Significance
We reveal species-specific changes in penguin trophic responses to historic shifts in krill availability over the last century by applying new molecular isotope techniques to historic penguin museum specimens. Generalist foraging gentoo penguins, whose population increased 6-fold in the last 40 y, showed adaptive shifts in trophic position in concert with changes in Antarctic krill availability following historic exploitation of marine mammals and recent climate change. In contrast, chinstrap penguins maintained a consistent krill diet despite changes in krill availability and concurrent population declines. These results highlight how responses to shared environmental change can vary substantially among closely related species, supporting ecological niche theory that specialists will be more sensitive to environmental change than their generalist counterparts.
Keywords: Antarctica, ecogeochemistry, environmental change, historical ecology, krill surplus
Abstract
The Southern Ocean is in an era of significant change. Historic overharvesting of marine mammals and recent climatic warming have cascading impacts on resource availability and, in turn, ecosystem structure and function. We examined trophic responses of sympatric chinstrap (Pygoscelis antarctica) and gentoo (Pygoscelis papua) penguins to nearly 100 y of shared environmental change in the Antarctic Peninsula region using compound-specific stable isotope analyses of museum specimens. A century ago, gentoo penguins fed almost exclusively on low-trophic level prey, such as krill, during the peak of historic overexploitation of marine mammals, which was hypothesized to have resulted in a krill surplus. In the last 40 y, gentoo penguin trophic position has increased a full level as krill declined in response to recent climate change, increased competition from recovering marine mammal populations, and the development of a commercial krill fishery. A shifting isotopic baseline supporting gentoo penguins suggests a concurrent increase in coastal productivity over this time. In contrast, chinstrap penguins exhibited no change in trophic position, despite variation in krill availability over the past century. The specialized foraging niche of chinstrap penguins likely renders them more sensitive to changes in krill availability, relative to gentoo penguins, as evinced by their declining population trends in the Antarctic Peninsula over the past 40 y. Over the next century, similarly divergent trophic and population responses are likely to occur among Antarctic krill predators if climate change and other anthropogenic impacts continue to favor generalist over specialist species.
Ecological responses to shared environmental change can vary substantially among species, even those that are closely related (1–4). Understanding species-specific responses to environmental change is critical to predicting the resilience and adaptation of species to disturbances associated with changes in climate or human–environment interactions. A species’ ecological niche, defined as a multidimensional hypervolume that includes axes relating to trophic dynamics, habitat use, and other life history requirements (5, 6), can be a key predictor of species’ responses to changes in their environment (1, 7). For example, specialist species use a narrow window of resources and are thought to be highly sensitive to environmental change (8, 9). In contrast, generalist species have broad or flexible resource use and are predicted to be more resilient to disturbances and/or changes in resource availability associated with environmental change (7, 10).
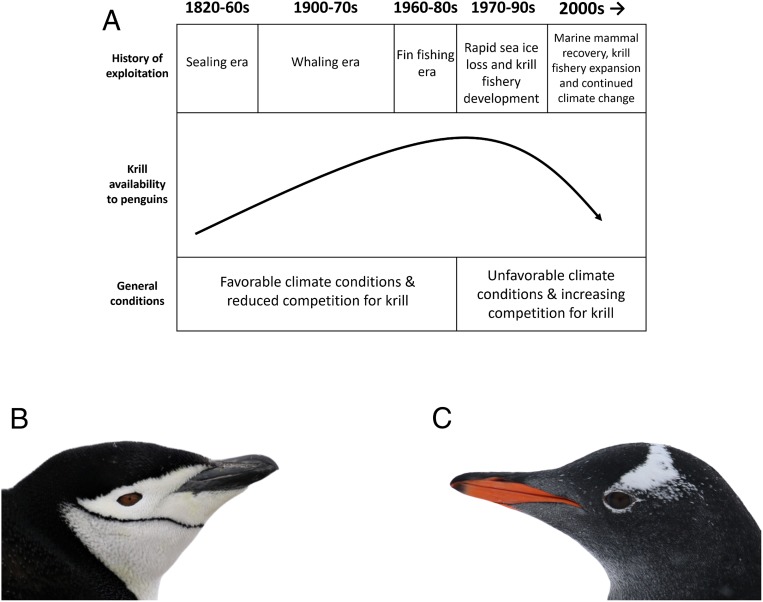
Chinstrap (Pygoscelis antarctica) and gentoo (Pygoscelis papua) penguins co-occur in the Antarctic Peninsula region and provide an opportunity to test explicit hypotheses about sympatric species' responses to environmental change. These sympatric species use similar nesting habitats, have similar phenology and breeding biology, are both considered pagophobic (ice-avoiding) unlike their pagophilic congener the Adélie penguin (Pygoscelis adeliae), and strongly overlap in dietary utilization of Antarctic krill (Euphausia superba) (11, 12). However, over the last 40 y, chinstrap penguin populations within the Antarctic Peninsula region have decreased by ∼30 to 53% (13, 14), while those of gentoo penguins have had more than a 6-fold increase (14–16). For example, along the South Orkney Islands, South Shetland Islands, and the Western Antarctic Peninsula, chinstrap penguin populations are estimated to have declined from as much as 3.1 million breeding pairs in the 1970s and 1980s to as few as 1.4 million breeding pairs in the 2010s (13), while gentoo penguins in this region increased from ∼31,312 to 243,316 breeding pairs during this same time period (15, 16). These divergent population trajectories may be, at least partially, explained by key differences in their trophic niches, which are thought to facilitate ecological niche segregation and promote coexistence (5, 17, 18). Chinstrap penguins have a narrow trophic niche with a specialized diet dominated by Antarctic krill (17, 18). Gentoo penguins, conversely, are generalist foragers with a broader and more flexible trophic niche relative to chinstrap penguins (18, 19). Trivelpiece et al. (20) proposed a hypothetical framework, invoking dietary reliance on krill, to explain recent population trends in Pygoscelis penguins as a function of the synergistic interaction of historic overexploitation of marine mammals, coupled with recent global climate change, on krill availability in the Antarctic Peninsula region (Fig. 1).
Fig. 1.
Drivers of krill availability in the Antarctic Peninsula region. (A) A conceptual summary of the historic and recent ecosystem perturbations in the Antarctic Peninsula region and their hypothesized implication for the availability of Antarctic krill to penguins in the genus Pygoscelis including (B) chinstrap (P. antarctica) and (C) gentoo (P. papua) penguins. (A) Adapted with permission from ref. 20.
The sequential overharvesting of seals, baleen whales, and finfish from the early 19th to the mid-20th century (12, 21, 22) is hypothesized to have resulted in a surplus of Antarctic krill available for the remaining krill predators, such as Pygoscelis penguins (23–25). For example, removal of whales alone in the mid-20th century is calculated to have led to an ∼150 million metric ton surplus of krill annually (21, 23). However, over the past 70 y, regional climate change, including ocean warming, sea ice decline, and ocean acidification, have acted in concert to negatively impact the abundance, distribution, and recruitment of krill (26–29), although see refs. 30–32. Recent recovery of whale and seal populations (33, 34) and a growing Antarctic krill fishery (35) have likely further decreased the prior krill surplus and increased competition among Southern Ocean krill predators, including penguins (Fig. 1).
While compelling, the hypothetical framework proposed by Trivelpiece et al. (20) has been challenging to test due to limited data on Pygoscelis penguin diets prior to the 1980s. To test the krill surplus hypothesis and shed light on species-specific responses to recent environmental stressors in the Southern Ocean, this study compares the trophic responses of sympatric chinstrap and gentoo penguins over nearly 100 y of shared environmental change. Accordingly, we applied molecular ecogeochemistry (see SI Appendix for further description) to historic penguin museum specimens dating back to the 1930s. We hypothesized that chinstrap penguins, with their specialized dietary niche and strong reliance on krill, would show little change in trophic position over time regardless of krill availability. In contrast, given the more plastic dietary niche of gentoo penguins, we hypothesized that this species’ trophic position has fluctuated over time in response to the proposed climate and harvesting-related changes in the availability of Antarctic krill, i.e., lower trophic positions indicative of higher dietary contribution of krill in the first half of the 20th century during the proposed krill surplus and higher trophic positions reflecting a switch away from krill during the krill decline of the second half of the 20th century.
To test these hypotheses, we analyzed the compound-specific stable nitrogen isotope values of individual amino acids (AAs) in archived penguin feathers to reconstruct the baseline food web nitrogen cycling and penguin trophic positions through time. The source AA phenylalanine δ15N value (δ15NPhe) exhibits minimal trophic discrimination, providing a proxy for the isotopic signature of nitrogen cycling at the base of the food web, while the trophic AA glutamic acid δ15N value (δ15NGlu) exhibits strong trophic discrimination [reviewed in McMahon and McCarthy (36)]. Together, these differentially fractionating AAs provide a measure of trophic position (TP) that is internally indexed to the nitrogen isotope value of the base of the food web (36). This approach is particularly valuable for examining biogeochemical cycling and trophic dynamics in a historical context when it is challenging, if not impossible, to a priori characterize the isotopic baseline of past ecosystems.
Results and Discussion
We identified divergent trophic responses in two congeneric, sympatric krill-predatory seabirds during nearly 100 y of shared environmental change in the Antarctic Peninsula region. Over the past century, gentoo penguins increased a full trophic position, shifting from a nearly exclusive krill diet in the 1930s to a diet with significantly more upper trophic level prey, likely fish and squid, in the modern system. At the same time, the nitrogen isotope baseline supporting the gentoo penguin food web also increased, suggesting that gentoo penguins have shifted from offshore to inshore foraging over the past 60 y and/or there has been an increase in coastal productivity. In contrast, chinstrap penguins showed no change in trophic dynamics over the past century, indicative of consistent offshore foraging on krill despite the recent decline in krill availability. The timing and direction of these divergent trophic responses support the krill surplus hypothesis and further highlight the sensitivity of specialist chinstrap penguin populations to anthropogenic and climatic forcings that drive changes in krill availability in the Antarctic Peninsula region.
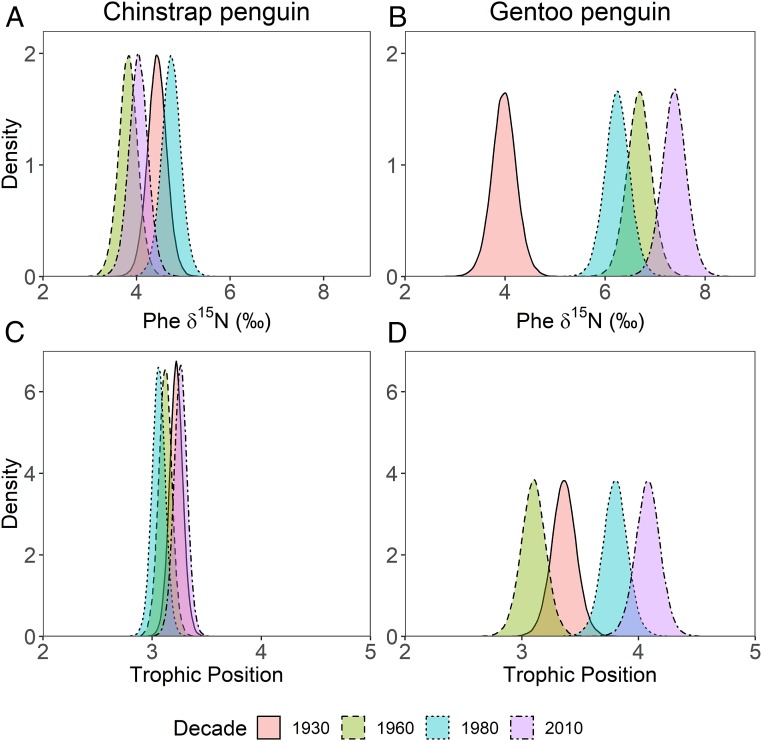
Our results provide support for the hypothetical framework proposed by Trivelpiece et al. (20), as temporal variation in the dietary generalist gentoo penguins’ trophic position was correlated with proposed historic shifts in krill availability. For example, gentoo penguin trophic position was lowest in the 1930s and 1960s during the proposed krill surplus and highest in the 1980s and 2010s when krill stocks are proposed to have declined (Bayesian analysis of variance [BANOVA] all PP > 0.99; Figs. 1 and 2). The trophic position of gentoo penguins in the 1930s (median [2.5 to 97.5% credible intervals]: TP = 3.4 [3.1 to 3.6]) and 1960s (TP = 3.1 [2.9 to 3.3]) was similar to those of the krill specialist chinstrap penguins (1930s TP = 3.2 [3.1 to 3.3] [BANOVA PP = 0.91], 1960s TP = 3.1 [3.0 to 3.2] [BANOVA PP = 0.42]), indicating a diet dominated by herbivorous zooplankton, such as Antarctic krill (37). Our data suggest that at the peak of the proposed krill surplus in the early to mid-20th century, owing to the sequential exploitation and depletion of krill-dependent Antarctic fur seals (Arctocephalus gazella), baleen whales, and finfish (Fig. 1 and refs. 20 and 21), gentoo and chinstrap penguins both consumed almost exclusively krill.
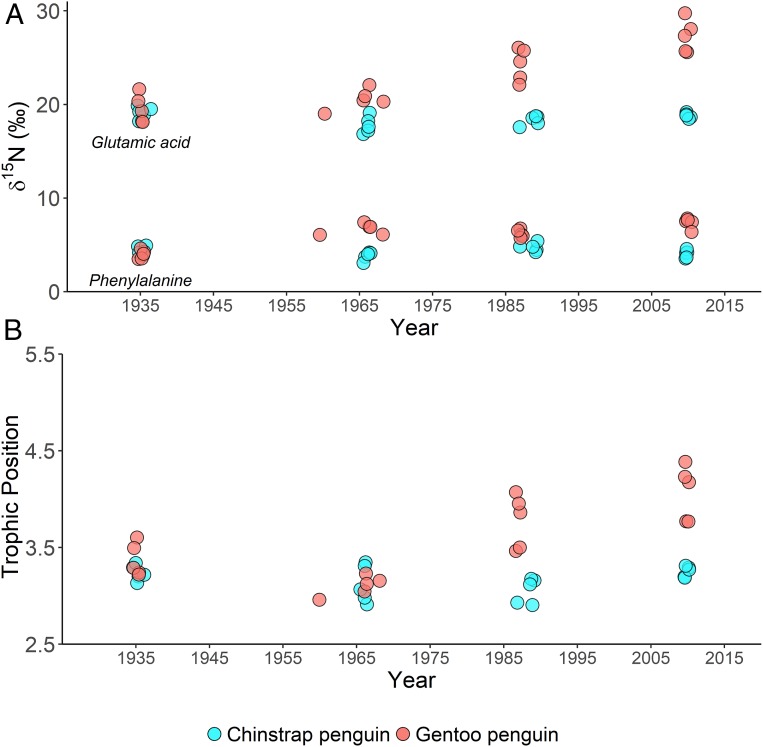
Fig. 2.
Penguin feather amino acid nitrogen stable isotope values. (A) Feather δ15N values of phenylalanine and glutamic acid and (B) the calculated trophic position of historic, archival museum specimens of chinstrap and gentoo penguins collected in the Antarctic Peninsula region in the 1930s, 1960s, 1980s, and 2010s.
Between the 1960s (TP = 3.1 [2.9 to 3.3]) and today (TP = 4.1 [3.9 to 4.3]), the trophic position of gentoo penguins in the northern Antarctic Peninsula increased by a full trophic level (Figs. 2 and 3). Modern gentoo penguin TP equates to a mixed diet of Antarctic krill (∼66%) and fish/squid (∼34%), assuming TP = 2.5 for Antarctic krill and TP = 4.1 for common prey fish and squid (37–39). This predicted modern diet composition agrees with recent studies using stomach content and bulk tissue stable isotope analyses (18, 19).
Fig. 3.
Density distribution of feather stable isotope parameters. Density distributions, estimated from BANOVA models, of feather δ15N values of phenylalanine (δ15NPhe) and calculated trophic position (TPTDF-multi) of archived historic museum specimens of (A and C) chinstrap and (B and D) gentoo penguins collected in the Antarctic Peninsula region in the 1930s, 1960s, 1980s, and 2010s.
The timing of the gentoo penguin trophic shift away from krill dominance aligns with recent declines in the abundance and recruitment of Antarctic krill since the 1970s (Fig. 1) (20, 27). Since the mid-20th century, the western Antarctic Peninsula has warmed faster than any region on Earth [6 °C average midwinter temperature increase since 1950 (40)] as a direct result of anthropogenic climate change (41, 42). Increases in sea surface temperature have led to substantial declines in the duration, extent, and thickness of sea ice (43, 44), which have been directly linked to major declines in krill abundance and recruitment since the 1970s (20, 26, 27, 29). Regional ocean acidification due to rising CO2 levels has further compounded environmental degradation for krill (45). Over this same time period, the recovery of whale and seal populations in response to the marine mammal conservation efforts of the International Convention for the Regulation of Whaling and the development of a commercial krill fishery (32) have likely increased competition for krill among Southern Ocean predators, including penguins. Our results provide long-term historical support for the conclusions drawn from a natural experiment in the western Ross Sea, where a short-term polynya brought penguins and whales together in a confined area, resulting in alterations to Adélie penguin diet and foraging behavior (46). That study concluded that competition for krill between Adélie penguins and minke whales resulted in the switch of penguins’ prey from krill to Antarctic silverfish (Pleuragramma antarctica), as opposed to a formerly hypothesized seasonal decrease in sea ice cover (47).
In contrast to the dynamic increase in trophic position of gentoo penguins over the past century, chinstrap penguins exhibited a consistent krill-based trophic position since the 1930s (TP ≅ 3.2 [range: 3.1 to 3.3] [BANOVA all PP < 0.99]), despite declines in krill abundance and recruitment in the northern Antarctic Peninsula in recent decades (20, 29, 48). As hypothesized, the divergent trophic responses of specialist-foraging chinstrap and generalist-foraging gentoo penguins aligned with known differences in their respective ecological niches (18, 19). Concurrent with these divergent trophic dynamics were major shifts in penguin population dynamics. Chinstrap penguin populations across the Antarctic Peninsula region decreased by 30 to 53% between 1979 and 2010, while gentoo penguin populations increased 6-fold during this same time period (13–16). Our trophic dynamics data, coupled with these recent population trends, agree with ecological theory that predicts climate change favors consumers in generalized niches over specialized niches, meaning that dietary specialists are more severely affected by climate change than generalists (7). We hypothesize that the specialized trophic niche of chinstrap penguins, if it remains static as in the last century, will increasingly negatively impact their population growth as they remain highly sensitive to declines in the abundance of Antarctic krill (18, 20) and potentially increased competition (49). In contrast, increasing population trends in gentoo penguins and their trophic plasticity relative to shifts in krill availability over the past century highlight this species’ ability to adapt to changing environmental conditions. Other life history traits, such as flexible phenology (50) and increased parental investment in chicks (51), may also buffer gentoo penguin populations relative to chinstrap penguin populations.
The rarity of museum specimens often imbues an opportunistic nature to retrospective studies. In this study, the availability of samples from the Antarctic Peninsula region varied both temporary and spatially (e.g., differing years and/or locations within decades for each species; SI Appendix, Tables S1 and S2). For example, in the 1980s samples were obtained from 2 years (1987 and 1989) and two locations (King George Island and Deception Island). Nevertheless, the chinstrap penguin sample from King George Island in 1987 (TP = 2.9) was within the range of trophic positions found in chinstrap penguins from Deception Island in 1989 (TP = 2.9 to 3.2) and outside the range observed in gentoo penguins from King George Island in 1987 (TP = 3.5 to 4.1). Similarly, variation in feather δ15N values and calculated trophic positions was consistently greater between species and among decades relative to interannual or among site variation (Fig. 2 and SI Appendix, Tables S1 and S2). This is predicted as body feathers synthesized with resources consumed during the postbreeding, premolt period (52) represent regional, as opposed to colony-specific, resource availability (35, 53). While obtaining additional historic museum samples is unlikely, future studies collecting feathers from current breeding populations at higher samples sizes over time could provide a more robust assessment of interannual and/or spatial variation.
Observed shifts in penguin trophic dynamics in the northern Antarctic Peninsula over the last century were overlain on apparent shifts in biogeochemical cycling at the base of the food web. δ15N values of the penguin source AA phenylalanine (δ15NPhe), which serve as a proxy for the sources and cycling of nitrogen at the base of the food web [reviewed in McMahon and McCarthy (36)], varied over the ∼100-y record for both penguin species, although not in parallel. In the 1930s, both gentoo (median = 4.0‰ [2.5 to 97.5%, credible intervals: 3.5 to 4.5‰]) and chinstrap penguins (4.4‰ [3.9 to 5.0‰]) had similarly low δ15NPhe values indicative of foraging in similar biogeochemical systems (BANOVA PP = 0.09). Over the next century, chinstrap penguin δ15NPhe values oscillated but remained relatively low (between 3.8 and 4.7‰), while gentoo penguin δ15NPhe values increased significantly between the 1930s (4.0‰ [3.5 to 4.5‰]) and the 1960s (6.7‰ [6.2 to 7.2‰]) and then again between the 1980s (6.2‰ [5.7 to 6.7‰]) and the 2010s (7.4‰ [6.9 to 7.9‰] [all BANOVAs PP > 0.99]). This increase in gentoo penguin δ15NPhe values over the last century, which was not found in chinstrap penguin δ15NPhe values, likely reflects changes in gentoo penguin foraging location, nearshore vs. offshore, regional increases in productivity in nearshore waters, or both. A shift in diet alone, from krill in the early to mid-20th century to more fish and squid today, cannot explain the gentoo penguin δ15NPhe trend as the shift in baseline preceded the shift in diet and trophic position (SI Appendix).
Body feather δ15NPhe values of the adult penguins sampled in this study (SI Appendix, Table S1 and S2) reflect the foraging habitats of penguins during the postbreeding, premolt period (52). Movement data from both the breeding and postbreeding periods, including limited data during the premolt period, indicate seasonally consistent differences in the foraging habitats of chinstrap and gentoo penguins (17, 35). In the Antarctic Peninsula region, breeding chinstrap penguins typically forage in oceanic waters (>200 m depth) off the continental shelf (77% of dives), whereas gentoo penguins primarily forage in nearshore waters (71% of dive locations), often near the benthos (28% of dives) (54). Outside of the breeding season, gentoo penguins increase their maximum foraging range but remain in coastal waters (53) regularly returning to land following 4- to 12-h bouts of foraging (55). In contrast, during premolt, chinstrap penguins forage in offshore, pelagic waters and spend their winter months at sea (35). These distinct nearshore (gentoo) and offshore (chinstrap) foraging habitats also have distinct baseline δ15N values as a function of NO3− utilization efficiency (higher utilization in nearshore systems = higher particulate organic matter [POM] δ15N values) (56, 57). Modern chinstrap penguin δ15NPhe values of 4.0‰ (3.6 to 4.5‰) align well with foraging within the offshore waters of the northwestern Antarctic Peninsula based on euphausiid-derived nitrogen isoscapes for the region (4.1 ± 0.8‰) (57). Similarly, modern gentoo penguin δ15NPhe values (7.4‰ [6.9 to 7.9‰]) suggest foraging within nearshore productive waters of the Antarctic Peninsula (NO3− δ15N = 7 to 10‰ during periods of maximum NO3− drawdown) (58). One possible explanation for the low δ15NPhe values of gentoo penguins in the 1930s, relative to later decades, is that during this time period, gentoo penguins foraged further offshore, overlapping with chinstrap penguins. However, this hypothesis seems less likely, given that past studies support the idea that gentoo penguins are nonmigratory, diurnal foragers that retain the use of nearshore foraging habitats year-round (35, 53, 55).
Alternatively, assuming no changes in foraging distributions, the increasing gentoo penguin δ15NPhe values over the past century could reflect an increase in primary productivity in nearshore waters. As primary productivity increases, the δ15N value of the residual pool of NO3– increases as NO3– is consumed, resulting in an increase in the δ15N value of newly formed plankton biomass (59). Few data are available for the δ15N values of POM from nearshore zones from the first half of the 20th century (60); however, Kim, et al. (61) reported increasing nearshore phytoplankton biomass in the South Shetland Islands and Western Antarctic Peninsula between 1992 and 2016, and Moreau et al. (62) found a strong correlation between the clear increase in summer sea surface temperature from 1990 to 2010 and water column primary production in the same region, likely driven by meltwater stabilization of the water column reducing light limitation (63). If this hypothesis is correct, our data would suggest that modern observations of increasing coastal productivity are part of a longer-term trend of increasing coastal productivity in the northern Antarctic Peninsula since the early 20th century. In contrast, between 1978 and 2006, chlorophyll a concentrations declined in the offshore, oceanic waters of the northern Antarctic Peninsula (64) where chinstrap penguin typically forage (54), which may explain the observed 0.7‰ decrease in chinstrap penguin δ15NPhe values over this same time period.
Our comparative trophodynamic approach using molecular geochemistry on museum archived penguins supports the hypothesis that historic anthropogenic exploitation and recent climate change have shifted the availability of Antarctic krill to Pygoscelis penguins, and by extension other krill predators, over the past century (20, 65). We predict that the trophic responses of the Pygoscelis penguin species in our study are emblematic of a larger ecosystem response to historic shifts in krill availability. For example, Emslie and Patterson (24) observed an abrupt shift in the bulk tissue δ15N values of radiocarbon-dated eggshells from pagophilic Adélie penguins sometime within the last 200 y. While the exact timing of the shift observed by Emslie and Patterson (24) was unclear due to uncertainty in the marine-carbon reservoir effect, we hypothesize that this bulk isotope shift reflects a trophic response of Adélie penguins to changing krill availability, similar to the shift observed in gentoo penguins in our study. Future analyses on Adélie penguin eggshell AA δ15N values could be used to test this hypothesis. Regardless, rising temperatures in the Antarctic Peninsula region over the next century, as well as krill harvesting if not managed properly (66), are predicted to continue to negatively impact krill biomass and lead to declines in populations of krill-dependent predators (67, 68). Over the next century the Antarctic Peninsula region will remain a hot spot with respect to multiple, superimposed climate change processes and other anthropogenic impacts (e.g., ocean warming, sea ice loss, ocean acidification, krill harvesting, tourism, and species introductions) likely creating synergistic challenges for Pygoscelis penguins and other specialist and generalist marine predators in Antarctica (68–70).
Materials and Methods
Chinstrap and gentoo penguin feather samples were obtained from specimens collected from the Antarctic Peninsula region (i.e., the Antarctic Peninsula, including the South Shetland and South Orkney Islands) during historic and more recent Antarctic explorations and curated at natural history museums (SI Appendix, Tables S1 and S2). Five individuals per species were sampled from four discrete time periods (i.e., 1930s, 1960s, 1980s, and 2010s) for a total of 40 specimens.
Three breast feathers per specimen were acid hydrolyzed, derivatized, and analyzed for compound-specific nitrogen (δ15N) stable isotope analysis (CSIA) of 12 individual AAs [McMahon et al. (71)] (SI Appendix). Feather samples were analyzed in triplicate along with a homogeneous laboratory algal standard (>100 repeat injections) and a mixed AA standard of known isotopic composition (Sigma-Aldrich Co.) (mean δ15N reproducibility: ±0.3‰ for laboratory algal standard and mixed AA standard). We calculated individual penguins’ trophic positions (TPTDF-multi) using a multi-trophic discrimination factor (TDF) equation following McMahon et al. (71):
where δ15NGlu and δ15NPhe represent the stable nitrogen isotope values of penguin Glu and Phe, respectively; β represents the difference in δ15N between Glu and Phe of primary producers [3.4‰ for aquatic microalgae (72–74)]; TDF(Glu-Phe) average represents an average TDF of 6.3‰ (Δ15NGlu − Δ15NPhe) characteristic of planktonic marine food webs [see metaanalysis by McMahon and McCarthy (36)]; and TDF(Glu-Phe) penguin represents the Pygoscelis penguin-specific TDFGlu-Phe value (3.5‰) derived from a controlled feeding experiment on Pygoscelis penguins (71). Alternative methods of calculating TP from AA δ15N values resulted in similar trends in TPCSIA over time and between species (SI Appendix, Fig. S1).
The mean and variance of δ15NGlu, δ15NPhe, and TPTDF-multi across each time period were estimated separately using BANOVA for gentoo and chinstrap penguins. Equal variance among time periods and species was assumed for each analysis. Parameter estimates (unbiased) were obtained with Markov chain Monte Carlo and Just Another Gibbs Sampler (JAGS). Vague priors were used for the mean (normal distributions with a mean of 0 and variance of 1,000) and the variance (uniform distribution between 0 and 100). Pairwise comparison among time periods and between species are expressed as Bayesian posterior probabilities (PP > 0.99 as a conservative indication of significant differences) that the posterior distributions of δ15NGlu, δ15NPhe, and TPTDF-multi from one group (time period or species) are different in a 1-way comparison with another group. All statistics were performed in R version 3.5.0 (75).
Ethics.
Field work was conducted via Antarctic Conservation Act permits (ACA 2006-001 and 2013-007), and animal use was approved by Woods Hole Oceanographic Institution Institutional Animal Care and Use Committee (27071382).
Data Accessibility.
Data are available in SI Appendix, Tables S1 and S2.
Supplementary Material
Acknowledgments
We thank the American Museum of Natural History, the National Museum of Natural History (United States), Swedish Museum of Natural History, Royal Belgian Institute of Natural Sciences, Natural History Museum at Tring (United Kingdom), Biodiversity Research and Teaching Collections of Texas A&M University, University of North Carolina Wilmington Natural History Collections, and Quark Expeditions for specimen collections. We thank Steven D. Emslie, Heather J. Lynch, the PNAS editor, and two anonymous reviewers for valuable assistance and comments on this manuscript. This work was funded by US National Science Foundation Office of Polar Programs awards to M.J.P. (ANT-1443585), K.W.M. (ANT-1826712), and the Antarctic Science Bursary (M.J.P.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1913093116/-/DCSupplemental.
References
- 1.Walther G.-R., et al. , Ecological responses to recent climate change. Nature 416, 389–395 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Hinke J. T., Salwicka K., Trivelpiece S. G., Watters G. M., Trivelpiece W. Z., Divergent responses of Pygoscelis penguins reveal a common environmental driver. Oecologia 153, 845–855 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Kroeker K. J., Micheli F., Gambi M. C., Martz T. R., Divergent ecosystem responses within a benthic marine community to ocean acidification. Proc. Natl. Acad. Sci. U.S.A. 108, 14515–14520 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younger J. L., Emmerson L. M., Miller K. J., The influence of historical climate changes on Southern Ocean marine predator populations: A comparative analysis. Glob. Change Biol. 22, 474–493 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Hutchinson G. E., Homage to Santa Rosalia or why are there so many kinds of animals? Am. Nat. 93, 145–159 (1959). [Google Scholar]
- 6.Hutchinson G., Concluding remarks. Cold Spring Harb. Symp. Quant. Biol. 22, 415–427 (1957). [Google Scholar]
- 7.Lurgi M., López B. C., Montoya J. M., Novel communities from climate change. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 2913–2922 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clavel J., Julliard R., Devictor V., Worldwide decline of specialist species: Toward a global functional homogenization? Front. Ecol. Environ. 9, 222–228 (2011). [Google Scholar]
- 9.Moritz C., Agudo R., The future of species under climate change: Resilience or decline? Science 341, 504–508 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Menéndez R., et al. , Species richness changes lag behind climate change. Proc. Biol. Sci. 273, 1465–1470 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trivelpiece W. Z., Trivelpiece S. G., Volkman N. J., Ecological segregation of Adélie, gentoo, and chinstrap penguins at King George Island, Antarctica. Ecology 68, 351–361 (1987). [Google Scholar]
- 12.Fraser W. R., Trivelpiece W. Z., Ainley D. G., Trivelpiece S. G., Increases in Antarctic penguin populations: Reduced competition with whales or a loss of sea ice due to environmental warming? Polar Biol. 11, 525–531 (1992). [Google Scholar]
- 13.Trivelpiece W. Z., Trivelpiece S. G., “Chinstrap Penguin” in Penguins: Natural History and Conservation, Borborolgu P. G., Boersma P. D., Eds. (University of Washington Press, 2013), pp. 59–71. [Google Scholar]
- 14.Lynch H. J., Naveen R., Trathan P. N., Fagan W. F., Spatially integrated assessment reveals widespread changes in penguin populations on the Antarctic Peninsula. Ecology 93, 1367–1377 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Humphries G., et al. , Mapping application for penguin populations and projected dynamics (MAPPPD): Data and tools for dynamic management and decision support. Polar Rec. 53, 160–166 (2017). [Google Scholar]
- 16.MAPPPD , Mapping Application for Penguin Populations and Projected Dynamics. http://www.penguinmap.com/. Accessed 27 September 2019.
- 17.Miller A. K., Kappes M. A., Trivelpiece S. G., Trivelpiece W. Z., Foraging-niche separation of breeding gentoo and chinstrap penguins, South Shetland Islands, Antarctica. Condor 112, 683–695 (2010). [Google Scholar]
- 18.Polito M. J., et al. , Contrasting specialist and generalist patterns facilitate foraging niche partitioning in sympatric populations of Pygoscelis penguins. Mar. Ecol. Prog. Ser. 519, 221–237 (2015). [Google Scholar]
- 19.Miller A. K., Karnovsky N. J., Trivelpiece W. Z., Flexible foraging strategies of gentoo penguins Pygoscelis papua over 5 years in the South Shetland Islands, Antarctica. Mar. Biol. 156, 2527–2537 (2009). [Google Scholar]
- 20.Trivelpiece W. Z., et al. , Variability in krill biomass links harvesting and climate warming to penguin population changes in Antarctica. Proc. Natl. Acad. Sci. U.S.A. 108, 7625–7628 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laws R., Seals and whales of the Southern Ocean. Philos. Trans. R. Soc. Lond. B Biol. Sci. 279, 81–96 (1977). [Google Scholar]
- 22.Kock K.-H., Jones C. D., Fish stocks in the southern Scotia Arc region—A review and prospects for future research. Rev. Fish. Sci. 13, 75–108 (2005). [Google Scholar]
- 23.Laws R. M., The ecology of the Southern Ocean. Am. Sci. 73, 26–40 (1985). [Google Scholar]
- 24.Emslie S. D., Patterson W. P., Abrupt recent shift in δ 13C and δ 15N values in Adélie penguin eggshell in Antarctica. Proc. Natl. Acad. Sci. U.S.A. 104, 11666–11669 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trathan P. N., Hill S. L., “The importance of krill predation in the Southern Ocean” in Biology and Ecology of Antarctic Krill, Siegel V., Ed. (Springer, 2016), pp. 321–350. [Google Scholar]
- 26.Atkinson A., Siegel V., Pakhomov E., Rothery P., Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 432, 100–103 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Meredith M. P., King J. C., Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys. Res. Lett. 32, L19604 (2005). [Google Scholar]
- 28.Flores H., et al. , Impact of climate change on Antarctic krill. Mar. Ecol. Prog. Ser. 458, 1–19 (2012). [Google Scholar]
- 29.Atkinson A., et al. , Krill (Euphausia superba) distribution contracts southward during rapid regional warming. Nat. Clim. Chang. 9, 142–147 (2019). [Google Scholar]
- 30.Cox M. J., et al. , No evidence for a decline in the density of Antarctic krill Euphausia superba Dana, 1850, in the Southwest Atlantic sector between 1976 and 2016. J. Crustac. Biol. 38, 656–661 (2018). [Google Scholar]
- 31.Hill S. L., Atkinson A., Pakhomov E. A., Siegel V., Evidence for a decline in the population density of Antarctic krill Euphausia superba still stands. A comment on Cox et al. J. Crustac. Biol. 39, 316–322 (2019). [Google Scholar]
- 32.Cox M. J., et al. , Clarifying trends in the density of Antarctic krill Euphausia superba Dana, 1850 in the South Atlantic. A response to Hill et al. J. Crustac. Biol. 39, 323–327 (2019). [Google Scholar]
- 33.Hucke-Gaete R., Osman L., Moreno C., Torres D., Examining natural population growth from near extinction: The case of the Antarctic fur seal at the South Shetlands, Antarctica. Polar Biol. 27, 304–311 (2004). [Google Scholar]
- 34.Reilly S., et al. , Biomass and energy transfer to baleen whales in the South Atlantic sector of the Southern Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 51, 1397–1409 (2004). [Google Scholar]
- 35.Hinke J. T., et al. , Identifying risk: Concurrent overlap of the Antarctic krill fishery with krill-dependent predators in the Scotia Sea. PLoS One 12, e0170132 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMahon K. W., McCarthy M. D., Embracing variability in amino acid δ15N fractionation: Mechanisms, implications, and applications for trophic ecology. Ecosphere 7, e01511 (2016). [Google Scholar]
- 37.Schmidt K., Atkinson A., “Feeding and food processing in Antarctic krill (Euphausia superba Dana)” in Biology and Ecology of Antarctic Krill, Siegel V., Ed. (Springer, 2016), pp. 175–224. [Google Scholar]
- 38.Mintenbeck K., Trophic Interactions within High Antarctic Shelf Communities-Food Web Structure and the Significance of Fish (University of Bremen, 2008). [Google Scholar]
- 39.Stowasser G., et al. , Food web dynamics in the Scotia Sea in summer: A stable isotope study. Deep Sea Res. Part II Top. Stud. Oceanogr. 59, 208–221 (2012). [Google Scholar]
- 40.McClintock J., Ducklow H., Fraser W., Ecological responses to climate change on the Antarctic Peninsula: The Peninsula is an icy world that’s warming faster than anywhere else on Earth, threatening a rich but delicate biological community. Am. Sci. 96, 302–310 (2008). [Google Scholar]
- 41.Mulvaney R., et al. , Recent Antarctic Peninsula warming relative to Holocene climate and ice-shelf history. Nature 489, 141–144 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Schmidtko S., Heywood K. J., Thompson A. F., Aoki S., Multidecadal warming of Antarctic waters. Science 346, 1227–1231 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Massom R. A., Stammerjohn S. E., Antarctic sea ice change and variability—Physical and ecological implications. Polar Sci. 4, 149–186 (2010). [Google Scholar]
- 44.Stammerjohn S., Massom R., Rind D., Martinson D., Regions of rapid sea ice change: An inter-hemispheric seasonal comparison. Geophys. Res. Lett. 39, L05502 (2012). [Google Scholar]
- 45.Kawaguchi S., et al. , Risk maps for Antarctic krill under projected Southern Ocean acidification. Nat. Clim. Chang. 3, 843 (2013). [Google Scholar]
- 46.Ainley D. G., Ballard G., Dugger K. M., Competition among penguins and cetaceans reveals trophic cascades in the western Ross Sea, Antarctica. Ecology 87, 2080–2093 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Ainley D. G., et al. , Spatial and temporal variation of diet within a presumed metapopulation of Adélie penguins. Condor 105, 95–106 (2003). [Google Scholar]
- 48.Siegel V., Loeb V., Gröger J., Krill (Euphausia superba) density, proportional and absolute recruitment and biomass in the Elephant Island region (Antarctic Peninsula) during the period 1977 to 1997. Polar Biol. 19, 393–398 (1998). [Google Scholar]
- 49.Hinke J. T., et al. , Spatial and isotopic niche partitioning during winter in chinstrap and Adélie penguins from the South Shetland Islands. Ecosphere 6, 1–32 (2015). [Google Scholar]
- 50.Hinke J. T., Polito M. J., Reiss C. S., Trivelpiece S. G., Trivelpiece W. Z., Flexible reproductive timing can buffer reproductive success of Pygoscelis spp. penguins in the Antarctic Peninsula region. Mar. Ecol. Prog. Ser. 454, 91–104 (2012). [Google Scholar]
- 51.Polito M. J., Trivelpiece W. Z., Transition to independence and evidence of extended parental care in the gentoo penguin (Pygoscelis papua). Mar. Biol. 154, 231 (2008). [Google Scholar]
- 52.Polito M. J., Abel S., Tobias C. R., Emslie S. D. J. P. B., Dietary isotopic discrimination in gentoo penguin (Pygoscelis papua) feathers. Polar Biol. 34, 1057–1063 (2011). [Google Scholar]
- 53.Wilson R., et al. , The movements of gentoo penguins Pygoscelis papua from Ardley Island, Antarctica. Polar Biol. 19, 407–413 (1998). [Google Scholar]
- 54.Kokubun N., Takahashi A., Mori Y., Watanabe S., Shin H.-C., Comparison of diving behavior and foraging habitat use between chinstrap and gentoo penguins breeding in the South Shetland Islands, Antarctica. Mar. Biol. 157, 811–825 (2010). [Google Scholar]
- 55.Hinke J. T., Trivelpiece W. Z. J. P. b., Daily activity and minimum food requirements during winter for gentoo penguins (Pygoscelis papua) in the South Shetland Islands, Antarctica. Polar Biol. 34, 1579–1590 (2011). [Google Scholar]
- 56.Schmidt K., et al. , Trophic relationships among Southern Ocean copepods and krill: Some uses and limitations of a stable isotope approach. Limnol. Oceanogr. 48, 277–289 (2003). [Google Scholar]
- 57.Brault E. K., et al. , Carbon and nitrogen zooplankton isoscapes in West Antarctica reflect oceanographic transitions. Mar. Ecol. Prog. Ser. 593, 29–45 (2018). [Google Scholar]
- 58.Henley S. F., et al. , Macronutrient supply, uptake and recycling in the coastal ocean of the west Antarctic Peninsula. Deep Sea Res. Part II Top. Stud. Oceanogr. 139, 58–76 (2017). [Google Scholar]
- 59.Sigman D., Altabet M., McCorkle D., Francois R., Fischer G., The δ15N of nitrate in the Southern Ocean: Consumption of nitrate in surface waters. Global Biogeochem. Cycles 13, 1149–1166 (1999). [Google Scholar]
- 60.Leventer A., et al. , Productivity cycles of 200–300 years in the Antarctic Peninsula region: Understanding linkages among the sun, atmosphere, oceans, sea ice, and biota. Geol. Soc. Am. Bull. 108, 1626–1644 (1996). [Google Scholar]
- 61.Kim H., et al. , Inter-decadal variability of phytoplankton biomass along the coastal West Antarctic Peninsula. Philos. Trans. A Math. Phys. Eng. Sci. 376, 20170174 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moreau S., et al. , Climate change enhances primary production in the western Antarctic Peninsula. Glob. Change Biol. 21, 2191–2205 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Smith R. C., Dierssen H. M., Vernet M., Phytoplankton biomass and productivity in the western Antarctic Peninsula region. Antarct. Res. Ser. 70, 333–356 (1996). [Google Scholar]
- 64.Montes-Hugo M., et al. , Recent changes in phytoplankton communities associated with rapid regional climate change along the western Antarctic Peninsula. Science 323, 1470–1473 (2009). [DOI] [PubMed] [Google Scholar]
- 65.Hückstädt L., et al. , Diet of a specialist in a changing environment: The crabeater seal along the western Antarctic Peninsula. Mar. Ecol. Prog. Ser. 455, 287–301 (2012). [Google Scholar]
- 66.Nicol S., Foster J., “The fishery for Antarctic krill: Its current status and management regime” in Biology and Ecology of Antarctic Krill, Siegel V., Ed. (Springer, 2016), pp. 387–421. [Google Scholar]
- 67.Klein E. S., Hill S. L., Hinke J. T., Phillips T., Watters G. M., Impacts of rising sea temperature on krill increase risks for predators in the Scotia Sea. PLoS One 13, e0191011 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siegert M. J., et al. , The Antarctic Peninsula under a 1.5° C global warming scenario. Front. Environ. Sci. 7, 102 (2019). [Google Scholar]
- 69.Aronson R. B., Thatje S., McClintock J. B., Hughes K. A., Anthropogenic impacts on marine ecosystems in Antarctica. Ann. N. Y. Acad. Sci. 1223, 82–107 (2011). [DOI] [PubMed] [Google Scholar]
- 70.Gutt J., et al. , The Southern Ocean ecosystem under multiple climate change stresses—An integrated circumpolar assessment. Glob. Change Biol. 21, 1434–1453 (2015). [DOI] [PubMed] [Google Scholar]
- 71.McMahon K. W., Polito M. J., Abel S., McCarthy M. D., Thorrold S. R., Carbon and nitrogen isotope fractionation of amino acids in an avian marine predator, the gentoo penguin (Pygoscelis papua). Ecol. Evol. 5, 1278–1290 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McClelland J. W., Montoya J. P., Trophic relationships and the nitrogen isotopic composition of amino acids in plankton. Ecology 83, 2173–2180 (2002). [Google Scholar]
- 73.Chikaraishi Y., Ogawa N. O., Ohkouchi N., “Further evaluation of the trophic level estimation based on nitrogen isotopic composition of amino acids” in Earth, Life, Isotopes, Ohkouchi N., Tayasu I., Koba K., Eds. (Kyoto University Press, 2010), pp. 37–51. [Google Scholar]
- 74.McCarthy M. D., Lehman J., Kudela R., Compound-specific amino acid δ15N patterns in marine algae: Tracer potential for cyanobacterial vs. eukaryotic organic nitrogen sources in the ocean. Geochim. Cosmochim. Acta 103, 104–120 (2013). [Google Scholar]
- 75.R Core Team , R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2018). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in SI Appendix, Tables S1 and S2.