Key Teaching Points.
-
•
Fast and irregular ventricular tachycardia with hemodynamic compromise could occur in a structurally normal heart.
-
•
Use of noninvasive ablation with radiotherapy on the right ventricular (RV) free wall is novel.
-
•
Ablation of the RV free wall using radiotherapy is safe and effective in the short term.
-
•
Stereotactic body radiotherapy could carry the risk of thromboembolism; hence anticoagulation needs to be considered.
Introduction
We present the case of a patient who presented in a dire situation with ventricular tachycardia (VT) storm, which did not respond to intubation, antiarrhythmics, or catheter ablation. This presentation is in the background of palliatively treated metastatic malignancy. We needed a strategy to treat VT to enable the step-down from intensive care and to restart systemic palliation. To that end we employed a strategy of electroanatomical mapping–guided stereotactic body radiotherapy (SBRT) with good effect.
Case report
Our patient is a 34-year-old woman suffering from neuroendocrine tumor of paranasal sinus complicated by distant metastasis. She had received previous palliative radiotherapy to the head and neck and unfortunately has a grim prognosis, with life expectancy estimated in months.
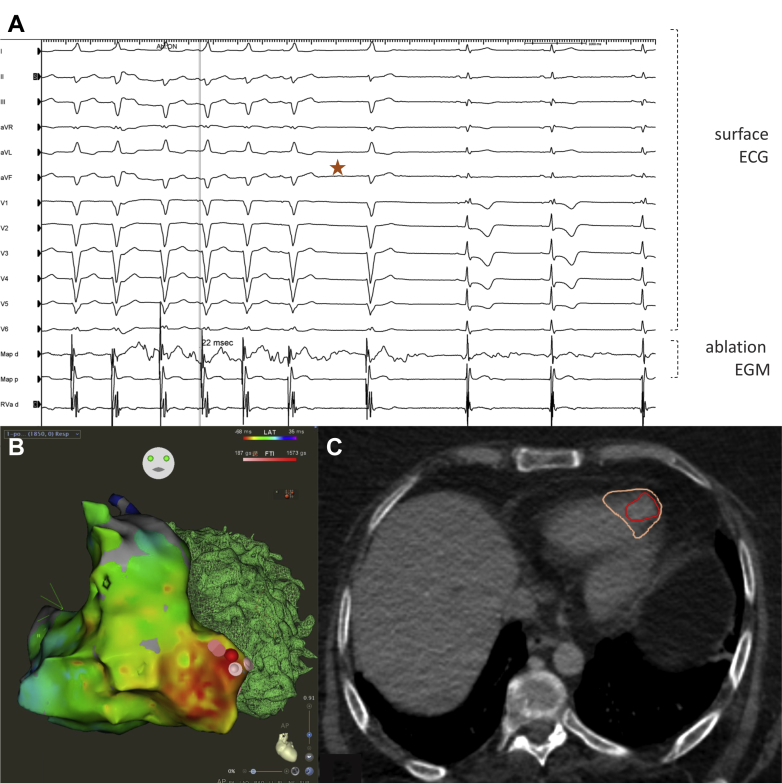
She presented in VT storm at 190–200 beats per minute (bpm) with hemodynamic compromise requiring intubation and ventilation. Multiple DC shocks were unsuccessful. A combination of intravenous amiodarone and procainamide controlled the VT rate to 130–180 bpm. The clinical VT was irregular, monoform, left bundle branch block morphology with superior, left axis deviation and late transition at V6, suggestive of the exit site emanating from the right ventricular (RV) apex (Figure 1A). The QRS duration was 130 ms with a steep initial QRS deflection. Cardiac magnetic resonance imaging (day 13) was unremarkable. Biventricular function was normal.
Figure 1.
A: Ventricular tachycardia (VT) termination during catheter ablation (indicated by star). ECG = electrocardiogram; EGM = electrogram. B: Electroanatomical map of successful (catheter) VT ablation at right ventricular apex. C: Stereotactic body radiotherapy target volume annotated in preprocedure contrast computed tomography.
Cardiac mapping and VT ablation
The patient was brought to the cardiac electrophysiological laboratory for VT ablation using CARTO electroanatomical mapping and PentaRay (Biosense Webster, Diamond Bar, CA) mapping catheter. The VT was persistent and was mapped to the RV apical free wall, where it was 22 ms ahead of surface QRS. Ablation was performed using a force-sensing Surround Flow DF Nav catheter (Biosense Webster), resulting in termination of VT during ablation, and VT was noninducible thereafter with programmed ventricular stimulation during isoproterenol infusion (Figure 1A). The clinical VT recurred an hour after the procedure at 130 bpm. Noninvasive ablation using SBRT was chosen for the second attempt because of the concern of thin RV free wall perforation. The patient was aware that this procedure was offered on compassionate grounds.
Noninvasive VT ablation
The patient was brought with intensive care unit support to the Princess Margaret Cancer Center and a computed tomography (CT) simulation with contrast and respiration-correlated CT scan (4-dimensional CT) was performed in supine position with external thermoplastic shoulder immobilization. The radioablation target was delineated in breathing phases using the electroanatomic map of the RV arrhythmogenic focus and included the contrast-enhancing previous endocardial ablation site. The clinical target volume included the RV apex, the full thickness of the RV, and the adjacent septum, along with 5–8 mm margin to include adjacent myocardial tracts. The resultant internal target volume was expanded by 5 mm to account for setup and delivery uncertainty to a final planning target volume, which measured 52 cubic centimeters. A volumetric arc therapy radiation plan was created to deliver 25 Gy in a single fraction to the planning target volume using a 6 MV flattening filter–free photon beam. The resultant plan underwent physics quality assurance a day prior to delivery. On the day of treatment, a dynamic multi-frame volumetric cine was obtained using the Canon Genesis 320-slice scanner (Canon, Tustin, CA) to confirm margins were adequate for both cardiac and respiratory motion. Lung and nontarget cardiac tissue were contoured away. On the treatment unit, a cone beam CT was performed to confirm patient localization and the patient tolerated the 5-minute treatment delivery without issue and only 1 radiation dose was given, on a True Beam Linear Accelerator (Varian, Palo Alto, CA). Delivery of 25 Gy was prescribed to the entire target volume; however, there was some heterogeneity within the treated volume.
Treatment response
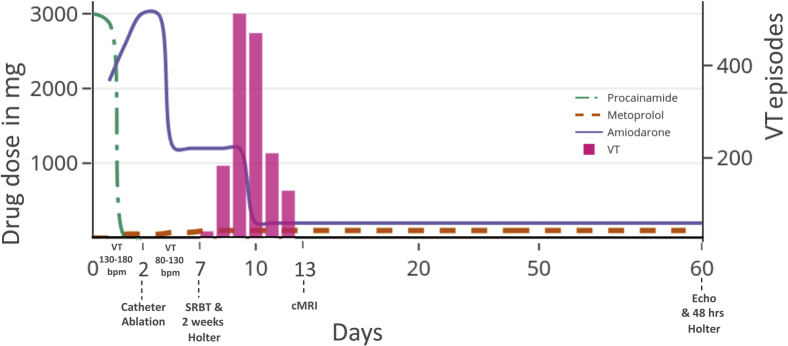
For the first 2 days, the patient was in incessant VT on intravenous antiarrhythmics (Figure 2). Catheter VT ablation was performed on the second day. The incessant VT at 130–180 bpm improved to frequent VT at 80–150 bpm with the same morphology. Epicardial ablation was considered; however, her comorbidities and her wishes were not encouraging. Hence we did not proceed with repeat invasive ablation.
Figure 2.
Medication and ventricular arrhythmia timelines. The patient presented with incessant ventricular tachycardia (VT) at 130–180 beats per minute (bpm). Catheter ablation was performed on the second day, following which VT slowed to 80–130 bpm. Holter monitoring for 2 weeks was started on day 7, simultaneously with stereotactic body radiotherapy (SBRT). VT was completely eliminated 6 days after SBRT until follow-up Holter monitoring at 60 days. Echocardiogram at 60 days was unremarkable. cMRI = cardiac magnetic resonance imaging.
SBRT was performed on the seventh day and Holter monitoring was commenced. On the 12th day (sixth day after SBRT) the VT burden decreased from 600 events/hour to nil—a 100% reduction in events, as recorded with Holter monitoring. She suffered a segmental pulmonary embolism 3 days post SBRT, which was managed conservatively. The pulmonary embolism was located in the right posterobasal segmental vein, which was distant from the radiotherapy site. Contrast echo did not show any RV clot. Echocardiogram and 48-hour Holter monitoring at 60 days did not reveal any abnormalities. Currently she is asymptomatic on metoprolol 50 mg twice a day and amiodarone 200 mg once a day. As she had a good response to ablation, tachycardia device therapy was not considered.
Discussion
The novelty of our case report lies in the target location of SBRT, which was the RV free wall. This is now proven to be safe and effective in the acute and subacute phase (2 months). The possibility of VT response being a late response to catheter ablation was considered unlikely, as the VT recurred soon after ablation and responded in a subacute manner to radiotherapy. Even though SBRT has been shown to be effective in VT ablation, the long-term safety of this novel modality is currently unclear. There has been a report of SBRT worsening mitral regurgitation.1 Safety needs to be demonstrated in other anatomical sites, such as outflow tract, and also in proximity to coronaries. The early response to SBRT within a week, as opposed to 4–6 weeks, is an interesting finding. Most studies have used a blanking period of multiple weeks, which could explain why an early response was not noticed. The contrast enhancement of radiofrequency lesion in the pre-SBRT CT scan has not been reported before. The other novel aspect of our case relates to the application of SBRT in a systemically palliated patient for the final definitive ablation therapy. From our experience and 1 case of stroke reported by Cuculich and colleagues,2 the risk of thromboembolism associated with SBRT needs to be further evaluated.
The mapping system used to localize VT prior to SBRT needs further study. Electrocardiography-based systems and scar targeting by imaging, which have been reported before, provide only estimates of arrhythmic regions of the heart.2, 3, 4 Our intraoperative VT mapping data demonstrated that the VT epicardial exit site localized by the electrocardiography-based system need not represent the critical sites in scar VT.5 We suggested that this issue could be resolved with electroanatomical mapping prior to radiotherapy, similar to the technique we used in the current case. This method is fast becoming an accepted pre-SBRT mapping strategy.1,6
Acknowledgments
Dr Patrick R. Lawler, Dr Mahmoud Bokhari, Dr Rahul Samanta, and Dr Andrea Daly, Toronto General Hospital, University Health Network, Toronto, Canada, helped in the clinical care of the patient.
Footnotes
Conflict of interest: There is no conflict of interest.
References
- 1.Neuwirth R., Cvek J., Knybel L. Stereotactic radiosurgery for ablation of ventricular tachycardia. Europace. 2019;21:1088–1095. doi: 10.1093/europace/euz133. [DOI] [PubMed] [Google Scholar]
- 2.Cuculich P.S., Schill M.R., Kashani R. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N Engl J Med. 2017;377:2325–2336. doi: 10.1056/NEJMoa1613773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson C.G., Samson P.P., Moore K.M. Phase I/II trial of electrophysiology-guided noninvasive cardiac radioablation for ventricular tachycardia. Circulation. 2019;139:313–321. doi: 10.1161/CIRCULATIONAHA.118.038261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loo B.W., Soltys S.G., Wang L. Stereotactic ablative radiotherapy for the treatment of refractory cardiac ventricular arrhythmia. Circ Arrhythm Electrophysiol. 2015;8:748–750. doi: 10.1161/CIRCEP.115.002765. [DOI] [PubMed] [Google Scholar]
- 5.Bhaskaran A., Nayyar S., Porta-Sánchez A. Exit sites on the epicardium rarely subtend critical diastolic path of ischemic VT on the endocardium: implications for noninvasive ablation. J Cardiovasc Electrophysiol. 2019;30:520–527. doi: 10.1111/jce.13843. [DOI] [PubMed] [Google Scholar]
- 6.Scholz E.P., Seidensaal K., Naumann P., André F., Katus H.A., Debus J. Risen from the dead: cardiac stereotactic ablative radiotherapy as last rescue in a patient with refractory ventricular fibrillation storm. HeartRhythm Case Rep. 2019;5:329–332. doi: 10.1016/j.hrcr.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]