Summary
GABAA and glycine receptors are thought to compete for gephyrin-binding sites at mixed inhibitory synapses. Changes in the occupancy of one receptor type are therefore expected to have opposite effects on the clustering of the other receptors. This does not explain, however, whether different receptors can be regulated independently from one another. Here we show that cAMP-dependent signaling reduces gephyrin phosphorylation at residue S270 in spinal cord neurons. Although no ultrastructural changes of the synaptic scaffold were detected using super-resolution imaging, gephyrin de-phosphorylation was associated with a selective increase in GABAAR diffusion and the loss of the receptors from synapses. As opposed to the PKA-dependent dispersal of α3-containing GlyRs, the regulation of gephyrin phosphorylation and GABAAR dynamics acts via non-canonical EPAC signaling. Subtype-specific changes in receptor mobility can thus differentially contribute to changes in inhibitory synaptic strength, such as the disinhibition of spinal cord neurons during inflammatory processes.
Subject Areas: Molecular Neuroscience, Cellular Neuroscience, Sensory Neuroscience
Graphical Abstract
Highlights
-
•
Differential signaling downstream of cAMP regulates mixed inhibitory synapses
-
•
EPAC reduces gephyrin S270 phosphorylation but not the copy numbers at synapses
-
•
Gephyrin de-phosphorylation reduces GABAAR binding and increases receptor diffusion
-
•
PKA phosphorylation of GlyRα3L at residue S346 mobilizes synaptic receptors
Molecular Neuroscience; Cellular Neuroscience; Sensory Neuroscience
Introduction
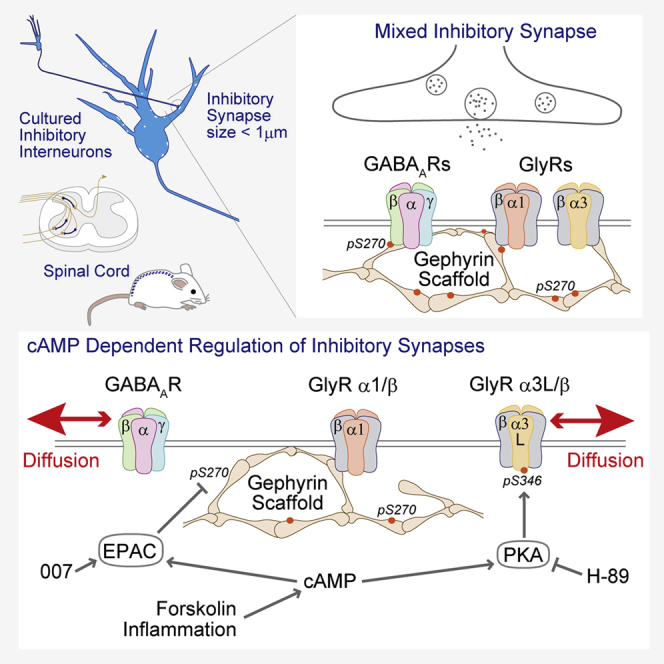
Inhibitory neurotransmission in the spinal cord is shaped by the balance between GABAA and glycine receptors that co-localize at the majority of synapses (Dumoulin et al., 2000, Geiman et al., 2002, Todd et al., 1996) and that are activated by the co-release of the two neurotransmitters from presynaptic vesicles (Aubrey and Supplisson, 2018, Jonas et al., 1998, Shrivastava et al., 2011). GABAARs and GlyRs bind to the same region of the synaptic scaffold protein gephyrin (Kim et al., 2006, Kowalczyk et al., 2013, Maric et al., 2011, Tretter et al., 2011), which is thought to create strong competition between the receptors for synaptic binding sites. As a consequence, the main parameters controlling receptor trapping at mixed inhibitory synapses are the number of receptors, the number of available binding sites, and the relative affinity of the receptors for these sites (discussed in Specht, 2019). Plastic changes in the strength of receptor-gephyrin interactions are expected to shift the equilibrium between GABAARs and GlyRs and alter the functional profile of mixed inhibitory synapses.
Post-translational modifications of gephyrin regulate the clustering of the scaffold protein and hence the number of receptor binding sites at synapses (Groeneweg et al., 2018). Phosphorylation of amino acid residue S270 of gephyrin appears to be particularly important for the clustering of gephyrin at GABAergic synapses, causing a variety of changes in the number, size, and intensity of synaptic clusters (Battaglia et al., 2018, Ghosh et al., 2016, Kalbouneh et al., 2014, Kuhse et al., 2012, Tyagarajan et al., 2011, Tyagarajan et al., 2013). The synaptic copy numbers of gephyrin and receptor complexes often change in synchrony owing to their reciprocal stabilization at synapses; however, it is unclear whether S270 phosphorylation acts on gephyrin oligomerization or on receptor-gephyrin binding (Specht, 2019). From a mechanistic point of view, gephyrin is known to assume various conformational states (Sander et al., 2013) that could mediate the downstream effects of S270 phosphorylation.
The intracellular domains (ICDs) of GABAARs and GlyRs are subject to post-translational modifications that target the receptors' gephyrin-binding motifs (Mukherjee et al., 2011, Specht et al., 2011). It was recently shown that receptor-gephyrin interactions are also modulated by conformational changes associated with receptor activity (Gouzer et al., 2014, Patrizio et al., 2017). These studies suggest that in addition to the primary interaction domains, the strength of receptor-gephyrin binding is controlled by mechanisms that are related to the channel function. To test this idea, we set out to investigate whether the cyclic AMP (cAMP)-dependent reduction of glycinergic transmission during inflammation (Harvey et al., 2004) is accompanied by changes in receptor mobility. We found that phosphorylation of GlyRα3 at amino acid residue S346 by protein kinase A (PKA) indeed disrupted receptor trapping at spinal cord synapses. Unexpectedly, we also identified a non-canonical cAMP signaling pathway that controls the phosphorylation status of gephyrin without affecting the size of gephyrin clusters and that leads to a specific reduction in GABAAR clustering. These observations lend support to the concept that diverging signaling processes downstream of cAMP differentially regulate GABAAR and GlyR numbers at mixed inhibitory synapses.
Results
Reduction of GABAAR Levels at Spinal Cord Synapses by Forskolin
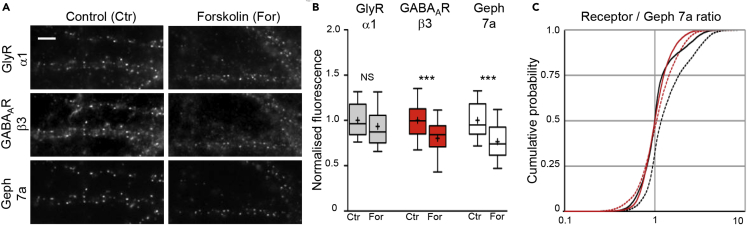
To investigate the regulation of inhibitory synapses by cAMP-dependent mechanisms, we carried out immunocytochemical analysis of endogenous GlyRs, GABAARs, and the scaffold protein gephyrin in two-week-old dissociated rat spinal cord neurons. cAMP-dependent signaling was induced with 20 μM forskolin for 30 min, followed by fixation of the neurons and triple labeling with specific antibodies against GlyRα1, GABAARβ3, and gephyrin (Figure 1A). Quantification of the integrated fluorescence intensity of synaptic puncta did not reveal any significant differences of GlyR levels between control cultures treated with vehicle (0.2% ethanol, see Methods for details) and neurons exposed to forskolin (Figure 1B). We did, however, observe a strong reduction of both GABAAR and gephyrin immunoreactivity in response to forskolin treatment. The effect of forskolin was similar in magnitude for GABAARβ3 and gephyrin (p > 0.05, ANOVA), with a mean reduction to 80.2% ± 2.8% and 76.6% ± 3.0% (mean ± SEM, not background corrected) of the control value, respectively, suggesting that the two effects could be linked (Figure 1B).
Figure 1.
Forskolin Reduces GABAAR and Gephyrin Immunoreactivity at Spinal Cord Synapses
(A) Triple immunolabeling of endogenous GlyRs, GABAARs, and gephyrin in cultured rat spinal cord neurons (DIV16). Cells were treated with 20 μM forskolin (For) or kept under control conditions for 30 min (Ctr; vehicle 0.2% ethanol), fixed, and stained with antibodies against the GlyRα1 and GABAARβ3 subunits, and with the rat gephyrin antibody mAb7a (Geph 7a, Synaptic Systems). Scale bar, 5 μm.
(B) Quantification of the integrated fluorescence intensity at synaptic GlyR puncta in the three channels (cell-by-cell analysis, arbitrary units normalized to the control condition in each channel; data are represented as 10%, 25%, 50% (median), 75%, and 90% percentiles; the mean is indicated as a cross; nCtr = 86, nFor = 79 cells from 6 coverslips and 3 independent experiments; ***p < 0.001, ANOVA).
(C) Cumulative probability of the ratio of the GlyR (black traces) or GABAAR fluorescence intensities (red traces) relative to the Geph 7a immunoreactivity at synapses (control: solid lines, nCtr = 1.4 × 104; forskolin: dotted lines, nFor = 1.2 × 104 synapses).
Synapse-by-synapse analysis of the relative changes in receptor and gephyrin immunoreactivity confirmed that forskolin treatment had little effect on the GABAARβ3/gephyrin ratio (Figure 1C, red traces). However, the apparent increase in the GlyRα1/gephyrin ratio in the presence of forskolin (black dotted line) was difficult to interpret, given that gephyrin levels directly control the number of receptor-binding sites (Specht et al., 2013). It appeared doubtful to us how GlyR levels could be maintained in the face of a 25% loss in the number of binding sites. One explanation could be that the internalized fraction of GlyRs is increased (Breitinger et al., 2018), without changing the total fluorescence intensity at synapses. We therefore carried out live surface labeling of GlyRα1 (Figure S1A). However, we did not observe any significant difference between the surface GlyRα1 levels in control conditions and after 30 min of 20 μM forskolin application (Figure S1B).
Forskolin Alters mAb7a Immunoreactivity but Not the Total Gephyrin at Synapses
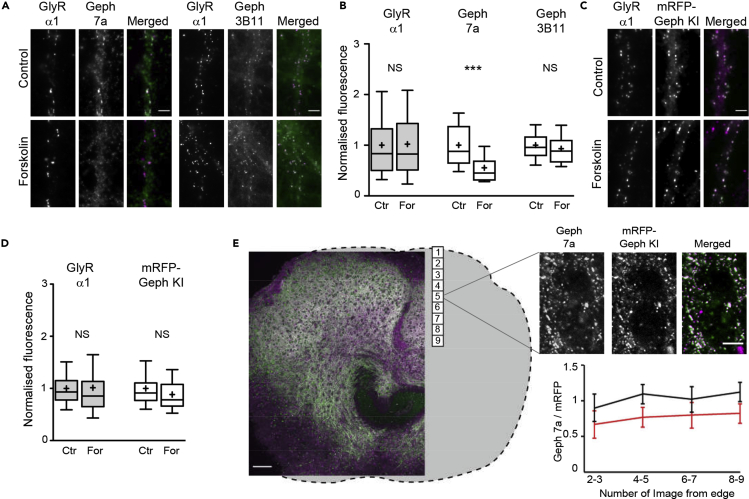
The monoclonal gephyrin antibody mAb7a that was used in the above experiments is widely employed to quantify gephyrin levels at inhibitory synapses, despite the fact that it recognizes an epitope in the central domain of gephyrin that includes the phosphorylated serine residue S270 (Kuhse et al., 2012). To verify the observed reduction of gephyrin labeling after forskolin treatment we performed experiments with a different monoclonal antibody that binds to the gephyrin E-domain (3B11; Smolinsky et al., 2008). To our surprise, gephyrin immunoreactivity with the antibody 3B11 was not different in control and forskolin-treated neurons (p > 0.05, ANOVA, Figures 2A and 2B), whereas mAb7a labeling was strongly reduced (55.3% ± 4.2% of control, mean ± SEM, p < 0.001). Note that the data were corrected for the background, which explains the stronger reduction than in the previous experiment (Figure 1B, see methods for data analysis). Endogenous GlyRα1 labeling was not significantly different between the two conditions (p > 0.05), in agreement with our earlier data.
Figure 2.
Gephyrin Protein Levels at Synapses Are Not Altered by Forskolin
(A) Sample images showing the effect of forskolin treatment on GlyRα1 (magenta in the merged image) and two different gephyrin antibodies (mAb7a and 3B11, green). Fluorescent signals from Geph 7a were reduced by forskolin, whereas GlyRα1 and Geph 3B11 were not affected. Scale bar, 5 μm.
(B) Integrated fluorescence intensity of endogenous gephyrin at rat spinal cord synapses was quantified in GlyRα1-positive puncta (background-corrected data). Geph 7a labeling was decreased by 45% ± 4% after 30-min treatment with 20 μM forskolin (For) compared with the control (***p < 0.001, ANOVA), whereas no significant changes (NS) were observed with Geph 3B11 (GlyRα1: nCtr = 149, nFor = 144 cells, 5 experiments; Geph 7a: nCtr = 60, nFor = 56, 2 experiments; Geph 3B11: nCtr = 89, nFor = 88 cells, 3 experiments). Data are represented as 10%, 25%, 50%, 75%, and 90% percentiles; the mean is indicated as a cross.
(C) Dissociated spinal cord cultures prepared from an mRFP-gephyrin knock-in mouse strain (mRFP-Geph KI, magenta) were treated with or without forskolin and then immunolabeled for GlyRα1 (green). Scale bar, 5 μm.
(D) No significant change in mRFP-Geph KI fluorescence and GlyRα1 immunoreactivity was observed (nCtr = 39; nFor = 40 cells, 1 experiment).
(E) Organotypic spinal cord cultures made from mRFP-gephyrin KI mice were treated at DIV21 with 20 μM forskolin for 60 min and fixed, labeled, and analyzed by confocal microscopy. Geph 7a immunoreactivity (green in merged image) and mRFP fluorescence (magenta) were quantified in a consecutive series of nine single-plane images taken from the dorsal edge of the slice toward the center (nCtr = 44, nFor = 57 images from 7–8 slices and 2 independent experiments; ***p < 0.0001 for the pooled data points for each condition, t test; data are shown as mean ± SD). The sample images represent a non-treated control slice; the gray outline illustrates the shape of the complete organotypic slice. Scale bar, 100 μm; zoomed images, 5 μm.
These observations raised our suspicion that the reduction in gephyrin labeling could be due to the mAb7a antibody and did not reflect any real change in the total gephyrin amount at synapses. We tested this hypothesis in spinal cord cultures derived from a knock-in mouse strain that expresses endogenous mRFP-tagged gephryin (Machado et al., 2011). Neither GlyRα1 nor the total mRFP-gephyrin levels at synapses were altered by bath application of forskolin (Figures 2C and 2D; pGlyRα1 > 0.05, pmRFP-Geph > 0.05, ANOVA), confirming that the treatment did not have a noticeable effect on the gephyrin molecule numbers at synapses.
To directly compare the effects of forskolin on mAb7a immunoreactivity and total mRFP-gephyrin levels in a more integrated system, we also conducted experiments in organotypic slice cultures from knock-in animals. Three-week-old spinal cord slice cultures were treated with 20 μM forskolin for 60 min, fixed, immunolabeled, and analyzed with confocal microscopy (Figure 2E). The mAb7a and mRFP-gephyrin fluorescence at synapses was quantified in single image planes that were taken from the dorsal edge of the slice toward the central region. We observed that the ratio of mAb7a/mRFP-gephyrin was reduced after forskolin treatment across all regions, demonstrating that mAb7a immunoreactivity is systematically reduced in different neuronal populations, and is not representative of the total synaptic gephyrin levels in our experimental paradigm.
Forskolin Reduces Gephyrin Phosphorylation of Residue S270
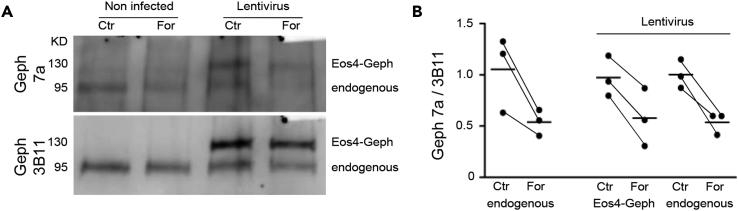
On the molecular level, the reduction in mAb7a immunoreactivity could be due either to the de-phosphorylation of gephyrin at residue S270 and/or to conformational changes induced by forskolin that render the epitope inaccessible for the antibody. To distinguish between these possibilities, we analyzed the gephyrin phosphorylation status by Western blotting (Figure 3). Dissociated rat spinal cord cultures were kept in medium containing 20 μM forskolin for 30 min, total protein fractions were collected in the presence of phosphatase inhibitors, and samples were separated by SDS-PAGE. Western blot membranes were sequentially probed with mAb7a antibody and with 3B11 that served as a loading control for total gephyrin (Figure 3A, first two lanes). Quantification of the mAb7a/3B11 ratio showed that forskolin treatment reduces mAb7a immunoreactivity by about 50% compared with the control condition (Figure 3B). It should be noted, however, that the mAb7a antibody is not well suited for Western blotting and produces only weak chemiluminescence signals.
Figure 3.
Forskolin Reduces Gephyrin Phosphorylation of Residue S270
(A) Western blotting of total protein fractions of rat spinal cord cultures exposed to forskolin (For) or kept in control condition (Ctr). Sequential labeling of the membranes with the gephyrin antibodies mAb7a and 3B11 identified the endogenous protein (95 kDa) and lentivirus-expressed mEos4b-gephyrin (130 kDa).
(B) For each Western blot membrane, we measured the intensity ratio of mAb7a/3B11 for all corresponding bands (endogenous gephyrin and recombinant mEos4b-gephyrin). Forskolin treatment reduced S270 phosphorylation of gephyrin by 45.8% as judged by the lower mAb7a/3B11 ratio (n = 9 bands per condition from 3 independent experiments, p < 0.001, paired t test).
To confirm the identity of the detected 95-kDa bands, we repeated the same experiment using cultures that had been infected with lentivirus driving the expression of mEos4b-gephyrin. The detection of an additional band at 130 kDa in these samples demonstrates the specificity of the mAb7a antibody (Figure 3A, lanes 3 and 4). What is more, the signals of both bands were weaker in the forskolin-treated sample, showing that the phosphorylation of both endogenous gephyrin and recombinant mEos4b-gephyrin are reduced by forskolin (Figure 3B). Across both pairs of samples and three independent experiments, the reduction in mAb7a/3B11 ratio was highly significant (mean reduction 45.8%, p < 0.001, paired t test, n = 9 bands per condition). These data indicate that forskolin treatment reduces S270 phosphorylation, although they do not exclude the fact that gephyrin could undergo concomitant conformational changes (Sander et al., 2013).
PKA-Independent Effect of Forskolin on Gephyrin Phosphorylation
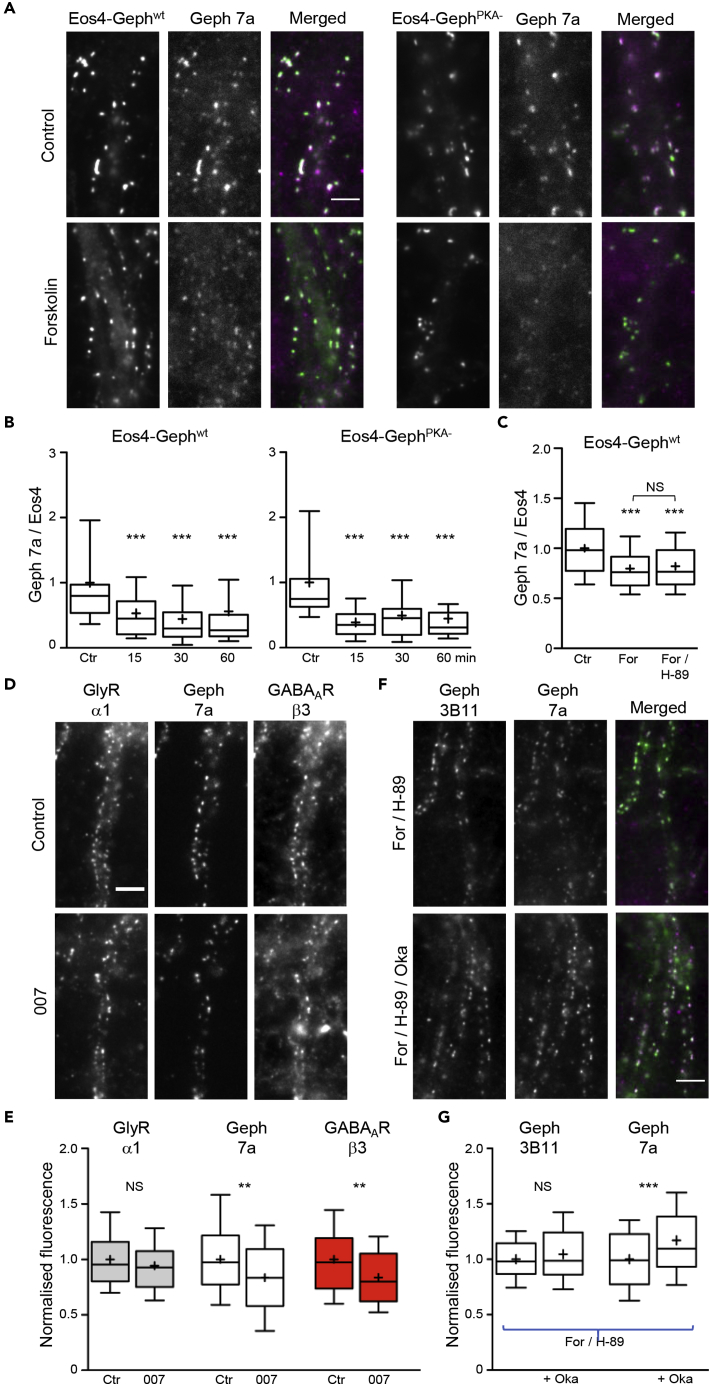
PKA is considered as the main effector downstream of cAMP signaling. To characterize the mechanism by which forskolin leads to the de-phosphorylation of residue S270 of gephyrin, we tested the involvement of known in vivo PKA phosphorylation sites of gephyrin. Wild-type mEos4b-gephyrin and a PKA-phosphorylation-deficient variant carrying the amino acid substitutions S294A/S295A/S303A/S305A/S319A (Eos4-GephPKA-) were expressed in spinal cord neurons by lentivirus infection (Figures 4A and S1C), and synaptic gephyrin levels were quantified after treatment of the neurons with forskolin for different durations (15, 30, 60 min).
Figure 4.
PKA-Independent Effect of Forskolin on Gephyrin Phosphorylation
(A) mEos4b-tagged wild-type (wt) and PKA-insensitive (PKA-) gephyrin were expressed in rat spinal cord neurons using lentiviral infection (green in merged image). Cells treated without (Ctr) or with forskolin (For) were labeled with Geph 7a antibody (magenta) and GlyRα1 antibody (shown in Figures S1D and S1E). Scale bar, 5 μm.
(B) Time course of the ratio of Geph 7a/Eos4-Geph fluorescence intensity (normalized for each construct to the control condition in each experiment) after forskolin application of up to 60 min (n > 40 cells for each construct and time point from 2 experiments, ***p < 0.001 against control, KW test).
(C) After 30-min exposure to forskolin, the Geph 7a/Eos4-Geph ratio was consistently reduced, regardless of the presence of the PKA inhibitor H-89 (n = 60 cells per condition, 2 experiments, ***p < 0.001 against control, ANOVA).
(D) Triple immunostaining of GlyRα1, Geph 7a, and GABAARβ3 with or without the EPAC agonist 007 for 30 min. Scale bar, 5 μm.
(E) Normalized fluorescence intensity of 007-treated neurons (nCtr = 90, n007 = 83 cells from 3 experiments). EPAC activity significantly reduced Geph 7a and GABAARβ3 labeling, but not GlyRα1 (**p < 0.01, ANOVA).
(F and G) Spinal cord neurons were treated for 30 min with forskolin and H-89 in the presence or absence of 40 nM okadaic acid (Oka). Blockade of phosphatase PP1/PP2A increased the Geph 7a signal (magenta), but not Geph 3B11 (green), at the synapses (nFor/H89 = 86 and nFor/H89+Oka = 84 cells from 3 experiments, ***p < 0.001, t test). Scale bar, 5 μm. Data are represented as 10%, 25%, 50%, 75%, and 90% percentiles; the mean is indicated as a cross.
Immunolabeling with mAb7a antibody confirmed that S270 phosphorylation was decreased relative to the total Eos4-Gephwt levels at synapses (mAb7a/Eos4 ratio, p < 0.001 at all time points versus control, Kruskal-Wallis (KW) test; Figure 4B, see also Figures S1D and S1E). Surprisingly, Eos4-GephPKA--expressing neurons showed the same temporal profile, suggesting that forskolin did not act directly via any of the mutated PKA phosphorylation sites. We also applied forskolin together with the PKA inhibitor H-89 (Figure 4C). The mAb7a labeling was reduced to a similar level as in Eos4-Gephwt-expressing neurons treated with forskolin without H-89 (p > 0.05, ANOVA). Therefore the effect of forskolin on gephyrin phosphorylation did not appear to be mediated by PKA.
We then considered the involvement of other cAMP-dependent signaling proteins, namely, the exchange proteins directly activated by cAMP (EPAC). Immunolabeling of EPAC shows a punctate distribution in spinal cord neurons that partially overlaps with gephyrin clusters (Figures S2A and S2B). Co-expression of N- and C-terminally tagged EPAC2 together with mRFP-gephyrin substantiated the presence of EPAC at inhibitory synapses (Figure S2C). The EPAC-specific agonist 007-AM led to a reduction of mAb7a labeling of endogenous gephyrin and of β3-containing GABAARs (p < 0.01, ANOVA), but did not have any effect on GlyRα1 levels (Figures 4D and 4E). These effects were very similar to what had been observed with forskolin (Figure 1B). Together, the results indicate that EPAC and not PKA is responsible for the changes in gephyrin phosphorylation.
As EPAC has been reported to form a complex and act in concert with protein phosphatase PP2a (Hong et al., 2008), we treated cells for 15 min with or without 40 nM okadaic acid, an inhibitor of PP1/PP2a, and then added forskolin together with H-89 for further 30 min (Figures 4F and 4G). Okadaic acid reversed the forskolin effect, resulting in a significant increase in mAb7a, but not 3B11, labeling (pmAb7a < 0.001, p3B11 = 0.20, t test). This is in agreement with an earlier study that has implicated PP1/PP2a in the de-phosphorylation of gephyrin at position S270 (Kalbouneh et al., 2014).
Single Molecule Localization Microscopy of Synaptic Gephyrin Clusters
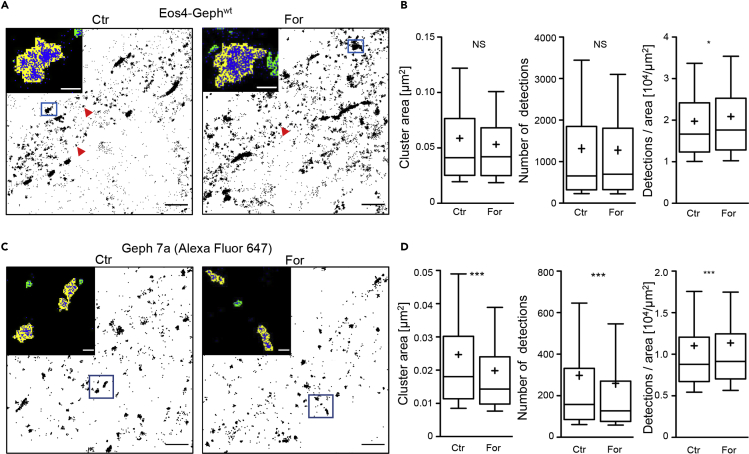
To explore the downstream consequences of S270 de-phosphorylation on the organization of the gephyrin scaffold at inhibitory synapses, we did super-resolution single-molecule localization microscopy (SMLM) imaging of recombinant gephyrin tagged with the photoconvertible protein mEos4b in fixed spinal cord neurons (Figure 5A). Owing to the high spatial resolution of the technique, differences in the ultrastructure of the synapse and in the distribution of scaffold proteins can be identified on the nanometer scale. As judged from pointillist images of the single fluorophore detections, Eos4-Gephwt is densely packed in synaptic clusters along the dendrites and on the somata of infected neurons. These clusters can be easily distinguished by their size and the number of detections from non-synaptic gephyrin molecules that are diffusely distributed throughout the cell (Figure 5A, arrowheads).
Figure 5.
SMLM Super-resolution Imaging of Synaptic Gephyrin Clusters
(A) Single molecule detections (shown as black dots, or blue in the zoomed images) of recombinant mEos4b-gephyrin expressed in spinal cord neurons in control condition (Ctr) or after forskolin application (For). Arrowheads indicate the positions of small, non-synaptic clusters arising from the repetitive detection of single mEos4b fluorophores. Dense synaptic clusters were identified based on cluster size and detection number in the pointillist images (yellow areas in the zoomed images, see Methods). Clusters with less than 200 detections were not considered (green areas). Scale bar, 1 μm; insert, 100 nm.
(B) Quantification of synaptic cluster areas, detection numbers, and detection densities (nCtr = 1,237, nFor = 894 clusters from 32 cells per condition and 3 independent experiments, *p < 0.05, MW test).
(C) dSTORM imaging of endogenous gephyrin labeled with mAb7a and secondary Alexa Fluor 647-coupled antibodies. Cluster analysis was done as in (A), with a lower threshold of 50 detections per cluster. Scale bar, 1 μm; insert, 100 nm.
(D) Quantification of the cluster area and the number of Alexa 647 detections shows a correlated decrease in the forskolin-treated neurons compared with control cells (nCtr = 8,162 clusters in 69 cells, nFor = 7,010 clusters in 68 cells, 3 experiments, ***p < 0.001, MW test). Data are shown as 10%, 25%, 50%, 75%, and 90% percentiles and the mean (cross).
The quantification of gephyrin cluster sizes did not reveal any differences between control and forskolin-treated neurons (synapse-by-synapse analysis, median areaCtr: 0.041 μm2, areaFor: 0.042 μm2, p = 0.20, Mann-Whitney U-test [MW] test). Similarly, the number of mEos4b detections per cluster was not altered (Figure 5B). These data show that forskolin treatment did not affect the clustering of gephyrin molecules at synapses. The fact that the number of detections was the same in both experimental conditions was expected, because the mEos4b detections reflect the total gephyrin content of the synapse. Due to the large sample size (n > 800 synapses per condition), there was a significant difference in the detection density (Figure 5B, right panel). However, cell-by-cell analysis of the same data did not reveal any obvious difference of the absolute detection densities (Figure S3A).
Experiments were also carried out using dSTORM to identify changes in the distribution of phosphorylated pS270-gephyrin (Figure 5C). Here, endogenous gephyrin in fixed spinal cord neurons was labeled with mAb7a and Alexa Fluor 647-coupled secondary antibodies. Pointillist images of Alexa 647 detections were analyzed in the same way as the mEos4b images, but with an adjusted threshold for cluster detection (see Methods). Both cluster size and detection numbers showed a significant reduction after forskolin treatment (median areacontrol: 0.018 μm2, areaforskolin: 0.014 μm2, p < 0.001, MW test, see Table S2). Interestingly, the detection density of pS270-gephyrin clusters was not noticeably changed by forskolin (Figures 5D and S3B). Our interpretation of these data is that the de-phosphorylation of gephyrin occurs within specific sub-synaptic domains, but that it has no influence on the overall organization of the gephyrin scaffold.
cAMP-Dependent Regulation of GABAAR Diffusion
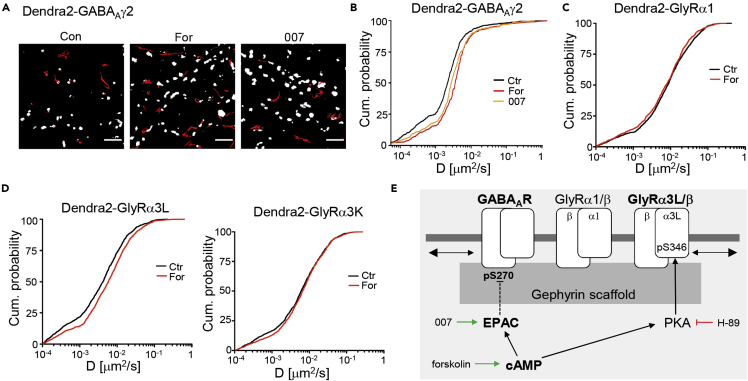
As the S270 phosphorylation status did not appear to have any effect on gephyrin clustering as such, we explored possible consequences of S270 de-phosphorylation on receptor binding at synapses. Single particle tracking with quantum dots was carried out in infected rat spinal cord neurons expressing different Dendra2-tagged receptor subunits. Diffusion coefficients were calculated from receptor trajectories at synapses as well as in the extrasynaptic plasma membrane (Figure 6A).
Figure 6.
cAMP-Dependent Regulation of GABAAR and GlyR Diffusion
(A) Quantum dot (QD) trajectories of Dendra2-GABAARγ2-containing receptor complexes (red traces) in control neurons and in the presence of either forskolin or 007-AM. Synapses were identified with FM4-64 labeling (white areas). Scale bar, 5 μm.
(B) Cumulative probabilities of QD-tagged Dendra2-GABAARγ2 diffusion coefficients at synapses in the three experimental conditions (median D values: DCtr = 0.0022 μm2/s, DFor = 0.0031, D007 = 0.0028, nCtr = 730 trajectories from 41 cells, nFor = 318 trajectories from 42 cells, n007 = 384 trajectories from 40 cells, 7 coverslips per condition, 3 experiments).
(C) The median diffusion coefficients of Dendra2-GlyRα1 at spinal cord synapses were similar in the control and forskolin condition (in the absence of the drug, after a 30-min forskolin incubation; DCtr = 0.0083 μm2/s, DFor = 0.0076, n > 1,000 trajectories from 5 experiments).
(D) Synaptic diffusion coefficients of Dendra2-GlyRα3L and α3K splice variants. Forskolin application specifically increased the speed of diffusion of Dendra2-GlyRα3L at synapses (α3L: DCtr = 0.0041, DFor = 0.0058, n > 1,000; α3K: DCtr = 0.0060 μm2/s, DFor = 0.0066, n > 900 QD trajectories; 5 experiments).
(E) Model of cAMP-dependent pathways regulating inhibitory receptor dynamics at mixed spinal cord synapses, including the pharmacological agents used in this study (see text for details).
In the presence of forskolin, the diffusion of Dendra2-GABAARγ2 receptors at synapses was considerably faster compared with control neurons (Figure 6B; p < 0.001, KW test). The same was true for neurons treated with the EPAC agonist 007-AM (p < 0.001). In contrast, we did not observe any changes in the diffusion of GlyRα1-containing receptors following a 30-min exposure to forskolin (Figure 6C). These data indicate that cAMP-dependent signaling via EPAC acts specifically on GABAAR-gephyrin binding, which is reflected in an increased mobility of the receptor at synapses.
Neurons in the dorsal horn of the spinal cord relay nociceptive signals from primary sensory neurons to the brain. The release of prostaglandin E2 (PGE2) during inflammation leads to the central disinhibition of the nociceptive pathways by blocking GlyRα3-containing receptor complexes in a PKA-dependent manner (Harvey et al., 2004). Given the known regulation of GlyRα3 by PKA in the context of inflammatory pain, we therefore looked at receptor diffusion in neurons expressing the splice variants Dendra2-GlyRα3L and α3K. The variant GlyRα3L contains a 15-amino acid insertion in the proximity of the PKA phosphorylation site S346 (Harvey et al., 2004, Nikolic et al., 1998). The mobility of GlyRα3K-containing receptors was not changed, whereas the GlyRα3L variant was accelerated significantly after forskolin application (p < 0.001, KW test; Figure 6D), suggesting that forskolin weakened the gephyrin binding of GlyRα3L-containing receptor complexes. This fits with immunocytochemical data that showed a reduction of Dendra2-GlyRα3L levels at synapses in response to forskolin (Figure S4). Moreover, mutagenesis of GlyRα3L at position S346 abolished the PKA-dependent reduction of synaptic receptor levels (Figure S5). In conclusion, separate cAMP pathways act on GABAARs and GlyRα3L/β complexes at mixed inhibitory synapses through post-translational modifications targeting gephyrin as well as individual receptor subtypes (Figure 6E).
Discussion
The main result of this study is that cAMP-dependent EPAC signaling reduces gephyrin phosphorylation at the amino acid residue S270, weakening the GABAAR-gephyrin interaction and leading to the selective dispersal of GABAARs from mixed inhibitory synapses in spinal cord neurons.
Gephyrin Clustering and the Role of S270 Phosphorylation
Labeling of gephyrin with the widely used antibody mAb7a showed that immunoreactivity of synaptic gephyrin clusters was substantially reduced in response to forskolin. As the antibody is specific for an epitope that includes the phosphorylated residue S270 of gephyrin (Kuhse et al., 2012), these data indicated that forskolin treatment causes a marked change in the phosphorylation status of endogenous and recombinant gephyrin in cultured spinal cord neurons as well as in organotypic slices.
The phosphorylation of gephyrin at residue S270 has been implicated in the regulation of gephyrin clustering at GABAergic synapses (Groeneweg et al., 2018). Several studies suggested that the phosphorylation-deficient gephyrin variant S270A gives rise to a higher number (Tyagarajan et al., 2011, Tyagarajan et al., 2013) or greater intensity and size of synaptic gephyrin clusters (Battaglia et al., 2018). We did not observe any significant changes in the total synaptic gephyrin levels, suggesting that S270 phosphorylation does not affect gephyrin clustering as such. This is consistent with the data of Kuhse and colleagues, who did not see any obvious changes of gephyrin clustering by S270 de-phosphorylation following the inhibition of Cdk5 (Kalbouneh et al., 2014, Kuhse et al., 2012).
Moreover, we did not detect any ultrastructural changes of the synaptic gephyrin scaffold using SMLM of mEos4b-tagged gephyrin in spinal cord cultures treated with forskolin. Endogenous pS270-gephyrin occupied a smaller synaptic area than total Eos4-Gephwt as judged by dSTORM imaging. Upon forskolin treatment, the pS270 area was further reduced, whereas the detection density remained unchanged. These data are at odds with the observed differences in the density of phosphorylation variants of gephyrin that were overexpressed in primary hippocampal neurons (Battaglia et al., 2018). Even though the overall clustering of gephyrin was not dependent on its phosphorylation, our super-resolution images suggest that the pS270 form is concentrated in specialized sub-synaptic domains (Pennacchietti et al., 2017). However, it cannot be ruled out that these results are influenced by the stochasticity of dSTORM imaging (Yang and Specht, 2020).
A possible explanation for the conflicting findings is that gephyrin residue S270 is the target of converging signaling pathways, which means that its phosphorylation could involve additional, as yet unidentified post-translational modifications. After all, gephyrin contains more than 50 known in vivo modifications (see PhosphoSitePlus database, www.phosphosite.org; Hornbeck et al., 2015). For example, the phosphorylation of S268 by ERK1/2 (Tyagarajan et al., 2013), SUMOylation, or acetylation of gephyrin (Ghosh et al., 2016) could shape the responses downstream of S270 phosphorylation. In our experiments, the silencing of multiple putative PKA sites of gephyrin (including residues S303 and S305; Flores et al., 2015) did not induce any changes in cluster intensity (IGeph PKA- = 1.001 ± 0.087 of the wild-type construct, mean ± SEM, n = 42 cells, 2 experiments), ruling out that the phosphorylation of these sites by PKA could have counteracted an effect of pS270 on gephyrin clustering.
We therefore conclude that S270 phosphorylation does not regulate gephyrin clustering, that is to say that it has no effect on gephyrin-gephyrin binding. Instead, our data point to a model whereby S270 phosphorylation promotes the GABAAR-gephyrin interaction (Figure 6E). This is in line with the loss of GABAAR clustering in response to S270 de-phosphorylation after Cdk5 inhibition (Kalbouneh et al., 2014). The mobilization of GABAARs does not have major structural consequences at mixed inhibitory synapses in the spinal cord, where GlyR-gephyrin interactions maintain the stability of the synaptic scaffold. In contrast, GABAAR dispersal is followed by a rapid loss of gephyrin at purely GABAergic synapses owing to the reciprocal stabilization of receptors and scaffold proteins (e.g., Niwa et al., 2012). A higher fraction of pS270 may also explain how synaptic GABAAR levels can be maintained during long-term application of diazepam, despite a substantial reduction in total gephyrin content (Lorenz-Guertin et al., 2019). In our view, this model can therefore reconcile some of the variable experimental results that have been reported.
Independence of GABAAR and GlyR Binding at Mixed Inhibitory Synapses
The reduction of GABAAR-gephyrin binding by pS270 de-phosphorylation is remarkable in that it regulates receptor diffusion in a subtype-specific manner by targeting the gephyrin scaffold itself. Post-translational modifications of the ICDs of GABAARs and GlyRs have been shown to control the strength of individual receptor-gephyrin interactions (Mukherjee et al., 2011, Petrini et al., 2014, Specht et al., 2011). As the inhibitory receptors bind to overlapping sites of gephyrin (Maric et al., 2011, Tretter et al., 2011) they directly compete for synaptic binding sites. This is exemplified by the opposite changes of α2- versus α5-containing GABAAR dynamics at hippocampal synapses (Gerrow and Triller, 2014), where the reduction in the affinity of one receptor type allows another receptor to occupy the liberated binding sites.
The fact that forskolin treatment specifically interfered with the clustering of GABAARs (and GlyRα3) at synapses, but did not affect GlyRα1, shows that receptor levels can also be regulated separately, without prompting a compensatory effect (Figure 6E). Long-term blockade of network activity by tetrodotoxin likewise reduced GABAAR levels independently of GlyRs (Specht et al., 2013). These observations imply that there is only a limited direct competition between receptors at mixed inhibitory synapses. Given the non-identity of the gephyrin-binding motifs (e.g., Grunewald et al., 2018, Kowalczyk et al., 2013, Maric et al., 2014), it is feasible that S270 de-phosphorylation and potentially associated conformational changes could only affect the binding of certain receptor subtypes. This raises the new concept that the synaptic gephyrin scaffold displays distinct, receptor-specific binding modes.
If different receptors can be regulated independently from one another, what then is the fate of the liberated binding sites? Although we cannot exclude that GABAARs with a different subunit composition replace the β3-containing receptors, the most likely explanation is that the excessive binding sites are lost over time through the dissociation of gephyrin molecules from the synaptic cluster. This is consistent with the observation of a delayed reduction in mEos4b-gephyrin levels during long forskolin applications (Figures S1D and S1E). There was also a trend that GlyRs decline after prolonged exposure to forskolin, suggesting that S270 de-phosphorylation could possibly affect GlyR-gephyrin binding. In view of the high affinity of the GlyRβ-gephyrin interaction, however, these processes are expected to take place on a much longer timescale and to a lesser degree (Specht, 2019).
Another possibility for the relative independence of GABAAR clustering is the implication of alternative binding mechanisms. GABAARγ2-containing receptors, for instance, can be recruited by neuroligin-2 via Lhfpl4 binding (Davenport et al., 2017, Yamasaki et al., 2017). The slight mismatch in the relative changes of GABAARs and pS270 levels (Figure 1C, red traces) could indeed point to the existence of a gephyrin-independent mechanism. Yet the overall correspondence between GABAAR and S270 phosphorylation levels suggests that GABAAR clustering at mixed inhibitory synapses in spinal cord neurons relies predominantly on a gephyrin-related mechanism.
Integration of cAMP-Dependent Pathways Regulating Receptor Trapping at Synapses
The effect of forskolin on GABAAR diffusion at synapses led us to assume initially that the mechanism would be dependent on protein kinase A. There is ample evidence that PKA-dependent phosphorylation regulates GABAAR function and trafficking, often producing divergent effects (reviewed in Nakamura et al., 2015). Unexpectedly, we found that neither mutagenesis of PKA phosphorylation sites of gephyrin nor the PKA blocker H-89 inhibited pS270 de-phosphorylation and GABAAR dispersal. It turned out that the GABAAR loss was in fact caused by a different process, namely, the activation of EPAC (Robichaux and Cheng, 2018). The presence of this cAMP-regulated guanine nucleotide exchange factor at inhibitory synapses (Figure S2) acting in parallel to PKA could have an important role in the regulation of inhibitory neurotransmission. In other words, cAMP could simultaneously trigger PKA and EPAC signaling processes, leading to complex downstream effects at inhibitory synapses.
For instance, PGE2 is known to inhibit glycinergic currents in a PKA-dependent manner during inflammatory pain (Harvey et al., 2004). The GlyRα3 specificity of this effect comes from the presence of a PKA phosphorylation site at residue S346 in the ICD that is not present in GlyRα1. Phosphorylation of this site was shown to induce global conformational changes that extend to the agonist-binding pocket (Han et al., 2013). We found that forskolin treatment of Dendra2-GlyRα3L-expressing spinal cord neurons increased the mobility of the receptors, whereas no change was observed for GlyRα1. This suggests that changes in receptor-scaffold interaction parallel the changes in receptor activity (Harvey et al., 2004, Rajalu et al., 2009). In other words, the PKA-dependent inhibition of α3-containing GlyRs is associated with a weakening of the receptor-gephyrin interaction, lending further support to the hypothesis that the α-subunits of the GlyR can influence the strength of GlyRβ-gephyrin binding (Patrizio et al., 2017).
In addition, GABAARβ subunits contain conserved PKA sites in their ICDs, the phosphorylation of which regulates GABAAR function (McDonald et al., 1998), internalization (Kittler et al., 2005), and possibly gephyrin binding (Bohnsack et al., 2016, Kowalczyk et al., 2013). How these different signaling processes are integrated at mixed inhibitory synapses is not yet understood. Nonetheless, our data demonstrate that cAMP signaling has a significant impact on the trapping of GABAARγ2- and GlyRα3L-containing receptors (Figure 6E). Given that α1-containing GlyRs cannot compensate for this loss, a reduction of inhibitory neurotransmission seems inevitable. The dispersal of GABAARs is likely to shift the inhibitory phenotype from a mixed to a purely glycinergic profile, with a fast, monophasic decay and lower charge transfer (Aubrey and Supplisson, 2018). This effect could exacerbate the disinhibition observed during pathological processes such as inflammatory pain. On a more positive note, the independence of GABAAR and GlyR regulation may also enable the development of specific pharmacological approaches that promote functional compensation.
Limitations of the Study
Different regulatory processes at inhibitory synapses may engage multiple and overlapping phosphorylation sites in the central domain of gephyrin. The phospho-specific antibody mAb7a therefore has to be seen as a sensitive tool that reports on one of these modifications that could be implicated in several regulatory mechanisms with different downstream consequences. This also raises concerns about the use of mAb7a antibody as a synaptic marker.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We are grateful to Xiaojuan Yang, Sabrina Colasse, Astou Tangara, Xavier Marques, Thomas Chapdelaine, and Marianne Renner (IFM, Paris) for technical help and Volker Eulenburg (University of Leipzig) for cDNA clones. We would also like to thank Hans Michael Maric for insightful discussions. This research has been funded by the European Research Council (ERC, Plastinhib), Agence Nationale de la Recherche (ANR, Synaptune and Syntrack), Labex (Memolife), and France-BioImaging (FBI). F.N. was supported by grants from TOYOBO Biotechnology Foundation and Bourses du Gouvernement Français. A.P. was supported by a Marie-Curie ITN network grant (NPlast) and Memolife.
Author Contributions
F.N., A.P., A.T., and C.G.S. planned the experiments; F.N., A.P., and C.G.S. performed the experiments and analyzed the data; F.N., A.P., and C.G.S. wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: December 20, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.11.013.
Data and Code Availability
The raw data of this article are available from the corresponding author upon request.
Supplemental Information
References
- Aubrey K.R., Supplisson S. Heterogeneous signaling at GABA and glycine co-releasing terminals. Front. Synaptic Neurosci. 2018;10:40. doi: 10.3389/fnsyn.2018.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia S., Renner M., Russeau M., Come E., Tyagarajan S.K., Levi S. Activity-dependent inhibitory synapse scaling is determined by gephyrin phosphorylation and subsequent regulation of GABAA receptor diffusion. eNeuro. 2018;5:1–20. doi: 10.1523/ENEURO.0203-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack J.P., Carlson S.L., Morrow A.L. Differential regulation of synaptic and extrasynaptic alpha4 GABA(A) receptor populations by protein kinase A and protein kinase C in cultured cortical neurons. Neuropharmacology. 2016;105:124–132. doi: 10.1016/j.neuropharm.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitinger U., Bahnassawy L.M., Janzen D., Roemer V., Becker C.M., Villmann C., Breitinger H.G. PKA and PKC modulators affect ion channel function and internalization of recombinant alpha1 and alpha1-beta glycine receptors. Front. Mol. Neurosci. 2018;11:154. doi: 10.3389/fnmol.2018.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport E.C., Pendolino V., Kontou G., McGee T.P., Sheehan D.F., Lopez-Domenech G., Farrant M., Kittler J.T. An essential role for the tetraspanin LHFPL4 in the cell-type-specific targeting and clustering of synaptic GABAA receptors. Cell Rep. 2017;21:70–83. doi: 10.1016/j.celrep.2017.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin A., Levi S., Riveau B., Gasnier B., Triller A. Formation of mixed glycine and GABAergic synapses in cultured spinal cord neurons. Eur. J. Neurosci. 2000;12:3883–3892. doi: 10.1046/j.1460-9568.2000.00271.x. [DOI] [PubMed] [Google Scholar]
- Flores C.E., Nikonenko I., Mendez P., Fritschy J.M., Tyagarajan S.K., Muller D. Activity-dependent inhibitory synapse remodeling through gephyrin phosphorylation. Proc. Natl. Acad. Sci. U S A. 2015;112:E65–E72. doi: 10.1073/pnas.1411170112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiman E.J., Zheng W., Fritschy J.M., Alvarez F.J. Glycine and GABA(A) receptor subunits on Renshaw cells: relationship with presynaptic neurotransmitters and postsynaptic gephyrin clusters. J. Comp. Neurol. 2002;444:275–289. doi: 10.1002/cne.10148. [DOI] [PubMed] [Google Scholar]
- Gerrow K., Triller A. GABAA receptor subunit composition and competition at synapses are tuned by GABAB receptor activity. Mol. Cell Neurosci. 2014;60:97–107. doi: 10.1016/j.mcn.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Ghosh H., Auguadri L., Battaglia S., Simone Thirouin Z., Zemoura K., Messner S., Acuna M.A., Wildner H., Yevenes G.E., Dieter A. Several posttranslational modifications act in concert to regulate gephyrin scaffolding and GABAergic transmission. Nat. Commun. 2016;7:13365. doi: 10.1038/ncomms13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzer G., Specht C.G., Allain L., Shinoe T., Triller A. Benzodiazepine-dependent stabilization of GABA(A) receptors at synapses. Mol. Cell Neurosci. 2014;63:101–113. doi: 10.1016/j.mcn.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Groeneweg F.L., Trattnig C., Kuhse J., Nawrotzki R.A., Kirsch J. Gephyrin: a key regulatory protein of inhibitory synapses and beyond. Histochem. Cell Biol. 2018;150:489–508. doi: 10.1007/s00418-018-1725-2. [DOI] [PubMed] [Google Scholar]
- Grunewald N., Jan A., Salvatico C., Kress V., Renner M., Triller A., Specht C.G., Schwarz G. Sequences flanking the gephyrin-binding site of GlyRbeta tune receptor stabilization at synapses. eNeuro. 2018;5:1–17. doi: 10.1523/ENEURO.0042-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L., Talwar S., Wang Q., Shan Q., Lynch J.W. Phosphorylation of alpha3 glycine receptors induces a conformational change in the glycine-binding site. ACS Chem. Neurosci. 2013;4:1361–1370. doi: 10.1021/cn400097j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R.J., Depner U.B., Wassle H., Ahmadi S., Heindl C., Reinold H., Smart T.G., Harvey K., Schutz B., Abo-Salem O.M. GlyR alpha3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science. 2004;304:884–887. doi: 10.1126/science.1094925. [DOI] [PubMed] [Google Scholar]
- Hong K., Lou L., Gupta S., Ribeiro-Neto F., Altschuler D.L. A novel Epac-Rap-PP2A signaling module controls cAMP-dependent Akt regulation. J. Biol. Chem. 2008;283:23129–23138. doi: 10.1074/jbc.M800478200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbeck P.V., Zhang B., Murray B., Kornhauser J.M., Latham V., Skrzypek E. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res. 2015;43:D512–D520. doi: 10.1093/nar/gku1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P., Bischofberger J., Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Kalbouneh H., Schlicksupp A., Kirsch J., Kuhse J. Cyclin-dependent kinase 5 is involved in the phosphorylation of gephyrin and clustering of GABAA receptors at inhibitory synapses of hippocampal neurons. PLoS One. 2014;9:e104256. doi: 10.1371/journal.pone.0104256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.Y., Schrader N., Smolinsky B., Bedet C., Vannier C., Schwarz G., Schindelin H. Deciphering the structural framework of glycine receptor anchoring by gephyrin. Embo J. 2006;25:1385–1395. doi: 10.1038/sj.emboj.7601029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler J.T., Chen G., Honing S., Bogdanov Y., McAinsh K., Arancibia-Carcamo I.L., Jovanovic J.N., Pangalos M.N., Haucke V., Yan Z. Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proc. Natl. Acad. Sci. U S A. 2005;102:14871–14876. doi: 10.1073/pnas.0506653102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk S., Winkelmann A., Smolinsky B., Forstera B., Neundorf I., Schwarz G., Meier J.C. Direct binding of GABA(A) receptor beta2 and beta3 subunits to gephyrin. Eur. J. Neurosci. 2013;37:544–554. doi: 10.1111/ejn.12078. [DOI] [PubMed] [Google Scholar]
- Kuhse J., Kalbouneh H., Schlicksupp A., Mukusch S., Nawrotzki R., Kirsch J. Phosphorylation of gephyrin in hippocampal neurons by cyclin-dependent kinase CDK5 at Ser-270 is dependent on collybistin. J. Biol. Chem. 2012;287:30952–30966. doi: 10.1074/jbc.M112.349597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz-Guertin J.M., Bambino M.J., Das S., Weintraub S.T., Jacob T.C. Diazepam accelerates GABAAR synaptic exchange and alters intracellular trafficking. Front. Cell Neurosci. 2019;13:163. doi: 10.3389/fncel.2019.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado P., Rostaing P., Guigonis J.M., Renner M., Dumoulin A., Samson M., Vannier C., Triller A. Heat shock cognate protein 70 regulates gephyrin clustering. J. Neurosci. 2011;31:3–14. doi: 10.1523/JNEUROSCI.2533-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maric H.M., Kasaragod V.B., Hausrat T.J., Kneussel M., Tretter V., Stromgaard K., Schindelin H. Molecular basis of the alternative recruitment of GABA(A) versus glycine receptors through gephyrin. Nat. Commun. 2014;5:5767. doi: 10.1038/ncomms6767. [DOI] [PubMed] [Google Scholar]
- Maric H.M., Mukherjee J., Tretter V., Moss S.J., Schindelin H. Gephyrin-mediated gamma-aminobutyric acid type A and glycine receptor clustering relies on a common binding site. J. Biol. Chem. 2011;286:42105–42114. doi: 10.1074/jbc.M111.303412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald B.J., Amato A., Connolly C.N., Benke D., Moss S.J., Smart T.G. Adjacent phosphorylation sites on GABAA receptor beta subunits determine regulation by cAMP-dependent protein kinase. Nat. Neurosci. 1998;1:23–28. doi: 10.1038/223. [DOI] [PubMed] [Google Scholar]
- Mukherjee J., Kretschmannova K., Gouzer G., Maric H.M., Ramsden S., Tretter V., Harvey K., Davies P.A., Triller A., Schindelin H. The residence time of GABA(A)Rs at inhibitory synapses is determined by direct binding of the receptor alpha1 subunit to gephyrin. J. Neurosci. 2011;31:14677–14687. doi: 10.1523/JNEUROSCI.2001-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Darnieder L.M., Deeb T.Z., Moss S.J. Regulation of GABAARs by phosphorylation. Adv. Pharmacol. 2015;72:97–146. doi: 10.1016/bs.apha.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic Z., Laube B., Weber R.G., Lichter P., Kioschis P., Poustka A., Mulhardt C., Becker C.M. The human glycine receptor subunit alpha3. Glra3 gene structure, chromosomal localization, and functional characterization of alternative transcripts. J. Biol. Chem. 1998;273:19708–19714. doi: 10.1074/jbc.273.31.19708. [DOI] [PubMed] [Google Scholar]
- Niwa F., Bannai H., Arizono M., Fukatsu K., Triller A., Mikoshiba K. Gephyrin-independent GABA(A)R mobility and clustering during plasticity. PLoS One. 2012;7:e36148. doi: 10.1371/journal.pone.0036148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrizio A., Renner M., Pizzarelli R., Triller A., Specht C.G. Alpha subunit-dependent glycine receptor clustering and regulation of synaptic receptor numbers. Sci. Rep. 2017;7:10899. doi: 10.1038/s41598-017-11264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennacchietti F., Vascon S., Nieus T., Rosillo C., Das S., Tyagarajan S.K., Diaspro A., Del Bue A., Petrini E.M., Barberis A. Nanoscale molecular reorganization of the inhibitory postsynaptic density is a determinant of GABAergic synaptic potentiation. J. Neurosci. 2017;37:1747–1756. doi: 10.1523/JNEUROSCI.0514-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini E.M., Ravasenga T., Hausrat T.J., Iurilli G., Olcese U., Racine V., Sibarita J.B., Jacob T.C., Moss S.J., Benfenati F. Synaptic recruitment of gephyrin regulates surface GABAA receptor dynamics for the expression of inhibitory LTP. Nat. Commun. 2014;5:3921. doi: 10.1038/ncomms4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajalu M., Muller U.C., Caley A., Harvey R.J., Poisbeau P. Plasticity of synaptic inhibition in mouse spinal cord lamina II neurons during early postnatal development and after inactivation of the glycine receptor alpha3 subunit gene. Eur. J. Neurosci. 2009;30:2284–2292. doi: 10.1111/j.1460-9568.2009.07018.x. [DOI] [PubMed] [Google Scholar]
- Robichaux W.G., 3rd, Cheng X. Intracellular cAMP sensor EPAC: physiology, pathophysiology, and therapeutics development. Physiol. Rev. 2018;98:919–1053. doi: 10.1152/physrev.00025.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander B., Tria G., Shkumatov A.V., Kim E.Y., Grossmann J.G., Tessmer I., Svergun D.I., Schindelin H. Structural characterization of gephyrin by AFM and SAXS reveals a mixture of compact and extended states. Acta Crystallogr. D Biol. Crystallogr. 2013;69:2050–2060. doi: 10.1107/S0907444913018714. [DOI] [PubMed] [Google Scholar]
- Shrivastava A.N., Triller A., Sieghart W. GABA(A) receptors: post-synaptic co-localization and cross-talk with other receptors. Front. Cell Neurosci. 2011;5:7. doi: 10.3389/fncel.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolinsky B., Eichler S.A., Buchmeier S., Meier J.C., Schwarz G. Splice-specific functions of gephyrin in molybdenum cofactor biosynthesis. J. Biol. Chem. 2008;283:17370–17379. doi: 10.1074/jbc.M800985200. [DOI] [PubMed] [Google Scholar]
- Specht C.G. Fractional occupancy of synaptic binding sites and the molecular plasticity of inhibitory synapses. Neuropharmacology. 2019:107493. doi: 10.1016/j.neuropharm.2019.01.008. [DOI] [PubMed] [Google Scholar]
- Specht C.G., Grunewald N., Pascual O., Rostgaard N., Schwarz G., Triller A. Regulation of glycine receptor diffusion properties and gephyrin interactions by protein kinase C. EMBO J. 2011;30:3842–3853. doi: 10.1038/emboj.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht C.G., Izeddin I., Rodriguez P.C., El Beheiry M., Rostaing P., Darzacq X., Dahan M., Triller A. Quantitative nanoscopy of inhibitory synapses: counting gephyrin molecules and receptor binding sites. Neuron. 2013;79:308–321. doi: 10.1016/j.neuron.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Todd A.J., Watt C., Spike R.C., Sieghart W. Colocalization of GABA, glycine, and their receptors at synapses in the rat spinal cord. J. Neurosci. 1996;16:974–982. doi: 10.1523/JNEUROSCI.16-03-00974.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V., Kerschner B., Milenkovic I., Ramsden S.L., Ramerstorfer J., Saiepour L., Maric H.M., Moss S.J., Schindelin H., Harvey R.J. Molecular basis of the gamma-aminobutyric acid A receptor alpha3 subunit interaction with the clustering protein gephyrin. J. Biol. Chem. 2011;286:37702–37711. doi: 10.1074/jbc.M111.291336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagarajan S.K., Ghosh H., Yevenes G.E., Imanishi S.Y., Zeilhofer H.U., Gerrits B., Fritschy J.M. Extracellular signal-regulated kinase and glycogen synthase kinase 3beta regulate gephyrin postsynaptic aggregation and GABAergic synaptic function in a calpain-dependent mechanism. J. Biol. Chem. 2013;288:9634–9647. doi: 10.1074/jbc.M112.442616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagarajan S.K., Ghosh H., Yevenes G.E., Nikonenko I., Ebeling C., Schwerdel C., Sidler C., Zeilhofer H.U., Gerrits B., Muller D. Regulation of GABAergic synapse formation and plasticity by GSK3beta-dependent phosphorylation of gephyrin. Proc. Natl. Acad. Sci. U S A. 2011;108:379–384. doi: 10.1073/pnas.1011824108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki T., Hoyos-Ramirez E., Martenson J.S., Morimoto-Tomita M., Tomita S. GARLH family proteins stabilize GABAA receptors at synapses. Neuron. 2017;93:1138–1152.e6. doi: 10.1016/j.neuron.2017.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Specht C.G. Practical guidelines for two-color SMLM of synaptic proteins in cultured neurons. In: Okada Y., Yamamoto N., editors. Single Molecule Microscopy in Neurobiology. Springer; 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data of this article are available from the corresponding author upon request.