Abstract
Circadian clocks usually run with a period close to 24 h, but are also plastic and can be entrained by external environmental conditions and internal physiological cues. Two key nutrient metabolites, glucose and vitamin B3 (nicotinamide), can influence the circadian period in both mammals and plants; however, the underlying molecular mechanism is still largely unclear. We reveal that the target of rapamycin (TOR) kinase, a conserved central growth regulator, is essential for glucose- and nicotinamide-mediated control of the circadian period in Arabidopsis. Nicotinamide affects the cytosolic adenosine triphosphate concentration, and blocks the effect of glucose-TOR energy signaling on period length adjustment, meristem activation, and root growth. Together, our results uncover a missing link between cellular metabolites, energy status, and circadian period adjustment, and identify TOR kinase as an essential energy sensor to coordinate circadian clock and plant growth.
Keywords: TOR, glucose, nicotinamide, circadian clock, Arabidopsis
For adaptation to the daily rotation of our planet, almost all organisms have evolved an internal circadian clock to drive daily rhythms in behavior, metabolism, and growth. While regulation of metabolic output by the circadian clock is well established, there is increasing evidence that metabolite fluctuations can also contribute to circadian clock adjustments (1–3). Decreasing sugar availability or treating with high dosage of nicotinamide can significantly lengthen the circadian period in both mammals and plants (2–5). Recently, an influence of sugar on the circadian clock through the transcription factor BASIC LEUCINE ZIPPER63 was suggested in plants (6). There is evidence of nicotinamide regulating plant circadian clock through the cyclic adenosine diphosphate ribose-Ca2+ signaling, and another study revealed Ca2+ signaling might be transduced through CALMODULIN-LIKE24 to modulate the circadian period (5, 7). How the metabolite status is sensed to allow for adjustment of the circadian period and whether there is any intimate link between these metabolites, however, is still largely unclear (5, 8, 9).
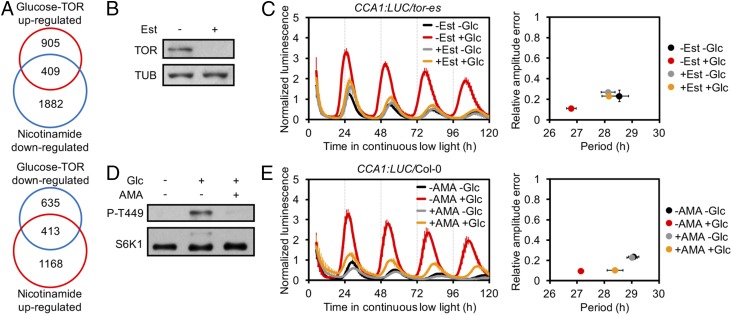
Recent studies have suggested a pivotal role for target of rapamycin (TOR) kinase in circadian period regulation under hypoxia or magnesium oscillations in mammals (10, 11). Whether TOR is involved in the plant circadian clock network has not yet been explored. We previously revealed a role for TOR in glucose energy signaling in meristem activation through extensive transcriptome reprogramming in Arabidopsis thaliana, and identified thousands of glucose-TOR−regulated genes (12). By comparing this transcriptome with the 10,615 publicly available Arabidopsis microarray datasets, using Genevestigator (13), we found statistically significant overlaps with a nicotinamide-regulated transcriptome from a circadian clock study (Gene Expression Omnibus [GEO] accession no. GSE19271) (Fig. 1A and Dataset S1; TOR up-regulated versus nicotinamide down-regulated: P = 1.43 × 10−106; TOR down-regulated versus nicotinamide up-regulated: P = 2.90 × 10−214, hypergeometric test). These overlaps (409 and 413 transcripts, Fig. 1A) are remarkably larger than the 131 and 73 transcripts expected for a chance overlap of 2 similarly sized datasets selected randomly from the Arabidopsis genome, respectively, indicating that there might be a link connecting glucose-TOR signaling, nicotinamide, and circadian clock in Arabidopsis.
Fig. 1.
Glucose regulates circadian period through energy–TOR signaling. (A) Venn diagram analysis of glucose-TOR– and nicotinamide-controlled transcriptome. (B) Protein blot analysis of TOR level in tor-es without or with estradiol (Est). Tubulin (TUB) as loading control. (C) (Left) Luminescence from the CCA1:LUC reporter lines. (Right) Mean period against mean RAE. Glucose (Glc)-shortened period without Est (1.7 h, P < 0.001) vs. with Est (−0.1 h, P = 0.999) treatment, P < 0.01. (D) Protein blot analysis of TOR activity in 35S:S6K1-HA seedlings; 2-h Glc recovery. TOR activity was monitored by P-T449 of S6K1; anti-HA (S6K1) was used as loading control. (E) Glc-shortened period without AMA (2.0 h, P < 0.001) vs. with AMA (0.6 h, P < 0.05) treatment, P < 0.01. For C and E, mean ± SEM, 2-way ANOVA P for multiple period comparisons, t test P for period change comparisons, n = 3, and each replicates with 8 to 10 seedlings.
We first investigated whether glucose controls the circadian period through TOR signaling in Arabidopsis. To avoid embryonic lethality of tor null mutants, we used estradiol-inducible RNA interference tor transgenic plants (tor-es) in which TOR protein could be efficiently diminished by estradiol (12) (Fig. 1B), and crossed the line with the core clock gene CIRCADIAN CLOCK ASSOCIATED1 (CCA1) promoter-driven luciferase line (3) to generate the CCA1:LUC/tor-es line. We depleted the endogenous sugars by growing the seedlings in photosynthesis-restrained (with limited CO2) and sugar-free liquid medium (12) for 2 d under 12-h light (40 μmol ⋅m−2⋅s−1):12-h dark (12L:12D) conditions before transferring to continuous low light (10 μmol⋅m−2⋅s−1) in the same liquid medium. Depletion of endogenous sugars led to inhibition of TOR activity, based on the phosphorylation of T449 in the TOR substrate protein S6 kinase 1 (S6K1) as a conserved indicator of endogenous TOR kinase activity (12), and lengthening of the period (Fig. 1 C and D). Exogenously applied glucose reactivated TOR and shortened the period by a mean of 1.7 h (Fig. 1 C and D). Estradiol-induced TOR silencing significantly lengthened the period, and completely blocked the recovery of period shortening by exogenous glucose (Fig. 1C). The low relative amplitude error (RAE) values indicated that the circadian clock maintained robust rhythmicity under these treatments despite the reduction in amplitude (Fig. 1C). In a previous study, we revealed that glucose activates TOR via mitochondria energy relays (12). We tested the effect of antimycin A (AMA), an inhibitor of mitochondria electron transport. AMA treatment significantly compromised the glucose-regulated TOR activation and period shortening (Fig. 1 D and E). Together, these data indicate the glucose-TOR energy signaling plays an important role in adjustment of the circadian period.
We next examined whether nicotinamide also regulates the circadian period via TOR. Nicotinamide rapidly inhibited TOR activity even in the presence of exogenous glucose, and this was unlikely due to an osmotic effect (Fig. 2A). Nicotinamide treatment increased the period length by a mean of 1.5 h (Fig. 2B); however, this period regulation was compromised in TOR silencing seedlings, and the circadian clock still maintained a good robustness (Fig. 2B). This suggests that nicotinamide-regulated circadian period is also dependent on TOR signaling.
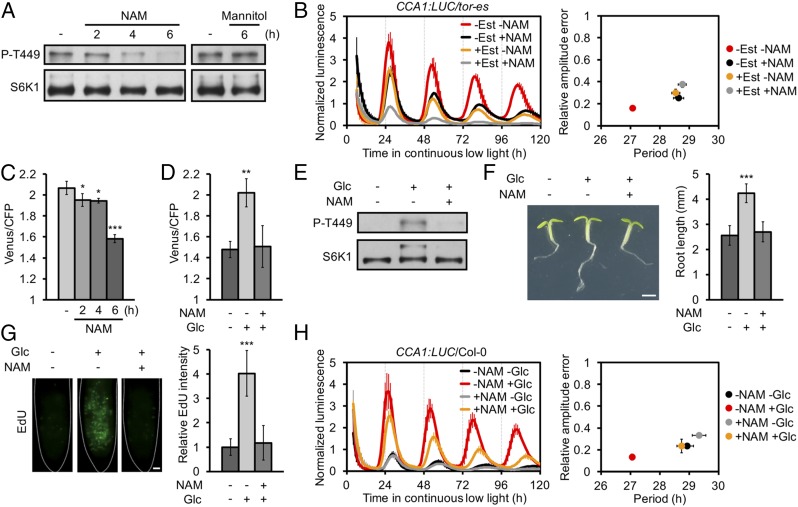
Fig. 2.
Nicotinamide blocks Glc-TOR energy signaling on root growth and circadian period regulation. (A) Protein blot analysis of TOR activity under nicotinamide (NAM) and mannitol treatment. (B) NAM lengthened period without Est (1.5 h, P < 0.001) vs. with Est (0.2 h, P = 0.252) treatment, P < 0.01. (C) Cytosolic ATP concentration reduction under NAM treatment was indicated by the Förster resonance energy transfer ratio (Venus/cyan fluorescent protein [CFP]) of ATeam seedlings (15). (D) NAM abolished the Glc-promoted ATP concentration. (E) NAM abolished the Glc-promoted TOR activity; 2-h Glc recovery. (F) NAM abolished the Glc-promoted root growth; 24-h Glc recovery. (Scale bar, 1 mm.) (G) NAM abolished the Glc-promoted cell proliferation at the root apical meristem; 2-h Glc recovery. EdU was used for in situ detection of S-phase entry. (Scale bar, 10 μm.) (H) Glc-shortened period without NAM (1.8 h, P < 0.001) vs. with NAM (0.6 h, P < 0.05) treatment, P < 0.01. For B and H, mean ± SEM, 2-way ANOVA P for multiple period comparisons, t test P for period change comparisons, n = 3, each replicates with 8 to 10 seedlings. For C and D, mean ± SEM, n = 3, each replicates with 6 seedlings, *P < 0.05, **P < 0.01, ***P < 0.001, 1-way ANOVA. For F and G, mean ± SD, n ≥ 12, ***P < 0.001, 1-way ANOVA.
Nicotinamide is the key precursor/byproduct for nicotinamide adenine dinucleotide, a redox cofactor that is particularly abundant in the mitochondria, where it supports respiration (14). Taking advantage of Arabidopsis lines expressing the fluorescent biosensor ATeam1.03-nD/nA, which provides a proxy for dynamic changes in the cytosolic magnesium adenosine triphosphate (MgATP2−) concentration (15), we found that nicotinamide significantly decreased ATP concentration (Fig. 2C). The kinetics with which the ATP concentration decreases correlated with the nicotinamide-induced decrease in TOR activity (Fig. 2 A and C). Glucose-TOR energy signaling is essential for root meristem activation and primary root growth (12). Interestingly, nicotinamide treatment largely impaired glucose-induced increase in ATP concentration (Fig. 2D), TOR activation (Fig. 2E), primary root growth (Fig. 2F), root meristem activation (Fig. 2G), and period shortening (Fig. 2H), indicating that nicotinamide may interfere with glucose-TOR energy signaling, although the osmotic effect is not fully excluded (5). Together, these results reveal an intimate relationship between energy status, as influenced by glucose and nicotinamide, and TOR to regulate circadian period, growth, and physiology.
Materials and Methods
In Figs. 1B and 2 A–C, the seeds were germinated in 1 mL of 0.5× Murashige and Skoog (MS) liquid medium with 5 mM glucose in 6-well plates for 6 d under 12L (40 μmol⋅m−2⋅s−1):12D conditions, then transferred to continuous low light (10 μmol⋅m−2⋅s−1) in the presence of 5 mM glucose. To study the effect of glucose (Figs. 1 C–E and 2 D, E, and H), the seeds were germinated in 1 mL of 0.5× MS liquid medium with 5 mM glucose for 4 d, and in photosynthesis-restrained (CO2 limited) sugar-free liquid medium for another 2 d under 12L (40 μmol⋅m−2⋅s−1):12D conditions (12) before being transferred to the same liquid medium under continuous low-light conditions (10 μmol⋅m−2⋅s−1) with/without 5 mM glucose. AMA (10 µM) and nicotinamide (10 mM) were treated 12 h before transferring unless otherwise stated. Estradiol (0.5 µM) was added at the beginning of germination. Mannitol (10 mM) was used as an osmotic control. For Fig. 2 A and C, all samples were collected at the same time. Luminescence was imaged by a cold charge-coupled device camera. Period and RAE were determined from ZT24 to ZT120 using Biological Rhythms Analysis Software System (BRASS). Root length and 5-ethynyl-2’-deoxyuridine (EdU) staining assay (Fig. 2 F and G) were performed as previously described (12). The ATH1 microarray data of nicotinamide treatment (GEO accession no. GSE19271, 69-h nicotinamide treatment, robust multiarray average, P value < 0.05; fold change > 2) were analyzed using the R Package limma (16).
Supplementary Material
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Grant 31870269 to Y.X.), the Chinese Academy of Sciences, and Fujian Agriculture and Forestry University.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1913095116/-/DCSupplemental.
References
- 1.Panda S., Poirier G. G., Kay S. A., tej defines a role for poly(ADP-ribosyl)ation in establishing period length of the Arabidopsis circadian oscillator. Dev. Cell 3, 51–61 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Nakahata Y., Sahar S., Astarita G., Kaluzova M., Sassone-Corsi P., Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324, 654–657 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haydon M. J., Mielczarek O., Robertson F. C., Hubbard K. E., Webb A. A., Photosynthetic entrainment of the Arabidopsis thaliana circadian clock. Nature 502, 689–692 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamia K. A., et al. , AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 326, 437–440 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dodd A. N., et al. , The Arabidopsis circadian clock incorporates a cADPR-based feedback loop. Science 318, 1789–1792 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Frank A., et al. , Circadian entrainment in Arabidopsis by the sugar-responsive transcription factor bZIP63. Curr. Biol. 28, 2597–2606.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martí Ruiz M. C., et al. , Circadian oscillations of cytosolic free calcium regulate the Arabidopsis circadian clock. Nat. Plants 4, 690–698 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X., et al. , Comment on “The Arabidopsis circadian clock incorporates a cADPR-based feedback loop.” Science 326, 230 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dodd A. N., et al. , Response to Comment on “The Arabidopsis circadian clock incorporates a cADPR-based feedback loop.” Science 326, 230 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feeney K. A., et al. , Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature 532, 375–379 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walton Z. E., et al. , Acid suspends the circadian clock in hypoxia through inhibition of mTOR. Cell 174, 72–87.e32 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiong Y., et al. , Glucose-TOR signalling reprograms the transcriptome and activates meristems. Nature 496, 181–186 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hruz T., et al. , Genevestigator v3: A reference expression database for the meta-analysis of transcriptomes. Adv. Bioinforma. 2008, 420747 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein L. R., Imai S., The dynamic regulation of NAD metabolism in mitochondria. Trends Endocrinol. Metab. 23, 420–428 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Col V., et al. , ATP sensing in living plant cells reveals tissue gradients and stress dynamics of energy physiology. eLife 6, e26770 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu D., et al. , The use of miRNA microarrays for the analysis of cancer samples with global miRNA decrease. RNA 19, 876–888 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.