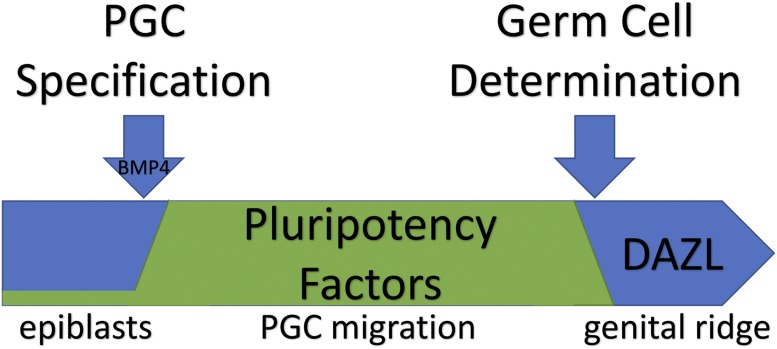
The most critical step of mammalian embryogenesis in securing the future of the species comes just days after fertilization, and immediately after implantation into the uterine wall. At this time, a small subset of epiblast cells receives an inductive signal from neighboring extraembryonic tissue, the germ lineage is specified, and the resulting primordial germ cells (PGCs) are given full potential to eclipse the lifetime of the developing embryo in which they reside (1). After specification, PGCs migrate along the hindgut and through the dorsal mesentery to the genital ridge, the precursor to the gonad. Once there, PGCs commit to their germ cell fate and become encompassed by the developing gonad, where germline stem cells proliferate and ultimately undergo meiosis to produce oocytes and sperm (Fig. 1). In PNAS, Nicholls et al. (2) describe events that transpire upon PGC colonization of the genital ridge.
Fig. 1.
Pluripotency factors are induced upon specification of primordial germ cells (PGCs) and remain on while PGCs migrate with and through the hindgut to the primitive gonad, or genital ridge. Upon their arrival, DAZL becomes expressed and turns off pluripotency factors, committing PGCs to a germ cell fate.
This journey between PGC specification and germ cell determination lasts 4 d in mice and about 3 wk in humans (3). During this transit, the fate of future generations depends on whether PGCs can preserve their identity by repressing cues that could trigger their somatic differentiation. Postimplantation epiblast cells have an epigenetic landscape poised for somatic gene expression, but inductive signals that specify PGCs from this cell population counter somatic differentiation by 1) reprogramming the epigenetic landscape, 2) silencing most transcription, and 3) expressing pluripotency factors that stifle differentiation (4). These PGC-expressed factors include SOX17, BLIMP1, PRDM14, Tfap2c, OCT4, Dnd1, and Nanos. While PGCs are protected and set apart, multiple lines of evidence suggest that the cell fate of PGCs remains undetermined as they can be easily coaxed to differentiate into all 3 germ layers (5–7).
Nicholls et al. describe how, upon arrival, PGCs stop expressing pluripotency markers, lose their somatic potential, and commit to a germ cell fate. To understand how this happens, they compare gene expression data taken from migratory and gonadal mouse and human PGCs, and then identify a conserved set of 10 germline-enriched genes that are up-regulated once cells reach the nascent gonad. Importantly, these 10 genes are up-regulated in both XX and XY germlines, suggesting they encode proteins that direct germ cell determination and not just sex-specific gamete development.
Within this set of 10 proteins are 4 that are known to localize exclusively to ribonucleoprotein assemblies in the germ plasm (DAZL, DDX4, MAEL, and TDRD12). This is a striking difference between the nuclear pluripotency factors expressed in migratory PGCs, which can also be found outside of the germline. Previous work showed that DAZL is required to isolate pluripotent embryonic germ cell lines (8) but also functions to limit the expression of pluripotency factors in vitro (9, 10). This study provides in vivo evidence that the germline expression of NANOG (a pluripotency marker) shuts down in PGCs when DAZL is expressed, as does the ability to derive pluripotent colonies from germlines. This pattern is reversed in Dazl-deficient mutants, where NANOG and the capacity to generate pluripotent colonies are retained, even 4 d after PGCs reach the genital ridge. Since Dazl-deficient mutants continue to express the set of genes that are up-regulated upon gonad colonization, the authors conclude that DAZL is shutting down germline pluripotency.
These findings have remarkable implications for testicular and ovarian cancer, and the origin of other germ cell tumors (GCTs). If PGCs do not reach their destination, if there are defects in the primitive gonad when they arrive, or if DAZL is not properly expressed, germline pluripotency factors will remain active and increase the risk of GCTs. Consistent with this, Nicholls et al. found that testicular teratomas increase in frequency from about 1% to about 30% in Dazl-deficient 129S4 mice. Ovarian tumors, which have not previously been observed in this mouse strain, were found in 10% of Dazl-deficient mice. They also found that these ovarian tumors arise from mitotic germline cells, suggesting they come from PGCs that failed to commit to a germ cell fate.
In humans, DAZL mutations are associated with low sperm count and premature ovarian failure (11). In these cases, the primary defect is the loss or reduction of germ cells instead of GCT formation, but DAZL does represent a susceptibility locus for the pathogenesis of GCTs in humans (12). One explanation is that, under normal conditions, uncommitted PGCs are actively cleared from the gonad through apoptosis. Nicholls et al. tested this theory by introducing a Dazl-null allele into Bax mutant mice. This increased the incidence of GCTs to 68% in males but did not change the incidence of GCTs in females. A conditional Dazl knockout showed that DAZL expression upon gonad colonization was sufficient to preserve germ cells through birth, although males (but not females) were sterile, suggesting a postnatal role for DAZL in males. Paralleling these findings, the authors found that most Dazl-deficient female pigs produce ovarian teratomas, while testicular teratomas were not observed in Dazl-deficient males. These findings from mice and pigs suggest that apoptosis plays a major role in clearing undetermined PGCs, primarily during spermatogenesis.
Concomitant with these observations is the question of why such a strategy for PGC specification and determination has been selected during mammalian evolution. Many invertebrate and nonmammalian vertebrates express cytoplasmic germ plasm determinants upon or soon after PGC specification instead of upon gonad colonization. Is it possible that the extended duration of mammalian PGC migration benefits from an uncommitted pluripotent state, possibly to antagonize somatic development? Does the pluripotent state of PGCs facilitate cell migration? Or, do mammalian PGCs defer germ cell determination to contribute to nongametogenic lineages? By demonstrating the conserved role of DAZL across mice, pigs, and humans, Nicholls et al. answer several outstanding questions about DAZL’s role in germ cell determination and open inquiries that pertain to the developmental role of specified, yet undetermined, migratory PGCs.
Acknowledgments
The author’s research is supported by NIH/National Institute of General Medical Sciences Grant R01 GM113933.
Footnotes
The author declares no competing interest.
See companion article on page 25677.
References
- 1.Kobayashi T., Surani M. A., On the origin of the human germline. Development 145, dev150433 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Nicholls P. K., et al. , Mammalian germ cells are determined after PGC colonization of the nascent gonad. Proc. Natl. Acad. Sci. U.S.A. 116, 25677–25687 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurimoto K., Saitou M., Epigenome regulation during germ cell specification and development from pluripotent stem cells. Curr. Opin. Genet. Dev. 52, 57–64 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Bleckwehl T., Rada-Iglesias A., Transcriptional and epigenetic control of germline competence and specification. Curr. Opin. Cell Biol. 61, 1–8 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Gross-Thebing T., et al. , The vertebrate protein dead end maintains primordial germ cell fate by inhibiting somatic differentiation. Dev. Cell 43, 704–715.e5 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Chatfield J., et al. , Stochastic specification of primordial germ cells from mesoderm precursors in axolotl embryos. Development 141, 2429–2440 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens L. C., Experimental production of testicular teratomas in the mouse. Int. J. Androl. 4 (suppl. s4), 54–59 (1981). [DOI] [PubMed] [Google Scholar]
- 8.Haston K. M., Tung J. Y., Reijo Pera R. A., Dazl functions in maintenance of pluripotency and genetic and epigenetic programs of differentiation in mouse primordial germ cells in vivo and in vitro. PLoS One 4, e5654 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung D., et al. , In vitro differentiation of human embryonic stem cells into ovarian follicle-like cells. Nat. Commun. 8, 15680 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H.-H., et al. , DAZL limits pluripotency, differentiation, and apoptosis in developing primordial germ cells. Stem Cell Reports 3, 892–904 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tung J. Y., et al. , Variants in Deleted in AZoospermia-Like (DAZL) are correlated with reproductive parameters in men and women. Hum. Genet. 118, 730–740 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Ruark E., et al. ; UK Testicular Cancer Collaboration (UKTCC) , Identification of nine new susceptibility loci for testicular cancer, including variants near DAZL and PRDM14. Nat. Genet. 45, 686–689 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]