Abstract
We report results from a multicenter, randomized clinical trial (N=535) of the effect of ω-3 supplementation, relative to placebo, on exploratory and minimally invasive outcome measures for moderate to severe dry eye disease.
The Dry Eye Assessment and Management (DREAM) Study was a multicenter (27 sites), randomized, double-masked, clinical trial for people with moderate to severe dry eye disease (DED).1 Between October 2014 and July 2016, 535 participants were assigned in a 2:1 ratio to either active omega-3 fatty acid daily supplements (2 gm eicosapentaenoic acid (EPA) and 1 gm docosahexaenoic acid (DHA)) or placebo (5 gm refined olive oil). One-year results showed no difference between ω-3 and placebo groups for the primary outcome of symptoms, as measured by the Ocular Surface Disease Index, or the traditional signs of DED (conjunctival and corneal staining, tear break-up time, and Schirmer’s II test results).1
Additional signs of DED acquired through use of devices were assessed in DREAM as exploratory outcome measures. Clinical staff completed a certification program including review of the protocol and instructional slides and a written test for each device. Measurements were made according to the manufacturer’s instructions.
Testing was performed on both eyes with the right eye first. Tear osmolarity was measured using the TearLab Osmolarity System (TearLab, San Diego, CA).The Keratograph 5M (Oculus, Arlington, WA) was used for non-invasive keratographic tear break up time (NIKBUT), tear meniscus height, bulbar conjunctival redness, and meibomian gland imaging. The examiner everted each eyelid and used the keratograph’s infrared photography system to capture images of meibomian glands. Examiners graded meibomian gland dropout on the Pult scale.2 When lid eversion or image quality was insufficient to judge dropout area, the result was “missing”. MMP-9 testing was performed with the Inflammadry system (RPS Diagnostics, Sarasota, Florida). Keratography and tear osmolarity testing was conducted only at centers equipped with the devices. Testing was at baseline, 6, and 12 months except for MMP-9 testing (screening and 3 months).
Differences between treatment groups were estimated with regression models using a generalized estimating equations approach to account for inter-eye correlation. Subgroups were defined based on the baseline values of the measures for signs, using category bounds to form tertiles or, for tear osmolarity, a previously defined threshold for abnormal (≥308 mOsms/L). Variation in treatment effects across subgroups was assessed with tests of interaction.
The DREAM study protocol was approved by each center’s institutional review board, was in compliance with HIPAA, and adhered to the tenets of the Declaration of Helsinki. Patients provided written informed consent. The trial was registered on ClinicalTrials.gov (NCT02128763).
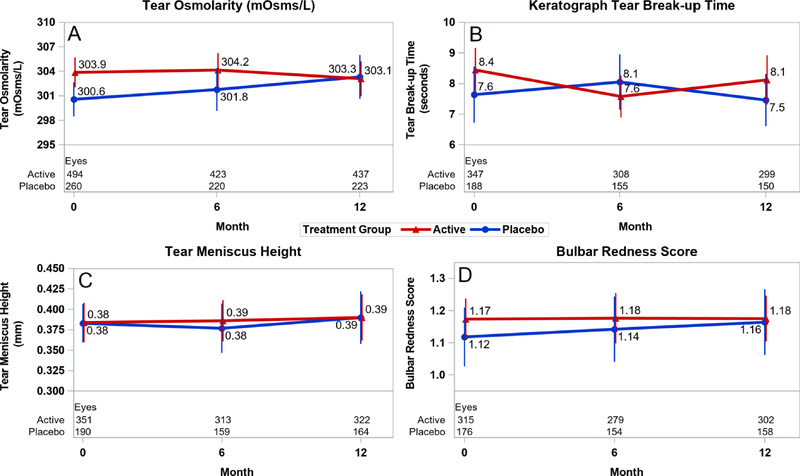
The baseline mean value [±SD] of tear osmolarity in the active group (303.9 [±17.2] mOsm/L) was higher than in the placebo group (300.6 [±14.5] mOsm/L; p=0.02; Table 1 (available at www.aaojournal.org)). The mean change was a decrease of 0.7 mOsm/L in the active group and an increase of 3.6 mOsm/L in the placebo group, yielding a difference of 4.3 mOsm/L (p=0.02; Table 2 (available at www.aaojournal.org); Figure 1A).
Figure 1.
Mean level of continuous exploratory outcomes at baseline and through 12 months by treatment group. Red line denotes the active group and blue line denotes the placebo group. Vertical bars denote 95% confidence intervals. A) tear osmolarity; B) keratograph tear break-up time; C) tear meniscus height; and D) bulbar conjunctival redness score.
The baseline keratography measurements were similar between treatment groups (Table 1). The mean NIKBUT decreased by 0.5 sec in each group (p=0.97; Table 2; Figure 1B). The change in mean tear meniscus height was near zero in the active (0.00 mm) and placebo (−0.01 mm) groups (p=0.71; Table 2; Figure 1C). The mean change in bulbar conjunctival redness score was near zero in the active (0.00) and placebo (−0.01) groups (p=0.81; Table 2; Figure 1D). The percentage of eyes with Pult scale scores indicating improvement, stability, or worsening by 1 or more categories was similar for the upper lid (p=0.34) and lower lid (p=0.21; Table 2).
At baseline, the MMP-9 test was positive for similar proportions of eye in the active (33%) and placebo (30%) groups. Between baseline and 3 months, 10% of eyes in the active group and 13% of eyes in the placebo group converted from negative to positive, and 13% of each group converted from positive to negative (p=0.69; Table 2).
Results of analyses of the mean difference between active and placebo groups within subgroups are displayed in Table 3 (available at www.aaojournal.org). None of the tests of interaction were statistically significant (all p≥0.39).
In this randomized, double-masked clinical trial, there were no significant differences between daily supplementation with ω-3 versus refined olive oil supplementation in NIKBUT, tear meniscus height, bulbar conjunctival redness, upper/lower lid meibography, and MMP-9 positivity (all p>0.21). Only the mean change in tear osmolarity yielded a statistically significant difference, with slight improvement in the active treatment group (−0.7 mOsm/L) when compared to the worsening in the placebo treatment group (+3.6 mOsm/L). The mean changes over time within each treatment group were small for keratography measures and the net change in classification of meibomian gland dropout and MMP-9 positivity was small. When subgroups were examined, there was no evidence of a greater benefit of ω-3 supplementation among eyes with more abnormal values at baseline.
Although a small improvement was observed in the mean change in tear osmolarity for the active group and a worsening in the placebo group, there was no difference between the active and placebo groups at 12 months (303.1 [±18.4] vs. 303.3 [±17.5] mOsm/L; P=0.90). These findings are difficult to interpret given the high variability among readings from the TearLab system and lack of correlation changes in tear osmolarity with changes in symptoms or corneal fluorescein staining.3,4
While several clinical trials have tested the efficacy of ω-3 in treating symptoms of DED, only three addressed the exploratory outcomes used in DREAM.5–7 Three studies measured tear osmolarity using the TearLab system, showing improvements relative to placebo after shorter periods (90 days) with lower doses of ω-3 supplementation than in DREAM. MMP-9 positivity and bulbar conjunctival redness were also measured in two small (n < 55) studies and showed improvement within 90 days of ω-3 supplementation.6,7
In conclusion, ω-3 supplementation was not beneficial relative to placebo for most of the exploratory measures. While there was a difference in the mean change in tear osmolarity in favor or the ω-3 group, the clinical significance of the difference is unclear. These findings are consistent with the results of no difference between ω-3 and placebo groups for the primary and secondary outcomes of the DREAM Study.
Supplementary Material
Acknowledgments
Supported by cooperative agreements U10EY022879 and U10EY022881 from the National Eye Institute, National Institutes of Health, Department of Health and Human Services. Additional support provided by the Office of Dietary Supplements National Institutes of Health, Department of Health and Human Services.
The sponsor participated in overseeing the conduct of the study.
EPAX Norway AS (Alesund, Norway) contributed the fish oil and the Access Business Group, LLC (Ada, MI) manufactured, packaged and delivered study supplements to the central pharmacy. OCULUS Inc. (Arlington, WA) provided discounted equipment, customized software, and user support for the keratograph used in the study. RPS Diagnostics, Inc. (Sarasota, FL) provided InflammaDry Detector test kits to the clinical centers for testing MMP-9.TearLab Corporation (San Diego, CA) provided a discount to the study for testing materials for their TearLab Osmolarity System.
Conflict of Interest: - Dr. Asbell reports personal fees from Santen, personal fees from Shire, grants and personal fees from Novartis, personal fees from Medscape, grants and personal fees from MC2 Therapeutics, grants, personal fees and non-financial support from Valeant, Bausch& Lomb, personal fees from Allergan, personal fees from ScientiaCME, grants and personal fees from Rtech, personal fees from Oculus, grants and personal fees from Miotech, personal fees and non-financial support from Shire, personal fees and non-financial support from Santen, personal fees and non-financial support from CLAO, personal fees from Vindico, outside the submitted work. Dr. Lin report, grants from Amorphex Therapeutics, grants from CooperVision, Inc, grants from Essilor USA, grants from GLIA LLC, grants from Johnson & Johnson, grants from Leo Lens, Inc, grants from Orinda Pharma, grants from Verily Life Science, grants from Viewpoint Pharmaceuticals, personal fees from Google X, personal fees from Health Advances, personal fees from Novartis, personal fees from Shire, during the conduct of the study. No disclosures from the other authors.
Abbreviations
- DREAM
Dry Eye Assessment and Management
- DED
dry eye disease
- NIKBUT
non-invasive keratography tear break-up time
- MMP
matrix metalloproteinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.The Dry Eye Assessment and Management Study Research Group. n-3 fatty acid supplementation for treatment of dry eye disease. N Engl J Med. 2018;378(18): 1681–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pult AH, Nichols JJ. A review of meibography. Optom Vis Sci. 2012;89(5):760–769. [DOI] [PubMed] [Google Scholar]
- 3.Szczesna-Iskander DH. Measurement variability of the TearLab Osmolarity System. Cont Lens Anterior Eye. 2016;39:353–38. [DOI] [PubMed] [Google Scholar]
- 4.Amparo F, Jin Y, Hamrah P, Schaumberg DA, Dana R. What is the value of incorporating tear osmolarity measurement in assessing patient response to therapy in dry eye disease? Am J Ophthalmol. 2014;157(1):69–77.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinnery HR, Naranjo Golborne C, Downie LE. Omega-3 supplementation is neuroprotective to corneal nerves in dry eye disease: a pilot study. Ophthalmic Physiol Opt. 2017;37(4):473–481. [DOI] [PubMed] [Google Scholar]
- 6.Deinema LA, Vingrys AJ, Wong CY, Jackson DC, Chinnery HR, Downie LE. A randomized, double-masked, placebo-controlled clinical trial of two forms of omega-3 supplements for treating dry eye disease. Ophthalmology. 2017;124(1):43–52. [DOI] [PubMed] [Google Scholar]
- 7.Epitropoulos AT, Donnenfeld ED, Shah ZA, et al. Effect of oral re-esterified omega-3 nutritional supplementation on dry eyes. Cornea. 2016;35(9):1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.