Significance
Insecticide resistance in Anopheles gambiae mosquitoes can derail malaria control programs, and to overcome it, we need to discover the underlying molecular basis. Here, we characterize 3 genes most often associated with insecticide resistance directly by their overproduction in genetically modified An. gambiae. We show that overexpression of each gene confers resistance to representatives of at least 1 insecticide class, and taken together, the 3 genes provide cross-resistance to all 4 major insecticide classes currently used in public health. These data validate the candidate genes as markers to monitor the spread of resistance in mosquito populations. The modified mosquitoes produced are also valuable tools to prescreen the efficacy of new insecticides against existing resistance mechanisms.
Keywords: functional analysis, cytochromes P450, glutathione-S-transferase, GAL4/UAS
Abstract
Resistance in Anopheles gambiae to members of all 4 major classes (pyrethroids, carbamates, organochlorines, and organophosphates) of public health insecticides limits effective control of malaria transmission in Africa. Increase in expression of detoxifying enzymes has been associated with insecticide resistance, but their direct functional validation in An. gambiae is still lacking. Here, we perform transgenic analysis using the GAL4/UAS system to examine insecticide resistance phenotypes conferred by increased expression of the 3 genes—Cyp6m2, Cyp6p3, and Gste2—most often found up-regulated in resistant An. gambiae. We report evidence in An. gambiae that organophosphate and organochlorine resistance is conferred by overexpression of GSTE2 in a broad tissue profile. Pyrethroid and carbamate resistance is bestowed by similar Cyp6p3 overexpression, and Cyp6m2 confers only pyrethroid resistance when overexpressed in the same tissues. Conversely, such Cyp6m2 overexpression increases susceptibility to the organophosphate malathion, presumably due to conversion to the more toxic metabolite, malaoxon. No resistant phenotypes are conferred when either Cyp6 gene overexpression is restricted to the midgut or oenocytes, indicating that neither tissue is involved in insecticide resistance mediated by the candidate P450s examined. Validation of genes conferring resistance provides markers to guide control strategies, and the observed negative cross-resistance due to Cyp6m2 gives credence to proposed dual-insecticide strategies to overcome pyrethroid resistance. These transgenic An. gambiae-resistant lines are being used to test the “resistance-breaking” efficacy of active compounds early in their development.
The number of worldwide malaria cases reduced steadily from 2000 until 2015, mainly due to the widespread rollout of insecticide-treated bed nets in endemic areas (1), which offer protection against bites from Plasmodium-infected Anopheles mosquitoes. Since then, the drop in malaria cases has stalled (2), which has been attributed partially to the increasing levels of insecticide resistance found in Anopheles vectors (3). Resistance in dominant African Anopheles vectors has been recorded to all major insecticide classes currently used in public health (pyrethroids, organochlorines, carbamates, and organophosphates [OPs]) (4). Therefore, understanding the mechanisms by which mosquitoes evolve resistance is critical for the design of mitigation strategies and in the evaluation of new classes of insecticides.
Research into the molecular mechanisms that give rise to resistance in mosquitoes has identified target site modifications and increased metabolic detoxification (detox) as the 2 main evolutionary adaptions (5) that often coexist in Anopheles gambiae. Families of detoxification enzymes, including cytochromes P450 (CYPs) and glutathione-S-transferases (GSTs), can provide phase I metabolism of insecticides and phase II conjugation reactions that alter the toxicity of compounds and increase polarity, enhancing excretion (6, 7).
To identify and characterize the role of the causative resistance genes from these detoxification families, a sequential process of transcriptomic, proteomic, and in vivo functional analysis is often applied (8). Candidate genes with up-regulated transcription or strong signatures of selection in resistant mosquitoes are typically expressed in bacteria to provide evidence of insecticide depletion and/or metabolism in vitro (9–19). Further studies have used the Drosophila melanogaster transgenic model to determine whether expression of single Anopheles genes confers increased tolerance to insecticides (13–18, 20).
This workflow has implicated a role in resistance of 2 CYP genes, Cyp6m2 and Cyp6p3, and a GST gene, Gste2, that are consistently up-regulated in resistant field populations found across Africa (21). However, there are often discrepancies in results from recombinant protein activity and transgenic D. melanogaster analyses. For example, while expression studies of Cyp6m2 and Cyp6p3 in Escherichia coli (10, 11) and D. melanogaster (15) suggest that both gene products can detoxify pyrethroids, the 2 systems produce conflicting results in respect to carbamate (15) and organochlorine insecticide detoxification (12, 15, 19). Moreover, the involvement of An. gambiae and Anopheles funestus Gste2 (AfGste2) orthologs in resistance to pyrethroid insecticides has produced contradictory results when explored in D. melanogaster (16, 20).
Clearly, functional validation of Anopheles genes directly in the mosquito would provide the benchmark approach to address these questions; however, to date, transgenic tools to perform such analysis have been limited. To this end, we have developed the GAL4/UAS expression system in An. gambiae (22–24), which allows genes to be overexpressed in a susceptible mosquito background and for resultant resistance phenotypes to be examined using the standard insecticide assays that have been developed for comparative analysis in mosquitoes by the World Health Organization (WHO) (25).
In vivo functional analysis in Anopheles can also help discover the mosquito tissues that are specifically involved in insecticide metabolism. Our previous research indicated high P450 activity in the midgut and oenocytes, since the essential P450 coenzyme, cytochrome P450 reductase (CPR), is highly expressed in these tissues, and RNA interference (RNAi) knockdown of Cpr increased mosquito sensitivity to a pyrethroid insecticide (26). Moreover, Cyp6m2 has been reported as enriched in the An. gambiae midgut (11), and Cyp6p3 was found up-regulated in midguts from pyrethroid-resistant populations (27).
Here, we have used the GAL4/UAS system to overexpress Cyp6m2 or Cyp6p3 genes in multiple tissues or specifically in the midgut or oenocytes of a susceptible An. gambiae strain and assayed the modified mosquitoes against representatives of each insecticide class available for public health use. In doing so, we determined the resistance profile generated for each gene and compared these results with those obtained in D. melanogaster and in vitro. We then analyzed the other major candidate, Gste2, to examine its role in conferring dichloro-diphenyl-trichloroethane (DDT) resistance and also, extended its testing to other classes of insecticides in which its role has yet to be tested in vivo.
In this work, we report the use of the GAL4/UAS system in An. gambiae as a benchmark to determine whether single candidate genes and/or expression in individual tissues are able to confer WHO-defined levels of resistance to the 4 public health classes of insecticides, including OPs. Crucially we find that, when assayed in An. gambiae, overexpression of Cyp6m2, Cyp6p3, or Gste2 produces cross-resistance phenotypes that encompass members of all 4 classes of insecticides currently used for malaria control.
Results
Mosquito Lines Generated for UAS-Regulated Expression of Cyp6m2 and Cyp6P3.
YFP-marked UAS-Cyp6m2 and -Cyp6p3 lines were created by site-directed recombination-mediated cassette exchange (RMCE) into the docking (CFP:2xattP) line A11 (24) to produce mosquitoes carrying transgene insertions in the same genomic site. By normalizing potential genomic position effect, this allows more reliable comparison of the consequences of Cyp6m2 and Cyp6p3 overexpression on resistance.
A summary of the screening and crossing strategy used to create the UAS responder lines is illustrated in Table 1. RMCE results in canonical cassette exchange in 2 potential orientations; however, integration of the whole donor transgene can also occur in either attP site. Fluorescent marker screening of F1 progenies from F0 pooled mosquitoes revealed that cassette exchange and integration events occurred in all experiments as shown by the recovery of individuals carrying single (YFP: exchange) or double (YFP/CFP: integration) markers (Table 1).
Table 1.
Summary of the screening and crossing strategy adopted to create and establish the UAS responder lines by RMCE
| Docking line (no. of embryos) and F0 pools (no. and sex) | F0 isofemale | F1 transgenics | Orientation of cassette exchange* | |
| YFP+ | YFP+/CFP+ | |||
| A11_UAS-Cyp6m2 (347) | ||||
| M2-1 (24 ♀) | G | 0 | 2 | N/A |
| J | 2♂ | 0 | 2 F1 ♂-A | |
| M2-2 (25 ♂) | N/A | 0 | 0 | N/A |
| A11_UAS-Cyp6p3 (460) | ||||
| P3-1 (28 ♀) | N/A | 7♀, 4♂ | 1 | 5 F1 ♀-A ×2, B ×3 |
| P3-2 (27 ♀) | N/A | 2♀, 8♂ | 2 | 2 F1 ♀-A, B |
| P3-3 (13 ♀) | N/A | 0 | 0 | N/A |
| P3-4 (56 ♂) | N/A | 10♀, 13♂ | 4 | 3 F1 ♀-A, B ×2 |
| Ubi-A10_UAS-Gste2 (208) | ||||
| E2-1 (10 ♂) | N/A | 0 | 0 | N/A |
| E2-2 (12 ♀) | N/A | 0 | 0 | N/A |
| E2-3 (19 ♂) | N/A | 2♂ | 36♀, 44 ♂ | 2 F1 ♂-A |
| E2-4 (24 ♀) | A | 3♀, 3♂ | (7)† | F2 progeny of 1 F1 ♂-B |
| E | 4♀, 3 ♂ | 2♀, 2♂ | 1 F1 ♀-A F2 progeny of 1 F1 ♂-A | |
Numbers in brackets after the docking line names in column 1 refer to number of eggs injected. M2, P3, and E2 refer to pools of single sex F0 adults (number of mosquitoes given in brackets) collected post injection that gave rise to F1 progeny. G, J, A, and E refer to F0 females laying eggs individually that gave rise to isofemale lines. N/A, not applicable. YFP+ and YFP+/CFP+ indicate the number and sex of F1 progeny showing YFP or YFP and CFP fluorescence, respectively.
As cassette exchange may occur in 2 different orientations with respect to the chromosome, designated A or B, orientation check was performed on F1 YFP+ individuals or on the F2 progeny derived from single YFP+ individuals.
Did not survive to adulthood.
Molecular analysis revealed one exchange orientation (A) in transgenic UAS-m2 individuals and both orientations for UAS-p3 transformation as indicated by diagnostic PCR (SI Appendix, Fig. S1). Overall, we found at least 2 events for UAS-m2 transformation, having equal efficiencies of 2% for cassette exchange and integration (1/49 F0 founders); for the UAS-p3 transformation, at least 9 transformation events (6 cassette exchanges, 3 in each orientation, and 3 transgene integrations) were detected, with a minimum cassette exchange efficiency of 5% (6/124 F0) and integration efficiency of 2% (3/124 F0). For comparative functional analysis, representative Cyp6 lines in orientation A were maintained and crossed with alternative GAL4 driver lines.
CYP6M2 or CYP6P3 Overexpression in Multiple Tissues Causes Distinct Profiles of Resistance to Pyrethroids and Bendiocarb.
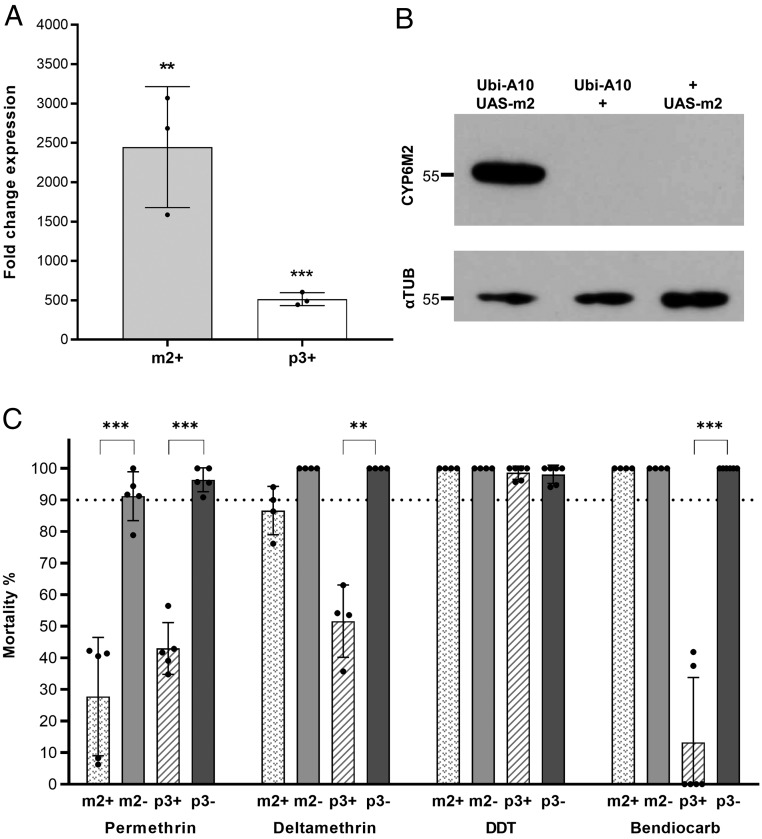
We previously described the production of a GAL4 driver line, Ubi-A10, directing widespread tissue expression (23). To quantify the overexpression achieved with this driver, we performed qRT-PCR in the progeny of Ubi-A10 driver and UAS-Cyp6 crosses. This revealed significant 2,447× (P = 0.005) and 513× (P < 0.001) increases of Cyp6m2 and Cyp6p3 transcript abundance, respectively, in adult females compared with native expression in respective controls (Fig. 1A). Western analysis also readily detected CYP6M2 in the adult female progeny of the Ubi-A10/UAS-m2 crosses but was beyond the level of detection in sibling controls (Ubi-A10/+ and +/UAS-m2) (Fig. 1B). No suitable antiserum was available for analysis of CYP6P3.
Fig. 1.
Multitissue Cyp6 gene up-regulation affects sensitivity to 2 pyrethroids and a carbamate insecticide. (A) Relative transcription levels of Cyp6m2 (m2+) and Cyp6p3 (p3+) in adult females where expression is driven by the Ubi-A10 driver compared with GAL4/+ controls. Bars represent SD (n = 3). Unpaired t test. **P < 0.01; ***P < 0.001. (B) Expression of CYP6M2 and α-tubulin in adult females from Ubi-A10 × UAS-m2 crosses with respective Ubi-A10/+ and +/UAS-m2 controls. Protein extract from the equivalent of 1/10 of a whole female mosquito was loaded in each lane. (C) Sensitivity to insecticides of GAL4/UAS (+) females overexpressing Cyp6m2 or Cyp6p3 ubiquitously under the control of the Ubi-A10 driver compared with GAL4/+ controls (−) measured by WHO tube bioassay. Bars represent SD (n = 4 to 6) (SI Appendix, Table S2). The dotted line marks the WHO 90% mortality threshold for defining resistance. Welch’s t test with P < 0.01 significance cutoff. **P < 0.01; ***P < 0.001.
WHO discriminating dose assays were then performed to assess the susceptibility of mosquitoes overexpressing Cyp6m2 or Cyp6p3 compared with their Ubi-A10/+ siblings. WHO tube bioassays are used to screen for the emergence of resistance in field populations and involve exposing mosquitoes to fixed concentration of insecticides (twice the lethal concentration that kills 99% of a susceptible population) for 60 min followed by a 24-h recovery period before recording mortality (25). The parental strains used here are susceptible (>90% mortality) to all of the insecticides tested; therefore, a decrease in mortality in test assays can be directly attributable to the overexpression of the specific candidate gene.
Mosquitoes overexpressing either Cyp6 gene under the Ubi-A10 driver showed resistance to permethrin (Cyp6m2: 28% mortality, P < 0.001; Cyp6p3: 43% mortality, P < 0.001) and deltamethrin (Cyp6m2: 88%, P = 0.04; Cyp6p3: 52%, P = 0.004) compared with controls (Fig. 1C). A significant difference in mortality was observed between mosquitoes overexpressing the 2 different Cyp6 genes for deltamethrin assays (P = 0.003), while no significant difference was observed for permethrin (P = 0.15). However, only Cyp6p3-overexpressing mosquitoes showed resistance to bendiocarb (13% mortality, P < 0.001) (Fig. 1C). No resistance to DDT was observed with either gene in conjunction with the Ubi-A10 driver (Fig. 1C).
CYP6M2 or CYP6P3 Multitissue Overexpression Increases Susceptibility to Malathion.
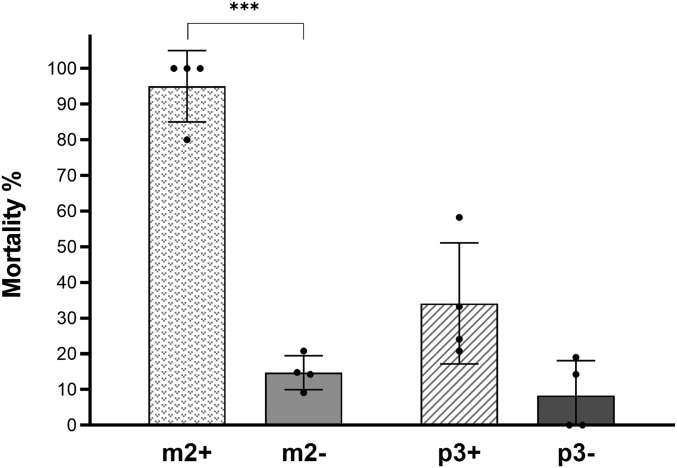
Malathion is an OP proinsecticide that is activated in vivo to the more toxic compound malaoxon through P450-based oxidative reactions (28). Preliminary analysis showed that a standard WHO diagnostic dose and 60-min exposure killed all test and control mosquitoes; however, during exposure, it was clear that Ubi-A10–directed Cyp6 overexpression induced more rapid knockdown compared with controls, suggesting malathion activation by these P450s. We, therefore, examined the relative sensitivity of mosquitoes overexpressing Cyp6m2 or Cyp6p3 when exposed to the same diagnostic dose of this OP for a shorter time (25 min) (Fig. 2). Under these conditions, mosquitoes overexpressing Cyp6m2 under the control of the Ubi-A10 driver showed significantly higher mortality rates compared with controls (95 vs. 15%, P < 0.001) and Ubi-A10/UAS-p3 mosquitoes (95 vs. 34%, P = 0.002), although the latter also showed a trend of increased mortality compared with Ubi-A10/+ controls (34 vs. 8%, P = 0.05).
Fig. 2.
Multitissue Cyp6 gene up-regulation increases sensitivity to the OP insecticide malathion (25-min exposure). Sensitivity to malathion of females overexpressing Cyp6m2 (m2+) or Cyp6p3 (p3+) ubiquitously under the control of the Ubi-A10 driver compared with respective GAL4/+ controls (m2−, p3−) measure by a modified WHO tube bioassay representing mortality rates after 25 min of exposure and 24-h recovery. Bars represent SD (n = 4) (SI Appendix, Table S2). Welch’s t test with P < 0.01 significance cutoff. ***P < 0.001.
Overexpression of GSTE2 in Multiple Tissues Causes Resistance to Diagnostic Doses of DDT and Fenitrothion.
To extend the analysis to the role of GSTE2 in insecticide resistance in An. gambiae, we utilized the previously described Ubi-A10 GAL4 line (23) as a docking line. Integration of the UAS cassette into a single docking site in this case would provide Ubi-A10GAL4 and UAS-Gste2 at the same locus (Ubi-A10GAL4:UAS-e2) and should natively overexpress Gste2 without the need for crossing separate lines. Alternatively, cassette exchange would generate a regular UAS-Gste2 responder line. After embryonic injections and screening, 3 exchange events (2 in orientation A and 1 in orientation B) (SI Appendix, Fig. S1) and 3 integration events were independently recovered with an overall transformation efficiency of 9% (6/65 F0), exchange efficiency of 5% (3/65 F0), and integration efficiency of 5% (3/65 F0) (Table 1).
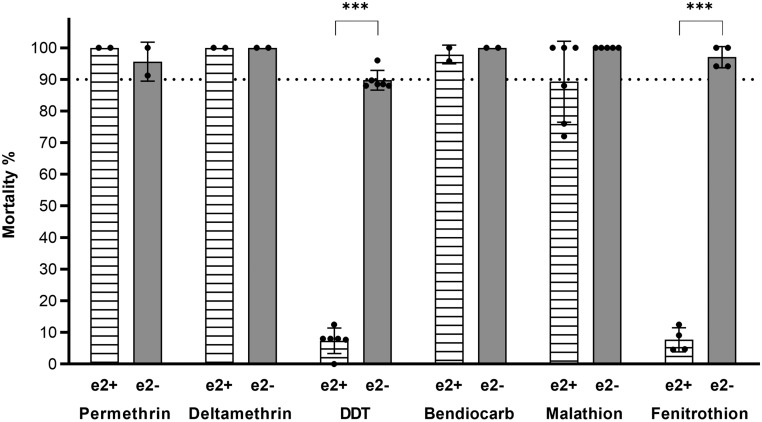
To obtain comparable data for Gste2 and the Cyp6 genes, we focused our analysis on the progeny from crosses between UAS-e2 and Ubi-A10GAL4 mosquitoes. When exposed to diagnostic doses of DDT, GSTE2-overexpressing mosquitoes showed a significantly lower mortality (7%, P < 0.001) compared with controls, while no significant difference in resistance was found when exposed to diagnostic doses of permethrin, deltamethrin, malathion, or bendiocarb (Fig. 3). A trend of increased tolerance was observed in mosquitoes overexpressing Gste2 against malathion (Fig. 3), and further analysis with the related OP fenitrothion indicated high resistance in Ubi-A10/UAS-e2 mosquitoes, showing 8% (P < 0.001) mortality (Fig. 3).
Fig. 3.
Multitissue overexpression of GSTE2 affects sensitivity to an organochlorine and an OP insecticide. Sensitivity to insecticides of adult female mosquitoes overexpressing Gste2 (e2+) ubiquitously under the control of the Ubi-A10 driver compared with Ubi-A10 controls (e2−) measured by WHO tube bioassay. Bars represent SD (n = 2 to 6) (SI Appendix, Table S2). The dotted line marks the WHO 90% mortality threshold for defining resistance. Welch’s t test with P < 0.01 significance cutoff. ***P < 0.001.
Preliminary analysis of Ubi-A10GAL4:UAS-e2 (integration) mosquitoes indicated the expected increase in GSTE2 protein in whole-body extracts compared with Ubi-A10 controls (SI Appendix, Fig. S2A) and a resistance phenotype against DDT in the F1 generation of transformed male and female mosquitoes (SI Appendix, Fig. S2B).
Oenocyte or Midgut-Specific Overexpression of CYP6M2 or CYP6P3 Does Not Confer Resistance to Insecticides.
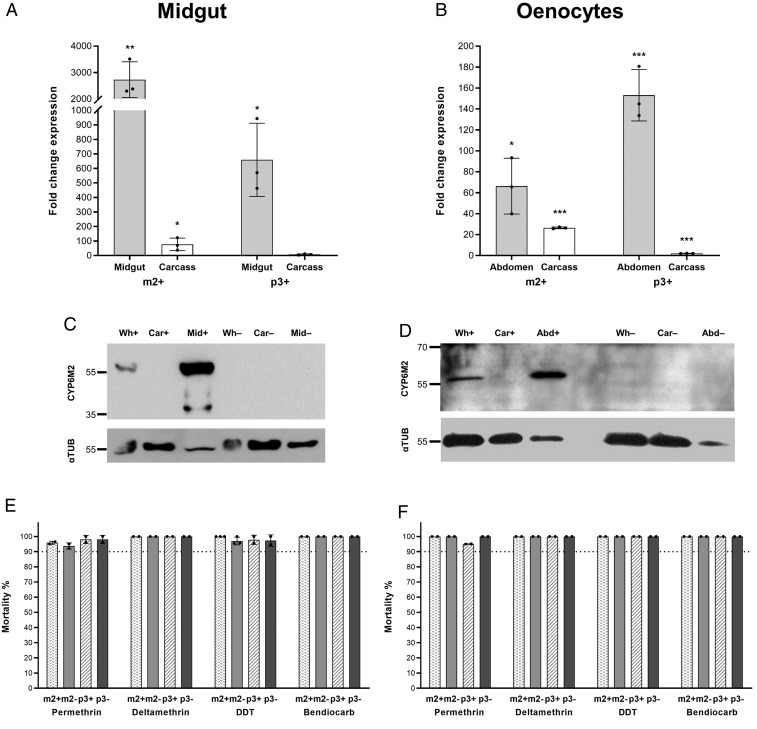
To examine the role of oenocytes and midgut tissues in P450-based metabolism of insecticides, we utilized previously published GAL4 driver lines to regulate tissue-specific expression. The specificity of these GAL4 lines to drive the respective tissue-specific expression has been established following crosses with UAS-regulated fluorescent gene reporter lines (22, 24). To examine the relative increase in Cyp6 gene expression, we performed qRT-PCR and western blot analysis in dissected body parts from progeny of alternative driver and Cyp6 responder crosses. Using the midgut driver (GAL4-mid), Cyp6m2 and Cyp6p3 transcripts were 2,730× (P = 0.002) and 659× (P = 0.011) more abundant, respectively, in midguts dissected from GAL4/UAS mosquitoes compared with controls (Fig. 4A). A low level of overexpression was detected in the remaining carcass of GAL4/UAS mosquitoes compared with that of controls (Cyp6m2: 77×, P = 0.038; Cyp6p3: 7×, P = 0.08). In GAL4-oeno crosses, Cyp6m2 and Cyp6p3 were specifically up-regulated in transgenic dissected abdomens (66×, P = 0.013 for Cyp6m2; 153×, P < 0.001 for Cyp6p3) where oenocytes are located (Fig. 4B). Background overexpression was also found in the remaining carcass of GAL4/UAS-m2 and -p3 adults compared with controls (26×, P < 0.001 and 2×, P < 0.001, respectively). In western blot analysis, CYP6M2 antiserum again only detected the target protein in GAL4/UAS mosquitoes. CYP6M2 was found exclusively in dissected midguts (and whole mosquitoes) from the progeny of GAL4 midcrosses but was not observed in GAL4/UAS carcasses or extracts from controls (Fig. 4C). Similarly, in GAL4-oeno crosses, CYP6M2 signal was only detected in whole adult female extracts and in dissected abdomen integument but not in the remaining carcass or control extracts (Fig. 4D).
Fig. 4.
Cyp6 gene up-regulation in the mosquito midgut or oenocytes does not affect sensitivity to insecticides. (A and B) Relative transcription levels of Cyp6m2 (m2+) and Cyp6p3 (p3+) in dissected midguts (A) and abdomens (B) of GAL4/UAS female mosquitoes compared with the equivalent body parts in GAL4/+ controls. Carcass is whole body without the relevant dissected part. Bars represent SD (n = 3). Unpaired t test. *P < 0.05; **P < 0.01; ***P < 0.001. (C and D) Expression of CYP6M2 and α-tubulin in females from the GAL4-mid × UAS-m2 (C) and GAL4-oeno × UAS-m2 (D) crosses. Abd, abdomen cuticle; Car, protein extract from 1/3 of a single female carcass–whole body without the midgut (C) or the abdomen (D); Mid, 2 dissected midguts; Wh, protein extract from 1/3 of a single whole female. +, GAL4/UAS-m2; −, GAL4/+. (E and F) Sensitivity to insecticides of GAL4/UAS females overexpressing (+) Cyp6m2 or Cyp6p3 in the midgut (E) or in the oenocytes (F) compared with GAL4/+ controls (−) measured by WHO tube bioassay. Bars represent SD (n = 2 to 3) (SI Appendix, Table S2). Dotted lines mark the WHO 90% mortality threshold for defining resistance. Welch’s t test with P < 0.01 significance cutoff.
Adult females overexpressing Cyp6m2 in the midgut (Fig. 4E) or in the oenocytes (Fig. 4F) showed complete susceptibility to permethrin, deltamethrin, DDT, and bendiocarb. Similar results were obtained with Cyp6p3 (Fig. 4 E and F); however, potential resistance (95% mortality, P = 0.013) was suggested in oenocyte-specific Cyp6p3-overexpressing mosquitoes when exposed to permethrin (Fig. 4F). Further analysis was performed to detect subtle differences in susceptibility by repeating the assays with reduced exposure time (SI Appendix, Fig. S3). However, no significant decrease (P < 0.01) was found in the mortality rates of mosquitoes overexpressing Cyp6m2 or Cyp6p3 in the midgut or oenocytes compared with their respective controls when exposed for 20 min to the same diagnostic doses of the 4 insecticides (SI Appendix, Fig. S3).
Finally, the 25-min reduced exposure bioassay for malathion showed no significant difference in the mortality of mosquitoes overexpressing Cyp6m2 or Cyp6p3 in midgut or oenocytes compared with controls (SI Appendix, Fig. S4).
Discussion
In vivo functional analysis is critical to provide evidence of causative links between candidate genes and their proposed phenotypes. Here, we demonstrate the utility of GAL4/UAS-based tools to characterize gene function directly in An. gambiae by reporting the use of the system to validate the ability of single candidate genes to confer WHO-defined resistance to different classes of insecticides. Overall, the transgenic analysis in An. gambiae is more in accordance with data generated from recombinant protein studies of insecticide metabolism rather than those obtained from D. melanogaster survival assays (Table 2).
Table 2.
In vitro (metabolism and/or depletion) and in vivo (An. gambiae and D. melanogaster) functional validation of An. gambiae Cyp6m2, Cyp6p3, and Gste2 genes
| Class, insecticide, and gene | In vitro | An. gambiae (this study) | D. melanogaster |
| Pyrethroids | |||
| Permethrin | |||
| Cyp6m2 | ✓ (11),* (19)† | ✓ | ✓ (15) |
| Cyp6p3 | ✓ (10),* (19)† | ✓ | ✓ (15) |
| Gste2 | N/A | ✗ | ✗ (20) |
| Deltamethrin | |||
| Cyp6m2 | ✓ (11),* (19)† | ✓ | ✓ (15) |
| Cyp6p3 | ✓ (10),* (19)† | ✓ | ✓ (15) |
| Gste2 | N/A | ✗ | N/A |
| Organochlorines | |||
| DDT | |||
| Cyp6m2 | ✗ (19),† ✓ (12)*,‡ | ✗ | ✓ (15) |
| Cyp6p3 | ✗ (19)† | ✗ | N/A |
| Gste2 | ✓ (9, 13)* | ✓ | ✓ (13, 20) |
| Carbamates | |||
| Bendiocarb | |||
| Cyp6m2 | ✗ (15, 19)† | ✗ | ✓ (15) |
| Cyp6p3 | ✓ (15, 19)† | ✓ | ✓ (15) |
| Gste2 | N/A | ✗ | N/A |
| Organophosphates | |||
| Malathion | |||
| Cyp6m2 | ✓ (28),* (19)† | ✓ | N/A |
| Cyp6p3 | ✓ (19)† | ✓ | N/A |
| Gste2 | N/A | ✗ | N/A |
| Fenitrothion | |||
| Cyp6m2 | ✓ (19)† | N/A | N/A |
| Cyp6p3 | ✓ (19)† | N/A | N/A |
| Gste2 | N/A | ✓ | N/A |
Presence (✓) or absence (✗) of in vitro activity or in vivo WHO-defined insecticide resistance (An. gambiae) or increased insecticide tolerance (D. melanogaster). N/A, no information available.
Data in reference indicated that metabolism of substrate had been observed through the production of metabolites.
Data in reference indicated that substrate had been depleted with no direct evidence of metabolite production.
In the presence of added cholate.
In Anopheles, multitissue overexpression of Cyp6m2 and Cyp6p3 demonstrated that resistance to permethrin and deltamethrin (types I and II pyrethroids, respectively) can be conferred by the sole overexpression of either Cyp6 gene. Cyp6p3 expression also conferred resistance to bendiocarb (carbamate), while the overexpression of either Cyp6 gene did not alter DDT (organochlorine) sensitivity. These phenotypes correlate with the profile of metabolism or substrate depletion of the respective insecticides for the 2 recombinant P450 enzymes (Table 2). More variable results have been observed using D. melanogaster as an in vivo model, with overexpression of Cyp6m2 surprisingly generating increased tolerance to bendiocarb compared with Cyp6p3, despite in vitro analysis not detecting activity against bendiocarb for Cyp6m2 (15, 19). DDT tolerance was also observed in Cyp6m2-overexpressing D. melanogaster, but data for Cyp6p3 could not be generated (15) (Table 2). In this study, DDT resistance was monitored by dose–response assays over a 24-h exposure time, while bendiocarb resistance was not observed when measured through such dose–response assays but was reported following 24-h exposure to a diagnostic dose. In the latter case, the controls used to compare Cyp6m2 and Cyp6p3 overexpression showed very different levels of sensitivity to bendiocarb, which seemed to contribute to the differences in resistance levels observed, while there were no data for the respective Cyp6p3 controls in the DDT analysis for comparison. It may thus be differences in the insecticide susceptibility of the control lines that give rise to the discrepant results observed in D. melanogaster. Since the D. melanogaster UAS-Cyp6 lines were also created by PhiC31 transformation, it seems unlikely that the differences are caused by position effects. However, it should also be noted that the different methods of insecticide bioassay performed in the D. melanogaster studies may not yield directly comparable results with the diagnostic WHO level of resistance in mosquitoes used in this study and extensively used to assess the emergence of resistance in endemic countries. Our data in mosquitoes unequivocally indicate, however, that the expression of single Cyp6 genes can confer resistance to different pyrethroids and that Cyp6p3 overexpression confers cross-resistance to prominent representatives of at least 2 classes of public health insecticides.
In contrast to our Cyp6 studies, increased An. gambiae Gste2 (AgGste2) expression generates clear DDT resistance, while resistance to bendiocarb and pyrethroids was not observed. These phenotypes again validate predictions from the DDT activity observed in vitro for recombinant AgGSTE2 (9, 13) as well as the increased DDT tolerance (13) and lack of pyrethroid tolerance (20) observed when overexpressed in D. melanogaster. The corresponding in vitro data for AgGSTE2 activity against bendiocarb and pyrethroids have not been reported, and here, bendiocarb resistance has been examined in vivo following Gste2 overexpression.
Although DDT tolerance was also observed in D. melanogaster overexpressing the orthologous AfGste2 (16, 18), conflicting results were reported about activity toward pyrethroids. For example, recombinant AfGSTE2 depleted permethrin but not deltamethrin in vitro, yet D. melanogaster acquired increased tolerance to both insecticides when AfGste2 was overexpressed (16, 18). RNAi analysis in deltamethrin-resistant Aedes aegypti of AaGste2 has also indicated a role in pyrethroid resistance (29). It is possible that the variation observed in resistance profiling is due to intrinsic differences in the activity of GSTE2s derived from the different mosquito species. In this context, it has been speculated that the predominant pyrethroid detoxification role of GSTs in some insects is sequestration or protection against oxidative stress rather than direct metabolism (30). Our results show that even high levels of AgGste2 overexpression do not confer WHO diagnostic levels of resistance to this class of insecticides in isolation. It is feasible that the level of glutathione cofactor is limiting in the mosquito; however, this seems unlikely, since the recombinant enzyme does not show pyrethroid metabolizing activity, even in the presence of excess glutathione (20). It is also possible that AgGste2 may need to work in concert with other genes that are not up-regulated in the sensitive genetic background of the An. gambiae transgenic lines to produce a pyrethroid resistance phenotype. Future work will test this hypothesis by coexpression of other UAS-regulated detoxification genes using the Ubi-A10GAL4:UAS-e2 (integration) line. Although beyond the scope of this work, this mosquito line expresses GAL4 and GSTE2 and can be crossed with other UAS lines to provide coexpression with other detoxification enzymes to examine additive or synergistic interactions.
Although GSTs have been associated with OP metabolism through biochemical studies (7), we report evidence that the expression of a single gene can provide OP resistance in mosquitoes. The high resistance shown toward fenitrothion by Gste2-overexpressing An. gambiae is intriguing. It is currently unclear if GSTE2 detoxifies fenitrothion by sequestration, by free radical protection, or directly through conjugation/modification. Evidence from early studies (31) suggests that Anopheles GST activity is associated with the conversion of fenitrothion to the nontoxic metabolite desmethyl fenitrooxon through an oxidized intermediate. Similar analysis in the Gste2-overexpressing lines would clarify which of these mechanisms is involved. Further investigation is also needed on the OP malathion, for which we report suspected resistance when Gste2 is overexpressed.
We have also demonstrated that Cyp6 overexpression increases susceptibility to malathion as well as conferring permethrin resistance, which may have direct implications on insecticide management, especially if replicated with other OPs that may be used for Anopheles control (32). Such sensitivity profiles are readily explained by the bioactivation of malathion to its more toxic metabolite malaoxon (33) by a P450-mediated mechanism (28). Direct evidence of activation has been shown by mass spectrometry analysis of in vitro CYP6M2 activity against malathion, which identified a major metabolite of 315 Da, corresponding to malaoxon (28). Here, we provide direct in vivo evidence that CYP6 enzymes can confer negative cross-resistance. Furthermore, there seems to be substrate specificity in the alternative P450-mediated reactions, since we observed higher mortality when assayed against Cyp6m2 overexpression compared with Cyp6p3. This may suggest that Cyp6m2 favors the higher steady-state production of the toxic intermediate compared with Cyp6p3.
Malathion activation by Cyp6m2 is also supported by recent evidence provided by Ingham et al. (34), who found that knockdown of the transcription factor Maf-S results in increased survival following malathion exposure. One of the P450s down-regulated by Maf-S knockdown was Cyp6m2, whereas Cyp6p3 transcription was not modified. Taken together, the results provide experimental evidence to support the use of OPs and potentially, other proinsecticides activated by CYP6 enzymes for Anopheles control in areas where pyrethroid resistance is also conferred by detoxification by the same enzyme(s). One such strategy involves combining the use of pyrethroid-based bed nets with OP-based residual wall spraying or impregnated hangings (32). This takes advantage of the additive effect of the 2 classes of insecticides while sensitizing Cyp6-based pyrethroid-resistant mosquitoes to malathion (35). In conjunction with recombinant enzyme assays, the modified mosquitoes described may thus become valuable tools to assess the susceptibility of public health proinsecticides (for example, chlorfenapyr [36]) to activation and detoxification by xenobiotic metabolizing P450 genes in Anopheles.
When validating resistance phenotypes conferred by transgenic overexpression, the spatial pattern of overexpression can give clues to the identity of key tissues of detoxification. The expression driven by Ubi-A10 is spread over multiple tissues, which makes it impossible to pinpoint which tissues are particularly important for generating the resistance phenotype. Here, we directly investigated the involvement of the midgut and oenocytes in conferring P450-mediated resistance. Critically, we did not observe clear resistance to any insecticide class when either Cyp6m2 or Cyp6p3 was specifically expressed in either of these tissues, despite achieving highly enriched expression and the knowledge that oenocytes and the midgut express abundant P450 coenzyme CPR (26). Furthermore, since our previous expression profiling of the Ubi-A10 driver indicated lack of expression in Malpighian tubules (23), yet resistance to multiple insecticides was observed with this driver, it would seem that the insecticides tested are not predominately metabolized in the Malpighian tubules either, and other unidentified tissues may be critical, alone or in combination, for detoxification. As described earlier, some evidence of tissue specificity of P450s associated with insecticide resistance has been derived from transcriptomic analysis of crude dissections of tissues and body segments from pyrethroid-resistant and -sensitive strains (27). This study indicated that Cyp6p3 is more highly expressed in the midgut of the resistant strain, whereas Cyp6m2 has a broader up-regulation in midgut, Malpighian tubules, and the abdomen (integument, fat body, and ovaries). The relevance of elevated Cyp6p3 levels in the midgut of the examined resistant strain is difficult to reconcile with the lack of a resistance phenotype when the same gene is overexpressed in this tissue with the GAL4/UAS system.
Previous D. melanogaster studies have shown that overexpression using drivers active in multiple tissues, such as actin5C-GAL4 (14–18) or tubulin-GAL4 (20), is generally needed to modify resistance. Nevertheless, there are few examples in which tissue-specific drivers have been used to validate Cyp6 gene-based resistance in D. melanogaster. Yang et al. (37) demonstrated the central role of Malpighian tubules for DmCyp6g1-mediated DDT resistance, while Zhu et al. (38) demonstrated the importance of neuronal expression to provide deltamethrin resistance in D. melanogaster expressing Tribolium castaneum Cyp6bq9. Even in this latter analysis, however, the neuronal driver showed leaky expression in other tissues, leading to the possibility that the observed phenotype results from expression in multiple tissues. Further work in D. melanogaster with alternative tissue-specific drivers could be exploited to provide insight into the involvement of particular tissues in Cyp6-mediated resistance. When similar tools become available in mosquitoes, a more definitive answer to which specific tissues are involved in the detoxification of insecticides in An. gambiae can be provided.
Conclusions
This work reports on functional analysis of mosquito insecticide resistance genes conducted in transgenic An. gambiae. The mosquitoes generated are resistant, in a solely metabolism-based manner, to at least 1 representative insecticide from the major classes used in public health and are, therefore, useful in prescreens of new and repurposed active compounds, including insecticides, proinsecticides, synergists, and sterilizing agents. The lines can also be used in combination with strains carrying genome-edited target sites (e.g., Kdr and Ace-1R) to examine the additive or synergistic effects of multiple resistance mechanisms. Similarly, it is possible to use the integration line carrying both Ubi-GAL4 and UAS-Gste2 to cross with other UAS detoxification genes to analyze metabolic interactions: for example, combining phases I and II metabolism. In addition, the Ubi-A10 driver is active in larval stages (23) and can thus be used to examine gene function in immature stages.
Importantly, for future work, there is growing evidence on the involvement in resistance of genes that are very difficult to test either in vitro, due to the lack of appropriate assays, or in D. melanogaster, since interacting partner proteins may be different or absent. These include genes coding for cuticle components (39), transcription factors (34), and other binding proteins (e.g., hexamerins and α-crystallins [21]), for which current transgenic tools, including GAL4/UAS, make An. gambiae the most relevant option for functional genetic analysis.
Materials and Methods
Plasmid Construction.
Responder plasmids were designed for the expression of the An. gambiae genes Cyp6m2 (AGAP008212), Cyp6p3 (AGAP002865), or Gste2 (AGAP009194) under the regulation of the UAS and carried a YFP marker gene regulated by the 3xP3 promoter. The coding sequences of Cyp6m2 (1,500 bp), derived from the susceptible strain Kisumu, were amplified from PB13:CYP6M2 (11) using primers M2fw and M2rv (SI Appendix, Table S1). The coding sequence of Cyp6p3 was obtained by amplifying a 193-bp fragment from Kisumu complementary DNA using primers P3fw1 and P3rv1 (SI Appendix, Table S1) and a 1,362-bp fragment from pCW:17α-Cyp6p3 (10) using primers P3fw2 and P3rv2 (SI Appendix, Table S1). P3fw1 and P3rv2 were then used to join the 2 fragments and obtain the 1,530-bp full-length Cyp6p3 coding sequence. The 666-bp Gste2-114T coding sequence derived from the DDT-resistant strain ZAN/U was amplified from the K1B plasmid (13) using primers Gste2k1bfor and Gste2k1brev (SI Appendix, Table S1). All coding sequences were cloned into the YFP-marked responder plasmid pSL*attB:YFP:Gyp:UAS14i:Gyp:attB (24) downstream of the UAS using EcoRV/XhoI (Cyp6) or EcoRI/NcoI (Gste2).
Creation of UAS Responder Lines by PhiC31-Mediated Cassette Exchange.
For creating responder lines carrying Cyp6 genes, embryos of the docking line A11 (24), which carries 2 inverted attP sites and is marked with 3xP3-driven CFP, were microinjected with 350 ng/µL of the responder plasmid and 150 ng/µL of the integrase helper plasmid pKC40 encoding the phiC31 integrase (40) as described in Pondeville et al. (41). The same protocol was followed to create the Gste2 responder line using embryos of the docking line Ubi-A10 (23), which carries 2 inverted attP sites and is marked with 3xP3-driven CFP. Emerging F0 was pooled into sex-specific founder cages and outcrossed with wild-type G3s. F1 progenies were screened for the expression of YFP (cassette exchange) and CFP/YFP (cassette integration) in the eyes and nerve cord. Orientation check to assess the direction of cassette exchange was performed on F1 YFP-positive individuals or on the F2 progeny deriving from single YFP-positive individuals. This was carried out by PCR using alternative combinations of 4 primers designed to give a product only in 1 of the orientations: Two pairs were diagnostic for orientation A, and two pairs were diagnostic for orientation B. Mosquito DNA with an insertion in orientation A gives products only with PiggyBacR-R2 + Red-seq4R (PCR1) and gene-specific primers M2intFW or P3intFW or Gste2_v1 + ITRL1R (PCR2). Mosquito DNA with an insertion in orientation B gives products only with PiggyBacR-R2 + gene specific primers M2intFW or P3intFW or Gste2_v2 (PCR3) and Red-seq4R + ITRL1R (PCR4). All definitive responder lines were created from individuals showing orientation of insertion A, which was chosen for consistency with previous RMCE lines created in this laboratory. Transformation efficiencies were calculated as the number of independent transgenic events (exchanges or integrations) over the number of surviving F0 adults.
Driver Lines and GAL4 × UAS Crosses.
Crosses for ubiquitous expression were established between the CFP-marked driver Ubi-A10 (23) and individuals of the responder lines marked with YFP. To obtain tissue-localized expression, dsRed-marked drivers specific for expression in the midgut (GAL4-mid) (22) or in the oenocytes (GAL4-oeno) (24) were used. Responder lines were kept as a mix of homozygous and heterozygous individuals so as to obtain GAL4/+ progeny to be used as transgenic blank controls.
Cyp6 Gene Expression Analysis.
To quantify Cyp6 gene expression in GAL4/UAS and GAL4/+ individuals, total RNA was harvested from pools of 2- to 5-d-old whole adults and their relevant dissected body part (midgut or abdomen cuticle). The adult tissues remaining after dissection constituted the carcass. Three biological replicates consisting of 5 mosquitoes (or body parts) each were collected from each mosquito population. RNA extraction was performed using the TRI Reagent protocol (Sigma). To remove genomic DNA contamination, samples were treated with the Turbo DNA-Free kit (Ambion). RNA was then reverse transcribed using the SuperScript III First-Strand Synthesis System (Life Technologies) following the oligo(dT) reaction protocol. qRT-PCR reactions were set up using 1× Brilliant III Ultra-Fast SYBR Green qPCR Master Mix (Agilent Technologies) and primers qM2fw and qM2rv for quantification of Cyp6m2 and qP3fw and qP3sub for Cyp6p3 (15) (SI Appendix, Table S1). The qP3sub primer bears a nucleotide substitution (A11G) to conform its sequence to that of the G3 strain template. Two housekeeping genes, the ribosomal protein S7 (AGAP010592) and the ribosomal protein L40/Ubiquitin (AGAP007927), were also quantified using primers qS7fw, qS7rv, qUBfw, and qUBrv (42) (SI Appendix, Table S1). Cyp6 transcription data obtained by qRT-PCR were analyzed using the ΔΔ cycle threshold (Ct) method as described in SI Appendix, Supplementary Method. Gene expression analysis was not performed to assess up-regulation of the Gste2 transcript.
CYP6 and GSTE2 Protein Expression Analysis.
To detect protein expression in GAL4/UAS and GAL4/+ individuals, total protein extracts were obtained from whole 2- to 5-d-old female adults and their dissected body parts. Whole mosquitoes and dissected body parts were directly homogenized in 1× Laemmli buffer (BioRad) containing 5% 2-mercaptoethanol and 1× protease inhibitors (cOmplete EDTA-free Protease Inhibitor Mixture; Sigma), incubated at 95 °C for 10 min, and then, centrifuged at 13,000 rpm for 5 min. Volumes of protein extract were separated by sodium dodecylsulphate/polyacrylamide gel electrophoresis to give the number of whole mosquito or body part equivalents as indicated.
Protein extracts equivalent to 1/3 of a mosquito or its body part were analyzed to detect CYP6 expression driven by tissue-specific drivers with the exception of midgut samples, for which 2 whole midguts were analyzed. The higher amount of midgut sample was required to visualize signal of the α-tubulin loading control. The equivalent of 1/10 of a single female mosquito was used to assess expression driven by ubiquitous drivers. CYP6s were probed using primary affinity-purified polyclonal peptide antibodies produced in rabbit against CYP6M2 or CYP6P3 (gifts from M. Paine Liverpool School of Tropical Medicine (LSTM), Liverpool, UK), while GSTE2 was probed with anti–GSTE2-28 rabbit primary antibodies (9). Secondary antibodies were anti-rabbit horseradish peroxidase (HRP)-tagged immunoglobulins G (IgG). (Bethyl Laboratories). Relative protein loading and blotting efficacy was verified by reprobing stripped membranes (Restore ThermoFisher) using primary mouse anti–α-tubulin antibodies (Sigma) and secondary goat anti-mouse HRP IgG antibodies (Abcam). Signal detection was carried out using SuperSignal West Dura Extended Duration Substrate (Life Technologies).
Assessment of Susceptibility to Insecticides.
Susceptibility to insecticides was assessed in mosquitoes overexpressing Cyp6 genes using the WHO tube bioassay (25). Pools of 20 to 25 GAL4/UAS and GAL4/+ adult female mosquitoes were exposed 2 to 5 d postemergence to standard discriminating doses of insecticides—0.75% permethrin, 0.05% deltamethrin, 0.1% bendiocarb, and 4% DDT—for 60 min, and mortality rates were assessed after a 24-h recovery period. For mosquitoes expressing Cyp6 genes in the midgut or oenocytes, a modified version of the standard WHO test was also performed, reducing the exposure time to 20 min (26). For assessing susceptibility to 5% malathion in mosquitoes overexpressing Cyp6 genes, the exposure time was decreased to 25 min. Mosquitoes overexpressing Gste2 were additionally tested for 1% fenitrothion using the recommended 2-h exposure time; 1 to 4 biological replicates were performed for each insecticide tested. A total of 2 to 8 technical replicate tubes were tested for each population. Welch’s t test was performed to determine statistical differences between mortality rates in GAL4/UAS and GAL4/+. Details on replicate numbers of insecticide bioassay experiments and statistical analysis are reported in SI Appendix, Table S2. All statistics were calculated using GraphPad Prism version 8.2.1 (GraphPad Software; https://www.graphpad.com/).
Data Availability.
All extant transgenic lines, primary antibodies, and plasmids described will be provided by G.J.L. on request. The raw bioassay data can be accessed at Figshare (43).
Supplementary Material
Acknowledgments
We acknowledge the LSTM (A. Adolfi) and Medical Research Council Grant MR/P016197/1 (to B.P.) for sponsoring PhD studentships and the Innovative Vector Control Consortium for follow-on funding to A. Adolfi. Thanks also go to Dave Weetman for very useful comments on the manuscript. We are grateful to the editor, Fred Gould, and the two anonymous reviewers for useful comments that improved the manuscript.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: We have supplied all of the raw insecticide bioassay data at Figshare, https://figshare.com/articles/Raw_data_Bioassays_xlsx/10000388/1.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1914633116/-/DCSupplemental.
References
- 1.Bhatt S., et al. , The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization , World Malaria Report 2018 (World Health Organization, Geneva, Switzerland, 2018). [Google Scholar]
- 3.Churcher T. S., Lissenden N., Griffin J. T., Worrall E., Ranson H., The impact of pyrethroid resistance on the efficacy and effectiveness of bednets for malaria control in Africa. eLife 5, e16090 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranson H., Lissenden N., Insecticide resistance in African anopheles mosquitoes: A worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 32, 187–196 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Hemingway J., Hawkes N. J., McCarroll L., Ranson H., The molecular basis of insecticide resistance in mosquitoes. Insect Biochem. Mol. Biol. 34, 653–665 (2004). [DOI] [PubMed] [Google Scholar]
- 6.David J.-P., Ismail H. M., Chandor-Proust A., Paine M. J., Role of cytochrome P450s in insecticide resistance: Impact on the control of mosquito-borne diseases and use of insecticides on Earth. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20120429 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enayati A. A., Ranson H., Hemingway J., Insect glutathione transferases and insecticide resistance. Insect Mol. Biol. 14, 3–8 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Donnelly M. J., Isaacs A. T., Weetman D., Identification, validation, and application of molecular diagnostics for insecticide resistance in malaria vectors. Trends Parasitol. 32, 197–206 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortelli F., Rossiter L. C., Vontas J., Ranson H., Hemingway J., Heterologous expression of four glutathione transferase genes genetically linked to a major insecticide-resistance locus from the malaria vector Anopheles gambiae. Biochem. J. 373, 957–963 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Müller P., et al. , Field-caught permethrin-resistant Anopheles gambiae overexpress CYP6P3, a P450 that metabolises pyrethroids. PLoS Genet. 4, e1000286 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevenson B. J., et al. , Cytochrome P450 6M2 from the malaria vector Anopheles gambiae metabolizes pyrethroids: Sequential metabolism of deltamethrin revealed. Insect Biochem. Mol. Biol. 41, 492–502 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Mitchell S. N., et al. , Identification and validation of a gene causing cross-resistance between insecticide classes in Anopheles gambiae from Ghana. Proc. Natl. Acad. Sci. U.S.A. 109, 6147–6152 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell S. N., et al. , Metabolic and target-site mechanisms combine to confer strong DDT resistance in Anopheles gambiae. PLoS One 9, e92662 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riveron J. M., et al. , Directionally selected cytochrome P450 alleles are driving the spread of pyrethroid resistance in the major malaria vector Anopheles funestus. Proc. Natl. Acad. Sci. U.S.A. 110, 252–257 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edi C. V., et al. , CYP6 P450 enzymes and ACE-1 duplication produce extreme and multiple insecticide resistance in the malaria mosquito Anopheles gambiae. PLoS Genet. 10, e1004236 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riveron J. M., et al. , A single mutation in the GSTe2 gene allows tracking of metabolically based insecticide resistance in a major malaria vector. Genome Biol. 15, R27 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riveron J. M., et al. , The highly polymorphic CYP6M7 cytochrome P450 gene partners with the directionally selected CYP6P9a and CYP6P9b genes to expand the pyrethroid resistance front in the malaria vector Anopheles funestus in Africa. BMC Genomics 15, 817 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riveron J. M., et al. , Genome-wide transcription and functional analyses reveal heterogeneous molecular mechanisms driving pyrethroids resistance in the major malaria vector Anopheles funestus across Africa. G3 (Bethesda) 7, 1819–1832 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yunta C., et al. , Cross-resistance profiles of malaria mosquito P450s associated with pyrethroid resistance against WHO insecticides. Pestic. Biochem. Physiol. 161, 61–67 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Daborn P. J., et al. , Using Drosophila melanogaster to validate metabolism-based insecticide resistance from insect pests. Insect Biochem. Mol. Biol. 42, 918–924 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Ingham V. A., Wagstaff S., Ranson H., Transcriptomic meta-signatures identified in Anopheles gambiae populations reveal previously undetected insecticide resistance mechanisms. Nat. Commun. 9, 5282 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynd A., Lycett G. J., Development of the bi-partite Gal4-UAS system in the African malaria mosquito, Anopheles gambiae. PLoS One 7, e31552 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adolfi A., Pondeville E., Lynd A., Bourgouin C., Lycett G. J., Multi-tissue GAL4-mediated gene expression in all Anopheles gambiae life stages using an endogenous polyubiquitin promoter. Insect Biochem. Mol. Biol. 96, 1–9 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Lynd A., et al. , Development of a functional genetic tool for Anopheles gambiae oenocyte characterisation: Application to cuticular hydrocarbon synthesis. bioRxiv:10.1101/742619 (28 August 2019).
- 25.World Health Organization , Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes (World Health Organization, Geneva, Switzerland, ed. 2, 2016). [Google Scholar]
- 26.Lycett G. J., et al. , Anopheles gambiae P450 reductase is highly expressed in oenocytes and in vivo knockdown increases permethrin susceptibility. Insect Mol. Biol. 15, 321–327 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Ingham V. A., et al. , Dissecting the organ specificity of insecticide resistance candidate genes in Anopheles gambiae: Known and novel candidate genes. BMC Genomics 15, 1018 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voice M., Kaaz A. W., Peet C. F., Paine M. J., Recombinant CYP6M2 inhibition by insecticides recommended by WHO for indoor residual spraying against malaria vectors. Drug Metab. Rev., 10.3109/03602532.2012.744573 (2012). [DOI] [Google Scholar]
- 29.Lumjuan N., McCarroll L., Prapanthadara L. A., Hemingway J., Ranson H., Elevated activity of an Epsilon class glutathione transferase confers DDT resistance in the dengue vector, Aedes aegypti. Insect Biochem. Mol. Biol. 35, 861–871 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Vontas J. G., Small G. J., Hemingway J., Glutathione S-transferases as antioxidant defence agents confer pyrethroid resistance in Nilaparvata lugens. Biochem. J. 357, 65–72 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ranson H., et al. , Identification of a novel class of insect glutathione S-transferases involved in resistance to DDT in the malaria vector Anopheles gambiae. Biochem. J. 359, 295–304 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wanjala C. L., et al. , Pyrethroid and DDT resistance and organophosphate susceptibility among Anopheles spp. mosquitoes, Western Kenya. Emerg. Infect. Dis. 21, 2178–2181 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen S. D., Mechanisms of toxicological interactions involving organophosphate insecticides. Fundam. Appl. Toxicol. 4, 315–324 (1984). [DOI] [PubMed] [Google Scholar]
- 34.Ingham V. A., Pignatelli P., Moore J. D., Wagstaff S., Ranson H., The transcription factor Maf-S regulates metabolic resistance to insecticides in the malaria vector Anopheles gambiae. BMC Genomics 18, 669 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin T., Ochou O. G., Vaissayre M., Fournier D., Organophosphorus insecticides synergize pyrethroids in the resistant strain of cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) from West Africa. J. Econ. Entomol. 96, 468–474 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Oxborough R. M., et al. , The activity of the pyrrole insecticide chlorfenapyr in mosquito bioassay: Towards a more rational testing and screening of non-neurotoxic insecticides for malaria vector control. Malar. J. 14, 124 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J., et al. , A Drosophila systems approach to xenobiotic metabolism. Physiol. Genomics 30, 223–231 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Zhu F., et al. , A brain-specific cytochrome P450 responsible for the majority of deltamethrin resistance in the QTC279 strain of Tribolium castaneum. Proc. Natl. Acad. Sci. U.S.A. 107, 8557–8562 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balabanidou V., et al. , Cytochrome P450 associated with insecticide resistance catalyzes cuticular hydrocarbon production in Anopheles gambiae. Proc. Natl. Acad. Sci. U.S.A. 113, 9268–9273 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ringrose L., Transgenesis in Drosophila melanogaster. Methods Mol. Biol. 561, 3–19 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Pondeville E., et al. , Efficient ΦC31 integrase-mediated site-specific germline transformation of Anopheles gambiae. Nat. Protoc. 9, 1698–1712 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Jones C. M., et al. , The dynamics of pyrethroid resistance in Anopheles arabiensis from Zanzibar and an assessment of the underlying genetic basis. Parasit. Vectors 6, 343 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adolfi A., et al. , Data from “Functional genetic validation of key genes conferring insecticide resistance in the major African malaria vector, Anopheles gambiae.” Figshare. https://figshare.com/articles/Raw_data_Bioassays_xlsx/10000388/1. Deposited 17 October 2019. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All extant transgenic lines, primary antibodies, and plasmids described will be provided by G.J.L. on request. The raw bioassay data can be accessed at Figshare (43).