Abstract
Current cancer therapies have encountered adverse response due to poor therapeutic efficiency, severe side effects and acquired resistance to multiple drugs. Thus, there are urgent needs for finding new cancer-targeted pharmacological strategies. In this review, we summarized the current understanding with THZ1, a covalent inhibitor of cyclin-dependent kinase 7 (CDK7), which demonstrated promising anti-tumor activity against different cancer types. By introducing the anti-tumor behaviors and the potential targets for different cancers, this review aims to provide more effective approaches to CDK7 inhibitor-based therapeutic agents and deeper insight into the diverse tumor proliferation mechanisms.
Keywords: THZ1, Cyclin-dependent kinase 7, Cancer therapy, Transcription, Super-enhancer
Introduction
Recent advances in the development of anticancer agents have largely improved the prognosis and survival rate of cancer patients. However, current cancer therapies sometimes encounter adverse response in some subsets of patients due to poor therapeutic efficiency, undesirable toxicity and acquired resistance, especially for the patients at advanced cancer stages. Thus, needs to find new pharmacological strategies for the intractable cancers remain nonnegligible. Basically, tumor oncogenes include transcription factors that cooperate with the general transcriptional machinery in order to sustain the oncogenic state.1 However, pharmacological inhibition aiming at transcription factors directly has been proven insufficient.2 This has led to the development of molecules that specifically inhibit various enzymatic cofactors of the transcriptional machinery, which have been found to be promising therapeutic candidates such as the cyclin-dependent kinases (CDKs).3 In this respect, different CDK inhibitors have been reported with anti-tumor properties based on the preclinical and clinical studies.4 CDK7, associated with the transcription initiation factor transcription factor II (TFII) H, has multiple functions including regulating cell cycle and mediating phosphorylation of RNA polymerase II (RNAP II) within the transcription machinery.5 In 2014, THZ1 has been firstly reported as a potent covalent inhibitor of CDK7, and exhibited unprecedented anti-proliferation behavior against tumor cells of different genotypes and phenotypes both in vitro and in vivo. The anti-tumor property of THZ1 has been discussed by Kwiatkowski et al6 through screening over 1000 cancer cell lines. THZ1 possesses half-maximum inhibitory concentration (IC50) values less than 200 nM against 53% of the cell lines tested. Notably, treatment with THZ1 was highly specific for only cancerous cell lines and demonstrated no obvious toxicity to normal human cell lines. The common genomic features from 527 THZ1-sensitive cancer cell lines have been illustrated via combined method of elastic net regression with gene ontology term enrichment analysis. The data reflected a strong enrichment of factors participating in RNAP II-driven transcriptional regulation and oncogenic transcription, which indicated that the dominant anti-tumor activity of THZ1 comes from the ability to modulate transcription of cancerous cells. Based on the biology and molecular structure of THZ1, various CDK7 inhibitors such as QS1189,7 SY-13658 and ICEC09429 have been developed and identified to have promising anticancer effects in mantle cell lymphoma, ovarian cancer and breast cancer respectively.
Aiming at emphasizing the importance and new possibilities brought by THZ1 in anticancer agent development, we reviewed and summarized the current understanding with THZ1, including the anti-tumor behaviors and the potential targets from different cancer types, with the purpose of providing more effective approaches to CDK7 inhibitor-based therapeutic agents and deeper insight into the diverse tumor proliferation mechanisms.
CDK7 inhibition: duel effects on cell-cycle and transcription regulation
Functions of cell-cycle CDKs depend on CDK-activating kinase (CAK) activity of CDK7
Cell cycle in all eukaryotic cells, including human cells is driven by sequential activation of cyclin-dependent kinases (CDKs).4 For tumors, normal cells are transformed to malignant clones directly or indirectly by the deregulation of cell cycle machinery. In some cases, the activity of CDKs is often increased due to the deactivation of CDKs inhibitor proteins by point mutations, gene deletions or silencing. Some CDKs themselves may be deregulated, such as the overexpression and amplification of CDK4 and CDK6 found in some tumors. Additionally, some CDKs such as CDK1 and CDK2 are activated by overexpression of their respective cyclins (A, B, D, E) instead of gene mutations. The overexpression of cell-cycle related CDKs allows hyperphosphorylation on multiple substrates which supports the tumor cells to proceed with cell cycles despite of unfavorable extra- or intra-cellular environment. CDKs that are responsible for cell-cycle require activation of T-loop phosphorylation for their full functions. CDK7 is a major CAK in mammals and participates in regulating the cell cycle. Despite that some evidence showed existence of other CAKs,10 removal or chemical inhibition of CDK7 blocks CAK activity of whole-cell extracts.11, 12 For example, selective inhibition of CDK7 in HCT116 colon cancer cells induced inactivation of both CDK1 and CDK2 and caused cell-cycle arrest at both G1/S and G2/M phase.13 Moreover, the CDK4 and CDK6 activation is reported to be enhanced by CDK7 T-loop phosphorylation, thus, CDK4 and CDK6 lose their functions immediately after CDK7 inhibition in human cells.14 Intense studies have demonstrated the dominant role of CDK7 in cell-cycle machinery.
CDK7 regulates cellular transcription
RNAP II (Pol II) is a protein complex which catalyzes DNA transcription in eukaryotes, to produce precursors of messenger RNA (mRNA), small nuclear RNA (snRNA) and microRNA. These processes are driven by general transcription factors (GTFs) including TFIIA, TFIIB, TFIID, TFIIE, TFIIF and TFIIH.15, 16 Among the GTFs, TFIIH have multiple functions in transcription of various protein-coding genes and nucleotide excision repair (NER) pathways of DNA. TFIIH is a protein complex containing 10 subunits, 7 of which form the core complex including xeroderma pigmentosum type D (XPD), xeroderma pigmentosum type B (XPB), p62, p52, p44, p34 and trichothiodystrophy group A (TTDA). The CAK subcomplex which consists of CDK7, menage a trois 1 (MAT1) and cyclin H, is linked with the core by XPD (ATP-dependent helicase) protein. During the initiation of transcription, TFIIH opens the core promoter DNA by helicase, while CDK7 phosphorylates the C-terminal domain (CTD) of RNAP II at Ser5 and Ser7 together with other transcription factors controlling the initiation-to-elongation transition.17 The phosphorylation of CTD is crucial in the recruitment of RNA processing factors and histone modification enzymes.18 Multiple studies have indicated CDK7 is essential for the timely execution of transcription in initiation, capping, pausing and productive elongation process.19 Thus, CDK7 inhibition may provide new targeted therapeutic method against cancer types with unsatisfying prognosis and overcome serious side effects accompanied by agents with broad anti-proliferation mechanisms.
A covalent inhibitor of CDK7: THZ1
The deregulation of CDKs is often observed in different types of tumor cells, which makes them potential targets in cancer therapies. A list of agents targeting different CDKs have been synthesized or extracted from natural products, and some of them have been introduced in early or advanced clinical trials.4 Kwiatkowski et al6 reported a covalent inhibitor of CDK7, THZ1, which demonstrated high selectivity of target and great potential in anti-tumor therapy against different types of cancer in vitro and in vivo.
Through mass spectrometry, the THZ1 binding site on CDK7 was identified to be at C312, which is a residue outside the kinase domain. Docking model of THZ1 with CDK7 crystal structure demonstrated that the C-terminal of CDK7 bearing C312 residue traverses the adenosine triphosphate (ATP) cleft in the kinase domain, where C312 directly connects to the acrylamide site of THZ1 possibly through Michael addition reaction. The binding site position of CDK7 was also confirmed by the fact that THZ1 lost its ability to inhibit RNAP II in an irreversible fashion when changing the CDK7 C312 residue to serine by mutation.6 In addition, the reactive acrylamide group on THZ1 was found essential for binding with nucleophilic C312 residual of CDK7. Altering the carbon-carbon double bond of acrylamide moiety of THZ1 to single bond afforded THZ1-R, which largely weakened its CDK7 inhibition activity.6 In summary, THZ1 is a highly rare inhibitor to target a residue out of the kinase domain, which provides covalent selectivity compared with other CDK inhibitors. Besides CDK7, THZ1 also demonstrated inhibition effect on CDK12/13 when with higher concentration. This may be related with structural similarity of CDK12/13 and CDK7, which also has a cysteine within four residues of C312.6, 20
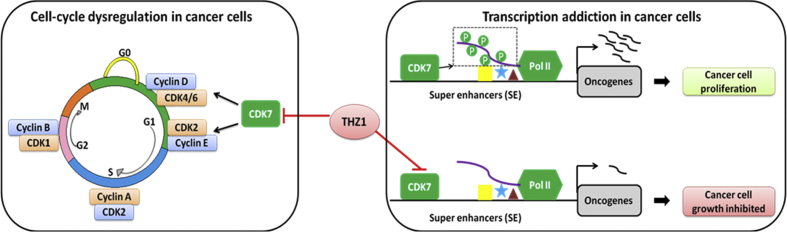
Further research has also been conducted by Nilson et al19 who looked into the detailed influence brought by THZ1 to each step of transcription process. By in vitro transcription analysis with nuclear extract, they have found THZ1 inhibited all essential phosphorylation of RNAP II large subunit CTD. THZ1 particularly inhibited capping of G21 transcripts and also downregulated capping of longer transcripts. Besides, a THZ1-sensitive factor was recognized from HeLa cell nuclear extract that modulated guanylylation of nascent RNAs. The pausing process was also inhibited by THZ1 through block of 5,6-dichloro-1-β-D-ribofuranosylbenzimidazole (DRB) sensitivity inducing factor (DSIF) and negative elongation factor (NELF) loading. Moreover, THZ1 downregulated the process of positive transcription elongation factor b (P-TEFb)-dependent transition into productive elongation, which was probably due to loss of DSIF. In conclusion, THZ1 negatively influenced multiple transcriptional processes including RNAP II phosphorylation, co-transcriptional capping, promoter proximal pausing and productive elongation via inhibition of CDK7. Generally, due to the multiple roles that CDK7 plays both in cell-cycle control and transcription process, the THZ1 anticancer mechanism proposed in the current reports can be summarized in two aspects (Fig. 1)19, 21: (1) THZ1 caused cell-cycle arrest by blocking the function of CDK7 as CAK to activate other CDK members which are essential in cell mitosis; (2) THZ1 downregulated the oncogene transcription on super-enhancer (SE) area by inhibition of CDK7.
Fig. 1.
Double influences brought by THZ1 in cell-cycle and transcription process. CDK: cyclin-dependent kinase; Pol II: RNA polymerase II.
Anti-tumor activity of THZ1: in vitro & in vivo
Human T-cell acute lymphoblastic leukaemia (T-ALL)
T-ALL represents approximately 12%–15% of all newly diagnosed acute lymphoblastic leukaemia (ALL) cases in pediatric patients with distinctive features of immunophenotypic diversity. A leukemic lymphoblast is created by several characteristic genetic changes including chromosomal translocations, intrachromosomal rearrangements, changes in the number of chromosomes in leukemic cells, and additional mutations in individual genes.22 T-ALL cell lines were screened against THZ1 and some were found to be extremely sensitive to THZ1. The drug treatment under 50 nM concentration (mostly 1 nM) within 72 hours profoundly decreased cellular proliferation of a T-ALL panel containing Jurkat, KOPTK1, Loucy and DND-41, while the non-transformed cell line, BJ fibroblasts and retinal pigment epithelial cells (RPE-1) demonstrated much higher tolerance against THZ1. The Jurkat and Loucy were monitored by fluorescent-activated cell sorting (FACS) analysis with annexin V and propidium iodide (PI) staining after being incubated with THZ1 at several time points which clearly indicated THZ1 induced apoptosis in both cancerous cell lines even at low compound concentrations. The immunoblot analysis results have also suggested that THZ1 inhibited RNAP II CTD phosphorylation and simultaneously reduced the anti-apoptotic proteins of Jurkat and Loucy cell lines, especially for myeloid cell leukemia-1 (MCL1) and X-linked inhibitor of apoptosis protein (XIAP).6
The cell-cycle progression of normal cell lines with THZ1 treatment was monitored by flow cytometer after permeabilization and PI-staining. The result indicated the insensitivity of normal cell lines against relatively high concentration of THZ1 by observing normal cell lines which only presented cell-cycle arrest instead of initiating apoptosis process.6
The strong toxicity THZ1 possessed even at a quite low concentration suggested the T-ALL cell lines are extremely sensitive to perturbations occurring in CDK7 kinase function and transcription. Consistent with the in vitro results, the in vivo anti-proliferation test against a bioluminescent xenografted mouse model bearing the human T-ALL cell line KOPTK1 turned out to be significantly efficient with THZ1 treatment at a dosage of 10 mg/kg and twice daily. Notably, the treatment demonstrated no obvious side effects based on the mouse behavior and body weight change, which is in accordance with the cell line test results.6
Kwiatkowski et al6 looked at the gene expression changes occurring in Jurkat cells and identified global mRNA downregulation in cells after THZ1 treatment. They have also found a group of genes that was particularly sensitive to low concentration of THZ1 in Jurkat cells, among which the RUNX1 gene was the most profoundly affected. Meanwhile, the transcripts downregulated by THZ1 showed significant similarity with the downregulated genes induced by RUNX1 knock-down. Actually, RUNX1 together with T-cell acute leukemia 1 (TAL1) and GATA binding protein 3 (GATA3), forms a feed-forward autoregulatory loop that maintains the oncogenic transcription program in T-ALL cells. Additionally, by SE analysis, RUNX1 in Jurkat cell was found to contain exceptionally large SE domain related with haematopoietic-cell-specific enhancer. The SE-regulated tumor oncogenes can be overexpressed while they are quite vulnerable to perturbation. This also gives an explanation for the extra THZ1 sensitivity of T-ALL cells out of other cancer types.
Human MYCN-amplified neuroblastoma (NB)
Many types of human cancers have been identified to rely on the overexpression of MYC oncoproteins which stimulate global gene transcriptional amplification and induce tumor proliferation through upregulating the gene expression involved in multiple processes. As a result, the MYC dependent cancers are usually aggressive and bring poor clinical outcome.23 The deactivation of MYC in cancerous cells has been proven to give multiple therapeutic benefits due to inhibition of tumor proliferation and causing tumor regression.24 The deregulation of MYC is usually realized by the formation of large tumor-specific SE in the region surrounding the MYC gene, which promotes intensive expression of various genes including MYC.25 This molecular mechanism gives us an important hint that the strategies disrupting the SE-related MYC transcription may be a promising therapeutic solution for diverse MYC-dependent cancers. Regarding to this, the function of CDK7 is known to activate CDKs, phosphorylate RNAP II and other transcription factors. Thus, the inhibitor of CDK7 may provide a possible method to block MYC-dependent transcriptional amplification of cancer cells.
Chipumuro et al26 investigated the anti-tumor activities of THZ1 against the MYC-deregulated cells using MYCN-amplified neuroblastoma as a typical model. To compare the anti-tumor efficiency of different CDK inhibitors, the toxicity of 11 candidates has been screened against MYCN-amplified cell lines. The CDK7 inhibitor THZ1 demonstrated the highest potency with IC50 ranging from 6 to 9 nM. This molecule was further tested with a larger library of cancer cell lines which can be divided by MYCN-amplified group and MYCN-non-amplified group. The IC50 of THZ1 against MYCN-amplified cell lines is about 10 times lower compared with MYCN-non-amplified group, while non-transformed cell lines NIH-3T3 and B6-MEFs turned out to be most insensitive. Interestingly, the reduced THZ1 derivative, THZ1-R also demonstrated some cytotoxicity against NB cell lines, however with less potency, which indicates the acrylamide group is not the only active site in the THZ1 molecular structure. In addition, MYCN-amplified cell lines underwent apoptotic process after treatment with THZ1 which has not been identified in MYCN-non-amplified group via the FACS analysis. The results suggested THZ1 possessed selective cytotoxicity with MYCN-amplified cell lines.
The tolerance of THZ1 was evaluated in non-tumor-bearing mice with a dose of 10 mg/kg intravenously twice daily. Within a duration of 4 weeks, no obvious toxicity was found. The anti-tumor efficiency was tested by employing xenograft models of MYCN-amplified human NB derived from subcutaneous flank injection of Kelly cells. The treatment with vehicle and THZ1 was started when the tumor volume reached suitable sizes. As a result, the tumor volume was profoundly reduced after 28-day treatment with THZ1 with no obvious side effects observed. Two mice out of 14 remained no tumor relapse at 35 and 128 days after treatment. The target engagement was confirmed through pull-down experiment of CDK7 in cell lysates from both vehicle and THZ1 treated mice with biotin labeled THZ1.26
By evaluating functions of CDK7, the influence brought by THZ1 was investigated. On one hand, CDK7 has effects on regulation of transcription by controlling RNAP II CTD phosphorylation and also affecting elongation through the CAK activity to stimulate other transcriptional CAKs.27, 28 The immunoblot analysis of RNAP II phosphorylation has demonstrated a dose-dependent decrease in the initiation-associated serine 5 (S5) and serine 7 (S7) and elongation-associated serine 2 (S2) RNAP II phosphorylation both in MYCN-amplified cells and in tumor cells from the animal model treated with THZ1. What's more, significant loss of antiapoptotic protein MCL1 in MYCN-amplified cells after THZ1 incubation was also observed. These findings were not identified in the MYCN non-amplified cells under the same circumstances, which strongly suggest the MYCN-overexpression cancer cell lines are sensitive to THZ1.26
On the other hand, CDK7 is also known to stimulate cell division by activating the T-loop phosphorylation of CDK1, 2, 4 and 6. The Kelly and IMR-32 cells treated with THZ1 demonstrated a time-dependent decrease in CDK2 phosphorylation and also in other proteins related with cell-cycle regulation including phosphorylated retinoblastoma protein (pRb) and cellular transcription factor (E2F). In conclusion, THZ1 affected MYCN-amplified cells by transcription inhibition and also cell-cycle process downregulation. Actually, the expression profiling of THZ1-incubated Kelly and IMR-32 cells indicated the percentage of actively transcribed genes downregulated by 63% and 68% respectively. Among the genes downregulated, the most affected gene sets include transcriptional and cell-cycle regulators including transcripts of CDKs and their partner cyclins. Notably, MYCN mRNA was ranked in the top 15% of significantly downregulated transcripts in NB cells. The disruption of MYCN function by THZ1 is also reflected by the profound decrease of binding with two MYCN transcription targets, MDM229 and MCL1.30 Besides, the expression profiles of the cancer cells treated with THZ1 have significant similarity with cells exposed to the general transcription inhibitor actinomycin D. These data altogether suggested the treatment of THZ1 against MYCN-amplified cells brought widespread transcriptional downregulation and also specifically suppressed the MYCN mRNA and protein levels.26
Based on the super-enhancer associated MYC transcriptional amplification machinery, Chipumuro et al26 investigated possible contribution of SEs to the high sensitivity of MYCN-amplified cells against THZ1. By employing chromatin immunoprecipitation coupled with high-throughput sequencing (ChIP-seq) analysis coupled with histone H3K27 acetylation and H3K4 monomethylation colocalization, strong enhancers with extra high signal have been recognized. From the result, the most activated SE is associated with the MYCN oncogene itself, and the remaining top-ranked SEs include PHOX2B,31 GATA2,32 HAND2,33 and DBH,34 which are transcription factors related with sympathetic neuronal development and cell identity. The receptor tyrosine kinase anaplastic lymphoma kinase (ALK) is also in the top SE rank, which is a known major oncogenic driver in NB cells.35
The ChIP-seq analysis of RNAP II was applied to both MYCN-amplified Kelly cells and MYCN non-amplified SH-SY5Y cells after THZ1 and dimethyl sulfoxide (DMSO) treatment separately. Both cell lines demonstrated higher RNAP II occupancy at SE associated gene region compared to the regular enhancer (RE) genes. The RNAP II occupancy of RE genes in both cell lines after THZ1 treatment has not been affected much, while the RNAP II binding with the SE-associated gene in MYCN-amplified Kelly cells rather than the MYCN non-amplified SH-SY5Y cells was significantly decreased. Based on these facts, the author argued the selectivity of THZ1 against MYCN-amplified cells is highly related with the SEs.26
In conclusion, THZ1 has potent anti-tumor effect against the MYCN-amplified NB cells and in xenograft mouse models of MYCN-amplified human NB. The THZ1 treatment inhibited MYCN-driven global transcription amplification and produced significant tumor regression without introducing detectable side-effects both in vitro and in vivo. The striking selectivity against MYCN-amplified cells is related with preferential downregulation of the SE-associated genes.
Small cell lung cancer (SCLC)
In general, about 10%–15% of lung cancers are diagnosed as small-cell lung cancer (SCLC). SCLC is the most malignant type of lung cancer with high mortality and rapid development of drug resistance. In clinical treatment of non-small-cell lung cancer (NSCLC), therapeutics targeting known oncogenetic drivers such as epidermal growth factor receptor (EGFR) and ALK are proven effective,36 while the therapeutic methodology of SCLC has no significant progress due to the poor understanding of the etiology of SCLC. Thus, numerous efforts have been put to discover efficient treatment targets. SCLC is defined by inactivation of p53 and retinoblastoma (RB),37 and developed mainly from pulmonary neuroendocrine cells (PNECs).38 The SCLC cells exhibit high expression level of many neuroendocrine genes, especially the transcription factors responsible for neuroendocrine development and differentiation of tissues. They play key roles in tumor-initiating, survival, tumor proliferation and growth, which includes neuroendocrine differentiation regulator achaete-scute homolog 1 (ASCL1),39 lineage-specific transcription factor NEUROD140 and proto-oncogene MYC, MYCN and MYCL.41 In addition, the Sry-related HMG box (SOX)-family genes, including SOX2 have also been found to be overexpressed in many SCLC cancer cells.42 These findings provided possible approaches that the inhibition of such factors would be the short-cut for SCLC targeted therapy.
Christensen et al43 have evaluated the sensitivity of SCLC by conducting a high-throughput cellular screening of a chemical library consisting of over 1000 small molecules that are at experimental or clinical stage for cancer therapies. Based on the screening results, the top 15 inhibitors with high efficiency have been identified. They can be mainly divided into three categories: (1) transcription inhibitors; (2) cell-cycle inhibitors; (3) the mammalian target of rapamycin (mTOR)-phosphatidylinositol 3-kinases (PI3K) pathway inhibitors. THZ1 was among the high ranking inhibitors, which also exhibited anti-tumor potency against T-ALL via downregulation of key oncogenic transcription factors. The authors also screened other CDK inhibitors including dinaciclib (CDK1, CDK2, CDK5 and CDK9) and flavopiridol (CDK1, CDK2, CDK4, CDK6, CDK7, CDK12 and CDK13) against murine SCLC cells and mutant Kras-driven murine NSCLC cell lines. THZ1 showed anti-tumor efficiency and good selectivity with IC50 at 75–100 nM for murine SCLC and about 750 nM for NSCLC cells. While dinaciclib and flavopiridol possessed similar IC50 with THZ1, however, they were lack of specificity to SCLC cell lines.43
The THZ1 anti-tumor efficiency was also tested in vivo by using mouse models with autochthonous SCLC disease and monitored at thorax region with magnetic resonance imaging (MRI). In order to compare with the standard clinical chemotherapy, another group of SCLC bearing mice was treated with cisplatin and etoposide (Cis-Eto). Within 2 weeks of treatment at a dose of 10 mg/kg, twice a day, the THZ1 treatment group showed significant suppression of tumor growth, and 2 out of 9 mice had more than 90% reduction in tumor size. Three mice showed partial response and 4 had stable disease. The result obtained with single-reagent THZ1 treatment is comparable with Cis-Eto at the response and survival rates. Importantly, THZ1 treatment-related toxicity was not recognized in reduced pigmentation (RP) mice model in contrast with chemotherapy. Notably, attempt that combines THZ1 and Cis-Eto did not show apparently improvement in the tumor treatment for the same mice model. Similar sensitivity against THZ1 was observed in SCLC lung tumor cells and liver metastases cells, indicating the efficiency against both primary and metastatic SCLC cells.43
Consistent with other cancer cells treated with THZ1 in the former discussion, the RNAP II CTD phosphorylation is also found decreased in SCLC tumor tissue. Thus, it is not hard to predict that the SCLC cells with amplification of MYC genes may respond to THZ1 very well as seen in the case of Jurkat cells. In fact, the human SCLC cells possessing amplifications in each of the MYC genes are confirmed to be significantly sensitive to THZ1. The low effective dose of THZ1 also suggested the chemorefractory NCl-H69, GLC16 and NCl-H82 cell lines are quite sensitive to THZ1, while their response to Cis-Eto is not good. The sphere-forming morphology of SCLC cells was disrupted after incubation with THZ1 for 12 hours in 3D culture.43
The gene expression profiling of human SCLC cell lines, HCl-H69, GLC16 and NCl-H82 treated with 100 nM THZ1 was investigated by gene ontology (GO) analysis, and the most sensitive transcripts turned out to be involved in DNA-dependent transcription. Gene enrichment analysis of transcription factor binding sites indicated the E2F, nuclear respiratory factor 1 (NRF1) and cyclic adenosine monophosphate (cAMP) response element binding protein (CREB) is especially suppressed by THZ1. These genes all contain transcription factors binding motifs. Interestingly, the most THZ1-downregulated E2F transcription factor binding sites are significantly enriched in SCLC cells in contrary to NSCLC cells. This phenomenon suggested E2F may play a dominant role in SCLC cell pathogenesis. By employing ChIP-seq analysis coupled with histone H3K27 acetylation in untreated human SCLC cells, strong enhancers with extra high signal have been recognized. From the result, the highly activated SE is associated with the RNAP II-mediated transcription, embryogenesis and neural development.43
The SEs associated with SOX2 and NFIB were found in both NCl-H69 and GLC16 cell lines, which have been reported as proto-oncogenic transcription factors in SCLC.42, 44 In the same cell lines, the neuroendocrine transcription factor ASCL145 was identified to be associated with one of the largest typical enhancers (TEs). Besides, insulinoma-associated protein 1 (INSM1) was also recognized to be an SE-associated transcription factor gene on the top of list. On the other type of SCLC cell line, HCl-H82, NEUROD1 gene was found to be associated with large TEs. SE at OTX2 gene demonstrated second high histone H3K27ac signal where MYC gene ranked at the top.43
Comparing the enrichment analysis of THZ1-sensitive transcripts with the SE-associated genes leads to the conclusion that THZ1-sensitive transcripts are enriched in the SE-associated gene list. Moreover, genes associated with SEs and large TEs exhibit extra vulnerability upon THZ1 treatment rather than genes associated with general enhancers, suggesting the downregulation effect of THZ1 may depend on the enhancer size. Different from the NB cell lines, THZ1 demonstrated similar toxicity against SCLC cell lines with or without MYCN, C-MYC or MYCL amplification. Instead of the MYC family, the master transcription factor of lung neuroendocrine differentiation, ASCL1 stood out as dominant oncogene candidate in SCLC based on the observation that ASCL1 was among the top 2% most decreased genes after THZ1 treatment.43
Recently, Cheng et al46 also reported the anti-tumor activity of THZ1 against NSCLC through in vitro investigations. The NSCLC cell line H1299 responded to THZ1 significantly with 48 h incubation at compound concentration of 50 nM, while other NSCLC cell lines H292, H23, A549 also demonstrated a dose-dependent inhibition at higher THZ1 concentrations. The author investigated the mRNA expression levels of the genes closely involved in cell-cycle regression of H1299 by real-time polymerase chain reaction (RT-PCR). The cell-cycle regulatory genes of G2/M phase were profoundly suppressed by 50 nM THZ1, including CDK1, CCNB and CDC25B, suggesting inhibition of CDK7 activity as CAK by THZ1 treatment.46
Triple-negative breast cancer (TNBC)
Similar to SCLC, triple-negative breast cancer (TNBC) also exhibits high genetic complexity with high rate of point mutation, gene amplification and deletion.47 In contrast with hormone receptor-positive breast cancers, TNBC is lack of common feature in gene expression except the tumor suppressor genes (INPP4B, TP53 and PTEN).48 Thus, finding a sensitive therapeutic target for TNBC is an urgent need. Based on former research with THZ1, the overexpression of oncogenic driver genes (including MYC, MYCN and RUNX1) has been identified in different types of cancer cells. These genes are highly dependent on the constant active transcription, which is often promoted by SEs. The striking vulnerability of these genes makes THZ1 selectively suppress tumor development factors through targeting transcription machinery before harming the non-cancerous cells.6, 43, 49
Wang et al50 investigated the THZ1 toxicity against a list of cell lines belonging to TNBC or estrogen and progesterone receptor positive (ER/PR+) breast cancer. The TNBC cell lines were significantly inhibited by THZ1 with IC50 less than 70 nM, while the ER/PR+ breast cancer was not affected even under micromolar concentration of THZ1. The THZ1 selectivity against only TNBC cell lines can be also confirmed by the cellular morphology of both types of breast cancer cell lines. THZ1 only induced apoptosis in TNBC cells without affecting the ER/PR+ cell line viability. Moreover, the CDK7 activity was equally suppressed by THZ1 in both types of cancer cells, reflected by the same level of CTD phosphorylation downregulation at Ser2, Ser5 and Ser7 position. This result suggested that the TNBC cell lines are more dependent on the CDK7 functions than ER/PR+ breast cancer. Another important function of CDK7 is regulating of cell-cycle as CDK-activating kinase (CAK). In MYCN-amplified NB cells, THZ1 was found to induce cell-cycle arrest at G2/M phase. However, the THZ1 treatment did not affect the mitosis process of TNBC cells according to the live-cell imaging analysis of MDA-MB-468 cell line. In conclusion, the sensitivity of TNBC to CDK7 inhibition is probably not from a role of CDK7 in directly regulating cell-cycle.50
The authors also investigated THZ1 toxicity against non-transformed BJ fibroblasts and retinal pigment epithelial cells. The immunoblotting analysis of TNBC, ER/PR+ breast cancer cell and normal human cell lines was conducted after 24 h of treatment with 100 nM THZ1. The only cell line undergoing apoptotic process indicated by the induced cleavage of poly-adenosine diphosphate-ribose polymerase (PARP) and cleaved caspase-3 (CC3) was TNBC while ER/PR+ breast cancer cell and normal cells were not affected. It is noteworthy that the RNAP II CTD phosphorylation was suppressed equally in all tested cell lines yet resulted in totally different cellular liability, suggesting again the TNBC proliferation is significantly dependent on transcription.50
Although THZ1 has shown selective potency against tumor cell growth, the short half-life of THZ1 in vivo (45 min in mouse plasma) will limit its clinical performance. To improve the pharmacokinetic stability of the THZ1, a molecule structural modification was made to change the acrylamide group from para- to meta- position affording THZ2. The modified molecule THZ2 exhibited 5 times longer half-life in mouse plasma and also selectively inhibited CDK7 activity. The anti-tumor potency is confirmed to be comparable with THZ1 to specifically inhibit TNBC without affecting ER/PR+ breast cancer and non-transformed cells.50
The anti-tumor efficiency of THZ2 in vivo was investigated with different mouse models including an orthotopic xenograft model with transplanted TNBC cell line MDA-MB-231 and two patient derived xenograft (PDX) models bearing TNBC cell line DFBC11-26 and DFBC13-11. The PDX models were the non-obese diabetic-severe combined immunodeficiency (NOD-SCID) mice transplanted with tumor fragments of patients with metastatic TNBC. Upon treatment of 25 days with 10 mg/kg of THZ2 twice daily, the tumor growth in xenograft model carrying MDA-MB-231 was significantly decreased. During this period, no side effect such as body weight loss was observed, suggesting THZ2 was well-tolerated in this model. Like THZ1, immunoblotting of tumor lysates harvested from MDA-MB-231 bearing mice after 2-day treatment with THZ2 showed downregulation of the CTD phosphorylation at S2, S5 and S7. However, the PDX models demonstrated body weight loss during THZ2 treatment, which indicated undesirable cytotoxicity. The PDX models were then tested with THZ1 in the same fashion, resulted in remarkable inhibition of tumor proliferation in both patient-derived cancer cell lines reflected by the loss of tumor cellularity and disease regression.50
In order to identify other transcriptional CDKs that could also be the therapeutic targets for TNBC besides CDK7, clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR associated protein 9 (Cas9) methodology was introduced to remove six known transcriptional CDKs (CDK7, 8, 9, 12, 13 and 19) separately in different breast cancer cell lines. The studies suggested CDK9 was another transcriptional CDK required by both TNBC and ER/PR+ breast cancer cell growth. Interestingly, ER/PR+ breast cancer cell line ZR-75-1 was not largely affected by removal of CDK7, which well explained the selective vulnerability of TNBC against CDK7 inhibitors.50
In addition, the author further proposed that certain genes overexpressed only in TNBC, but not in ER/PR+ breast cancer cells, are responsible for the specific sensitivity to THZ1. By comparing the genome-wide expression data of two TNBC cell lines with two ER/PR+ breast cancer cell lines, 451 genes were found to be overexpressed only in TNBC and especially sensitive to THZ1. Eventually, a list of genes associated with transcriptional regulators and signaling factors in TNBC were illustrated and named as “Achilles cluster”, which are possible mediators of the response to THZ1. Interestingly, 40% of genes included in Achilles cluster were associated with SEs in TNBC cells. The expression of SE associated genes in TNBC was typically sensitive to THZ1 treatment, according to microarray expression analysis. This phenomenon has been also observed in other cancer cells which demonstrated remarkable sensitivity against THZ1 such as MYCN-amplified NB and SCLC.50
In order to identify potential THZ1 therapeutic target in Achilles cluster, 8 SE-associated genes were chosen as candidates for CRISPR/Cas9-mediated gene editing analysis. Among which, EGFR, Fos-like antigen 1 (FOSL1), forkhead box C1 (FOXC1), MYC and sex-determination region Y (SRY)-related high mobility group (HMG)-box gene 9 (SOX9) turned out to be specifically essential for TNBC proliferation rather than ER/PR+ breast cancer cell line. Additionally, knock out of EGFR and SOX9 genes suppressed cellular viability and induced apoptosis in TNBC cells. EGFR was reported as a therapeutic target for TNBC by Corkery51 and Ueno52. Thus, three EGFR kinase inhibitors (erlotinib, gefitinib and lapatinib) were tested against TNBC cell lines together with THZ1. However, the anti-proliferation activity of EGFR kinase inhibitors was poor against TNBC. In fact, kinase inhibitors of EGFR did not provide satisfactory results in TNBC clinic trials either. In contrast, targeting transcription of EGFR by THZ1 seems to be much more efficient instead of directly suppressing the kinase activity of EGFR in TNBC therapy. Similar to formerly studied cancer types, SE-associated transcripts were found to be especially THZ1-sensitive in TNBC and appeared as key targets of THZ1 treatment.6, 26, 43, 50
Peripheral T-cell lymphomas (PTCL)
Peripheral T-cell lymphomas (PTCL) are a diverse group of aggressive lymphomas with poor response to chemotherapy. There are still no efficient targeted therapies to some subtypes of PTCL, as a result, PTCL patients usually suffered from severe side effects induced by combination of chemotherapy agents such as cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP).53 Thus, new therapeutic protocol or agent with specific targets and minimal toxicity is highly desirable.
Cayrol et al54 conducted drug efficacy screening over 101 known anti-cancer molecules and indentified 4 most potent agents for anti-proliferation of PTCL cell lines. They are: (1) romidepsin, inhibitor of lysine deacetylases (KDAC), (2) bortezomib and carfilzomib, inhibitors of proteasome and (3) actinomycin which suppresses transcription by inhibiting elongation of RNA chain.55 Taking clinical data into consideration, only about 25% of PTCL responded to agents targeting KDAC and proteasome pathway.56 Thus, it is reasonable to further explore inhibitors targeting transcription, which may provide new therapeutic methods. Despite the potent anti-proliferation activity to PTCL cell lines, actinomycin inhibits RNA elongation assisted by RNAP II, which blocks global transcription pathway and leads to serious side effects. Instead, THZ1 as a covalent inhibitor of CDK7, selectively downregulates RNAP II CTD phosphorylation, may provide a new solution to PTCL targeted therapy. In former discussion, THZ1 has exhibited promising anti-tumor potency against a diverse collection of cancers without obvious side effects observed in vitro and in vivo.
In order to identify if treatment targeting CDK7 could be effective against PTCL, two most commonly seen subtypes of PTCL, PTCL not otherwise specified (PTCL-NOS) and anaplastic lymphoma kinase-negative anaplastic large cell lymphoma (ALCL-ALKneg) were chosen for analysis. Among 18 PTCL-NOS and 17 ALCL-ALKneg patients, 50% and 100% were found with nuclear expression of CDK7 respectively. Overexpression of CDK7, together with another two components of TFIIH complex, MAT1 and cyclin H were also identified in PTCL-NOS and ALCL-ALKneg cell lines compared with normal T cells from tonsils. All of the results indicated most PTCL is highly dependent on the existence of CDK7.54
As expected, THZ1 demonstrated significant anti-proliferation effect against PTCL cell lines, OCI-Ly12 and OCI-Ly13.2 in time-dependent manner (within 72 h). The RNAP II CTD phosphorylation at Ser5 was downregulated by THZ1 treatment, which indicated the inhibition effect on CDK7. Notably, the Ser 2 downregulation also occurred after THZ1 incubation in immunoblot analysis. THZ1 induced apoptosis in both OCI-Ly12 and OCI-Ly13.2 lines, which was suggested by the induced cleavage of PARP and activation of caspases 3 and 7. The cell cycle distribution of OCI-Ly12 and OCI-Ly13.2 treated with 500 nM THZ1 was also monitored by flow cytometer considering that the CDK7 also possesses CAK effect on cell-cycle CDKs. However, the THZ1 treatment did not affect the cell cycle process of PTCL, indicating that the sensitivity of PTCL cells to CDK7 inhibition is probably not from regulating cell-cycle CDKs. All of these data suggested CDK7 transcriptional activity is essential for PTCL cells survival.54
Recent research has uncovered the presence of activating mutations on signal transducers and activators of transcription 3 (STAT3) or mutations of other genes that activate STAT3 (such as Janus kinase 1 [JAK1] and kinase fusions) is responsible for proliferation of ALCL-ALKneg and PTCL-NOS in patients.57 Consistently, Y640F mutation in the Src homology 2 (SH2) domain of STAT3 associated with higher dimer stability and transcriptional activity was found within OCI-Ly12 and OCI-Ly13.2 cell lines. In order to verify whether STAT3 transcriptional machinery could be affected by CDK7 inhibition, gene set enrichment analysis of STAT3 target genes58 was conducted with ranked gene expression changes after 3 and 6 h of 500 nM THZ1 treatment in OCI-Ly13.2 cells. The result demonstrated that STAT3 target genes are largely enriched among the THZ1 downregulated genes. The transcriptional complexes bearing Tyr705 phosphorylated STAT3 is known to target genes by cooperating with RNAP II.21 Thus, inhibition of CDK7 by THZ1 suppresses RNAP II activity, which leads to downregulation of STAT3 functions. In fact, 500 nM of THZ1 decreased STAT3-dependent luciferase activity to the similar level with 2.5 μM cryptotanshinone administration, a typical agent that blocks phosphorylation of STAT3. This is a strong evidence for the efficiency of THZ1 in suppressing STAT3 transcriptional program. Additionally, quantitative chromatin immunoprecipitation assay (Q-ChIP) of CDK7 and STAT3 on one of the STAT3 target gene MYC in OCI-Ly13.2 cells was conducted. On the MYC promoter region, significant enrichment of STAT3 and CDK7 was both identified, which indicated that the active chromatin was associated with both STAT3 and CDK7. Treatment of THZ1 largely decreased the signal of both STAT3 and CDK7 on MYC region. The MYC transcription was also suppressed at the same time. All the experimental data together indicated that THZ1 disassembled STAT3 mutant from MYC gene via inhibition of CDK7.54
If the inhibition of STAT3 is fully responsible for the anti-proliferation effect of THZ1 in PTCL, the same anti-tumor activity should be also achievable by other STAT3 inhibitors such as S31-201 (a STAT3 dimerization inhibitor) and cryptotanshinone (a STAT3 Tyr705 inhibitor). However, in OCI-Ly12 and OCI-Ly13.2 cells, the two agents did not exhibit significant anti-tumor efficacy, despite that they did block DNA binding of STAT3 by 40%–50%. The reason lies in that specific inhibition of STAT3 has led to activation of STAT1 and STAT5 in PTCL cell lines, which could make up for the STAT3 function loss. While the situation became different with THZ1, by immunoblotting analysis of OCI-Ly12 and OCI-Ly13.2 cell lines, THZ1 was found to decrease the existence of phospho-STAT1 Tyr701 and phospho-STAT5 Tyr694 and also suppress DNA binding of STAT1 and STAT5A (B) simultaneously. In addition, mRNA and protein level of JUND, a STAT5 target oncogene, were significantly decreased after only 3 h of THZ1 treatment in OCI-Ly13.2 cell line, suggesting downregulation of gene transcription should be the primary response of PTCL cells followed by downregulation of STAT phosphorylation and DNA binding.54
Currently, clinical therapeutic agents of PTCL include obatoclax, ABT-737 and venetoclax (ABT-199) which belong to the B-cell lymphoma 2 homologue 3 (BH3)-mimetic drug category. The mechanism of BH3-mimetic drug is to prevent the pro-apoptotic BH3 proteins from being sequestrated by anti-apoptotic BH3 proteins such as B-cell lymphoma 2 (BCL2), B-cell lymphoma-extra large (BCL-XL) and induced myeloid leukemia cell differentiation protein MCL1. The pro-apoptotic BH3 proteins are capable to release the mitochondrial outer membrane permeabilization (MOMP) effectors BCL2-associated X protein (BAX) and BCL2-homologous antagonist killer (BAK) so as to induce apoptotic program.59 However, in some cases the treatment of PTCL patients by BH3-mimetic agents encountered undesirable drug resistance which limits their applications. Exposure to 500 nM THZ1 after 24 h changed BH3 expression profile to decrease anti-apoptotic BH3 proteins while upregulating BH3 effectors in PTCL cells. As a result, the THZ1-treated cells turned more vulnerable to BH3-mimetic drugs.54
To investigate the efficacy of combining BH3 mimetic drugs with THZ1, three BH3 mimetic drugs, which are obatoclax, ABT-737 and venetoclax (ABT-199) were tested against OCI-Ly12 and OCI-Ly13.2 cell lines in different drug concentrations and ratios to THZ1. Eventually, the two cell lines were most sensitive to a combination of the pan-BH3 inhibitor obatoclax and THZ1. The ratio of obatoclax to THZ1 which gives the maximum potency against PTCL cells was illustrated from a response-surface analysis.60 Anti-proliferation effect of obatoclax was significantly improved at obatoclax to THZ1 ratio of 5:1. Notably, this combined treatment demonstrated promising potency without increased toxicity compared to both single-obatoclax and single-THZ1 treatment in the xenografts model of OCI-Ly12 and OCI-Ly13.2. Thus, THZ1 is promising to provide new solutions to increase the potency of targeted agent-based PTCL therapies.54
Epithelial ovarian cancer (OC)
In the cases of MYCN-amplified neuroblastoma, SCLC and TNBC, the significant tumor regression produced by THZ1 treatment was all proven to be related with the inhibition of MYC-driven global transcription amplification. Zeng et al20 turned their attention to high-grade serous ovarian cancer (HGSOC), which is also highly dependent on MYC amplification for oncogenic growth and responsible for the majority of death caused by all ovarian cancer subtypes. Despite good efficacies with platinum analog and PARP inhibitor (PARPi) treatment, the HGSOC patients suffer from over 90% relapse rate, and poor response to subsequent treatment.61 Thus, a novel therapeutic strategy with specific target is highly desired.
Studies focusing on the genetic profiling have suggested that MYC on 8q24 is most frequently amplified in ovarian carcinoma compared with the other cancer types. The overexpression of MYC has multiple influences on cell cycle control and cancerous cell survival.62 Based on the clonogenic growth survival assay, MYC-depleted (by CRISPR/Cas9) ovarian cancer cell lines KURAMOCHI and OVCAR8 showed significant decrease in cell proliferation, which also indicated MYC may be a promising therapeutic target for ovarian cancer. However, directly targeting MYC proteins is difficult due to its lack of binding site for small molecules. Instead, reagents that targeting the genesis of MYC transcription or protein turned out to be a possible solution. Among those candidates, JQ1 has demonstrated significant downregulation of MYC in different cancer cell lines through inhibition of bromodomain and extra-terminal motif (BET) proteins.63 Unfortunately, JQ1 did not work well with ovarian cancer cell lines as analyzed by immunoblotting, and the same result was also seen in the case of lung adenocarcinoma cells.64 Aiming at downregulation of MYC transcription in ovarian cancer cells, the author investigated the THZ1, which was found to be the most potent agent to inhibit MYC among the selected small molecules targeting different transcriptional and epigenetic components. The MYC protein abundance was significantly decreased by THZ1 in three different ovarian cancer cell lines. Concurrently, the phosphorylation of the RNAP II CTD at Ser 2, 5, 7 was significantly inhibited by treatment of 250 nM THZ1.20
In addition, the anti-apoptotic protein MCL1 was also suppressed by THZ1, which was probably a result of transcriptional inhibition of MYC and MCL1 genes. According to The Cancer Genome Atlas (TCGA), HGSOCs having both MYC and MCL1 amplification did not response well to clinical therapies and were often related to poor prognosis compared with other TCGA subtypes. In fact, the HGSOC cell line SKOV3 with low protein abundance of MYC and MCL1 was found to be most insensitive to THZ1 treatment among eight ovarian cancer cell lines. In brief, the ovarian cancer cells with high-level MYC and MCL1 expression significantly responded to THZ1 treatment.20
In order to investigate the effects of THZ1 on cellular transcription, global gene expression profiling was conducted with two THZ1-sensitive ovarian cancer cell lines, KURAMOCHI (Ku) and COV362 (Cov). As a result of incubation with 250 nM THZ1 for 6 h, both cell lines demonstrated profoundly downregulated gene expression, among which MYC and MCL1 were specifically decreased. Notably, the downregulation of MYC by THZ1 appeared to be highly selective, since an MYC co-amplified long non-coding RNA (lncRNA) gene on 8q24, PVT1, was not significantly suppressed through THZ1 treatment. The oncogenic pathways were identified from the overexpressed genes through gene set enrichment of MSigDB hallmarks of cancer. The G2M checkpoint and E2F targets were found to be most enriched hallmark gene sets. MYC_targets_V1 and MYC_targets_V2 hallmark gene sets were also identified.20
Considering the THZ1 chemistry, which not only inhibits CDK7, but also CDK12/13, it is necessary to clarify which target(s) are responsible for the anti-tumor activity of THZ1 in ovarian cancer. Thus, two inhibitors that specifically targeting only CDK7 (YKL-1-116) or CDK12/13 (THZ531) were chosen in parallel experiments.65, 66 Compared to THZ1, the CDK7 inhibitor YKL-1-116 demonstrated weaker downregulation effect on MYC and MCL1 expression, while the CDK12/13 inhibitor THZ531 showed opposite effects on MYC and MCL1 expression with different drug dosages. Interestingly, THZ531 functioned as a promoter at relatively low concentration but acted as a suppressor at concentration higher than 0.5 micromolar. The expression of MYC and MCL1 was profoundly decreased by combination of YKL-1-116 and THZ531, which indicated the anti-tumor efficacy of THZ1 was derived from both CDK7 and CDK12/13 suppression. The same conclusion could also be illustrated from the result that expression of THZ1-resistant mutant C312S on CDK7 was not sufficient for ovarian cancer cell survival.20
The therapeutic efficiency of THZ1 was evaluated in orthotopic ovarian PDX models. Looking for possibility of combination treatment with other anti-tumor agents, a PARP inhibitor, olaparib was also employed in the in vivo evaluation. olaparib is a FDA-approved drug for relapsed ovarian cancer irrespective of BRCA1/2 status. Similar with former reports of THZ1, the drug was well tolerated at a dose of 10 mg/kg twice daily and caused no overt toxicity in the treated models. The mice were divided to four groups and treated with vehicle, THZ1, olaparib and combination of THZ1 plus olaparib separately during 27 days. The tumor sizes were monitored by bioluminescent imaging upon luciferase gene transduced tumor cells. The tumor growth was significantly suppressed in THZ1 group while olaparib group only showed limited efficacy to slow down tumor growth. Models treated with combination of THZ1 and olaparib demonstrated unprecedented anti-tumor effect and tumor tissue was almost invisible after treatment. Thus, the combined treatment with THZ1 and olaparib showed huge potential in future therapeutic strategies of ovarian cancer.20
Conclusion
In summary, THZ1 has been developed as a covalent inhibitor of CDK7, demonstrating significant anti-proliferation efficacy against 53% of tumor cells with different genotypes and phenotypes out of over 1000 candidates.6 Among which, administration of THZ1 against several malignant tumors has been well studied, including human T-ALL, human MYCN-amplified NB, SCLC, TNBC, PTCL and epithelial OC. THZ1 exhibited not only potent anti-cancer effects in vitro, but also no obvious toxicity to non-transformed cell lines. THZ1 also largely inhibited tumor growth in different xenograft models without obvious side effects observed. Functions of THZ1 in cancer cells appeared to be suppressing expression of one or several transcription factors which is necessary for cell proliferation, such as RUNX1 in T-ALL, MYCN in NB, MYC family members and neuroendocrine lineage-specific factors in SCLC. Thus, THZ1 may provide highly selective treatment targeting the SE-driven transcriptional addiction seen in many tumor types. Further research on anti-tumor mechanisms of THZ1 suggested its negative influence on multiple transcriptional processes including RNAP II phosphorylation, co-transcriptional capping, promoter proximal pausing and productive elongation by inhibition of CDK7. THZ1 or its derivatives will probably bring new therapeutic methods as single agent or in combined treatment with other anti-tumor agents, especially to the cancer types with transcriptional complexity and limited treatment targets. The preclinical research related to THZ1 in the treatment of different types of cancers from 2014 to 2019 has been summarized for reference (Table 1).
Table 1.
The preclinical research related to THZ1 in the treatment of different types of cancers.
| Authors & year | Cancer type | Investigation methods | Proposed mechanisms |
|---|---|---|---|
| Kwiatkowski et al. 20146 | T-ALL | Resazurin based assay; GO term enrichment analysis; ChIP-seq analysis; bioluminescent images; gene set enrichment analysis; in vitro & in vivo assay | Downregulation of the RUNX1-driven transcriptional program |
| Chipumuro et al. 201426 | NB | Cell-cycle analysis; apoptosis analysis; immunohistochemical analysis; immunoblot analysis; qRT-PCR analysis; ChIP-qPCR analysis; ChIP-seq analysis; in vitro & in vivo assay | THZ1 targets the expression of both MYCN and MYCN-driven transcription amplification |
| Christensen et al. 201443 | SCLC | High-throughput small-molecule screen; MRI quantification; immunoblot analysis; proliferation assay; GO term enrichment analysis; ChIP-seq analysis; in vitro & in vivo assay | Downregulation of ASCL1, INSM1 and other oncogenetic transcription factors |
| Wang et al. 201550 | TNBC | Cell viability assay; immunoblot analysis; chemical molecular modification; drug in vivo stability analysis; H&E staining of tissue sections; gene set enrichment analysis; microarray expression analysis; quantitative PCR analysis; CRISPR/Cas9-mediated gene editing; in vitro & in vivo assay | Inhibition of CDK7 downregulates super-enhancer mediated oncogene transcription |
| Cayrol et al. 201754 | PTCL | Immunohistochemical analysis; immunoblot analysis; cell viability assay (Resazurin based); gene set enrichment analysis; Q-ChIP analysis; qRT-PCR analysis; response-surface analysis; RNA sequencing; in vitro & in vivo assay | THZ1 downregulates STAT-signaling dependent actively transcribed genes |
| Zeng et al. 201820 |
Ovarian cancer | CRISPR/Cas9-mediated gene editing; immunoblot analysis; qRT-PCR analysis; gene set enrichment analysis; cell proliferation assays; RNA sequencing; in vitro & in vivo assay | Downregulation of MYC and MCL1 which requires both CDK7 and CDK 12/13 inhibition |
| Cheng et al. 201846 | NSCLC | Cell proliferation assay; wound-healing assay; qRT-PCR analysis; immunoblot analysis; cell-cycle analysis; apoptosis analysis | THZ1 induced cell cycle arrest; blocked the glycolysis pathway; promoted ubiquitination and degradation of NUDT21 |
| Ning et al. 201967 | Cervical cancer and retinoblastoma | Cell-cycle analysis; apoptosis analysis; RT-PCR analysis; Immunoblot analysis; RNA sequencing; In vitro & in vivo assay | THZ1 prevents TGFβ2-induced EMT in human LECs;THZ1 inactivates the MAPK, ERK1/2 and PI3K/AKT signaling pathways; THZ1 caused cell-cycle arrest |
| Lu et al. 201968 | PDAC | Cell viability assay; apoptosis analysis; cell-cycle analysis; immunoblot analysis; immunohistochemical analysis; H&E staining of tissue sections; GO term enrichment analysis; RNA sequencing; in vitro & in vivo assay | THZ1 caused cell-cycle arrest; THZ1 inhibites TNF/NF-κB signaling pathway; THZ1 downregulates Hippo, MAPK signaling pathways |
T-ALL: T-cell acute lymphoblastic leukaemia; GO: gene ontology; ChIP-seq: chromatin immunoprecipitation coupled with high-throughput sequencing; RUNX1: Runt-related transcription factor 1; NB: neuroblastoma; qRT-PCR: quantitative real-time polymerase chain reaction; PCR: polymerase chain reaction; CHIP-qPCR: chromatin Immunoprecipitation-quantitative PCR; SCLC: small-cell lung cancer; MRI: magnetic resonance imaging; ASCL1: achaete-scute homolog 1; INSM1: insulinoma-associated 1; TNBC: triple-negative breast cancer; H & E: hematoxylin-eosin; CRISPR: clusters of regularly interspaced short palindromic repeats; Cas9: CRISPR associated protein 9; CDK7: cyclin-dependent kinase 7; PTCL: peripheral T-cell lymphomas; Q-ChIP: fast chromatin immunoprecipitation assay; RT-PCR: real-time PCR; RNA: ribonucleic acid; STAT: signal transducers and activators of transcription; NSCLC: non-small-cell lung cancer; NUDT21: nudix-type motif 21; RT-PCR: real-time PCR; TGFβ2: transforming growth factor beta 2; EMT: epithelial-to-mesenchymal transition; LECs: lens epithelial cells; ERK1/2: extracellular signal-regulated kinase1/2; PI3K: phosphatidylinositol 3-kinases; AKT: protein kinase B; PDAC: pancreatic ductal adenocarcinoma; TNF: tumor necrosis factor; NF-κB: nuclear factor-κB; MAPK: mitogen-activated protein kinase.
Despite the fact that THZ1 has shown selective potency against tumor cell growth, the short half-life of THZ1 in vivo (45 min in mouse plasma) may limit its clinical performance. To improve the pharmacokinetic stability of the THZ1, molecular structural modification or syntheses of new THZ1 derivatives will be an important research object. On the other hand, new drug delivery technology will also provide considerable solution regarding to the instability of THZ1. In addition, since transcription events in non-cancerous cells also depend on function of CDK7 as an RNAP II subunit, a sub-saturating amount of THZ1 may be essential to give an effective treatment in clinical trials, which should balance the treatment time, compound concentration, and the drug/target ratio. Finally, THZ1 may also be useful as an experimental protocol to explore detailed mechanisms and important driving factors involved in transcription process both in cancer cells and non-transformed cells.
Conflicts of interest
None.
Edited by Pei-Fang Wei
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.O'Neil J., Look A.T. Mechanisms of transcription factor deregulation in lymphoid cell transformation. Oncogene. 2007;26:6838–6849. doi: 10.1038/sj.onc.1210766. [DOI] [PubMed] [Google Scholar]
- 2.Berg T. Inhibition of transcription factors with small organic molecules. Curr Opin Chem Biol. 2008;12:464–471. doi: 10.1016/j.cbpa.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Sanso M., Fisher R.P. Pause, play, repeat: CDKs push RNAP II's buttons. Transcription. 2013;4:146–152. doi: 10.4161/trns.25146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vladimir K. Cyclin-dependent kinase inhibitors as anticancer drugs. Curr Drug Targets. 2010;11:291–302. doi: 10.2174/138945010790711950. [DOI] [PubMed] [Google Scholar]
- 5.Fisher R.P. Cdk7: a kinase at the core of transcription and in the crosshairs of cancer drug discovery. Transcription. 2019;10:47–56. doi: 10.1080/21541264.2018.1553483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwiatkowski N., Zhang T., Rahl P.B. Targeting transcription regulation in cancer with a covalent CDK7 inhibitor. Nature. 2014;511:616–620. doi: 10.1038/nature13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi Y.J., Kim D.H., Yoon D.H. Efficacy of the novel CDK7 inhibitor QS1189 in mantle cell lymphoma. Sci Rep. 2019;9:7193. doi: 10.1038/s41598-019-43760-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu S., Marineau J.J., Rajagopal N. Discovery and characterization of SY-1365, a selective, covalent inhibitor of CDK7. Cancer Res. 2019;79:3479–3491. doi: 10.1158/0008-5472.CAN-19-0119. [DOI] [PubMed] [Google Scholar]
- 9.Patel H., Periyasamy M., Sava G.P. ICEC0942, an orally bioavailable selective inhibitor of CDK7 for cancer treatment. Mol Cancer Ther. 2018;17:1156–1166. doi: 10.1158/1535-7163.MCT-16-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bisteau X., Paternot S., Colleoni B. CDK4 T172 phosphorylation is central in a CDK7-dependent bidirectional CDK4/CDK2 interplay mediated by p21 phosphorylation at the restriction point. PLoS Genet. 2013;9:e1003546. doi: 10.1371/journal.pgen.1003546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher R.P. Secrets of a double agent: CDK7 in cell-cycle control and transcription. J Cell Sci. 2005;118:5171–5180. doi: 10.1242/jcs.02718. [DOI] [PubMed] [Google Scholar]
- 12.Larochelle S., Amat R., Glover-Cutter K. Cyclin-dependent kinase control of the initiation-to-elongation switch of RNA polymerase II. Nat Struct Mol Biol. 2012;19:1108–1115. doi: 10.1038/nsmb.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larochelle S., Merrick K.A., Terret M.E. Requirements for Cdk7 in the assembly of Cdk1/cyclin B and activation of Cdk2 revealed by chemical genetics in human cells. Mol Cell. 2007;25:839–850. doi: 10.1016/j.molcel.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schachter M.M., Merrick K.A., Larochelle S. A Cdk7-Cdk4 T-loop phosphorylation cascade promotes G1 progression. Mol Cell. 2013;50:250–260. doi: 10.1016/j.molcel.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sims R.J., 3rd, Mandal S.S., Reinberg D. Recent highlights of RNA-polymerase-II-mediated transcription. Curr Opin Cell Biol. 2004;16:263–271. doi: 10.1016/j.ceb.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Thomas M.C., Chiang C.M. The general transcription machinery and general cofactors. Crit Rev Biochem Mol Biol. 2006;41:105–178. doi: 10.1080/10409230600648736. [DOI] [PubMed] [Google Scholar]
- 17.Akhtar M.S., Heidemann M., Tietjen J.R. TFIIH kinase places bivalent marks on the carboxy-terminal domain of RNA polymerase II. Mol Cell. 2009;34:387–393. doi: 10.1016/j.molcel.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heidemann M., Hintermair C., Voß K., Eick D. Dynamic phosphorylation patterns of RNA polymerase II CTD during transcription. Biochim Biophys Acta. 2013;1829:55–62. doi: 10.1016/j.bbagrm.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Nilson K.A., Guo J., Turek M.E. THZ1 reveals roles for Cdk7 in Co-transcriptional capping and pausing. Mol Cell. 2015;59:576–587. doi: 10.1016/j.molcel.2015.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng M., Kwiatkowski N.P., Zhang T. Targeting MYC dependency in ovarian cancer through inhibition of CDK7 and CDK12/13. Elife. 2018;7 doi: 10.7554/eLife.39030. pii:e39030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lerner L., Henriksen M.A., Zhang X., Darnell J.E., Jr. STAT3-dependent enhanceosome assembly and disassembly: synergy with GR for full transcriptional increase of the α2-macroglobulin gene. Genes Dev. 2003;17:2564–2577. doi: 10.1101/gad.1135003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raetz E.A., Teachey D.T. T-cell acute lymphoblastic leukemia. Hematol Am Soc Hematol Educ Progr. 2016;1:580–588. doi: 10.1182/asheducation-2016.1.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wasylishen A.R., Penn L.Z. Myc: the beauty and the beast. Genes Cancer. 2010;1:532–541. doi: 10.1177/1947601910378024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arvanitis C., Felsher D.W. Conditional transgenic models define how MYC initiates and maintains tumorigenesis. Semin Cancer Biol. 2006;16:313–317. doi: 10.1016/j.semcancer.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 25.Schuijers J., Manteiga J.C., Weintraub A.S. Transcriptional dysregulation of MYC reveals common enhancer-docking mechanism. Cell Rep. 2018;23:349–360. doi: 10.1016/j.celrep.2018.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chipumuro E., Marco E., Christensen C.L. CDK7 inhibition suppresses super-enhancer-linked oncogenic transcription in MYCN-driven cancer. Cell. 2014;159:1126–1139. doi: 10.1016/j.cell.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glover-Cutter K., Larochelle S., Erickson B. TFIIH-associated Cdk7 kinase functions in phosphorylation of C-terminal domain Ser7 residues, promoter-proximal pausing, and termination by RNA polymerase II. Mol Cell Biol. 2009;29:5455–5464. doi: 10.1128/MCB.00637-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palancade B., Bensaude O. Investigating RNA polymerase II carboxyl-terminal domain (CTD) phosphorylation. Eur J Biochem. 2003;270:3859–3870. doi: 10.1046/j.1432-1033.2003.03794.x. [DOI] [PubMed] [Google Scholar]
- 29.Slack A., Chen Z., Tonelli R. The p53 regulatory gene MDM2 is a direct transcriptional target of MYCN in neuroblastoma. Proc Natl Acad Sci U S A. 2005;102:731–736. doi: 10.1073/pnas.0405495102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Labisso W.L., Wirth M., Stojanovic N. MYC directs transcription of MCL1 and eIF4E genes to control sensitivity of gastric cancer cells toward HDAC inhibitors. Cell Cycle. 2012;11:1593–1602. doi: 10.4161/cc.20008. [DOI] [PubMed] [Google Scholar]
- 31.Pattyn A., Morin X., Cremer H., Goridis C., Brunet J.F. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature. 1999;399:366–370. doi: 10.1038/20700. [DOI] [PubMed] [Google Scholar]
- 32.Tsarovina K., Pattyn A., Stubbusch J. Essential role of Gata transcription factors in sympathetic neuron development. Development. 2004;131:4775–4786. doi: 10.1242/dev.01370. [DOI] [PubMed] [Google Scholar]
- 33.Howard M.J., Stanke M., Schneider C., Wu X., Rohrer H. The transcription factor dHAND is a downstream effector of BMPs in sympathetic neuron specification. Development. 2000;127:4073–4081. doi: 10.1242/dev.127.18.4073. [DOI] [PubMed] [Google Scholar]
- 34.Mercer E.H., Hoyle G.W., Kapur R.P., Brinster R.L., Palmiter R.D. The dopamine beta-hydroxylase gene promoter directs expression of E. coli lacZ to sympathetic and other neurons in adult transgenic mice. Neuron. 1991;7:703–716. doi: 10.1016/0896-6273(91)90274-4. [DOI] [PubMed] [Google Scholar]
- 35.George R.E., Sanda T., Hanna M. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature. 2008;455:975–978. doi: 10.1038/nature07397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.William W.N., Jr., Glisson B.S. Novel strategies for the treatment of small-cell lung carcinoma. Nat Rev Clin Oncol. 2011;8:611–619. doi: 10.1038/nrclinonc.2011.90. [DOI] [PubMed] [Google Scholar]
- 37.Sato M., Shames D.S., Gazdar A.F., Minna J.D. A translational view of the molecular pathogenesis of lung cancer. J Thorac Oncol. 2007;2:327–343. doi: 10.1097/01.JTO.0000263718.69320.4c. [DOI] [PubMed] [Google Scholar]
- 38.Sutherland K.D., Proost N., Brouns I., Adriaensen D., Song J.Y., Berns A. Cell of origin of small cell lung cancer: inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell. 2011;19:754–764. doi: 10.1016/j.ccr.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 39.Borges M., Linnoila R.I., van de Velde H.J. An achaete-scute homologue essential for neuroendocrine differentiation in the lung. Nature. 1997;386:852–855. doi: 10.1038/386852a0. [DOI] [PubMed] [Google Scholar]
- 40.Osborne J.K., Larsen J.E., Shields M.D. NeuroD1 regulates survival and migration of neuroendocrine lung carcinomas via signaling molecules TrkB and NCAM. Proc Natl Acad Sci U S A. 2013;110:6524–6529. doi: 10.1073/pnas.1303932110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peifer M., Fernández-Cuesta L., Sos M.L. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet. 2012;44:1104–1110. doi: 10.1038/ng.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rudin C.M., Durinck S., Stawiski E.W. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet. 2012;44:1111–1116. doi: 10.1038/ng.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christensen C.L., Kwiatkowski N., Abraham B.J. Targeting transcriptional addictions in small cell lung cancer with a covalent CDK7 inhibitor. Cancer Cell. 2014;26:909–922. doi: 10.1016/j.ccell.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dooley A.L., Winslow M.M., Chiang D.Y. Nuclear factor I/B is an oncogene in small cell lung cancer. Genes Dev. 2011;25:1470–1475. doi: 10.1101/gad.2046711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ito T., Udaka N., Yazawa T. Basic helix-loop-helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development. 2000;127:3913–3921. doi: 10.1242/dev.127.18.3913. [DOI] [PubMed] [Google Scholar]
- 46.Cheng Z.J., Miao D.L., Su Q.Y. THZ1 suppresses human non-small-cell lung cancer cells in vitro through interference with cancer metabolism. Acta Pharmacol Sin. 2019;40:814–822. doi: 10.1038/s41401-018-0187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumors. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abramson V.G., Lehmann B.D., Ballinger T.J., Pietenpol J.A. Subtyping of triple-negative breast cancer: implications for therapy. Cancer. 2015;121:8–16. doi: 10.1002/cncr.28914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greengard E.G. Molecularly targeted therapy for neuroblastoma. Children. 2018;5:142–158. doi: 10.3390/children5100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y., Zhang T., Kwiatkowski N. CDK7-dependent transcriptional addiction in triple-negative breast cancer. Cell. 2015;163:174–186. doi: 10.1016/j.cell.2015.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corkery B., Crown J., Clynes M., O'Donovan N. Epidermal growth factor receptor as a potential therapeutic target in triple-negative breast cancer. Ann Oncol. 2009;20:862–867. doi: 10.1093/annonc/mdn710. [DOI] [PubMed] [Google Scholar]
- 52.Ueno N.T., Zhang D. Targeting EGFR in triple negative breast cancer. J Cancer. 2011;2:324–328. doi: 10.7150/jca.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moskowitz A.J., Lunning M.A., Horwitz S.M. How I treat the peripheral T-cell lymphomas. Blood. 2014;123:2636–2644. doi: 10.1182/blood-2013-12-516245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cayrol F., Praditsuktavorn P., Fernando T.M. Corrigendum: THZ1 targeting CDK7 suppresses STAT transcriptional activity and sensitizes T-cell lymphomas to BCL2 inhibitors. Nat Commun. 2017;8:14747. doi: 10.1038/ncomms14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sobell H.M. Actinomycin and DNA transcription. Proc Natl Acad Sci U S A. 1985;82:5328–5331. doi: 10.1073/pnas.82.16.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coiffier B., Federico M., Caballero D. Therapeutic options in relapsed or refractory peripheral T-cell lymphoma. Cancer Treat Rev. 2014;40:1080–1088. doi: 10.1016/j.ctrv.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 57.Crescenzo R., Abate F., Lasorsa E. Convergent mutations and kinase fusions lead to oncogenic STAT3 activation in anaplastic large cell lymphoma. Cancer Cell. 2015;27:516–532. doi: 10.1016/j.ccell.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Durant L., Watford W.T., Ramos H.L. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32:605–615. doi: 10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Besbes S., Mirshahi M., Pocard M., Billard C. New dimension in therapeutic targeting of BCL-2 family proteins. Oncotarget. 2015;6:12862–12871. doi: 10.18632/oncotarget.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Foucquier J., Guedj M. Analysis of drug combinations: current methodological landscape. Pharmacol Res Perspect. 2015;3:e00149. doi: 10.1002/prp2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lord C.J., Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355:1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Staebler A., Karberg B., Behm J. Chromosomal losses of regions on 5q and lack of high-level amplifications at 8q24 are associated with favorable prognosis for ovarian serous carcinoma. Genes Chromosomes Cancer. 2006;45:905–917. doi: 10.1002/gcc.20356. [DOI] [PubMed] [Google Scholar]
- 63.Mertz J.A., Conery A.R., Bryant B.M. Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc Natl Acad Sci U S A. 2011;108:16669–16674. doi: 10.1073/pnas.1108190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lockwood W.W., Zejnullahu K., Bradner J.E., Varmus H. Sensitivity of human lung adenocarcinoma cell lines to targeted inhibition of BET epigenetic signaling proteins. Proc Natl Acad Sci U S A. 2012;109:19408–19413. doi: 10.1073/pnas.1216363109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kalan S., Amat R., Schachter M.M. Activation of the p53 transcriptional program sensitizes cancer cells to Cdk7 inhibitors. Cell Rep. 2017;21:467–481. doi: 10.1016/j.celrep.2017.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang T., Kwiatkowski N., Olson C.M. Covalent targeting of remote cysteine residues to develop CDK12 and CDK13 inhibitors. Nat Chem Biol. 2016;12:876–884. doi: 10.1038/nchembio.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ning J., Ma X., Long C. Anti-tumor drug THZ1 suppresses TGFβ2-mediated EMT in lens epithelial cells via notch and TGFβ/Smad signaling pathway. J Cancer. 2019;10:3778–3788. doi: 10.7150/jca.30359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu P., Geng J., Zhang L. THZ1 reveals CDK7-dependent transcriptional addictions in pancreatic cancer. Oncogene. 2019;38:3932–3945. doi: 10.1038/s41388-019-0701-1. [DOI] [PubMed] [Google Scholar]