Erratum in: Vesalius17, 41 (2011). [PubMed] [Google Scholar]
- 26.Procopius, Wars (Loeb Classical Library, Macmillan, London, 1914).
- 27.W. Witakowski (Translator), “Pseudo-Dionysius of Tel-Mahre, Chronicle Part III” in Translated Texts for Historians LUP (Liverpool University Press, 1996).
- 28.Michael the Syrian, Chronique de Michel le Syrien, Patriarche Jacobite d’Antioche, 1166-1199 (Leroux, Paris, 1901), vol. 2. [Google Scholar]
- 29.Procopius , Secret History (Loeb Classical Library, Macmillan, London, 1935), vol. 6. [Google Scholar]
- 30.Ambraseys N., Earthquakes in the Mediterranean and Middle East: A Multidisciplinary Study of Seismicity up to 1900 (Cambridge University Press, Cambridge, 2009). [Google Scholar]
- 31.Theophanes Confessor , The Chronicle of Theophanes Confessor: Byzantine and Near Eastern History, AD 284-813 (Clarendon Press, Oxford, 1997). [Google Scholar]
- 32.Agapios , Kitab al-’Unwan (Patrologia Orientalis, Firmin-Didot, Paris, 1912). [Google Scholar]
- 33.Dekkers E., Gaar E., Eds. Clavis Patrum Latinorum. Editio Tertia Aucta et Emendata (Steenbrugge, Abbatia S. Petri, 1995). [Google Scholar]
- 34.Frier B. W., et al., Eds., The Codex of Justinian : A New Annotated Translation, with Parallel Latin and Greek Text Based on a Translation by Justice Fred H. Blume (Cambridge University Press, Cambridge, 2016). [Google Scholar]
- 35.Honoré T., Tribonian (Duckworth, London, 1978). [Google Scholar]
- 36.Miller D., Sarris P., Eds., The Novels of Justinian: A Complete Annotated English Translation (Cambridge University Press, New York, 2018). [Google Scholar]
- 37.Bransbourg G., Capital in the sixth century: The dynamics of tax and estate in Roman Egypt. J. Late Antiq. 9, 305–414 (2016). [Google Scholar]
- 38.Papaconstantinou A., “Egypt” in The Oxford Handbook of Late Antiquity, Fitzgerald J. S., Ed. 2012), pp. 195–223. [Google Scholar]
- 39.Croke B., Ed. The Chronicle of Marcellinus: A Translation with Commentary (Brill, 1995). [Google Scholar]
- 40.Sallares R., “Ecology, evolution, and epidemiology of plague” in Plague and the End of Antiquity: The Pandemic of 541-750, Little L. K., Ed. (Cambridge University Press, Cambridge, 2007), pp. 231–289. [Google Scholar]
- 41.Maddicott J., “Plague in seventh-century England” in Plague and the End of Antiquity: The Pandemic of 541-750, Little L. K., Ed. (Cambridge University Press, Cambridge, 2007), pp. 171–214. [Google Scholar]
- 42.Ersgård L., “Change, desertion and survival: An archaeology of the late-medieval crisis” in Environment, Society and the Black Death: An Interdisciplinary Approach to the Late-Medieval Crisis in Sweden, Ersgård L., Ed. (Oxbow Books, Oxford, 2016), pp. 69–103. [Google Scholar]
- 43.Lagerås P., et al. , “Abandonment, agricultural change and ecology” in Environment, Society and the Black Death: An Interdisciplinary Approach to the Late-Medieval Crisis in Sweden, Lagerås P., Ed. (Oxbow Books, Oxford, 2016), pp. 30–68. [Google Scholar]
- 44.Yeloff D., Geel B. V., Abandonment of farmland and vegetation succession following the Eurasian plague pandemic of AD 1347–52. J. Biogeogr. 34, 575–582 (2007). [Google Scholar]
- 45.van Hoof T. B., Bunnik F. P. M., Waucomont J. G. M., Kürschner W. M., Visscher H., Forest re-growth on medieval farmland after the Black Death pandemic—Implications for atmospheric CO2 levels. Palaeogeogr. Palaeoclimatol. Palaeoecol. 237, 396–409 (2006). [Google Scholar]
- 46.Gogou A., et al. , Climate variability and socio-environmental changes in the northern Aegean (NE Mediterranean) during the last 1500 years. Quat. Sci. Rev. 136, 209–228 (2016). [Google Scholar]
- 47.Izdebski A., Koloch G., Słoczyński T., Exploring Byzantine and Ottoman economic history with the use of palynological data: A quantitative approach. Jahrbuch der Österreichischen Byzantinistik 65, 67–110 (2015). [Google Scholar]
- 48.Mensing S. A., et al. , Historical ecology reveals landscape transformation coincident with cultural development in central Italy since the Roman Period. Sci. Rep. 8, 2138 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Izdebski A., Koloch G., Słoczyński T., Tycner M., On the use of palynological data in economic history: New methods and an application to agricultural output in Central Europe, 0–2000AD. Explor. Econ. Hist. 59, 17–39 (2016). [Google Scholar]
- 50.Izdebski A., Średniowieczni Rzymianie i Przyroda. Interdyscyplinarna Historia Środowiskowa (Historica Iagiellonica, Kraków, 2018). [Google Scholar]
- 51.Roberts N., et al. , Not the end of the world? Post-classical decline and recovery in rural Anatolia. Hum. Ecol. 46, 305–322 (2018). [Google Scholar]
- 52.Izdebski A., A Rural Economy in Transition: Asia Minor from Late Antiquity into the Early Middle Ages (Warsaw: Fundacja im. Rafała Taubenschlaga, 2013). [Google Scholar]
- 53.England A., Eastwood W. J., Roberts C. N., Turner R., Haldon J. F., Historical landscape change in Cappadocia (central Turkey): A palaeoecological investigation of annually laminated sediments from Nar lake. Holocene 18, 1229–1245 (2008). [Google Scholar]
- 54.Cohn S. K., Jr, Epidemiology of the Black Death and successive waves of plague. Med. Hist. Suppl. 52, 74–100 (2008). [PMC free article] [PubMed] [Google Scholar]
- 55.Drancourt M., et al. , Genotyping, Orientalis-like Yersinia pestis, and plague pandemics. Emerg. Infect. Dis. 10, 1585–1592 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drancourt M., et al. , Yersinia pestis Orientalis in remains of ancient plague patients. Emerg. Infect. Dis. 13, 332–333 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Z., et al. , The user’s guide to comparative genomics with EnteroBase. Three case studies: Micro-clades within Salmonella enterica serovar Agama, ancient and modern populations of Yersinia pestis, and core genomic diversity of all Escherichia. bioRxiv:10.1101/613554 (18 April 2019).
- 58.Cui Y., et al. , Historical variations in mutation rate in an epidemic pathogen, Yersinia pestis. Proc. Natl. Acad. Sci. U.S.A. 110, 577–582 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Green M. H., Putting Africa on the Black Death map: Narratives from genetics and history. Afriques, 10.4000/afriques.2125 (2018).
- 60.Marciniak S., Poinar H., “Ancient pathogens through human history: A paleogenomic perspective” in Paleogenomics, Genome-Scale Analysis of Ancient DNA, Lindqvist C., Rajora O., Eds. (Springer, New York, 2018), pp. 115–138. [Google Scholar]
- 61.Dean K. R., et al. , Human ectoparasites and the spread of plague in Europe during the Second Pandemic. Proc. Natl. Acad. Sci. U.S.A. 115, 1304–1309 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Staskiewicz A., “The early medieval cemetery at Aschheim-Bajuwarenring–A Merovingian population under the influence of pestilence?” in Skeletal Series and Their Socio-Economic Context, Grupe G., Ed. (Documenta Archaeobiologiae, Marie Leidorf, Rahden/Westfalen, 2007), pp 35–56. [Google Scholar]
- 63.Rott A., Päffgen B., Haas-Gebhard B., Peters J., Harbeck M., Family graves? The genetics of collective burials in early medieval southern Germany on trial. J. Archaeol. Sci. 92, 103–115 (2018). [Google Scholar]
- 64.Kay J. E., Children’s burials in fifth-century Britain and connections to the Roman past. Child. Past 9, 86–108 (2016). [Google Scholar]
- 65.Kay J. E., “Old, new, borrowed, and buried: Burial practices in fifth-century Britain, 350–550 CE”, thesis, Boston College, Chestnut Hill, MA (2017).
- 66.Stoodley N., “Multiple burials, multiple meanings? Interpreting the early Anglo-Saxon multiple interment” in Burial in Early Medieval England and Wales, Lucy S., Reynolds A., Eds. (Society for Medieval Archaeology, London, 2002), pp. 103–121. [Google Scholar]
- 67.Crawford S., “Companions, co-incidences or chattels? Children in the early Anglo-Saxon multiple burial ritual” in Children, Childhood and Society, Crawford S., Shepherd G., Eds. (Archaeopress, Oxford, 2007), pp. 83–92. [Google Scholar]
- 68.Redfern R. C., DeWitte S. N., Pearce J., Hamlin C., Dinwiddy K. E., Urban-rural differences in Roman Dorset, England: A bioarchaeological perspective on Roman settlements. Am. J. Phys. Anthropol. 157, 107–120 (2015). [DOI] [PubMed] [Google Scholar]
- 69.Rohnbogner A., Listening to the kids: The value of childhood palaeopathology for the study of rural Roman Britain. Britannia 48, 221–252 (2017). [Google Scholar]
- 70.DeWitte S. N., Setting the stage for medieval plague: Pre-black death trends in survival and mortality. Am. J. Phys. Anthropol. 158, 441–451 (2015). [DOI] [PubMed] [Google Scholar]
- 71.Bonser W., The Medical Background of Anglo-Saxon England: A Study in History, Psychology, and Folklore (Wellcome Historical Medical Library, 1963). [Google Scholar]
- 72.Newfield T. P., Mysterious and mortiferous Clouds: The climate cooling and disease burden of Late Antiquity. Late Antiq. Archaeol. 12, 271–297 (2018). [Google Scholar]
- 73.Marciniak S., et al. , Plasmodium falciparum malaria in 1st-2nd century CE southern Italy. Curr. Biol. 26, R1220–R1222 (2016). [DOI] [PubMed] [Google Scholar]
- 74.Newfield T. P., Malaria and malaria‐like disease in the early Middle Ages. Early Mediev. Eur. 25, 251–300 (2017). [Google Scholar]
- 75.Benedictow O. J., The Black Death, 1346-1353: The Complete History (Boydell Press, Woodbridge, Suffolk, UK, 2004). [Google Scholar]
- 76.Varlık N., Plague and Empire in the Early Modern Mediterranean World: The Ottoman Experience, 1347–1600 (Cambridge University Press, Cambridge, 2015). [Google Scholar]
- 77.Campbell B., The Great Transition: Climate, Disease and Society in the Late-Medieval World (Cambridge University Press, Cambridge, 2016). [Google Scholar]
- 78.Haldon J., et al. , History meets palaeoscience: Consilience and collaboration in studying past societal responses to environmental change. Proc. Natl. Acad. Sci. U.S.A. 115, 3210–3218 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blume F. H., Annotated Justinian code, http://www.uwyo.edu/lawlib/blume-justinian/ajc-edition-2/novels/index.html. Accessed 15 January 2019.
- 80.Noailles P., Les Collections de Novelles de l’Empereur Justinien (Recueil Sirey, Paris, 1912). [Google Scholar]
- 81.Banaji J., “Rural Communities in the Late Empire–Economic and Monetary Aspects,” PhD thesis, University of Oxford, Oxford, UK (1992).
- 82.Mazza R., L’Archivio degli Apioni: Terra, Lavoro e Proprietà Senatoria nell’Egitto Tardoantico (Edipuglia, Bari, 2001). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.