Abstract
Although there are a number of effective treatments for posttraumatic stress disorder (PTSD), there is a need to develop more efficient evidence-based PTSD treatments to address barriers to seeking and receiving treatment. Written exposure therapy (WET) is a potential alternative that is a 5-session treatment without any between-session assignments. WET has demonstrated efficacy, and low treatment dropout rates. However, prior studies with WET have primarily focused on civilian samples. Identifying efficient PTSD treatments for military service members is critical given the high prevalence of PTSD in this population. The current ongoing randomized clinical trial builds upon the existing literature by investigating whether WET is equally efficacious as Cognitive Processing Therapy (CPT) in a sample of 150 active duty military service members diagnosed with PTSD who are randomly assigned to either WET (n = 75) or CPT (n = 75). Participants are assessed at baseline and 10, 20, and 30 weeks after the first treatment session. The primary outcome measure is PTSD symptom severity assessed with the Clinician Administered PTSD Scale for DSM-5. Given the prevalence of PTSD and the aforementioned limitations of currently available first-line PTSD treatments, the identification of a brief, efficacious treatment that is associated with reduced patient dropout would represent a significant public health development.
Keywords: Clinical trial, Posttraumatic stress disorder, Cognitive behavioral therapy, Written exposure therapy
Abbreviations
- AUDIT
Alcohol Use Disorders Identification Test
- B-IPF
Brief Inventory of Psychosocial Functioning
- CAPS-5
Clinician Administered PTSD Scale for DSM-5
- CEQ
Credibility/Expectancy Questionnaire
- CPT
Cognitive Processing Therapy
- DRRI-2
Deployment Risk and Resilience Inventory, second version
- DoD
Department of Defense
- DSI-SS
Depressive Symptoms Index – Suicidality Subscale
- DSM-5
Diagnostic and Statistical Manual of Mental Disorders, 5th Edition
- EBTs
evidence-based treatments
- FTND
Fagerstrom Test for Nicotine Dependence
- FTND-ST
Fagerstrom Test for Nicotine Dependence – Smokeless Tobacco Version
- GAD-7
Generalized Anxiety Disorder Screener
- IRB
Institutional Review Board
- ISI
Insomnia Severity Index
- ITT
intent-to-treat
- LEC
Life Events Checklist
- MINI
Mini International Neuropsychiatric Interview
- PCL-5
PTSD Checklist for DSM-5
- PE
Prolonged Exposure
- PHQ-9
Patient Health Questionnaire-9
- PI
principal investigator
- PTSD
posttraumatic stress disorder
- QDS
Quick Drinking Screen
- RCT
randomized clinical trial
- SITBI
Self-Injurious Thoughts and Behaviors Interview
- STOP
Snoring, Tired, Observed, Blood Pressure Sleep Apnea Screen
- STAXI-2
State-Trait Anger Inventory-2
- STRONG STAR
South Texas Research Organizational Network Guiding Studies on Trauma And Resilience
- Tx
treatment
- VA
Department of Veterans Affairs
- VR-12
Veterans RAND 12-Item Health Survey
- WET
Written Exposure Therapy
- WAI-SR
Working Alliance Inventory-Short Revised.
1. Introduction
Although trauma-focused treatments such as Cognitive Processing Therapy (CPT [1]) and Prolonged Exposure (PE [2]) have strong supporting evidence for their efficacy, many individuals suffering from posttraumatic stress disorder (PTSD) still experience clinically significant symptoms or even remain above the diagnostic threshold for caseness after treatment has ended [3]. These statistics are typically worse among military service members who receive CPT and PE to treat their PTSD (see 4, for a review), with only 40–50% losing their PTSD diagnosis after treatment [5,6], 50–60% demonstrating clinically significant changes in PTSD symptoms from baseline [7] and even fewer showing symptom change occurring no more than 5% of the time due to measurement error [6]. Attrition among service members who receive these treatments ranges from a low of 14% to a high of 45% [[5], [6], [7]], although attrition for CPT can be at the higher end of this range and loss to follow-up can be even higher.
From the provider perspective, many mental healthcare providers with the Departments of Defense (DoD) and Department of Veterans Affairs (VA) are not inclined to use these treatment approaches, even after being trained, due to time constraints and other implementation barriers [[8], [9], [10]]. More efficient PTSD treatments may also accelerate military readiness among affected service members and may address the barriers identified for implementing PTSD treatments. Taken together, these findings establish a pressing need to identify effective PTSD treatments that are more efficient for providers and patients and that promote greater treatment engagement.
One such treatment is Written Exposure Therapy (WET [11]), which involves repeatedly confronting a trauma memory through writing over the course of five, treatment sessions. Direct face-to-face contact between therapist and client is significantly reduced in WET, as the therapist reads the writing instructions aloud to the client who is then left alone to complete the writing. The therapist returns after the 30 min for writing has elapsed and briefly checks in with the patient (see detailed information about the treatment in Methods section). Prior research has indicated that WET is efficacious [12,13], and noninferior to CPT [14,15]. Notably, prior studies have consistently demonstrated that there is very low attrition among patients receiving WET (e.g., less than 10%). Although WET is now recommended as a first-line treatment in the most recently published VA/DoD PTSD Clinical Practice Guidelines [16]. Notably, there are no WET randomized controlled trials with active duty service members and it remains uncertain if WET will demonstrate the same efficacy with them as it has with civilians; such research is crucially important as civilians tend to have better PTSD treatment outcomes than active duty service members and veterans [4].
This study will compare PTSD treatment outcomes and attrition among active duty military service members diagnosed with PTSD. Based on prior findings with civilians [12,14], we are expecting WET will be at least as good as CPT, and observed treatment gains will be maintained at follow-up. In contrast, we expect WET will have significantly less attrition than CPT, even when comparing session attendance for only the first five sessions of CPT. The study is affiliated with the South Texas Research Organizational Network Guiding Studies on Trauma And Resilience (STRONG STAR Consortium), headquartered at the University of Texas Health Science Center at San Antonio (see www.STRONGSTAR.org), and is funded by the Department of Defense (W81XWH-15-1-0391).
2. Materials and methods
2.1. Participants
Participants (N = 150) are active duty United States military personnel stationed at bases in San Antonio or Fort Hood, Texas, who are seeking treatment for PTSD, ages 18–65. Up to 220 individuals will be consented and screened to obtain data for analysis from 150 participants (75 participants in each treatment condition).
Inclusion criteria include a PTSD diagnosis, determined by the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5 [17]). The diagnosis of PTSD may be indexed to a combat-related Criterion A event, or to another Criterion A event. In addition, participants must expect to remain in the local area for the 3 months following the first assessment. Participants who are taking psychotropic medications must agree to remain on a stable dose for at least 4 weeks. Few exclusion criteria were included in order to recruit a sample that was representative of the general population of individuals with a diagnosis of PTSD. Exclusion criteria include any recent manic episode or psychotic disorder (determined by the bipolar and psychosis sections of the Mini International Neuropsychiatric Interview [MINI, 18]), current alcohol dependence (determined by clinical interview and the Alcohol Use Disorders Identification Test [AUDIT, 19]), evidence of moderate or severe traumatic brain injury (determined by an inability to comprehend baseline screening questionnaires), current suicidal ideation severe enough to warrant immediate intervention (determined by the Depressive Symptoms Index – Suicidality Subscale [DSI-SS, 20] and corroborated by a clinical risk assessment by a credentialed provider), other psychiatric disorders severe enough to warrant designation as the primary disorder, or current engagement in evidence-based treatment (EBT) for PTSD. Concomitant medications are not exclusionary; all medication changes are monitored for the duration of the trial. Please refer to Table 1 for the list of inclusion and exclusion criteria and rationale.
Table 1.
Inclusion and exclusion criteria and rationale.
| Inclusion Criteria | Rationale |
|---|---|
| Active duty service member seeking treatment for PTSD | Population under study |
| Current PTSD diagnosis | Population under study |
| Stable medication dose for at least 4 weeks | Treatment confound |
|
Exclusion Criteria |
Rationale |
| Current psychotic disorder | Human subjects concern |
| Currently in EBP for treatment for PTSD | Treatment confound |
| Moderate to severe brain damage | Human subjects concern |
| Suicidal risk | Human subjects concern |
PTSD = posttraumatic stress disorder; EBT = evidence-based treatment.
2.2. Study design and procedures
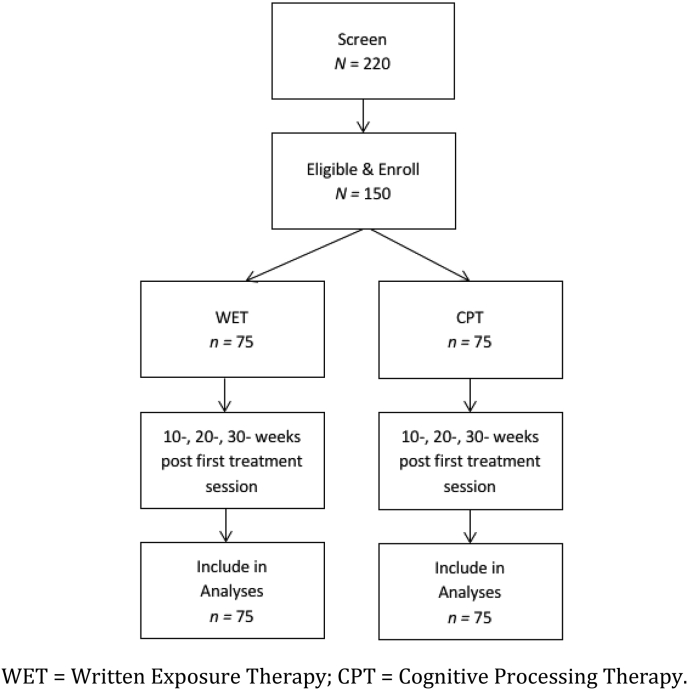
The study was designed in such a way as to permit us to examine whether WET is noninferior to CPT, a more time and resource intense treatment approach. Participants (N = 150) are randomly assigned to either WET (n = 75) or to CPT (n = 75). Recruitment is occurring over the course of 4 years. Because of the differences in treatment dosage, diagnostic assessments are scheduled to occur at pretreatment, as well as 10, 20, and 30 weeks following the first treatment session. Thus, assessments occur at the same time point for all participants regardless of treatment assignment; structuring the assessments in this manner controls for any possible effects of time. Participants are not compensated for any portion of the study. The entire study is anticipated to require 4 years to complete. See Fig. 1 for the planned participant flow.
Fig. 1.
Planned participant flow.
WET = Written Exposure Therapy; CPT = Cognitive Processing Therapy.
Following informed consent, baseline assessment includes a battery of psychological health questionnaires and interviews administered by an independent evaluator who is masked as to condition. Participants who are eligible based on the questionnaire and interview symptom assessment are randomized into a treatment arm: WET or CPT (see Fig. 1). Clinical assessments comprised of questionnaires and interviews occur at each assessment period. In addition, participants complete self-report measures assessing symptoms of PTSD, depression, and suicidal ideation at every other treatment session.
2.2.1. Ethical oversight
This study was reviewed and approved by the Institutional Review Boards (IRB) of Brooke Army Medical Center, VA Boston Healthcare System, Duke University, and the University of Texas Health Science Center at San Antonio. Clinical trial registration was completed at ClinicalTrials.gov (NCT03033602).
2.2.2. Randomization
The blocked randomization sequence, using a 1:1 ratio, was entered by a study statistician into a secure, web-based application using SAS version 9.4 (SAS Institute Inc.), which is accessed by the project coordinator upon enrollment of each participant. The project coordinator then informs the participant of his or her treatment assignment. The participant is then contacted by the assigned study therapist to schedule the first treatment session.
2.3. Measures
The primary outcome is PTSD severity, as measured by the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5 [17]), a structured diagnostic interview that assesses DSM-5 criteria for PTSD and yields information about PTSD symptom severity and PTSD diagnostic status. The CAPS-5 is administered at all major assessment points: at baseline and at 10, 20, and 30 weeks post first treatment session.
As part of a core battery of measures used by the STRONG STAR Consortium [21], secondary measures are included and administered at every assessment period. These measures include: Alcohol Use Disorders Identification Test (AUDIT [19]) Babor, Higgins-Biddle, Saunders, & Monteiro, 2001); Brief Inventory of Psychosocial Functioning (B-IPF [22]); Client Satisfaction Questionnaire (CSQ [23]); Credibility Expectancy Scale (CEQ [24]) Pre and Post; Demographics & Military Service Characteristics; Deployment Risk and Resilience Inventory (DRRI-2 [25]) Combat Experience and Postbattle Experience Sub-Scales; Depressive Symptom Index – Suicidality Subscale (DSI-SS [20]); Fagerstrom Test for Nicotine Dependence – Smokeless Tobacco Version (FTND-ST [26,27]); Fagerstrom Test for Nicotine Dependence (FTND [28]); Generalized Anxiety Disorder Screener (GAD-7 [29]); Health Questionnaire; History of Head Injuries [30,31]; Insomnia Severity Index (ISI [32]); Life Events Checklist-5 (LEC-5 [33]); Mini International Neuropsychiatric Interview (MINI 7.0) – Psychotic Module [18]; Patient Health Questionnaire (PHQ-9 [34]); PROMIS Sleep Disturbance and Sleep-Related Impairment [35]; PTSD Checklist for DSM-5 (PCL-5 [36]); Quick Drinking Screen (QDS) self-report version [37]; Self-Injurious Thoughts and Behaviors Interview (SITBI [38]); Snoring, Tired, Observed, Blood Pressure (STOP) Sleep Apnea Screen [39]; Veterans RAND 12-Item Health Survey (VR-12 [40]); Working Alliance Inventory-Short Revised (WAI-SR [41]; See Table 2).
Table 2.
Schedule of assessment measures.
| Measure | Baseline | Weekly During Tx | End of Tx | Week 10 | Week 20 | Week 30 | |
|---|---|---|---|---|---|---|---|
| Demographic Information | |||||||
| Demographics | X | ||||||
| PTSD Measures | |||||||
| LEC-5 | X | X | X | X | |||
| DRRI-2 | X | ||||||
| CAPS-5 | X | X | X | X | |||
| PCL-5 | X | X | X | X | X | ||
| Sleep Measures | |||||||
| ISI | X | X | X | X | |||
| STOP | X | ||||||
| PROMIS | X | X | X | X | |||
| Health Measures | |||||||
| HHI | X | X | X | X | |||
| VR-12 | X | X | X | X | |||
| FTND | X | X | X | X | |||
| FTND-ST | X | X | X | X | |||
| Health Questionnaire | X | X | X | X | |||
| Other Psychosocial Measures | |||||||
| MINI | X | ||||||
| PHQ-9 | X | X | X | X | X | ||
| DSI-SS | X | X | X | X | |||
| SITBI | X | X | X | X | |||
| GAD-7 | X | X | X | X | |||
| AUDIT | X | ||||||
| QDS | X | X | X | X | |||
| B-IPF | X | X | X | X | |||
| Therapy Process Measures | |||||||
| CEQ Pre and Post | X | X | |||||
| CSQ | X | ||||||
| WAI-SR | X | ||||||
Note: Tx = treatment; Demographics = Demographics & Military Service Characteristics; PTSD = posttraumatic stress disorder; LEC-5 = Life Events Checklist-5; DRRI-2 = Deployment Risk and Resilience Inventory Combat Experience and Postbattle Experience Sub-Scales; CAPS-5 = Clinician Administered PTSD Scale for DSM-5; PCL-5 = PTSD Checklist for DSM-5; ISI = Insomnia Severity Index; STOP = Snoring, Tired, Observed, Blood Pressure Sleep Apnea Screen; PROMIS = Patient Reported Outcomes Measurement Information System; HHI = History of Head Injuries; VR-12 = Veterans RAND 12-Item Short Form Health Survey; FTND = Fagerstrom Test for Nicotine Dependence; FTND-ST = Fagerstrom Test for Nicotine Dependence – Smokeless Tobacco; MINI = Mini International Neuropsychiatric Interview; PHQ-9 = Patient Health Questionnaire-9; DSI-SS = Depressive Symptom Index – Suicidality Subscale; SITBI = Self-Injurious Thoughts and Behaviors Interview short form; GAD = Generalized Anxiety Disorder Screener; AUDIT = Alcohol Use Disorders Identification Test; QDS = Quick Drinking Screen; B-IPF = Brief Inventory of Psychosocial Functioning; CEQ = Credibility and Expectancy Questionnaire; CSQ = The Client Satisfaction Questionnaire; WAI-SR = The Working Alliance Inventory, Short Form.
2.4. Treatment conditions
2.4.1. Written Exposure Therapy (WET)
The WET protocol was developed over the course of a systematic series of studies investigating the use of expressive writing for the treatment of PTSD [12,[42], [43], [44], [45]]. WET consists of five weekly treatment sessions, with the first session lasting 1 h and each subsequent session lasting approximately 40 min. In the first session, the therapist educates the participant about common reactions to trauma and provides the rationale for WET as a treatment for PTSD. The participant is then given general instructions for completing the trauma narratives, followed by specific instructions for completing the first session. The participant then completes the first (30 min) writing session. Participants are instructed to write about the same trauma during each session. This event is the same event identified as the index trauma during the baseline assessment session. The importance of delving into one's deepest emotions surrounding the traumatic event is emphasized, as well as the importance of providing detailed information about the event. In each WET session, the therapist reads the specific writing instructions for that session and then leaves the instructions with the participant, while the 30-min writing session is completed alone. After 30 min has elapsed, the therapist re-enters the room and asks the participant to stop writing. The therapist then checks in with the participant regarding how the writing session went. The discussion of the participant's reaction to the writing session is kept brief (i.e., less than 10 min).
Writing instructions begin with a focus on describing the details of the trauma as well as the emotions and thoughts that occurred during the traumatic event. They then change over the five sessions to focus more on the meaning of the trauma event (e.g., what the event has meant to the person, how it has changed the way the person views his or her life). No between-session homework assignments are included. Between sessions, the therapist reads the written narrative to make sure the participant followed the writing instructions. At the start of subsequent writing session, the therapist provides feedback to the participant regarding how well he or she followed the instructions.
Although the core aspect of WET involves written trauma narratives, we have not found educational level or IQ to moderate treatment outcome of WET [11]. This is not surprising as the purpose of the writing is to confront one's trauma memory; the quality of how one writes does not matter.
2.4.2. Cognitive Processing Therapy (CPT)
CPT consists of twelve, 60-min, twice weekly sessions that focus on challenging and changing distorted beliefs and self-blame regarding the traumatic event through Socratic questioning [1] using a progressive series of worksheets. The therapy consists of sequential cognitive therapy practice assignments that teach participants to examine and modify their thoughts about their traumatic events and the consequences.
The CPT protocol has evolved over time. The original protocol included a detailed traumatic account that was written between sessions and discussed within session. A dismantling study of CPT found that the protocol without the trauma accounts led to faster PTSD reductions [46]. Based on these findings, Resick and colleagues [1] now recommend using a version of CPT that does not include the written trauma narratives. This protocol version was previously referred to as CPT, cognitive only (CPT-C) but is now referred to as CPT. The protocol that includes written accounts was previously referred to as CPT, but is now referred to as CPT plus written accounts (CPT + A).
Sloan et al. [14] investigated whether WET was noninferior using the full CPT protocol (CPT + A). However, as previously described, CPT without written accounts led to faster PTSD reductions in one study [46]. Moreover, Sloan and colleagues noted that a substantial proportion of dropouts in the CPT condition occurred during the written account sessions. Thus, the dropout rate may be lower using the CPT version that does not include written accounts. Consequently, this study is using the protocol version that omits the written accounts. Consistent with Resick et al. [1], we use the term CPT to refer to the protocol version that omits the written accounts.
2.5. Quality control
2.5.1. Training and supervision of therapists
All study therapists hold either a master's or doctoral degree in psychology and have at least 1 year of experience in treating PTSD patients. Therapists are counterbalanced to treat participants across the two treatment conditions. Given the nature of the two treatment conditions in this study, the amount of training and supervision each treatment requires differs substantially. For CPT, a 2-day workshop is completed by all therapists. For WET, a 4-h training session is required. Following completion of the initial training, therapists receive 30–60 min weekly supervision or case consultation from psychologists who have extensive experience with the respective treatment protocol. All treatment sessions are audio-recorded and available for supervision and fidelity assessment.
2.5.2. Assessment of fidelity
Treatment fidelity is assessed by two individuals who are otherwise unaffiliated with the study. These two individuals are selected based on their familiarity with either the WET or the CPT protocol. For each treatment condition, 15% of the treatment sessions are randomly selected, reviewed, and rated, using the adherence and competence forms developed for each of the treatment conditions.
2.6. Safety protocol
To optimize participant safety, we do not include participants with high suicidal risk. At each screening visit, assessment, and therapy session, the therapist or independent evaluator assesses the degree to which a participant may be a danger to himself or others. Suicidal risk is determined by the Self-Injurious Thoughts and Behaviors Interview (SITBI), the Depressive Symptoms Index – Suicidality Subscale (DSI-SS [20]), and the Patient Health Questionnaire-9 (PHQ-9 [34]) at baseline and follow-up encounters. If potential risk is identified on self-report or standardized assessment, the participant will then be further evaluated by a STRONG STAR Risk Consultant. Risk Consultants are providers who are permitted to complete risk assessment and intervention, in accordance with that sites' requirements. An on-call schedule is maintained based on that site's specific needs to ensure coverage during any scheduled participant visit. Determination as to whether a participant who is identified as high risk may continue with or re-enter treatment is made on a case-by-case basis in accordance with the study protocol by the overall principal investigator (PI) with consultation from on-site PIs and STRONG STAR leadership as necessary. Participants are excluded if suicide risk warrants immediate psychiatric intervention. To monitor suicide risk during the treatment, the PHQ-9 is administered at each treatment session. If a participant endorses suicidal ideation at 2 or higher in response to item 9 (thoughts you would be better off dead or hurting yourself), the therapist will assess for suicide risk. Therapists assigned to participant care during the treatment phase will monitor fluctuations risk and provide management strategies to the participant as indicated (e.g., developing a crisis response plan).
The PTSD Checklist for DSM-5 (PCL-5 [36]) is administered at the beginning of every treatment session to monitor potential worsening of symptoms. Worsening of symptoms is defined by an increase from the initial assessment of at least 10 points on the PCL-5 that is sustained for at least three consecutive treatment sessions [47]. The PCL-5 is completed with reference to the identified Criterion A event established at the baseline assessment. If substantial worsening of symptoms occurs, the therapist talks with the participant about the possible reasons for symptom increase and whether study withdrawal, with appropriate referrals, is appropriate.
Adverse events are assessed at each assessment visit by inquiring whether any major change in mental or physical health has occurred since the participant's previous visit and whether any hospitalizations have occurred since the last visit. All serious adverse events are reported within 48 business hours to the local IRB as well as to the Data and Safety Monitoring Board that meets on a quarterly basis to monitor safety of participants enrolled in the study.
2.7. Data analytic strategy
2.7.1. General
The similarity of the patients in the two treatment conditions on key baseline variables (e.g., age sex, racial background, PTSD symptom severity, depression severity) will be examined using t-tests (e.g., sex), nonparametric equivalence, or chi-square tests, (racial background) depending on the type (continuous or dichotomous) and distribution (normal or non-normal) of the data. Any variable that statistically differs among groups will be used as covariates in the final analyses.
Missing Data. The proposed likelihood-based analyses are valid when the assumption that data is missing at random (MAR) holds. We will examine whether data are MAR using several approaches. First, we will perform [48] Missing Completely At Random test (MCAR). Second, we will compare those who are lost to outcome assessment with those available on baseline characteristics and response until dropout. If a statistical model predicting discontinuation can be developed, it can be the basis for inverse propensity weighting, a method that gives more weight to patients who are similar to those lost to follow-up [49]. On the other hand, Little [50] suggests that non-MAR situations are best handled by simple sensitivity analyses, where the assumptions are clear. For example, if a subset of the dropouts are thought to have an NMAR mechanism, the model might assume the mean of the predictive distribution of those values deviates from the distribution assumed under MAR by some specified amount, say 0.2 or 0.5 times the residual standard deviation given known variables for that case. The results from “tilting” the MAR model in this way can then be assessed.” Enders [51](p.289) describes a very similar process calling it “Rubin's ad hoc sensitivity analysis.” He suggests performing a series of multiple imputation analyses using a range of plausible constant values. This multiple imputation approach is now implemented in SAS/STAT version 9.4's PROC MI with the inclusion of a new NMAR option [52], which permits systematically shifting imputed values by a constant amount or percent. It also can base the imputation model either on the complete sample or on a subset of cases such as the control group, as suggested by Mallinckrodt et al.‘s [53] “worst case scenario.” In the end, Demirtas & Schafer [[54], p. 2574] conclude: “To us, an ignorability-based analysis that includes good predictors of attrition often seems more plausible than a non-ignorable model that assumes that the probability of dropout is a known function of the outcome being measured.”
2.7.2. Analysis of study aims
To test the hypothesis of the primary study aim that WET is noninferior to CPT analyses will be conducted using the intent-to-treat (ITT) sample. The ITT sample will consist of participants who are randomized. The primary outcome measure will be PTSD symptom severity as indexed by the CAPS-5 total score. Consistent with Sloan et al. [14], a difference of 10 points (or less) on the CAPS-5 will be used to define noninferiority. The study includes assessments at 10-, 20-, and 30-weeks post the first treatment session. In order to examine treatment change over time, each of the three assessments will be examined and it is anticipated that WET will be non-inferior to CPT at each of these assessments.
The second aim of the study is to examine whether treatment dropout rates are significantly lower in the WET condition relative to CPT. Treatment dropout is defined as dropping out of treatment before completion of the protocol. However, because we are interested in dropout related to tolerability of the treatment, participants will not be included as a dropout if they report that they dropped out of treatment before completion because they felt they had achieved sufficient treatment gain or if they dropped out due to military relocation. Moreover, for a fair comparison, dropout occurring by session 5 of CPT will be compared to dropouts occurring in the WET condition.
Analysis of proportions (i.e., dropout rate) is straightforward with contingency tables, chi-square tests, and logistic regression, but that approach requires unambiguous coding of outcomes for all cases. That is problematic for those pulled out or lost to follow-up for extraneous reasons (e.g., removed due to deployment). Excluding those cases will not bias analyses if their departure is assumed to be unrelated to treatment, but it may reduce power. Survival analysis deals well with cases lost to follow-up. The Kaplan-Meier (product limit) survival curve estimates the proportions of participants dropping out over time with log-rank, Wilcoxon and likelihood ratio tests of differences between groups in the survival functions. Cox proportional hazard regression is a flexible method of survival analysis to analyze of predictors of time to event that can include time-dependent predictors that change over time [55,56] compared four alternative data analysis methods for the study of time to event (in their case the event was recovery) and concluded that survival analysis was most powerful. Consequently, comparison of results with both approaches will most informative.
2.7.3. Power analysis to determine sample size
Primary aim. A power analysis was conducted with the focus being the primary study aim to test noninferiority based on the CAPS-5 PTSD total symptom severity score. Following the practice of Sloan et al. [14], an outcome difference of 10 points or more on the CAPS-5 total severity score was chosen as the “noninferiority margin.” Differences smaller than 10 points would be considered clinically insignificant, so noninferiority will be declared if the upper bound of the 95% one-sided confidence limit of the difference between group means is less than 10. Using the CAPS for DSM-IV, Schnurr et al. [57] reported the standard deviation of the CAPS to be 20, so this represents a standardized mean difference in Cohen's [58] terms of d = 0.50, a conventional medium effect.
Sample size was determined using the module for noninferiority tests in the NCSS/PASS power software [59]. Specifications were a 10-point noninferiority margin, a standard deviation of 20 [57], a true difference between treatment groups of zero, one-sided noninferiority test at p = .05, desired power = .80 and equal allocation to the two treatment groups. With these specifications, PASS indicated that N = 50 per group is required. This number was increased by 25% to account for unavoidable loss to follow-up. This is the basis for proposed recruitment of 126 participants. The sample size is consistent with other previously conducted PTSD noninferiority trials [60,61].
Secondary aim: Power analysis was also conducted for the second study aim testing dropout rate differences between the two treatment conditions. Sample size was determined using NCSS/PASS software [59]. Power would be at least 0.80 for a chi-square test of differences predicting dropout in CPT of 30–40% and 5–10% in WET. For survival analysis with a sample size of 126 and 20% attrition using Cox proposal hazard model or log-range test has power of 0.87 for the smallest of these values (i.e., 30%–10%). Finally, logistic regression with a sample size of 100 and a binary predictor (treatment) has power ranging from 0.75 to 0.90. Taken together, the sample size to be recruited in the study will provide sufficient power to test the second aim of the study.
3. Discussion
Written Exposure Therapy is a viable alternative treatment that addresses the time constraint barrier currently noted by both patients and mental health providers [[8], [9], [10]]. This issue is particularly salient in the military setting where rates of PTSD are especially high, yet PTSD treatment utilization and treatment dropout is a significant problem [62,63]. If the study finds that WET is noninferior to the more time intensive CPT, then a more efficient yet effective PTSD treatment approach for military service members will be identified.
This study is particularly important because WET is now listed as a first-line PTSD treatment in the most recent version of the VA/DoD Clinical Practice Guidelines [16]. Despite its inclusion in the practice guidelines, there has been no randomized controlled trial of the efficacy or effectiveness of WET with either a veteran or active duty military service member sample. Accordingly, it is critically important to conduct this study before widespread use of WET in DoD settings.
The second goal of the study is to examine whether WET has significantly lower dropout rates relative to CPT, even when considering only the first five treatment sessions. Even if findings indicate that WET is inferior to CPT, the degree of inferiority would need to be evaluated in light of the tolerability and brevity of the treatment.
A finding that, as expected, WET will have significantly lower attrition than CPT would have serious implications for treating service members with PTSD. Such findings would indicate that DoD could potentially reach and treat many more service members in need than ever before. Such circumstances would potentially improve military mission readiness, reduce the number of military veterans with PTSD in need of treatment following discharge, as well as lower the amount of money spent on compensating military service members and veterans with PTSD-related disabilities. Even if findings indicate that WET is inferior to CPT, the degree of inferiority would need to be evaluated in light of the tolerability and brevity of the treatment.
More broadly, this study will add to the growing literature concerning whether effective PTSD treatment can be obtained with a substantially smaller treatment dose. For instance, a brief version of PE was found to be efficacious in the treatment of service members with PTSD presenting to primary care in an initial pilot study [64] as well as an RCT [65]. Nacasch and colleagues [66] found that PE using a 60-min protocol with a 20-min imaginal exposure was noninferior to using the standard 90-min protocol. Galovski and colleagues [67] found that the majority of individuals receiving CPT needed fewer than the standard 12 sessions to achieve clinically meaningful gains. Although each of these three protocols are shorten versions of either PE or CPT, they are more time intensive than WET and include between session assignments. Nevertheless, these findings suggest that the dose of trauma-focused treatment can be reduced while maintaining the treatment outcome benefits of the treatments. WET was developed as a brief and tolerable PTSD intervention that could provide greater access to effective treatment in a variety of settings. We look forward to additional studies examining whether the efficacy findings of WET are replicated in other settings and with other populations.
Funding
This work is supported by an award to Denise M. Sloan (W81XWH-15-1-0391) by the U.S. Department of Defense.
Role of the funding sources
The funding source has had no involvement in the study design, the collection, analysis and interpretation of data, the writing of this report, or the decision to submit this article for publication.
Disclaimer
The views expressed in this article are solely those of the authors and do not reflect an endorsement by or the official policy of the U.S. Army, the Department of Defense, the Department of Veteran Affairs, or the U.S. Government.
Additional contributions
The authors of this manuscript would like to acknowledge and thank Julie R. Collins, BA, for providing editorial support for this manuscript.
Conflict of interest
Dr. Resick receives book royalties (Resick, Monson, & Chard 2016).
Drs. Sloan and Marx receive book royalties (Sloan & Marx, 2019).
Contributor Information
Denise M. Sloan, Email: denise.sloan@va.gov.
Brian P. Marx, Email: brian.marx@va.gov.
Patricia A. Resick, Email: patricia.resick@duke.edu.
Stacey Young-McCaughan, Email: youngs1@uthscsa.edu.
Katherine A. Dondanville, Email: dondanville@uthscsa.edu.
Jim Mintz, Email: mintz@uthscsa.edu.
Brett T. Litz, Email: brett.litz@va.gov.
Alan L. Peterson, Email: petersona3@uthscsa.edu.
References
- 1.Resick P.A., Monson C.M., Chard K.M. Guilford Press; New York: 2016. Cognitive Processing Therapy for PTSD: a Comprehensive Manual. [Google Scholar]
- 2.Foa E.B., Hembree E., Rothbaum B.O. Oxford University Press; New York: 2007. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences Therapist Guide. [Google Scholar]
- 3.Cusack K., Jonas D.E., Forneris C.A., Wines C., Sonis J., Middleton J.C., Feltner C. Psychological treatments for adults with posttraumatic stress disorder: a systematic review and meta-analysis. Clin. Psychol. Rev. 2016;43:128–141. doi: 10.1016/j.cpr.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Steenkamp M.M., Litz B.T., Hoge C.W., Marmar C.R. Psychotherapy for military-related PTSD: a review of randomized clinical trials. J. Am. Med. Assoc. 2015;314:489–500. doi: 10.1001/jama.2015.8370. [DOI] [PubMed] [Google Scholar]
- 5.Foa E.B., McLean C.P., Zang Y. Effect of prolonged exposure therapy delivered over 2 Weeks vs 8 Weeks vs present-centered therapy on PTSD symptom severity in military personnel: a randomized clinical trial. J. Am. Med. Assoc. 2018;319(4):354–364. doi: 10.1001/jama.2017.21242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Resick P.A., Wachen J.S., Dondanville K.A. Effect of group vs individual cognitive processing therapy in active-duty military seeking treatment for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2017;74(1):28–36. doi: 10.1001/jamapsychiatry.2016.2729. [DOI] [PubMed] [Google Scholar]
- 7.Resick P.A., Wachen J.S., Mintz J. A randomized clinical trial of group cognitive processing therapy compared with group present-centered therapy for PTSD among active duty military personnel. J. Consult. Clin. Psychol. 2015;83:1058–1068. doi: 10.1037/ccp0000016. [DOI] [PubMed] [Google Scholar]
- 8.Borah E.V., Wright E.C., Donahue D.A., Cedillos E.M., Riggs D.S., Isler W.C., Peterson A.L. Implementation outcomes of military provider training in cognitive processing therapy and prolonged exposure therapy for post-traumatic stress disorder. Mil. Med. 2013;178:939–944. doi: 10.7205/MILMED-D-13-00072. [DOI] [PubMed] [Google Scholar]
- 9.Finley E.P., Garcia H.A., Ketchum N.S., McGeary D.D., McGeary C.A., Stirman S.W., Peterson A.L. Utilization of evidence-based psychotherapies in Veterans Affairs posttraumatic stress disorder outpatient clinics. Psychol. Serv. 2015:73–82. doi: 10.1037/ser0000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilk J.E., West J.C., Farifteh D.F., Herrell R.K., Rae D.S., Hoge C.W. Use of evidence-based treatment for posttraumatic stress disorder in Army behavioral healthcare. Psychiatry Interpers. Biol. Process. 2013;76:336–348. doi: 10.1521/psyc.2013.76.4.336. [DOI] [PubMed] [Google Scholar]
- 11.Sloan D.M., Marx B.P. American Psychological Press; Washington, DC: 2019. Written Exposure Therapy for PTSD: a Brief Treatment Approach for Mental Health Professionals. [Google Scholar]
- 12.Sloan D.M., Marx B.P., Bovin M.J., Feinstein B.A., Gallagher M.W. Written exposure as an intervention for PTSD: a randomized clinical trial with motor vehicle accident survivors. Behav. Res. Ther. 2012;50:627–635. doi: 10.1016/j.brat.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sloan D.M., Lee D., Litwak S., Sawyer A.T., Marx B.P. Written exposure therapy for veterans diagnosed with PTSD: a pilot study. J. Trauma. Stress. 2013;26(6):776–779. doi: 10.1002/jts.21858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sloan D.M., Marx B.P., Lee D.J., Resick P.A. A brief exposure-based treatment vs cognitive processing therapy for posttraumatic stress disorder: a randomized noninferiority clinical trial. JAMA Psychiatry. 2018 doi: 10.1001/jamapsychiatry.2017.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson-Hollands J., Marx B.P., Lee D.J., Resick P.A., Sloan D.M. Long-term treatment gains of a brief exposure-based treatment for PTSD. Depress. Anxiety. 2018;35:985–991. doi: 10.1002/da.22825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Department of Veterans Affairs and Department of Defense VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. June 2017. https://www.healthquality.va.gov/guidelines/MH_ptsd/VADoDPTSDCPGFinal082917.pdf Published.
- 17.Weathers F.W., Blake D.D., Schnurr P.P., Kaloupek D.G., Marx B.P., Keane T.M. The clinician-administered PTSD scale for DSM-5 (CAPS-5). Instrument available from the National center for PTSD. 2013. www.ptsd.va.gov
- 18.Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 19.Babor T.F., Higgins-Biddle J.C., Saunders J.B., Monteiro M.G. second ed. World Health Organization; Geneva, Switzerland: 2001. The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care. [Google Scholar]
- 20.Metalsky G.I., Joiner T.E. The hopelessness depression symptom questionnaire. Cogn. Ther. Res. 1997;21:359–384. [Google Scholar]
- 21.Barnes J.B., Presseau C., Jordan A.H., Kline N.K., Young-McCaughan S., Keane T.M., Peterson A.L. The Consortium to Alleviate PTSD. Common data elements in the assessment of military-related PTSD research applied in the Consortium to Alleviate PTSD. Mil. Med. 2019;184:e218–e226. doi: 10.1093/milmed/usy226. [DOI] [PubMed] [Google Scholar]
- 22.Marx B.P. Development and validation of a PTSD-related impairment scale. 2013. www.dtic.mil/cgi-537_bin/GetTRDoc?AD=ADA585414 Retrieved from.
- 23.Larsen D., Attkisson C., Hargreaves W., Nguyen T. Assessment of client/patient satisfaction: development of a general scale. Eval. Program Plann. 1979;2:197–207. doi: 10.1016/0149-7189(79)90094-6. [DOI] [PubMed] [Google Scholar]
- 24.Devilly G.J., Borkovec T.D. Psychometric properties of the credibility/expectancy questionnaire. J. Beh. Ther. Experiment Psychiat. 2000;31:73–86. doi: 10.1016/s0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- 25.Vogt D.S., Smith B.N., King L.A., King D.W., Knight J.A., Vasterling J.J. Deployment Risk and Resilience Inventory-2 (DRRI-2): an updated tool for assessing psychosocial risk and resilience factors among service members and veterans. J. Trauma. Stress. 2013;26:710–717. doi: 10.1002/jts.21868. [DOI] [PubMed] [Google Scholar]
- 26.Ebbert J.O., Patten C.A., Schroeder D.R. The Fagerstrom test for nicotine dependence – smokeless tobacco (FTND-ST) Addict. Behav. 2006;31:1716–1721. doi: 10.1016/j.addbeh.2005.12.015. https://search.crossref.org/?q=The+Fagerstrom+test+for+nicotine+dependence+%E2%80%93+smokeless+tobacco+%28FTND-ST%29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferketich A.K., Wee A.G., Schultz J., Wewers M.E. A measure of nicotine dependence for smokeless tobacco users. Addict. Behav. 2007;9:1970–1975. doi: 10.1016/j.addbeh.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heatherton T.F., Kozlowski L.T., Frecker R.C., Fagerstrom K.O. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 29.Spitzer R.L., Kroenke K., Williams J.B., Lowe B. A brief measure for assessing Generalized Anxiety Disorder: the GAD-7. Arch. Intern. Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 30.Schwab K.A., Baker G., Ivins B., Sluss-Tiller M., Lux W., Warden D. The Brief Traumatic Brain Injury Screen (BTBIS): investigating the validity of a self-report instrument for detecting traumatic brain injury (TBI) in troops returning from deployment in Afghanistan and Iraq. Neurology. 2006;66(5):A235. Sup. 2. [Google Scholar]
- 31.Schwab K.A., Ivins B., Cramer G., Johnson W., Sluss-Tiller M., Kiley K. Screening for traumatic brain injury in troops returning from deployment in Afghanistan and Iraq: initial investigation of the usefulness of a short screening tool for traumatic brain injury. J. Head Trauma Rehabil. 2006;22:377–389. doi: 10.1097/01.HTR.0000300233.98242.87. [DOI] [PubMed] [Google Scholar]
- 32.Morin C.M. The Guilford Press; New York: 1993. Insomnia: Psychological Assessment and Management. [Google Scholar]
- 33.Weathers F.W., Blake D.D., Schnurr P.P., Kaloupek D.G., Marx B.P., Keane T.M. The life events checklist for DSM-5 (LEC-5). instrument available from the National center for PTSD. 2013. https://www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp
- 34.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu L., Buysse D.J., Germain A., Moul D.E., Stover A., Dodds N.E. Development of short forms from the PROMIS sleep disturbance and sleep-related impairment item banks. Behav. Sleep Med. 2011;10:6–24. doi: 10.1080/15402002.2012.636266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weathers F.W., Litz B.T., Keane T.M., Palmieri P.A., Marx B.P., Schnurr P.P. The PTSD checklist for DSM-5 (PCL-5). Scale available from the National center for PTSD. 2013. https://www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp
- 37.Sobell L.C., Agrawal S., Sobell M.B., Leo G.I., Young L.J., Cunningham J.A., Simco E.R. Comparison of a Quick drinking screen with the timeline followback for individuals with alcohol problems. J. Stud. Alcohol. 2003;64:858–861. doi: 10.15288/jsa.2003.64.858. [DOI] [PubMed] [Google Scholar]
- 38.Nock M.K., Holmberg E.B., Photos V.I., Michel B.D. Self-injurious thoughts and Behaviors interview: development, reliability, and validity in an adolescent sample. Psychol. Assess. 2007;19:309–317. doi: 10.1037/1040-3590.19.3.309. https://search.crossref.org/?q=Photos+VI%2C+%26+Michel+BD.+Self-injurious+thoughts+and+Behaviors+interview%3A+development%2C+reliability%2C+and+validity+in+an+adolescent+sample [DOI] [PubMed] [Google Scholar]
- 39.Chung F., Yegneswaran B., Liao P., Chung S.A., Vairavanathan S., Islam S. STOP Questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812–921. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 40.Kazis L.E., Selim A., Rogers W., Ren X.S., Lee A., Miller D.R. Veterans RAND 12-Item Health Survey (VR-12): a white paper summary. 2008. https://www.researchgate.net/publication/237314426_Veterans_RAND_12_Item_Health_Survey_VR12_A_White_Paper_Summary Retrieved from.
- 41.Tracey T.J., Kokotovic A.M. Factor structure of the working alliance inventory. Psychol. Assess. 1989;541:207–210. [Google Scholar]
- 42.Sloan D.M., Marx B.P. A closer examination of the structured written disclosure procedure. J. Consult. Clin. Psychol. 2004;72:165–175. doi: 10.1037/0022-006X.72.2.165. [DOI] [PubMed] [Google Scholar]
- 43.Sloan D.M., Marx B.P., Epstein E.M. Further examination of the exposure model underlying written emotional disclosure. J. Consult. Clin. Psychol. 2005;73:549–554. doi: 10.1037/0022-006X.73.3.549. [DOI] [PubMed] [Google Scholar]
- 44.Sloan D.M., Marx B.P., Epstein E.M., Lexington J. Does altering the instructional set affect written disclosure outcome? Behav. Ther. 2007;38:155–168. doi: 10.1016/j.beth.2006.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sloan D.M., Marx B.P., Greenberg E.M. A test of written emotional disclosure as an intervention for posttraumatic stress disorder. Behav. Res. Ther. 2011;49:299–304. doi: 10.1016/j.brat.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Resick P.A., Galovski T.E., Uhlmansiek M.O.B., Scher C.D., Clum G.A., Young-Xu Y. A randomized clinical trial to dismantle components of cognitive processing therapy for posttraumatic stress disorder in female victims of interpersonal violence. J. Consult. Clin. Psychol. 2008;76(2):243–258. doi: 10.1037/0022-006X.76.2.243. https://search.crossref.org/?q=A+randomized+clinical+trial+to+dismantle+components+of+cognitive+processing+therapy+for+posttraumatic+stress+disorder+in+female+victims+of+interpersonal+violence [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foa E.B., Zoellner L.A., Feeny N.C., Hembree E.A., Alvarez-Conrad J. Does imaginal exposure exacerbate PTSD symptoms? J. Consult. Clin. Psychol. 2002;70:1022–1028. doi: 10.1037//0022-006x.70.4.1022. [DOI] [PubMed] [Google Scholar]
- 48.Little R.A. A test of missing completely at random for multivariate data with missing values. J. Amer Stat. Assoc. 1988. 1988;83(404):198–1202. [Google Scholar]
- 49.Hirano K., Imbens G.W., Ridder G. Efficient estimation of average treatment effects using the estimated propensity score. Econometrica 2003. 2003;71:1161–1189. [Google Scholar]
- 50.Little R. Selection and pattern-mixture models. In: Fitzmaurice G., Davidian M., Verbeke G., Molenberghs G., editors. Longitudinal Data Analysis. Chapman & Hall/CRC; Boca Raton, FL: 2009. pp. 409–432. [Google Scholar]
- 51.Enders C.K. Guilford Press; New York: 2010. Models for Not Missing at Random Data. Applied Missing Data Analysis. [Google Scholar]
- 52.Yuan Y. Sensitivity Analysis in Multiple Imputation for Missing Data. Rockville, MD: SAS Institute, Inc.
- 53.Mallinckrodt C.H., Lin Q., Molenberghs M. A structured framework for assessing sensitivity to missing data assumptions in longitudinal clinical trials. Pharm Stat. 2012. 2012;12(1):1–6. doi: 10.1002/pst.1547. [DOI] [PubMed] [Google Scholar]
- 54.Demirtas H., Schafer J.L. On the performance of random-coefficient pattern-mixture models for non-ignorable drop-out. Stat. Med. 2003;22:2553–2575. doi: 10.1002/sim.1475. [DOI] [PubMed] [Google Scholar]
- 55.Therneau T.M., Grambsch P.M. Springer-Verlag.; New York: 2000. (Modeling Survival Data: Extending the Cox Model). [Google Scholar]
- 56.Nobler M.S., Sackeim H.A., Moeller J.R., Prudic J., Petkova E., Waternaux C. Quantifying the speed of symptomatic improvement with electroconvulsive therapy: comparison of alternative statistical methods. Convuls. Ther. 1997;13(4) 208-21. [PubMed] [Google Scholar]
- 57.Schnurr P.P., Friedman M.J., Foy D.W. Randomized trial of trauma-focused group therapy for posttraumatic stress disorder: results from a department of veterans affairs cooperative study. Arch. Gen. Psychiatr. 2003;60(5):481489. doi: 10.1001/archpsyc.60.5.481. [DOI] [PubMed] [Google Scholar]
- 58.Cohen J. second ed. Erlbaum; Hillsdale, NJ: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 59.Hintze J. NCSS Statistical Software; Kaysville, UT: 2019. NCSS and PASS, Number Cruncher Statistical Systems. [Google Scholar]
- 60.Markowitz J.C., Petkova E., Neria Y. Is exposure necessary? a randomized clinical trial of interpersonal psychotherapy for PTSD. Am. J. Psychiatry. 2015;172(5):430–440. doi: 10.1176/appi.ajp.2014.14070908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morland L.A., Mackintosh M.A., Rosen C.S. Telemedicine versus in-person delivery of cognitive processing therapy for women with posttraumatic stress disorder: a randomized noninferiority trial. Depress. Anxiety. 2015;32(11):811–820. doi: 10.1002/da.22397. [DOI] [PubMed] [Google Scholar]
- 62.Hoge C.W., Grossman S.H., Auchterlonie J.L., Riviere L.A., Milliken C.S., Wilk J.E. PTSD treatment for soldiers after combat deployment: low utilization of mental health care and reasons for dropout. Psychiatr. Serv. 2014;65(8):997–1004. doi: 10.1176/appi.ps.201300307. [DOI] [PubMed] [Google Scholar]
- 63.Kehle-Forbes S.M., Meis L.A., Spoont M.R., PolusnyMA Treatment initiation and dropout from prolonged exposure and cognitive processing therapy in a VA outpatient clinic. Psychol Trauma. 2016;8(1):107–114. doi: 10.1037/tra0000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cigrang J.A., Rauch S.A.M., Mintz J., Brundige A., Avila L., Bryan C.J. Treatment of active duty military with PTSD in primary care: a follow-up report. J. Anxiety Disord. 2015;36:110–114. doi: 10.1016/j.janxdis.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 65.Cigrang J.A., Rauch S.A., Mintz J., Brundige A.R., Mitchell J.A., Najera E. Moving effective treatment for posttraumatic stress disorder to primary care: a randomized controlled trial with active duty military. Fam. Syst. Health. 2017;35:450–462. doi: 10.1037/fsh0000315. [DOI] [PubMed] [Google Scholar]
- 66.Nacasch N., Huppert J.D., Yi-Jen S., Kivity Y., Dinshtein Y., Yeh R. Are 60-minute prolonged exposure sessions with 20-minute imaginal exposure to traumatic memories sufficient to successfully treat PTSD? A randomized noninferiority clinical trial. Behav. Ther. 2015;46:328–341. doi: 10.1016/j.beth.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 67.Galovski T.E., Blain L.M., Mott J.M., Elwood L., Houle T. Manualized therapy for PTSD: flexing the structure of cognitive processing therapy. J. Consult. Clin. Psychol. 2012;80:968–981. doi: 10.1037/a0030600. [DOI] [PMC free article] [PubMed] [Google Scholar]