Abstract
Although the association between childhood socioeconomic status (SES) and late-life cognition is well-established, the mechanisms underlying this association are less clear. One important potential mediator seldom examined is adolescent cognitive ability. To address this gap, we examined 5,880 respondents from the Wisconsin Longitudinal Study, which follows a random sample of high school students who graduated from Wisconsin high schools in 1957. Structural equation models were used to examine the direct and indirect effects of childhood SES on cognition in late midlife through adolescent cognitive ability, educational attainment, midlife economic condition, and midlife health. Cognitive function was measured as a latent variable composed of scores from 6 cognitive assessments including immediate and delayed recall, digit ordering, letter and category fluency, and a subset of the Wechsler Adult Intelligence Scale similarities test. We found that childhood SES predicts cognition in late midlife, and this association is largely mediated by adolescent cognitive ability and educational achievement and to a lesser extent by midlife economic condition and health. The findings underscore the long-arm of childhood SES in cognitive function in later life and highlight the complex life-course pathways underlying the association between childhood SES and cognition.
Keywords: childhood socioeconomic status, adolescent cognitive ability, education, midlife economic condition, Wisconsin Longitudinal study, cognitive function
Introduction
Studies of older adults have shown that people with low cognitive function have difficulty performing activities of daily living, have significant demands for formal and informal care, and face a higher risk of death (Institute of Medicine, 2015; Langa et al., 2001; Obisesan and Gillum, 2009). With the aging of the baby boomers and longer life expectancy in the United States, there is a growing interest in understanding life-course determinants of cognitive health in late life. In recent years, research has suggested that the origin of cognitive health in old age can be traced back to early-life conditions (Horvat et al., 2014; Richards and Deary, 2005). Studies have shown that higher childhood socioeconomic status (SES) is significantly associated with a higher level of cognitive function in late life (Beck et al., 2018; Kaplan et al., 2001; Richards and Wadsworth, 2004; Singh-Manoux et al., 2005; Zhang et al., 2018).
While the association between childhood SES and late-life cognition is well-established, less is known about the complex mechanisms underlying the association. Most previous studies have focused on adulthood SES as an important mediator of the association between childhood SES and late-life cognition (Luo and Waite, 2005; Lyu and Burr, 2016; Singh-Manoux et al., 2005). Empirical studies in this line of research primarily applied multiple regressions and did not take into consideration measurement errors associated with the observed variables such as childhood SES and late-life cognition. Few studies have used rigorous statistical methods such as structural equation models to examine the potential pathways linking childhood conditions and late-life cognitive function (see Horvat et al., 2014; Singh-Manoux et al., 2005 for exceptions). Moreover, due to data limitations, most prior studies did not consider adolescent cognitive ability, an important correlate of education, occupation, and late-life cognition (Plomin and Deary, 2015; Richards and Sacker, 2003). A few studies that did include early-life cognitive ability (i.e., at ages 8 or 11) all came from two British cohort studies (Johnson et al., 2010; Richards and Sacker, 2003) and it is not clear whether the findings can be generalized to populations from different socio-cultural contexts or historical periods. Almost all studies that used retrospective childhood SES relied heavily on older adults’ long-term recall of their childhood socioeconomic circumstances, a method which is often subject to bias and inaccuracies (Horvat et al., 2014; Johnson et al., 2010).
To address these limitations and extend previous research, we drew on data from the 1957–2004 Wisconsin Longitudinal Study (WLS) and used structural equation modeling to investigate the multiple life-course pathways from childhood SES to cognition in late midlife. WLS is particularly valuable for our study because the respondents were followed up for half of a century, and the survey collected childhood information including household income, parental education and occupation, and adolescent cognitive ability when the respondents were in high school.
Theoretical Background
Early-Life SES, Adulthood SES, Health, and Late-Life Cognition
Previous research has pointed to childhood SES as one of the major factors that affect cognitive function in late life. Childhood SES has been found to be positively associated with mid- and late-life cognitive function in the United States, European countries, and developing countries such as China (Beck et al., 2018; Melrose et al., 2015; Singh-Manoux et al., 2005; Turrell et al., 2002; Zhang et al., 2010). For example, Using the British 1946 birth cohort, Richards and Wadsworth (2004) found that exposure to poor material home conditions (e.g., dated structure, poor repair, uncleanliness, crowding, etc.) at age 4 was strongly associated with lower cognitive ability in childhood and adolescence; and the negative effects of poverty on measures of verbal ability, memory, speed, and concentration persisted into midlife (i.e., age 53). In addition, both father’s and mother’s education were also found to be positively associated with cognitive function among older adults age 65 and older (Glymour et al., 2012; González et al., 2013). Most of these studies defined childhood (or early life) as the period before the respondents reached age 18.
Explanations for the association between childhood SES and late-life cognition have been offered from two perspectives. One suggests that childhood SES has a direct association with late-life cognitive function. Families with fewer economic resources may not be able to purchase nutritious foods and provide a cognitively stimulating home environment for their children (Brito and Noble, 2014; Lyu and Burr, 2016). Consequently, this early socioeconomic adversity can affect brain development given that the prenatal period and the first three years of life are a critical period for brain growth, and the brain continues to grow throughout childhood and adolescence (Kim et al., 2003). Animal studies suggest that malnutrition could damage specific regions of the brain, such as the hippocampus, a region important for learning and memory (Abbott et al., 1998). Even small degrees of hippocampal damage can affect cognitive performance (Hertzman, 1999). Recent research in early-life exposure to famine has also suggested that severe nutritional deprivation in childhood has lasting negative effects on midlife cognition (ages between 48 and 53) in China (Xu et al., 2018). Studies of socioeconomic differences in brain structure revealed that children from lower-income families had smaller hippocampal volumes (Hanson et al., 2011; Luby et al., 2013). Furthermore, early-life impairment in brain development can produce a brain that functions less efficiently because of “less myelin, less branching of dendrites, and less developed connectivity patterns” (Moceri et al., 2000, p. 415). The negative effects of impaired brain development may be small until aggravated by the aging process (Haan and Wallace, 2004; Hall et al., 2000; Moceri et al., 2000). In sum, this perspective suggests that childhood socioeconomic disadvantage may have a long-lasting effect on late-life cognition, irrespective of adulthood conditions.
Another perspective suggests that childhood SES affects late-life cognition indirectly through adulthood factors such as adulthood SES and health. Early socioeconomic adversity often has negative impacts on educational achievement and occupation. A large body of research has shown that formal education and adult occupation provide cognitive stimulation that is conducive to the development and maintenance of cognitive abilities (National Research Council, 2000). For example, using data from a population-based study of men aged 58 and 64 from eastern Finland, Turrell et al. (2002) found that education and income made independent contributions to cognitive function in late middle age after adjusting for childhood socioeconomic position. There is also substantial evidence that working at a complex job was positively associated with adulthood cognition, although early-life cognitive ability was not controlled for in those studies (Andel et al., 2007; Hauser and Roan, 2007; Schooler, 1987; Schooler et al., 1999). Besides, a growing body of research has shown that childhood SES also affects adulthood health. For example, disadvantaged childhood background significantly increases the risk of chronic diseases such as cardiovascular disease and stroke in later life, both of which can have negative effects on cognition in later life (de la Torre, 2012; Ferraro et al., 2016; O’Rand and Hamil-Luker, 2005).
So far, there is support for both perspectives in empirical research, and both processes are likely at work simultaneously. Adulthood SES was found to mediate a significant portion of the association between childhood conditions and cognitive function in the United States and European populations, but most studies found that there were still net effects of childhood SES on late-life cognition after controlling for adulthood conditions (Case and Paxson, 2008; González et al., 2013; Luo and Waite, 2005). What remains unclear is the complex pathways linking childhood SES and early-life cognitive ability to late-life cognition, which we will discuss next.
Early-Life and Young Adulthood Cognitive Ability, Adulthood SES, Health, and Late-Life Cognition
It has long been recognized that early-life cognitive ability is correlated with cognition in later life. Early-life cognitive ability is influenced by many factors including genetic factors, the uterine environment, the postnatal family environment, and experiences in school (Beck et al., 2018; Osler et al., 2013; Richards and Deary, 2014). One of the earlier studies in the United States about the association between early-life cognitive ability and late-life cognition showed that low idea density and low grammatical complexity in autobiographies written at a mean age of 22 were associated with low cognitive test scores about 58 years later among Catholic sisters who were members of the School Sisters of Notre Dame congregation (Snowdon et al., 1996). Researchers from Britain reported that childhood cognition at age 11 not only affected educational and social class attainment in adulthood (Johnson et al., 2010) but also influenced adult cognition, net of father’s occupation, own educational achievement, and occupation (Richards and Sacker, 2003). In a four-decade longitudinal study in the United States, researchers found that cognition at age 20 largely mediated the association between childhood SES and late midlife cognition among male twins who served in the military during the Vietnam era (1965–1975). Midlife SES (measured by education and occupation) at the mean age of 56 was not a major mediator, a finding that contradicts earlier studies that did not include data on early-life or young adulthood cognition (Beck et al., 2018).
Few studies have examined adulthood health as a potential mediator between early-life cognitive ability and late-lite cognition. However, several studies have shown that childhood cognitive ability was associated with adulthood health outcomes via the pathway of adulthood SES (Hemmingsson et al., 2006; Wraw et al., 2015). For example, using the National Longitudinal Survey of Youth 1979 cohort, researchers found that higher early-life cognitive ability, measured by the Armed Forces Qualification Test at the ages of 15–23, were associated with a lower risk of common mental disorders such as depression at age 50. Adjusting for adulthood SES accounted for most of this association (Wraw et al., 2016). On the other hand, midlife health problems (e.g., hypertension, cardiovascular disease, diabetes, and depression) have been increasingly recognized as risk factors for cognitive impairment and dementia in later life (Hughes & Ganguli, 2009; Vos et al., 2017). Therefore, midlife health may be another potential mediator between childhood conditions and late-life cognition.
Finally, Foverskov et al. (2017) showed that the effects of childhood and adult SES on midlife cognition tended to be exaggerated when childhood cognitive ability at age 12 was not controlled for in a cohort of Danish men. Similarly, research showed that cognitive ability at age 11 was the strongest predictor of cognition at age 70, followed by education and social class in Scotland (Johnson et al., 2010). While these findings are important, most of these studies focused on men only. To our knowledge, no population-based studies in the United States have incorporated early-life cognitive ability in the analysis of childhood conditions and late-life cognition among older men and women.
The present study aims to extend our understanding of the specific pathways underlying childhood SES and cognitive function in later life by testing the following hypotheses: 1) Childhood SES is associated with cognition in late midlife; 2) The association between childhood SES and cognition in late midlife is largely mediated by adolescent cognitive ability, educational attainment, midlife economic condition, and health.
Data and Methods
Data
The WLS is a longitudinal study of a random sample of one-third of the high school students who graduated from Wisconsin high schools in 1957. The baseline included 10,317 men and women, and most were born in 1939. Subsequent waves of data were collected from the original respondents or their parents in 1964, 1975, 1993, 2004, and 2011. WLS used multiple modes of survey over time, which included in-person, phone, and mail surveys. Cognition data came from the 2004 wave when the comprehensive cognitive tests were first administered over the phone, and most respondents were about 65 years old. The analytic sample was restricted to those respondents who participated in both telephone and mail surveys in 1993, and in phone interviews in 2004 (n=5,976). We further excluded 94 respondents who had missing data on all cognitive tests and 2 respondents who were 68 years old. Our final sample included 5,880 respondents. Compared to the respondents who were included in our analytic sample, those who were excluded had lower adolescent cognitive ability (results not shown but available upon request).
Measures
Cognition in late midlife
Cognitive function was a latent variable in our analysis, which is composed of scores from several cognitive tests. A battery of cognitive tests was used to measure the respondents’ cognitive abilities in 2004, including vocabulary similarities, letter and category fluency, immediate word recall, delayed word recall, and digit ordering. All respondents were asked either a six- or nine-item subset of the Wechsler Adult Intelligence Scale (WAIS-R) similarities items. For example, respondents were asked, “In what way are an orange and a banana alike?” and scores of 0, 1, or 2 were assigned based on WAIS-R protocol. The respondents received 2 points if they used abstract reasoning and 1 point for concrete reasoning. For letter fluency, the respondents were asked to think of as many words as they can beginning with either an “L” or an “F”, which were randomly selected. In the category fluency test, respondents were asked to name either as many foods or as many animals as possible in one minute. For immediate and delayed word recall, the interviewer read a list of ten high-frequency words and asked respondents to repeat back as many words as they could. The number of correct words respondents repeated ranged from 0 to 10. After about 12 minutes, respondents were asked to recall as many words as they can from the original list. As for digit ordering, the respondents were asked to say the numbers back in ascending order after the interviewer read a series of three-digit numbers. The interviewer added an additional digit following each correct response, up to eight digits. Scores ranged from 2 to 8. To reduce costs and interview lengths, four of the five tests of cognition were randomly administered to 80% of the entire sample. Based on previous studies (Yonker et al., 2007), we used a single, second-order factor for general cognitive function. Specifically, cognitive function was a second-order factor loaded by three first-order factors: memory, abstract reasoning, and verbal fluency. Memory was loaded by immediate word recall, delayed word recall, and digit ordering. Abstract reasoning was loaded by the nine WAIS-R items, and verbal fluency was loaded by letter and category fluency.
Childhood SES
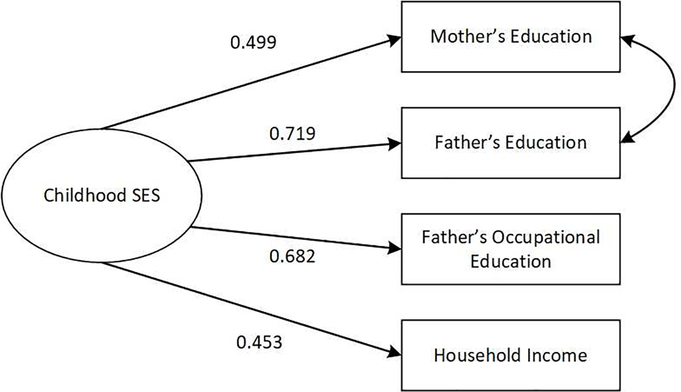
Childhood SES is a latent variable measured via four indicators: 1) father’s education was measured in years; 2) mother’s education was measured in years; 3) father’s occupational education was calculated by the percentage of persons in the 1970 Census in a given occupation who completed one or more years of college; and 4) childhood household income was measured by the family’s income in 1957, and the values were logged.
Adolescent cognitive ability
Adolescent cognition was mapped from raw Henmon-Nelson Mental Ability test scores in the Wisconsin State Testing Archive when respondents were in high school, most of whom in their junior year. The Henmon-Nelson test is a 30-minute, multiple-choice test designed to measure aspects of mental ability that are important for success in college. It was administered in all Wisconsin high schools from the 1930s to the 1960s (Hauser and Palloni, 2011).
Educational attainment
Education was measured as the total number of years of completed schooling.
Midlife economic condition
Midlife economic condition was measured by the respondents’ household income in the last 12 months when most respondents were aged 54 in 1993. To adjust for family size across households, we divided household income by the square root of the number of people in the household, and then logged the values (Glymour et al., 2008).
Midlife health
Midlife health was gauged by two questions asked in 1993 when they were about 54. The first question asked respondents how they would rate their health (on a scale from 1=very poor to 5=excellent). The second question asked how they would rate their health compared to other people their age and sex. The two items are highly correlated (α = 0.91). We took the mean of the two items to create a new variable that reflected respondents’ self-rated health around age 54.
Other socio-demographic covariates
We controlled for age in 2004 (in years), gender (female=1), marital status (divorced, widowed, and never married with “married” as the reference category), number of children (0 or 1, 4 or more, with 2 or 3 as the reference category).
Analytic strategy
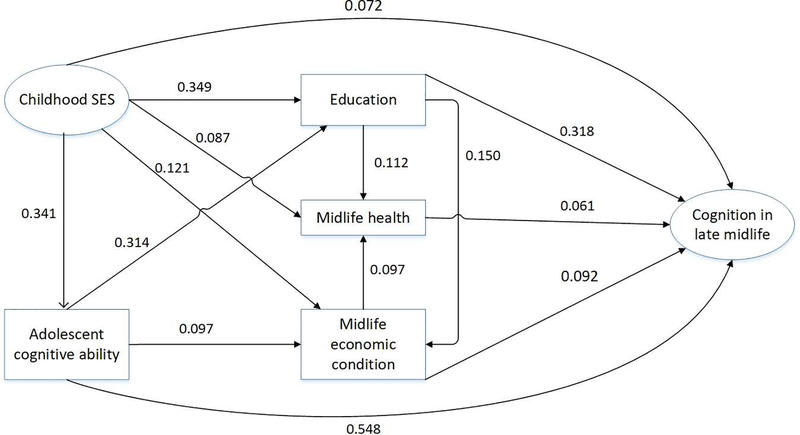
We applied the structural equation modeling (SEM) technique to analyze the pathways linking childhood conditions, adolescent cognitive ability, and cognition in late midlife. The SEM tests whether regression estimates fit into a theory-driven, pre-selected causal structure. As shown in Figure 1, childhood SES and cognition in late midlife were latent factors and enclosed in ellipses, and adolescent cognitive ability, educational attainment, midlife economic condition, and midlife health were observed variables enclosed in rectangles. To simplify the presentation, control variables and error terms were not shown in the figure. In the structural model, childhood SES was assumed to have a direct effect on cognition in late midlife and an indirect effect through adolescent cognitive ability, educational attainment, midlife economic condition, and midlife health. Specifically, both childhood SES and adolescent cognitive ability affected educational achievement and midlife economic condition in 1993, which in turn affected cognition in late midlife in 2004. Educational attainment also affected midlife economic condition. Another pathway linking childhood SES and cognition in late midlife was through midlife health: Childhood SES affected midlife health, which in turn affected cognition in late midlife. Adolescent cognitive ability also predicted cognition in late midlife. In preliminary analyses, we included bidirectional effects between midlife health and midlife economic condition, but the model did not converge. Although there are still debates on the nature of the causal relationship between adulthood SES and health, more recent research has shown that adulthood SES affected multiple health outcomes but not vice versa (Warren, 2009). Therefore our final model only included a path from midlife economic condition to midlife health but not vice versa. Besides, we have also tried to include a path from adolescent cognitive ability to midlife health, but the effect was not statistically significant and adding it did not improve the model fit, therefore we did not include that path in our final model.
Figure 1.
Standardized path coefficients from the structural equation model. Latent constructs are shown in ellipses, and observed variables are shown in rectangles. All coefficients are statistically significant at p < .01. Model fit statistics: RMSEA=0.026, CFI=0.945.
We used Mplus version 7.4 for the analysis (Muthén and Muthén, 2010). Overall, the variables had a modest amount of missing (<6%) except for a few cognitive measures including letter fluency and category fluency (missing by design to reduce interview length and control costs). Because some of the observed cognitive variables are categorical, we used the WLSMV estimator in Mplus which handles missing data using pairwise deletion (Asparouhov and Muthén, 2010). In additional sensitivity analyses (results not shown but available upon request), we also used multiple imputation based on Bayesian estimation and listwise deletion to handle missing data, and the findings are similar to those reported here. We also conducted multiple group analysis to examine potential gender differences in the pathways linking childhood SES and cognition in late midlife but found few significant gender differences (results not shown but available upon request). We used the root mean square error of approximation (RMSEA) and comparative fit index (CFI) to assess model fit. A model can be considered a plausible approximation of the data when the value of RMSEA is smaller than .05 and the value of CFI greater than .90 (Horvat et al., 2014; Hu and Bentler, 1999; Kline, 2005; Raykov and Marcoulides, 2006).
Results
Descriptive Results
Table 1 presents summary statistics of the analytic variables. The mean age of respondents was about 65 years old, and 54.3% were female. The majority were married (79.4%) with 9.3% divorced, 7.4% widowed and 3.9% never married. About 14% of the respondents had fewer than 2 children, 54.6% had 2 or 3 three children, 31.4% had 4 or more children. As for early-life conditions, fathers’ education level was around 10 years of schooling, and on average father worked in an occupation where 21% of employees had completed one or more years of college. Mother’s education was similar to that of the father. On average, respondents’ childhood family income was approximately $6,000 in 1957. The mean score of childhood cognitive ability was about 102.6, and respondents on average had completed almost 14 years of schooling. In 1993 when they were about 54 years old, their household income was about $71,600, and most of them reported very good health.
Table 1.
Sample Distribution of Variables, Wisconsin Longitudinal Study (WLS)
| Mean or % | SD | Range | |
|---|---|---|---|
| Childhood SES | |||
| Father’s education (years) | 9.8 | 3.5 | 0–26 |
| Father’s occupational education (%) | 21.1 | 22.5 | 0–99.3 |
| Mother’s education (years) | 10.5 | 2.8 | 0–20 |
| Household income (in thousands) | 6.0 | 3.3 | 0–15 |
| Adolescent cognitive ability | 102.6 | 14.5 | 61–145 |
| Education | 13.8 | 2.3 | 12–21 |
| Midlife economic condition in1993 (in thousands) | 71.6 | 54.9 | 0.03–300 |
| Midlife health in 1993 (1–5) | 4.2 | 0.7 | 1–5 |
| Number of Children in 1993 (%) | |||
| 0–1 | 14.0 | 0–1 | |
| 2–3 | 54.6 | 0–1 | |
| 4 or more | 31.4 | 0–1 | |
| Age in 2004 (years) | 64.8 | 0.7 | 63–67 |
| Female (%) | 54.3 | 0–1 | |
| Marital status in 2004 | |||
| Married | 79.4 | 0–1 | |
| Divorced/separated | 9.3 | 0–1 | |
| Widowed | 7.4 | 0–1 | |
| Never married | 3.9 | 0–1 |
Notes: N=5,880. SES=socioeconomic status. SD=standard deviation.
Results from Structural Equation Models
The standardized factor loadings for the latent variable of childhood SES and the latent variable of cognition are reported in Figure 2 and Figure 3 respectively. All the factor loadings are statistically significant at p <.001. Both measurement models have good fits (for childhood SES, RMSEA=0.042 and CFI=0.997; for cognitive function, RMSEA=0.021 and CFI=0.977). Both latent constructs have high levels of internal consistency (α=0.70 for childhood SES and α=0.73 for cognitive function).
Figure 2.
Standardized factor loadings for childhood SES, WLS. Latent constructs are shown in ellipses, and observed variables are shown in rectangles. The error between mother’s education and father’s education is correlated. All factor loadings are statistically significant at p < .001. Model fit statistics: RMSEA=0.042, CFI=0.997.
Figure 3.
Standardized factor loadings for cognition in late midlife, WLS, 2004. Latent constructs are shown in ellipses, and observed variables are shown in rectangles. The error between immediate and delayed recall is correlated. Model fit statistics: RMSEA=0.021 and CFI=0.977.
Table 2 shows the standardized path coefficients from the structural models, which were also graphically illustrated in Figure 1. Both Table 2 (the upper panel) and Figure 1 indicate that childhood SES had a direct effect on cognition in late midlife (β = 0.072, p <0.01). Furthermore, results in Table 2 (the middle panel) and Figure 1 show that multiple indirect pathways linked childhood SES and cognition in late midlife. First, childhood SES had a significant effect on adolescent cognitive ability (β =0.341, p < .001), which in turn had a significant effect on cognitive function in late midlife (β = 0.548, p < .001) — suggesting an indirect effect of childhood SES on late-life cognition through adolescent cognitive ability. Second, childhood SES had significant effects on one’s educational attainment (β = 0.349, p < .001) and midlife economic condition (β = 0.121, p < .001); and both educational attainment (β = 0.318, p < .001) and midlife economic condition (β = 0.092, p < .001) had significant effects on cognition in late midlife. These results suggest that childhood SES affected cognition in late midlife through both education attainment and midlife economic condition. In addition, adolescent cognitive ability also had a significant effect on education (β = 0.314, p < .001) and midlife economic condition (β = 0.097, p < .001). Midlife health was another pathway linking childhood SES and cognition in later life: Childhood SES had a significant effect on midlife health (β = 0.087, p < .001), which in turn affected cognition in later life (β = 0.061, p < .001).
Table 2.
Standardized Path Coefficients for the Effects of Childhood SES on Cognitive Function in Late Midlife, WLS, 2004
| β | SE | |
|---|---|---|
| Direct effects | ||
| Childhood SES → adolescent cognitive ability | 0.341*** | 0.014 |
| Childhood SES → education | 0.349*** | 0.013 |
| Childhood SES → midlife economic condition | 0.121*** | 0.015 |
| Childhood SES → cognition in late midlife | 0.072** | 0.023 |
| Childhood SES → midlife health | 0.087*** | 0.018 |
| Adolescent cognitive ability → education | 0.314*** | 0.012 |
| Adolescent cognitive ability → midlife economic condition | 0.097*** | 0.010 |
| Adolescent cognitive ability → cognition in late midlife | 0.548*** | 0.018 |
| Education → midlife economic condition | 0.150*** | 0.011 |
| Education → midlife health | 0.112*** | 0.016 |
| Education → cognition in late midlife | 0.318*** | 0.020 |
| Midlife economic condition → midlife Health | 0.097*** | 0.012 |
| Midlife economic condition → cognition in late midlife | 0.092*** | 0.017 |
| Midlife Health → cognition in late midlife | 0.061*** | 0.016 |
| Indirect effects | ||
| Childhood SES on cognition in late midlife | 0.362*** | 0.014 |
| Adolescent cognitive ability on cognition in late midlife | 0.116*** | 0.008 |
| Education on cognition in late midlife | 0.021*** | 0.003 |
| Midlife economic condition on cognition in late midlife | 0.006*** | 0.002 |
| Total effects | ||
| Childhood SES on cognition in late midlife | 0.434*** | 0.021 |
| Adolescent cognitive ability on cognition in late midlife | 0.664*** | 0.017 |
| Education on cognition in late midlife | 0.340*** | 0.020 |
| Midlife economic condition on cognition in late midlife | 0.098*** | 0.017 |
| Midlife health on cognition in late midlife | 0.061*** | 0.016 |
| Model fit statistics: χ2 (df)=1647.88(329), RMSEA=0.026 [0.025–0.027], CFI=0.945 | ||
Note: SE=standard error; RMSEA=the root mean square error of approximation; CFI=the comparative fit index. The model controls for age, gender, marital status, and the number of children.
p<0.05
p<0.01
p<0.001.
The middle panel of Table 2 further shows the total indirect effects of childhood SES, adolescent cognitive ability, education, and midlife economic condition on cognition in late midlife. The indirect effect of childhood SES (β = 0.362, p < .001) was statistically significant and much larger than its direct effect on cognition in midlife. Among the 14 indirect paths linking childhood SES and cognition in late midlife (results not shown in Table 2 and available upon request), the path through adolescent cognitive ability was the largest (β =0.187, p < .001), followed by the pathway through educational attainment (β = 0.111, p < .001). All other indirect paths were statistically significant but had relatively small effect sizes with βs ranging from 0.000 to 0.034. On the other hand, the direct effect of childhood cognitive ability on cognition in late midlife (β = 0.548, p < .001) was greater than its indirect effect (β = 0.116, p < .001). Similarly, the direct effect of education on cognition (β = 0.318, p < .001) was greater than its indirect effect (β = 0.021, p < .001). The bottom panel of Table 2 shows the total effects of various childhood and midlife characteristics on late-life cognition, suggesting that about 83% (0.362/0.434=83.4%) of the total effect of childhood SES on cognition in late midlife was mediated by adolescent cognitive ability, education, midlife economic condition, and midlife health, controlling for age, gender, marital status and the number of children.
Discussion and Conclusion
The primary goal of this study is to examine the associations between early-life characteristics and cognition in later life and the multiple pathways through which childhood conditions affect late-life cognition. Using data from the 1957–2004 WLS, we conducted structural equation modeling analysis to examine whether the effect of childhood SES on late-life cognition is mediated through adolescent cognitive ability, educational attainment, midlife economic condition, and midlife health.
Our findings have extended prior studies in several ways. First, we found that childhood SES had both direct and indirect effects on cognition in late midlife among WLS respondents. Therefore, both of our hypotheses were supported. Previous studies have provided mixed evidence on direct effects of childhood SES on adulthood cognition with most studies finding statistically significant direct effects (e.g., Kaplan et al., 2001; Luo and Waite, 2005; Turrell et al., 2002) while a few found little evidence of the direct effect (e.g., Beck et al., 2018; Singh-Manoux et al., 2005). Our results are consistent with the former and add evidence to this mixed literature. The reasons for the inconsistent results are not clear and can be partially explained by studies using different samples, different mediators, and different statistical methods. Nonetheless, all studies in the western countries have confirmed that most of the effects of childhood SES on cognition in later life are indirect (e.g., Beck et al., 2018; Luo and Waite, 2005).
Second, to our knowledge, we are among the first to find that adolescent cognitive ability is an important mediator between childhood SES and cognition in late midlife for both men and women based on population-based data from the United States. Previous research suggests that variations in brain function and cognitive ability are partially influenced by interactions between genetic variations and environmental experiences, and there are strong correlations between early-life cognitive ability and adulthood cognition when childhood SES, educational attainment, and adulthood SES are controlled for (Beck et al., 2018; Bouchard and McGue, 1981; Richards and Deary, 2014). Our results are consistent with this line of findings: We find that adolescent cognitive ability exerted strong direct effects on cognition in late midlife. Furthermore, it also exerted significant effects on educational attainment and midlife economic condition. Because most previous studies did not include early-life cognitive ability in their analyses of the association between childhood SES and late-life cognition, they may have overestimated the effects of other mediators including education, income, and occupation (Foverskov et al., 2017).
Third, our results show that even after taking adolescent cognitive ability into consideration, educational attainment and midlife economic condition still have significant effects on cognition in late midlife. These results suggest that childhood, as well as young adulthood and midlife, are all crucial periods for cognitive health in later life. Previous studies have consistently demonstrated that higher SES — indicated by higher education and higher socioeconomic position — is associated with better general health because it increases both a sense of personal control over one’s life and learned effectiveness in accessing health care and health information. Compared to individuals with lower SES, those with higher SES are more likely to exercise, abstain from tobacco use, maintain a healthy weight, and make good use of health care services that improve overall health, perhaps also including cognitive health (Link and Phelan, 1995; Mirowsky and Ross, 2003). Our study adds to the growing evidence on the importance of life-course SES in producing disparities in late-life cognitive function.
Finally, we found that midlife health was also a mediator between childhood SES and cognition in late life: Childhood SES had both a direct and an indirect effect on midlife health, which in turn affected cognition in late life. This finding is consistent with previous studies suggesting that childhood SES has a significant impact on adulthood health (Crimmins, 2005), which in turn affects cognition in later life (Zhang et al., 2018).
There are a few caveats to the conclusions in our study. First, our data included high-school graduates in Wisconsin, a relatively advantaged group of respondents, most of whom were whites. It is likely that children from extremely poor backgrounds were not included in our sample. Moreover, we looked at cognition around age 65, and a significant proportion of the original cohorts (43%) were not in our analytic sample due to nonresponse in the 1993 mail survey, panel attrition, and mortality. It is possible that we may have underestimated the effect of early-life factors on cognition in later life as adolescents from poor childhood conditions and with lower cognitive function were less likely to survive to old age than those from advantaged backgrounds (Hauser, 2010; Hauser and Palloni, 2011). In this sense, our estimates may be conservative. Second, the data is from Wisconsin and not nationally representative. Future research should replicate the study in a nationally representative sample and see whether the results can be generalized to other more disadvantaged individuals, including high-school dropouts and minority groups.
Third, although we used longitudinal data which limited reverse causality (i.e., we used ancestor variables from earlier waves to predict subsequent-wave descendent variables), our results cannot be used to draw causal conclusions because there are still unmeasured confounding variables (e.g., parental cognitive ability, parental health, genetic factors) that may influence both childhood SES and cognition in later life. In addition, we want to acknowledge that although our goal is to investigate causal processes linking childhood SES and cognition in late midlife, we cannot make strong causal claims due to limitations in our data and method. Specifically, SEM is an inference engine that uses 2 inputs: qualitative causal assumptions and empirical data; and the quantitative causal claims produced in SEM are conditional on the input qualitative assumptions, which are often based on prior studies, logical arguments, and temporal order (Bollen and Pearl, 2013; Glymour and Cooper, 1999; Rubin, 2004). In this paper, we followed previous studies and made some causal assumptions (as depicted in Figure 1). We also adopted widely used model-fit indices (e.g., the comparative fit index, the root mean squared error of approximation) to evaluate consistency between the causal assumptions of our SEM model and our data. We found that the results supported the causal assumptions made in our SEM model.
Four, our measure of childhood SES has limitations because it did not take into consideration other important factors such as household wealth. Thus, we may have underestimated the effect of childhood SES on cognition in later life due to our imperfect measure. Our measure of midlife economic condition has similar limitations, and as a result, we may have underestimated the mediating role of midlife economic condition and overestimated the direct effect of childhood SES on cognition in later life. Finally, in our study we only examined the association between childhood SES and a global measure of cognition; future research is needed to examine whether childhood SES affects multiple domains of cognition. A few studies suggested that childhood SES was more strongly associated with language and executive functioning than with visual and spatial cognition among young children (Noble et al., 2005).
Finally, because we only studied cognitive function at age 65 instead of cognitive decline over time (a crucial factor in the diagnosis of dementia), we cannot directly assess the extent to which childhood conditions affect dementia risk in later life. However, previous research has found that a low level of cognitive function in adulthood was a strong predictor of dementia and Alzheimer’s disease in late life (Kuller et al., 2003). For example, one study found that older white respondents with low cognitive test scores at baseline had 2.7 times the risk of dementia as those with high scores in the follow-up (Shadlen et al., 2006).
Despite these limitations, this is one of the first cohort studies in the U.S. to examine the complex mechanisms linking childhood SES, adolescent cognitive ability, and cognition in later life. We advanced this line of research by using high-quality SES collected across the life course, considering adolescent cognitive ability, and applying structural equation models to formally test multiple pathways linking childhood conditions and cognition in late midlife. From a policy perspective, our study suggests that interventions should focus on the entire life course, the formative years in particular. More government and societal attention and resources should be devoted to improving the schools and communities that our kids and young adults live, learn and work, as these investments can make a long-term positive effect on their cognitive health in later life, and ultimately on their overall health and longevity. At the same time, our study also suggests that adulthood economic condition also affects cognition in late midlife. Socioeconomic policies and programs that alleviate adults’ economic stress and improve their financial well-being will also promote cognitive health in their later years.
Research highlights.
Applies SEM technique to examine pathways linking childhood SES and cognition in late midlife.
Adolescent cognitive ability mediates the association between childhood SES and cognition.
Education, midlife economic condition, and midlife health are also important mediators.
Acknowledgments
This work was supported in part by a WLS pilot grant from the Center for Demography of Health and Aging at the University of Wisconsin-Madison. This research uses data from the Wisconsin Longitudinal Study, funded by the National Institute on Aging (R01 AG009775; R01 AG033285).
Footnotes
Declarations of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Zhenmei Zhang, Department of Sociology, Michigan State University.
Hui Liu, Department of Sociology, Michigan State University.
Seung-won Choi, Department of Sociology, Anthropology, and Social Work, Texas Tech University
Reference
- Abbott RD, White LR, Ross GW, Petrovitch H, Masaki KH, Snowdon DA, Curb JD, 1998. Height as a marker of childhood development and late–life cognitive function: the Honolulu–Asia aging study. Pediatrics 102, 602–609. [DOI] [PubMed] [Google Scholar]
- Andel R, Kåreholt I, Parker MG, Thorslund M, Gatz M, 2007. Complexity of primary lifetime occupation and cognition in advanced old age. J. Aging Health 19, 397–415. [DOI] [PubMed] [Google Scholar]
- Asparouhov T, Muthén B, 2010. Weighted least squares estimation with missing data. Mplus Technical Appendix, 1–10. [Google Scholar]
- Beck A, Franz CE, Xian H, Vuoksimaa E, Tu X, Reynolds CA,… Kremen WS, 2018. Mediators of the effect of childhood socioeconomic status on late midlife cognitive abilities: a four decade longitudinal study. Innov. Aging 2 (1), igy003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen KA, Pearl J, 2013. Eight myths about causality and structural equation models, in: Morgan SL (Ed.), Handbook of causal analysis for social research. Springer, Dordrecht, pp. 301–328. [Google Scholar]
- Bouchard TJ, McGue M, 1981. Familial studies of intelligence: a review. Science 212, 1055–1059. [DOI] [PubMed] [Google Scholar]
- Brito NH, Noble KG, 2014. Socioeconomic status and structural brain development. Front. Neurosci. 8, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case A, Paxson C, 2008. Height, health, and cognitive function at older ages. Ame. Econ. Rev. 98, 463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins EM, 2005. Childhood conditions and late-life health: introduction. Soc Biol, 52(3–4), 89–93. [PubMed] [Google Scholar]
- de la Torre JC, 2012. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol 1–15. doi: 10.1155/2012/367516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro KF, Schafer MH, Wilkinson LR, 2016. Childhood disadvantage and health problems in middle and later life: Early imprints on physical health? Am. Sociol. Rev 81(1), 107–133. doi: 10.1177/0003122415619617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foverskov E, Mortensen EL, Holm A, Pedersen JLM, Osler M, Lund R, 2019. Socioeconomic position across the life course and cognitive ability later in life: the importance of considering early cognitive ability. J. Aging Health 31(6), 947–966. [DOI] [PubMed] [Google Scholar]
- Fritsch T, McClendon MJ, Smyth KA, Lerner AJ, Friedland RP, Larsen JD, 2007. Cognitive functioning in healthy aging: the role of reserve and lifestyle factors early in life. Gerontologist. 47, 307–322. [DOI] [PubMed] [Google Scholar]
- Glymour C, Cooper G, 1999. Computation, Causation, and Discovery. AAAI/MIT Press, Menlo Park, CAMenlo Park. [Google Scholar]
- Glymour MM, Tzourio C, Dufouil C, 2012. Is cognitive aging predicted by one’s own or one’s parents’ educational level? results from the three-city study. Am. J. Epidemiol. 175, 750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González HM, Tarraf W, Bowen ME, Johnson-jennings MD, Fisher GG, 2013. What do parents have to do with my cognitive reserve? life course perspectives on twelve-year cognitive decline. Neuroepidemiology 41, 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan MN, Wallace R, 2004. Can dementia be prevented? Brain aging in a population-based context. Annu. Rev. Public Health 25, 1–24. [DOI] [PubMed] [Google Scholar]
- Hall KS, Gao S, Unverzagt FW, Hendrie HC, 2000. Low education and childhood rural residence risk for Alzheimer’s disease in African Americans. Neurology 54, 95–95. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Chandra A, Wolfe BL, Pollak SD, 2011. Association between income and the hippocampus. PLoS One 6, e18712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayat SA, Luben R, Dalzell N, Moore S, Hogervorst E, Matthews FE,… Khaw KT, 2018. Understanding the relationship between cognition and death: a within cohort examination of cognitive measures and mortality. Eur. J. Epidemiol 33(11), 1049–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser RM, Palloni A, 2011. Adolescent IQ and survival in the Wisconsin longitudinal study. J. Gerontol.: Soc. Sci 66 (Suppl. 1), i91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser RM, Roan CL, 2007. Work complexity and cognitive functioning at midlife: cross-validating the Kohn-Schooler hypothesis in an American cohort. Center for Demography and Ecology, University of Wisconsin–Madison, Working Paper No. 2007–08, 1–34. [Google Scholar]
- Hauser RM, 2010. Causes and consequences of cognitive functioning across the life course. Educ. Res 39, 95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmingsson T, Melin B, Allebeck P, Lundberg I, 2006. The association between cognitive ability measured at ages 18–20 and mortality during 30 years of follow-up--a prospective observational study among Swedish males born 1949–51. Int. J. Epidemiol 35(3), 665–670. [DOI] [PubMed] [Google Scholar]
- Hertzman C, 1999. The biological embedding of early experience and its effects on health in adulthood. Ann. N. Y. Acad. Sci 896, 85–95. [DOI] [PubMed] [Google Scholar]
- Horvat P, Richards M, Malyutina S, Pajak A, Kubinova R, Tamosiunas A, … Bobak M, 2014. Life course socioeconomic position and mid-late life cognitive function in Eastern Europe. J. Gerontol.: Soc. Sci 69, 470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L.t., Bentler PM 1999. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Modeling 6, 1–55. [Google Scholar]
- Hughes TF, & Ganguli M (2009). Modifiable midlife risk factors for late-life cognitive impairment and dementia. Curr. Psychiatry Rev, 5(2), 73–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine, 2015. Cognitive Aging: Progress in Understanding and Opportunities for Action. The National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- Johnson W, Brett CE, Deary IJ 2010. The pivotal role of education in the association between ability and social class attainment: a look across three generations. Intelligence 38, 55–65. [Google Scholar]
- Kaplan GA, Turrell G, Lynch JW, Everson SA, Helkala E-L, Salonen JT, 2001. Childhood socioeconomic position and cognitive function in adulthood. Int. J. Epidemiol. 30, 256–263. [DOI] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Shin IS, Yoon JS, 2003. Limb length and dementia in an older Korean population. J. Neurol. Neurosurg. Psychiatry 74, 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB, 2005. Principles and practice of structural equation modeling. Guilford Press, New York, NY. [Google Scholar]
- Kuller LH, Lopez OL, Newman A, Beauchamp NJ, Burke G, Dulberg C,… Haan MN, 2003. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology 22(1), 13–22. [DOI] [PubMed] [Google Scholar]
- Langa KM, Chernew ME, Kabeto MU, Regula Herzog A, Ofstedal MB, Willis RJ, … Fendrick AM, 2001. National estimates of the quantity and cost of informal caregiving for the elderly with dementia. J. Gen. Intern. Med 16, 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link BG, Phelan J, 1995. Social conditions as fundamental causes of disease. J. Health Soc. Behav. (Extra issue), 80–94. [PubMed] [Google Scholar]
- Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, Nishino T, Barch D, 2013. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr. 167, 1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Waite LJ, 2005. The impact of childhood and adult SES on physical, mental, and cognitive well-being in later life. J. Gerontol.: Soc. Sci 60, S93–S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu J, Burr JA, 2016. Socioeconomic status across the life course and cognitive function among older adults: an examination of the latency, pathways, and accumulation hypotheses. J. Aging Health 28, 40–67. [DOI] [PubMed] [Google Scholar]
- Melrose RJ, Brewster P, Marquine MJ, MacKay–Brandt A, Reed B, Farias ST, Mungas D, 2015. Early life development in a multiethnic sample and the relation to late life cognition. J. Gerontol.: Soc. Sci 70, 519–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirowsky J, Ross CE, 2003. Education, Social Status, and Health. Aldine de Gruyter, New York, NY. [Google Scholar]
- Moceri VM, Kukull WA, Emanuel I, van Belle G, Larson EB, 2000. Early-life risk factors and the development of Alzheimer’s disease. Neurology 54, 415–415. [DOI] [PubMed] [Google Scholar]
- Muthén L, Muthén B, 2010. Version 6 Mplus User’s Guide. Muthén & Muthén, Los Angeles, CA. [Google Scholar]
- National Research Council, 2000. The Aging Mind: Opportunities in Cognitive Research. National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- Noble KG, Norman MF, Farah MJ, 2005. Neurocognitive correlates of socioeconomic status in kindergarten children. Dev. Sci 8(1), 74–87. [DOI] [PubMed] [Google Scholar]
- Obisesan TO, Gillum R, 2009. Cognitive function, social integration and mortality in a U.S. national cohort study of older adults. BMC Geriatr. 9, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rand AM, Hamil-Luker J, 2005. Processes of cumulative adversity: childhood disadvantage and increased risk of heart attack across the life course. J. Gerontol.: Soc. Sci 60(S2), 117–124. [DOI] [PubMed] [Google Scholar]
- Osler M, Avlund K, Mortensen EL, 2013. Socio-economic position early in life, cognitive development and cognitive change from young adulthood to middle age. Eur. J. Public Health 23, 974–980. [DOI] [PubMed] [Google Scholar]
- Plomin R, Deary IJ, 2015. Genetics and intelligence differences: five special findings. Mol. Psychiatry 20, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raykov T, Marcoulides GA, 2006. On multilevel model reliability estimation from the perspective of structural equation modeling. Struct. Equ. Modeling 13, 130–141. [Google Scholar]
- Richards M, Deary IJ, 2005. A life course approach to cognitive reserve: a model for cognitive aging and development? Ann. Neurol 58, 617–622. [DOI] [PubMed] [Google Scholar]
- Richards M, Deary IJ, 2014. A life course approach to cognitive capability In: Kuh D, Cooper R, Hardy R, Richards M, Ben-Shlomo Y (Eds.), A Life Course Approach to Healthy Ageing. Oxford University Press, Oxford, pp. 32–45. [Google Scholar]
- Richards M, Sacker A, 2003. Lifetime antecedents of cognitive reserve. J. Clin. Exp. Neuropsychol. 25, 614–624. [DOI] [PubMed] [Google Scholar]
- Richards M, Wadsworth MEJ, 2004. Long term effects of early adversity on cognitive function. Arch. Dis. Child 89, 922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB, 2004. Direct and indirect causal effects via potential outcomes. Scand. J. Stat. 31(2), 161–170. [Google Scholar]
- Schooler C, 1987. Cognitive effects of complex environments during the life span: a review and theory In: Schooler C, Schaie KW (Eds.), Cognitive Functioning and Social Structure Over the Life Course. Ablex Pub, Norwood, NJ, pp. 24–49. [Google Scholar]
- Schooler C, Mulatu MS, Oates G, 1999. The continuing effects of substantively complex work on the intellectual functioning of older workers. Psychol. Aging 14, 483–506. [DOI] [PubMed] [Google Scholar]
- Shadlen MF, Siscovick D, Fitzpatrick AL, Dulberg C, Kuller LH, Jackson S, 2006. Education, cognitive test scores, and black-white differences in dementia risk. J. Am. Geriatr. Soc 54(6), 898–905. [DOI] [PubMed] [Google Scholar]
- Singh–Manoux A, Richards M, Marmot M, 2005. Socioeconomic position across the lifecourse: How does it relate to cognitive function in mid–life? Ann. Epidemiol 15, 572–578. [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, Markesbery WR, 1996. Linguistic ability in early life and cognitive function and Alzheimer’s disease in late life: findings from the nun study. JAMA 275, 528–532. [PubMed] [Google Scholar]
- Vos SJB, van Boxtel MPJ, Schiepers OJG, Deckers K, de Vugt M, Carriere I,… Kohler S 2017. Modifiable risk factors for prevention of dementia in midlife, late life and the oldest-old: validation of the LIBRA Index. J. Alzheimers Dis 58(2), 537–547. [DOI] [PubMed] [Google Scholar]
- Warren JR, 2009. Socioeconomic status and health across the life course: A test of the social causation and health selection hypotheses. Soc Forces 87(4), 2125–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraw C, Deary IJ, Gale CR, Der G, 2015. Intelligence in youth and health at age 50. Intelligence, 53, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraw C, Deary IJ, Der G, Gale CR, 2016. Intelligence in youth and mental health at age 50. Intelligence, 58, 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Zhang Z, Li L, Liu J, 2018. Early life exposure to China’s 1959–61 famine and midlife cognition. Int. J. Epidemiol 47 (1), 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonker JA, Hauser RM, Freese J, 2007. The dimensionality and measurement of cognitive functioning at age 65 in the Wisconsin Longitudinal Study: Preliminary findings. Center for Demography and Ecology, University of Wisconsin–Madison; Working Paper No. 2007–06. [Google Scholar]
- Zhang Z, Gu D, Hayward MD, 2010. Childhood nutritional deprivation and cognitive impairment among older Chinese people. Soc. Sci. Med 71 (5), 941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Liu J, Li L, Xu H, 2018. The long arm of childhood in china: Early-life conditions and cognitive function among middle-aged and older adults. J. Aging Health 30, 1319–1344. [DOI] [PubMed] [Google Scholar]