Abstract
Noise pollution remains a pervasive health hazard that people encounter especially in large commercial metropolis and has been implicated in many adverse non-auditory health conditions such as hypertension, atherosclerosis, vascular (endothelial) dysfunction and metabolic disorders. There is a growing body of evidence showing that chronic noise exposure is associated with an increased risk of hypercholesterol, adiposity and development of type 2 diabetes. The present study investigated the effect of noise stress on parameters of glucose homeostasis in male rats and possible recovery after noise cessation. Twenty-four (24) adult male Sprague-Dawley rats were designated into four groups (n = 6 per group). All rats except the control group were exposed to 95dB noise using a noise generator for 28 consecutive days. A group of rats was investigated immediately after 28 days of noise exposure (NE28), while others were left to recover from noise stress for 7 days (NER7) or 14 days (NER14). OGTT and ITT were performed using standard methods. Plasma levels of triglyceride (TRIG), total cholesterol (CHOL), low density lipoprotein (LDL) and high-density lipoprotein (HDL) were determined. Serum level of insulin, corticosterone (CORT) and corticosterone-releasing-factor (CRF) were determined using ELISA. Homeostasis model assessment-insulin resistance (HOMA-IR) and glycogen content in liver as well as gastrocnemius muscle were also determined.
Although glucose tolerance remained unchanged in the noise-exposed groups, insulin sensitivity was however significantly reduced compared with control. There was significant increase (P < 0.05) in the level of CHOL, LDL and HDL. Noise also increased (P < 0.05) both insulin and CORT levels; and elicited a higher HOMA-IR index in NE28 rats. Hepatic and myocytic glycogen content were lower (P < 0.05) in NE28 rats relative to control. The reported changes above were reversed following a 14-day noise withdrawal period. Noise-induced insulin resistance may result from dysregulation of the stress axis and appears to be reversible with noise cessation.
Keywords: Biochemistry, Physiology, Systems biology, Oxidative stress, Diabetes, Insulin, Metabolism, Noise, Stress, Insulin sensitivity, Corticosterone, Glucose, Lipids, Glycogen
Biochemistry; Physiology; Systems biology; Oxidative stress; Diabetes; Insulin; Metabolism; Noise; Stress, Insulin sensitivity; Corticosterone; Glucose; Lipids; Glycogen
1. Introduction
Noise pollution remains a pervasive health hazard that people encounter especially in commercial metropolis and has been implicated in many adverse non-auditory health conditions such as hypertension, atherosclerosis, vascular (endothelial) dysfunction and metabolic disorders. In fact, there is growing body of evidence showing that chronic noise exposure is associated with an increased risk of hypercholesterolaemia (Mohammadi et al., 2016), adiposity (Christensen et al., 2016; Foraster et al., 2018) and development of type 2 diabetes (T2D) (Clark et al., 2017; Eze et al., 2017; Kempen et al., 2018).
Exposure to noise has been shown to result in perturbations in glucose homeostasis in both animal and human subjects. In an animal study designed to evaluate the glucometabolic consequences of noise, Cui and co-workers showed that chronic noise exposure increased blood glucose and impaired hepatic insulin production (Cui et al., 2016), which indicates that prolonged noise exposure increased the risk and accelerate the onset of T2D development. Furthermore, acute noise exposure elicited transient glucose intolerance and insulin resistance while chronic noise exposure led to prolonged insulin resistance in male mice (Liu et al., 2016); thereby manifesting a time course difference in the two windows of exposure. Meanwhile and perhaps more importantly, human study mirrored the findings in animal experiments with exposure to nocturnal noise shown to impair glucose tolerance and insulin sensitivity in normal subjects (Sorensen et al., 2013).
Glucocorticoid release in response to stress has been well characterized in rodents (Klenerova et al., 2003; Agrawal et al., 2011). One of the critical physiological functions of corticosterone, a major glucocorticoid is to increase gluconeogenesis and hepatic glycogenolysis in rats, resulting in increased availability of metabolic substrates to cope with stressful conditions (Solin and Lysashev, 2014). In addition to glucose mobilization from the energy store, there are indication that corticosterone may act antagonistically to the actions of insulin, the primary hormone responsible for glucose uptake in the body (Dallman et al., 1993). Current evidence suggests that glucocorticoids counteract the effects of insulin-sensitive tissues where insulin performs its major anabolic actions (hepatocytes, adipocytes, and muscle tissue) by exerting gluconeogenic effects to increase plasma glucose levels (Dallman et al., 1993; Strack et al., 1995). Interestingly, glucocorticoid during stress also has the potential to mobilize lipids from adipose tissue, which supports gluconeogenesis.
Insulin resistance (IR), a state where normal concentrations of insulin elicit a suboptimal biological response (Kahn, 1978), manifests in metabolically active tissues such as skeletal muscle, adipose tissue and liver. IR can become worse and wear out the pancreatic beta-cells if exposure to the insult is continuous and unabating. Noise exposure has been shown to be associated with IR development, however, additional studies explaining the noise-induced IR (Liu et al., 2016) and especially potential for improvement after cessation of noise exposure are still warranted. Therefore, this study sought to evaluate perturbations associated with noise-induced IR and possible reversibility of noise-induced metabolic indices such as glucose tolerance, insulin sensitivity, lipid profile, glycogen content and corticosterone hormone after a period of withdrawal from the stressor.
2. Methods
2.1. Animals
A total of 24 male adult rats of the Sprague-Dawley strain (150–220 g body weight) were used for the study. They were obtained from the animal breeding facility of the College of Medicine of the University of Lagos, Lagos, Nigeria. All procedures for care and use of animals were carried out according to the criteria outlined by the National Academy of Science published by the National Institute of Health (NIH, 1985). This was approved by the Animal and Human Use in Research Committee of the institution. The animals were handled humanely, kept in plastic cages, placed in a well ventilated and hygienic rat house under suitable conditions of temperature, humidity and natural photoperiod of 12 h light and 12 h dark cycle. They also had free access to food (standard rodent chow) and water. After a 2-week acclimatization period, the animals were randomly assigned into one control group and three noise groups (6 rats per group). The animals in the noise groups were exposed to white noise for 28 consecutive days (4 h per day, from 0900 to 1300 h) and classified according to the time points of noise exposure. One of the noise groups was assessed immediately after 28 days of exposure (NE28), while the remaining groups of noise-exposed rats were left to recover from noise stress for 7 days (NER7) or 14 days (NER14) before assessing their metabolic function.
2.2. Noise exposure setup
Noise was generated using a noise generator that was mounted on the rat cage. The noise level was set at 95 dB and monitored using a digital sound meter (NKTech). Variations in noise level was 2 dB within the space available to the animals and the noise generator was off automatically after 1 h and then restarted 10 min later. The method used was adapted with modifications from previous study of Cui et al. (2016).
2.3. Oral glucose tolerance test
After overnight fasting for 12 h, initial fasting blood glucose levels were estimated prior to glucose loading. Thereafter, the animals were administered a glucose (1 g/kg of body weight) dissolved in water by gavage. Blood glucose concentrations were determined from the tail vein with an Accu-check at 30, 60, 90, and 120 min afterwards. The incremental area under the curve (AUC-GTT) was also calculated.
2.4. Insulin tolerance test
Rats that had been fasted for 4 h were injected i.p. with regular human insulin (Humulin, 0.75 U/kg body weight). The blood glucose concentrations were monitored before (0 min) and 15, 30, 45, 60, 90, and 120 min after insulin injection. The AUC for the blood glucose–time function (AUC-ITT) was calculated.
2.5. Insulin, corticosterone and corticosterone-releasing-factor levels
To determine the concentration of insulin, corticosterone (CORT) and corticosterone-releasing-factor (CRF), commercially available ELISA kit (Elabscience Biotechnology Co., Ltd., Wuhan, China) which are designed as a competitive immunoassay for the quantitative determination of respective hormone in body fluids were used according to the manufacturer's instructions. Briefly, serum samples were added to 96-well plates containing biotinylated primary antibody and incubated for 45 min at 37 °C. Thereafter, plates were washed, and horseradish peroxidase-conjugated streptavidin solution was added to the wells and incubated for another 30 min at 37 °C. The plates were then washed, substrate reagent was added, and the plates were incubated for an additional 15 min at 37 °C. Finally, stop solution was added to the wells to terminate the reaction. The resultant absorbance was measured at 450 nm using an ELISA microplate reader (Biobase Bioindustry Co. Ltd., Shandong, China). The concentration of insulin, CORT or CRF was determined using their respective standard curve. Homeostasis model assessment-insulin resistance (HOMA-IR) was also calculated to measure the insulin sensitivity of the rats (Hanley et al., 2002): [Fasting plasma insulin (μg/L) × Fasting blood glucose (mg/dL)]/22.5.
2.6. Blood lipids
Plasma lipid levels of TG, TC, LDL-C, and HDL-C after treatment were determined by automatic biochemistry analyzer (BT, 2000 Plus, Germany) using diagnostic kits for each, purchased from BioSystems® (S.A Costa Brava of Barcelona, Spain).
2.7. Tissue isolation
After the last glucose level was determined, the animals were fasted overnight and then sacrificed by cervical dislocation. Tissue samples of the liver and gastrocnemius muscle were carefully dissected over ice, rinsed with 1.15 % KCl, blotted and weighed.
2.8. Muscle and liver glycogen determination
Glycogen contents in hepatocytes and myocytes were determined as described in our previous study (Morakinyo et al., 2018). By this method, liver and gastrocnemius muscles of experimental animals were harvested and cleaned immediately before known weight were homogenized in ice-cold trichloroacetic acid (deproteinizing) solution and incubated for 15 min in water-bath. After discarding the precipitate, the supernatant was mixed with sulphuric acid and heated for 5 min and the absorbance read with ELISA reader (Biobase Bioindustry Co. Ltd., Shandong, China) at 520 nm wavelength. A standard glycogen (Sigma; St. Louis, MO, USA) was also prepared and employed for the standard curve.
2.9. Statistical analysis
All results are presented as the mean ± standard error of mean (SEM). Statistical analyses were conducted using GraphPad Prism Software (GraphPad, Inc., La Jolla, CA, USA). Data analyses were performed by one-way analysis of variance (ANOVA) with post hoc Tukey's multiple comparison test. Statistical significance was set at p < 0.05.
3. Results
3.1. Effect of noise exposure on fasting glucose level
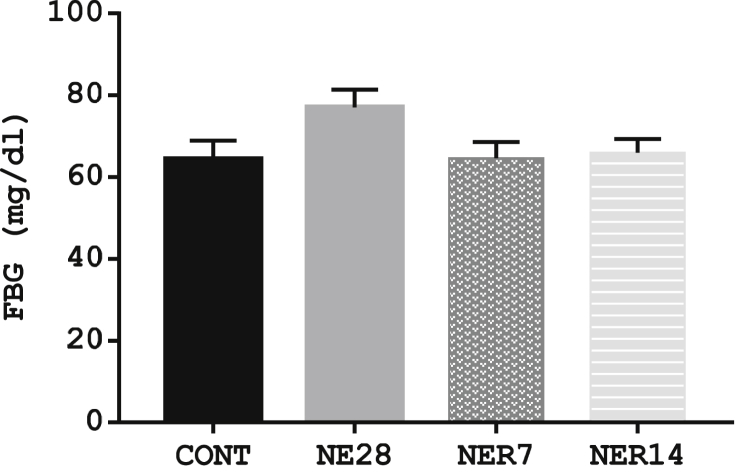
The fasting blood glucose concentration in NE28 rats was higher than control group (64.4 ± 4.49 vs. 77.0 ± 4.34 mmol/L) but the difference was not statistically significant (P = 0.17). Meanwhile, there was no significance difference (P = 0.99) in the glucose concentration in NER7 and NER14 rats (64.6 ± 4.49 and 66.0 ± 3.3 respectively) compared with the control (77.0 ± 4.34) following 7 and 14 days recovery period (Figure 1).
Figure 1.
Fasting blood glucose level in Sprague-Dawley rats exposed to noise stress. Values are expressed as mean ± SEM, n = 6 per group.
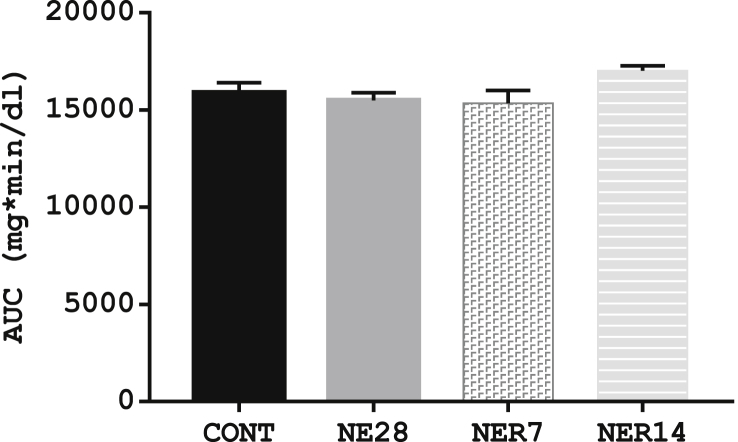
3.2. Effect of noise exposure on glucose tolerance
The blood glucose concentrations during the oral glucose tolerance test in noise-exposed groups and the control are shown in Table 1. The GTT reflects the efficiency with which the body handles glucose after an oral glucose load and results from this test showed no significant difference (P > 0.05) in the glucose tolerance of NE28, NER7 and NER14 rats when compared with control rats, as indicated by a similar AUC-glucose (Figure 2). Nonetheless, NE28 rats had a generally blunted response to the glucose load with a 31.7 %, 11.2 %, -1.8 % and -17.1 % rise in glucose concentration compared with 66.3 %, 85.9 %, 18 % and -1.3 % in control rats at 30-, 60-, 120- and 180-min post-glucose load respectively (see Table 1).
Table 1.
Blood glucose lebel during oral glucose tolerance test in male Sprague-Dawley rats exposed to noise stress.
| Time (min) | Control | NE28 | NER7 | NER14 |
|---|---|---|---|---|
| 0 | 63.8 ± 5.62 | 77.0 ± 4.34 | 64.6 ± 3.96 | 66.0 ± 3.30 |
| 30 | 106.2 ± 8. 45 (66.3 %) | 101.4 ± 7.74 (31.7 %) | 103.8 ± 18.43 (60.7 %) | 102.2 ± 7.94 (54.9 %) |
| 60 | 118.7 ± 8.43 (85.9 %) | 85.6 ± 7.82 (11.2 %) | 106.0 ± 13.58 (64.09 %) | 112.4 ± 4.74 (70.3 %) |
| 120 | 75.3 ± 7.87 (18.0 %) | 75.6 ± 6.10 (-1.8 %) | 77.4 ± 7.89 (19.8 %) | 93.6 ± 2.58 (41.8 %) |
| 180 | 63.0 ± 5.32 (-1.3 %) | 63.8 ± 4.62 (-17.1 %) | 62.0 ± 5.48 (-4.0 %) | 76.4 ± 3.06 (15.8 %) |
Data presented as mean ± SEM (n = 6 per group; unit – mg/dl). Values in parenthesis represent percent change in blood glucose level relative to 0 min.
Figure 2.
AUC of glucose tolerance test in Sprague-Dawley rats exposed to noise stress. Each point represents the mean with SEM (n = 6 per group). Data are expressed as mean ± SEM, n = 6 per group.
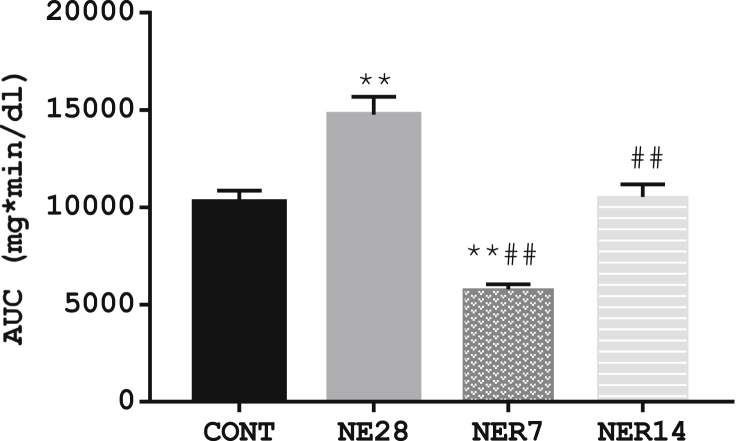
3.3. Effect of noise exposure on insulin tolerance
The results shown in Table 2 depicts changes in blood glucose in response to insulin challenge and demonstrate the effect of noise exposure on insulin tolerance. The rate of glucose disappearance in NE28 rats (-12.9 %) in response to the insulin challenge was remarkably lower than control rats (-21.6 %) at the 15-min post i.p. insulin and the trend continued throughout the serial measurement timeline. As depicted by Figure 3, noise stress appears to reduce insulin sensitivity in NE28 with a significant increase (P < 0.01) in AUC-insulin (14760 ± 929) when compared with control (10275 ± 581). However, insulin sensitivity was improved in NER7 rats with a significantly reduced AUC-insulin (5998 ± 239) compared with both control and NE28 rats.
Table 2.
Blood glucose level during insulin tolerance test in male Sprague-Dawley rats exposed to noise stress.
| Time (min) | Control | NE28 | NER7 | NER14 |
|---|---|---|---|---|
| 0 | 104.0 ± 2.99 | 105.4 ± 12.37 | 85.8 ± 8.79 | 109.5 ± 4.37 |
| 15 | 81.5 ± 5.75 (-21.6 %) | 91.8 ± 13.14 (-12.9 %) | 29.0 ± 4.48 (-66.2 %) | 86.3 ± 10.01 (-21.2 %) |
| 30 | 52.7 ± 8.67 (-49.4 %) | 74.6 ± 12.65 (-29.2 %) | 32.4 ± 3.50 (-62.2 %) | 62.3 ± 13.33 (-43.2 %) |
| 60 | 49.5 ± 8.98 (-52.4 %) | 74.8 ± 13.09 (-29.0 %) | 31.0 ± 2.65 (-63.9 %) | 48.0 ± 13.03 (-56.2 %) |
| 120 | 47.5 ± 10.12 (-54.3 %) | 85.0 ± 17.68 (-19.4 %) | 29.3 ± 4.87 (-65.9 %) | 47.5 ± 13.25 (-56.6 %) |
| 180 | 67.0 ± 6.40 (- 35.6 %) | 81.6 ± 15.20 (-22.6 %) | 28.0 ± 4.81 (-67.4 %) | 67.0 ± 6.00 (38.8 %) |
Data presented as mean ± SEM (n = 6 per group; units – mg/dl). Values in parenthesis represent percent change in blood glucose level relative to 0 min after insulin challenge.
Figure 3.
AUC of insulin tolerance test in Sprague-Dawley rats exposed to noise stress. Each point represents the mean with SEM (n = 6 per group). Data are expressed as mean ± SEM, **p < 0.01 vs control, ##p < 0.01 vs NE28, bp<0.01 vs NE7, n = 6 per group.
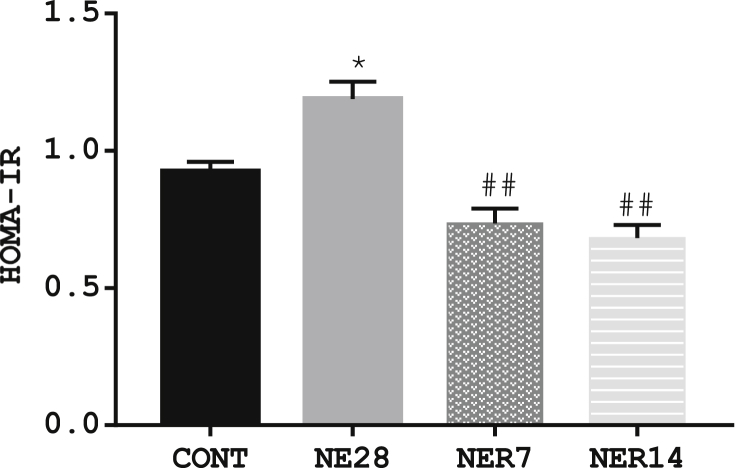
3.4. Effect of noise exposure on insulin sensitivity using HOMA-IR
We examined the changes on insulin sensitivity in noise-exposed rats and their control counterparts. HOMA-IR was significantly increased in NE28 (1.19 ± 0.035) but not in NER7 (0.74 ± 0.053) and NER14 (0.68 ± 0.047) compared with control rats (0.92 ± 0.035). Moreover, both values in NER7 and NER14 were within the control range (Figure 4).
Figure 4.
HOMA-IR indices in Sprague-Dawley rats exposed to noise stress. Data are expressed as mean ± SEM, *p < 0.05 vs control; ##p < 0.01 vs NE28, n = 6 per group.
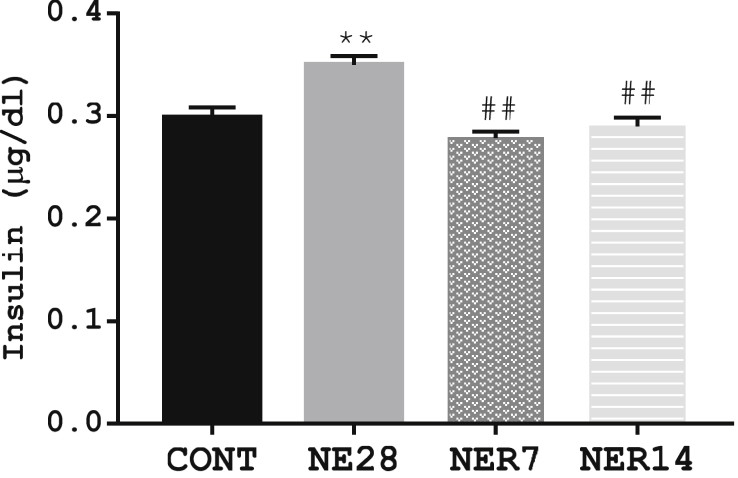
3.5. Effect of noise exposure on insulin concentration
Noise stress caused a significant (P = 0.002) increase in insulin concentration in NE28 group (0.35 ± 0.009) when compared with the control (0.30 ± 0.01). However, NER7 (0.28 ± 0.007) and NER14 (0.29 ± 0.009) rats had significantly (P ≤ 0.01) reduced concentration of insulin compared with NE28 rats (Figure 5).
Figure 5.
Effect of noise stress on the serum insulin concentration in Sprague-Dawley rats. Data are expressed as mean ± SEM, **p < 0.01 vs control; ##p < 0.01 vs NE28, n = 6 per group.
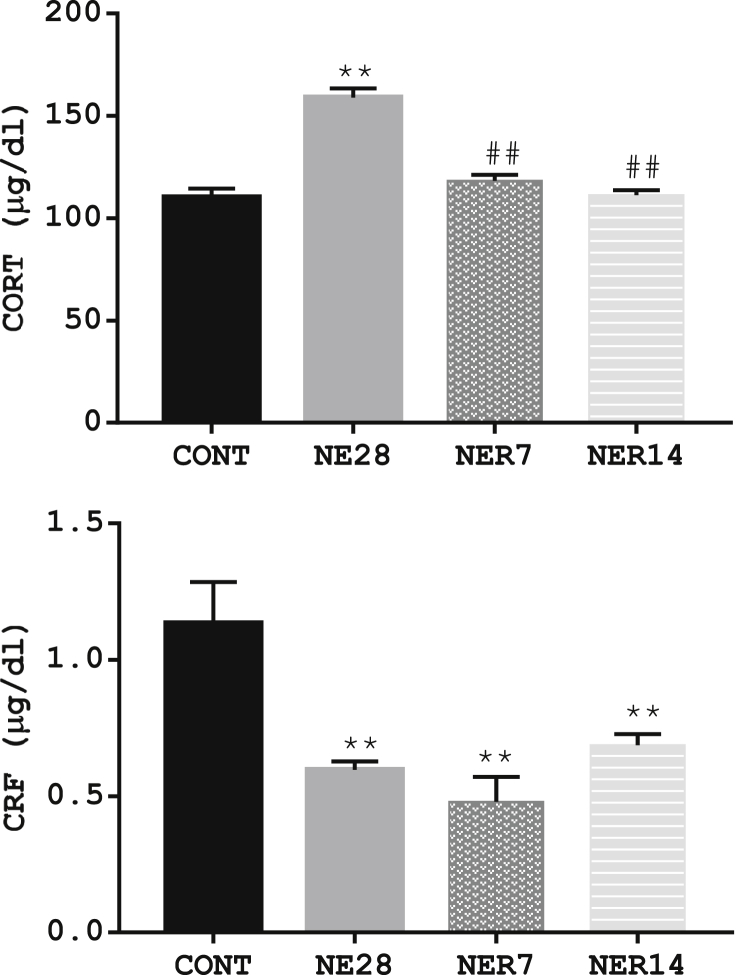
3.6. Effect of noise exposure on CORT and CRF concentrations
The level of CORT and CRF in rats exposed to noise stress and their control counterpart is depicted in Figure 6. There was a significant increase in CORT level in NE28 (159 ± 4.32) compared with control rats (110 ± 4.34. However, following a period of withdrawal from noise stress for 7 and 14 days, CORT concentration was significantly reduced (P = 0.0001) in NER7 and NER14 rats (118 ± 2.75 and 111 ± 2.39 respectively) compared to NE28 (159 ± 4.32). Conversely, the concentration of CRF was significantly (P ≤ 0.01) lower in NE28 (0.60 ± 0.03) as well as in NER7 (0.48 ± 0.09) and NER14 (0.69 ± 0.04) rats when compared with control (1.13 ± 0.15).
Figure 6.
Effect of noise exposure on the serum levels of CORT (A) and CRF (B) in Sprague-Dawley rats. Data are expressed as mean ± SEM, n = 6 per group, **p < 0.01 vs control, n = 6 per group.
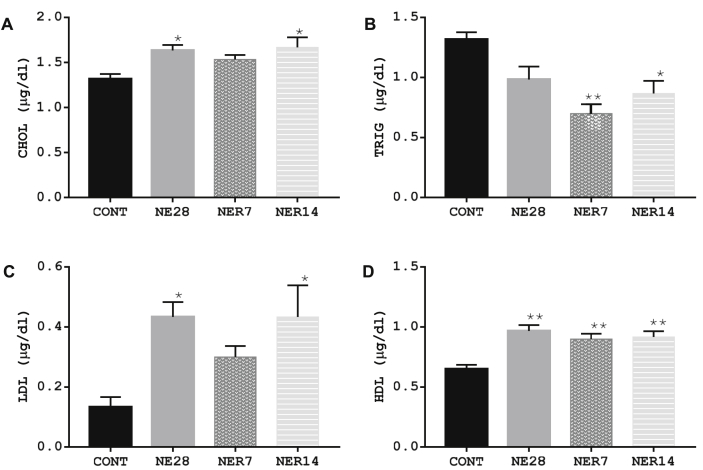
3.7. Effect of noise exposure on lipid profile
We examined the effect of noise stress on serum lipid profiles of male Sprague-Dawley rats. Figure 7 shows that the rats exposed to noise stress for 28 days (NE28 rats) had significantly higher levels of CHOL, LDL and HDL but TRIG level was similar to that seen in control rats. An apparent effect of 7 days recovery from stress was observed on CHOL and LDL values in NER7 rats, however HDL level was significantly higher (P = 0.003) in NER7 rats when compared with control. The pattern of lipid profile observed in NER14 rats was similar to that seen in NE28 rats, particularly with respect to CHOL, LDL and HDL levels which were significantly higher in NE14 rats compared to control rats. In both NER7 and NER14 rats, the levels of TRIG were significantly lower (P = 0.001 and P = 0.01 respectively) relative to the control group.
Figure 7.
CHOL (A), TRIG (B), LDL (C) and HDL (D) profile of Sprague-Dawley rats exposed to noise stress. Data are expressed as mean ± SEM, *p < 0.05 vs control; **p < 0.01 vs control; n = 6 per group.
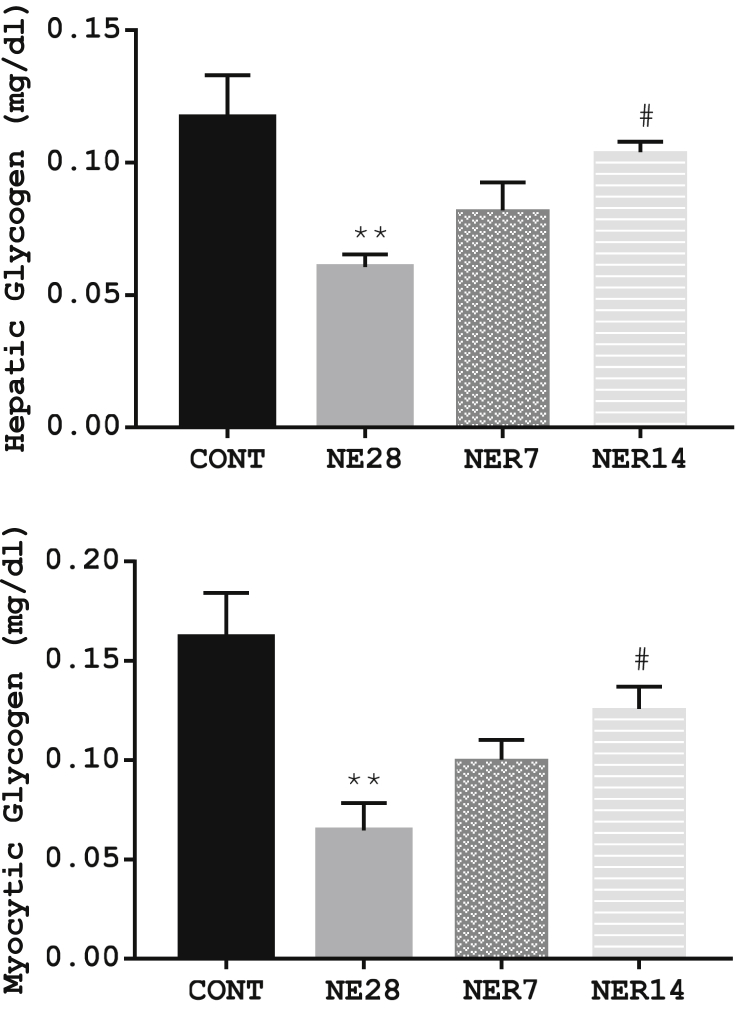
3.8. Effect of noise exposure on hepatic and myocytic glycogen storage
The contents of hepatic and myocytic glycogen storage in rats exposed to noise stress is shown in Figure 8. Rats exposed to noise stress for 28 days had a significantly lower hepatic and myocytic glycogen content than rats from control group. The reduced glycogen content due to stress exposure was reversed to control values 14 days post-recovery from stress as seen in NER14 rats, which had a significantly higher levels of glycogen in hepatic and myocytic tissues than NE28 rats.
Figure 8.
Effect of noise stress on hepatic glycogen (A) and myocytic glycogen (B) storage in Sprague-Dawley rats. Data are expressed as mean ± SEM, **p < 0.01 vs control, #p < 0.05 vs NE28, n = 6 per group.
4. Discussion
There is a general shift in the global burden of disease from communicable to non-communicable causes in the last decade, and recent evidence suggests that environmental factors contribute to the development of chronic non-communicable diseases (Lim et al., 2012). The study of noise as an environmental stressor is becoming more and more important in our industrialized world especially traffic noise from road, crowd and workplace among others (Munzel et al., 2014). Numerous studies have demonstrated that noise plays a significant role in the development of metabolic diseases (Munzel et al., 2017), including insulin resistance which is a key contributor to the development of Type 2 diabetes mellitus. However, limited work has been done to elucidate the mechanisms underlying the effect of noise stress in the development of these metabolic disorders.
Several studies have reported significant increases in plasma stress hormone levels during and after exposure to different forms of noise (Samson et al., 2007; Cui et al., 2016). Noise-exposed rats in the present study demonstrated elevated corticosterone level suggesting sustained response to noise stress. However, the concentration of corticosterone declined in the reversal groups of rats suggesting that withdrawal from exposure to noise leads to an abatement of noise stress-induced perturbation of hypothalamo-pituitary adrenal axis. This result follows the pattern of cortisol secretion in humans exposed to night noise (Maschke, 2003) and gives evidence of persistent rise in stress hormone occasioned by chronic nocturnal noise exposure (Ising and Braun, 2000). Cortisol provides the body with glucose under stressful conditions via increased gluconeogenesis in the liver (Dallman et al., 1993; Strack et al., 1995). However, elevated cortisol over a long term consistently produces glucose, leading to hyperglycaemia.
Furthermore, the increase in the FBG recorded in noise-exposed rats is an indication of disturbance of glucose homeostasis which was however reversed after a period of non-exposure to noise. Elevated level of stress hormones has been suggested to be responsible for altered glycaemic control (Knudsen et al., 2014) via catecholamines influence on hepatic glucose output (Yuen et al., 2013) and suppression of insulin-mediated glucose transport into skeletal muscle (Hunt and Ivy, 2002). It thus appears that the improvement in FBG in the recovery group is due to absence of the noise. Meanwhile, when the rats were challenged with glucose load during GTT, glucose response in noise-exposed rats were not remarkably different from the control animals although the glucose level trended higher in noise-exposed rats. We argue though that increased level of corticosterone without a notable deterioration of glucose tolerance capacity in rats exposed to noise stress might imply a delayed response in glucose tolerance to the insult or perhaps the exposure requires an extended period.
Interestingly, other endpoints of glucose homeostasis appear to be worsening in the noise-exposed rats within the same exposure window. Rats exposed to noise demonstrated a significant increase in serum insulin level. Insulin works as a key signal of blood glucose levels and mean plasma insulin level is used to indicate the relative degree of insulin sensitivity (Morakinyo et al., 2018). A higher insulin level in noise-exposed rats may be connected with the development of insulin resistance and worsening insulin function (Ferrannini and Mari, 1998). In addition, elevated insulin level might not be unconnected with the rising level of corticosterone in the animals. Cortisol is known to hinder insulin function and render the cells insulin resistant thereby potentially increasing the risk for type-2 diabetes mellitus (Ramamoorthy and Cidlowski, 2016; Magomedova and Cummins, 2016). Meanwhile a remarkable decline in insulin levels in the recovery groups following a period of noise withdrawal, is indicative of the possible recovery of insulin receptor functions in the uptake of glucose from the bloodstream into the body tissues.
In response to exogenous insulin challenge, both control and noise-exposed animals produced similar reaction in the first 15-minutes of insulin challenge with marked fall in glucose levels; however, the insulin-stimulated glucose uptake was attenuated in noise-exposed rats from the 30-minutes mark resulting in an elevated blood glucose level at 120-minutes post-insulin administration. This implies that noise stress predisposes the animals to insulin insensitivity. A significantly reduced level of insulin sensitivity is known as insulin resistance; a metabolic disorder in which cells are less responsive to the effect of circulating insulin, so that higher insulin concentrations are required to achieve euglycemia with a given glucose load (Cefalu, 2006). This view is substantiated by HOMA-IR data from the different groups which showed that noise-exposed rats demonstrated relatively higher HOMA-IR compared to control rats. HOMA-IR is an index of insulin resistance and a useful non-invasive way of determining insulin sensitivity in rodents (Andrikopoulos et al., 2008). Higher HOMA-IR relates to the level of insulin resistance such that the higher the number, the more resistant the tissues are to insulin. Taken together, the coincidence of increased fasting glucose and fasting insulin is symptomatic of noise-induced insulin resistance. This therefore provide some rationale for noise-stress induced gluco-metabolic dysfunction which may not be unrelated to the role of noise in the induction type 2 diabetes (Ozougwu et al., 2013).
Environmental stressors contribute to metabolic disorders such as hyperglycaemia and hyperlipidaemia (Babisch, 2002). It has been well established that adipose tissue plays a dynamic and critical role in the regulation of glucose and lipid metabolism as well as energy homeostasis (Bays et al., 2004). Based on this fact, it was deemed fit to evaluate the lipid profile of rats exposed to noise in the present study. A lipid profile is a direct measure of four blood components namely; total cholesterol (CHOL), triglycerides (TRIG), high-density lipoproteins cholesterol (HDL) and low-density lipoprotein cholesterol (LDL) (Schaefer et al., 2000). Noise-exposed rats had occurrence of pronounced lipid disturbance (increased LDL only) compared with the controls in the present study. The most likely cause of dyslipidaemia in noise-exposed animal is the increased free fatty-acid flux, secondary to insulin resistance. Insulin plays a critical role in the control of lipid metabolism. Besides facilitating the entry of glucose into adipocytes and providing more material for triglyceride synthesis within the adipocyte, insulin also inhibits the breakdown of fat by suppressing the adipose triglyceride lipase that hydrolyses triglycerides to release fatty acids (Park et al., 2015). However, this ability of insulin to inhibit lipolysis and reduce the plasma FFA concentration is markedly impaired in insulin resistance (Roder et al., 1998; Kasuga, 2006). We therefore reasonably interpret the significantly higher LDL level after 28 days of noise exposure as a metabolic biomarker of the gradual progression of glucometabolic disturbance in noise-exposed rats.
The liver is a major metabolic organ, and among its many functions, is the production of glucose (via gluconeogenesis and glycogenolysis) and its release into circulation (Radziuk and Pye, 2001). Overall, it is a principal organ in controlling blood sugar, because an imbalance in glucose release from the liver and uptake from peripheral tissues can lead to the persistent hyperglycaemia; a major contributing factor to the development of diabetes (Meyer et al., 2002). In noise-exposed rats, we observed that both hepatic glycogen synthesis and glycogen synthesis in skeletal muscle by insulin were stunted. It therefore appears that with progressing insulin resistance in noise-stressed rats, insulin action may be repressed at the liver and/or peripheral tissues, leading to increased hepatic glycogenolysis, suppressed glucose uptake into peripheral tissues and increased blood glucose levels.
In conclusion, the present study provide evidence that exposure to noise led to a dysregulated hypothalamo-pituitary adrenal axis activity with high corticosterone read-out, which appears to blunt the action of insulin in peripheral tissues; and, consequently limits glucose uptake as well as glycogen deposition in hepatic and gastrocnemius muscle of Sprague-Dawley rats. However, these adverse effects were reversible after a period of withdrawal from stress factor.
Declarations
Author contribution statement
Ayodele Olufemi MORAKINYO, Titilola Aderonke SAMUEL, Funmileyi Olubajo AWOBAJO: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Daniel Abiodun ADEKUNBI: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Idowu Olufemi OLATUNJI, Fortune Ucheonye BINIBOR, Adedotun Felicia ONI: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the University of Lagos Research Grant (CRC No. 2017/05).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Agrawal A., Jaggi A.S., Singh N. Pharmacological investigations on adaptation in rats subjected to cold water immersion stress. Physiol. Behav. 2011;103:321–329. doi: 10.1016/j.physbeh.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Andrikopoulos S., Blair A.R., Deluca N., Fam B.C., Proietto J. Evaluating the glucose tolerance test in mice. Am. J. Physiol. Endocrinol. Metab. 2008;295(6):E1323–E1332. doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- Babisch W. The noise/stress concept, risk assessment and research needs. Noise Health. 2002;4(16):1–11. [PubMed] [Google Scholar]
- Bays H., Mandarino L., DeFronzo R.A. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J. Clin. Endocrinol. Metab. 2004;89(2):463–478. doi: 10.1210/jc.2003-030723. [DOI] [PubMed] [Google Scholar]
- Cefalu W.T. Animal models of type 2 diabetes: clinical presentation and pathophysiological relevance to the human condition. ILAR J. 2006;47(3):186–198. doi: 10.1093/ilar.47.3.186. [DOI] [PubMed] [Google Scholar]
- Christensen J.S., Raaschou-Nielsen O., Tjonneland A., Overvad K., Nordsborg R.B., Ketzel M., Sorensen T., Sorensen M. Road traffic and railway noise exposures and adiposity in adults: a cross-sectional analysis of the Danish diet, cancer, and health cohort. Environ. Health Perspect. 2016;124(3):329–335. doi: 10.1289/ehp.1409052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C., Sbihi H., Tamburic L., Brauer M., Frank L.D., Davies H.W. Association of long-term exposure to transportation noise and traffic-related air pollution with the incidence of diabetes: a prospective cohort study. Environ. Health Perspect. 2017;125(8) doi: 10.1289/EHP1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui B., Gai Z., She X., Wang R., Xi Z. Effects of chronic noise on glucose metabolism and gut microbiota-host inflammatory homeostasis in rats. Sci. Rep. 2016;6:36693. doi: 10.1038/srep36693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman M.F., Strack A.M., Akana S.F., Bradbury M.J., Hanson E.S., Scribner K.A., Smith M. Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front. Neuroendocrinol. 1993;14:303–347. doi: 10.1006/frne.1993.1010. [DOI] [PubMed] [Google Scholar]
- Eze I.C., Foraster M., Schaffner E., Vienneau D., Heritier H., Rudzik F., Thiesse L., Pieren R., Imboden M., von Eckardstein A., Schindler C., Brink M., Cajochen C., Wunderli J.M., Roosli M., Probst-Hensch N. Long-term exposure to transportation noise and air pollution in relation to incident diabetes in the SAPALDIA study. Int. J. Epidemiol. 2017;46(4):1115–1125. doi: 10.1093/ije/dyx020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E., Mari A. How to measure insulin sensitivity. J. Hypertens. 1998;16(7):895–906. doi: 10.1097/00004872-199816070-00001. [DOI] [PubMed] [Google Scholar]
- Foraster M., Eze I.C., Vienneau D., Schaffner E., Jeong A., Heritier H., Rudzik F., Thiesse L., Pieren R., Brink M., Cajochen C., Wunderli J.M., Roosli M., Probst-Hensch N. Long-term exposure to transportation noise and its association with adiposity markers and development of obesity. Environ. Int. 2018;121(Pt 1):879–889. doi: 10.1016/j.envint.2018.09.057. [DOI] [PubMed] [Google Scholar]
- Hanley A.J., Williams K., Stern M.P., Haffner S.M. Homeostasis model of assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care. 2002;25(7):1177–1184. doi: 10.2337/diacare.25.7.1177. [DOI] [PubMed] [Google Scholar]
- Hunt D.G., Ivy J.L. Epinephrine inhibits insulin-stimulated muscle glucose transport. J. Appl. Physiol. 2002;93:1638–1643. doi: 10.1152/japplphysiol.00445.2002. [DOI] [PubMed] [Google Scholar]
- Ising H., Braun C. Acute and chronic endocrine effects of noise : review of the research conducted at the Institute for water, soil and air hygiene. Noise Health. 2000;2(7):7–24. [PubMed] [Google Scholar]
- Kahn C.R. Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metabolism. 1978;27(12 Suppl 2):1893–1902. doi: 10.1016/s0026-0495(78)80007-9. [DOI] [PubMed] [Google Scholar]
- Kasuga M. Insulin resistance and pancreatic beta cell failure. J. Clin. Investig. 2006;116(7):1756–1760. doi: 10.1172/JCI29189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempen E.V., Casas M., Pershagen G., Foraster M. WHO environmental noise guidelines for the European region: a systematic review on environmental noise and cardiovascular and metabolic effects: a summary. Int. J. Environ. Res. Public Health. 2018;15(2) doi: 10.3390/ijerph15020379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenerova V., Jurcovicova J., Kaminsky O., SõÂda P., Krejcõ I., HlinaÂk Z. Combined restraint and cold stress in rats: effects on memory processing in passive avoidance task and on plasma levels of ACTH and corticosterone. Behav. Brain Res. 2003;142:143–149. doi: 10.1016/s0166-4328(02)00401-1. [DOI] [PubMed] [Google Scholar]
- Knudsen S.H., Karstoft K., Pedersen B.K., van Hall G., Solomon T.P.J. The immediate effects of a single bout of aerobic exercise on oral glucose tolerance across the glucose tolerance continuum. Phys. Rep. 2014;2:e12114. doi: 10.14814/phy2.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S.S., Vos T., Flaxman A.D., Danaei G., Shibuya K., Adair-Rohani H., Amann M. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Wang F., Lu H., Cao S., Du Z., Wang Y., Feng X., Gao Y., Zha M., Guo M., Sun Z., Wang J. Effects of noise exposure on systemic and tissue-level markers of glucose homeostasis and insulin resistance in male mice. Environ. Health Perspect. 2016;124(9):1390–1398. doi: 10.1289/EHP162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magomedova L., Cummins C.L. Glucocorticoids and metabolic control. Handb. Exp. Pharmacol. 2016;233:73–93. doi: 10.1007/164_2015_1. [DOI] [PubMed] [Google Scholar]
- Maschke C. Stress hormone changes in persons exposed to simulated night noise. Noise Health. 2003;5(17):35–45. [PubMed] [Google Scholar]
- Meyer C., Dostou J.M., Welle S.L., Gerich J.E. Role of human liver, kidney, and skeletal muscle in postprandial glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 2002;282(2):E419–E427. doi: 10.1152/ajpendo.00032.2001. [DOI] [PubMed] [Google Scholar]
- Mohammadi H., Alimohammadi I., Roshani S., Pakzad R., Abdollahi M.B., Dehghan S.F. The effect of occupational noise exposure on blood and biochemical parameters: a case study of an insulator manufacturer in Iran. Electron. Physician. 2016;8(1):1740–1746. doi: 10.19082/1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morakinyo A.O., Iranloye B.O., Ogunsola O.A. Glucometabolic effects of single and repeated exposure to forced-swimming stressor in Sprague-Dawley rats. Endocr. Regul. 2018;52(2):85–92. doi: 10.2478/enr-2018-0010. [DOI] [PubMed] [Google Scholar]
- Munzel T., Gori T., Babisch W., Basner M. Cardiovascular effects of environmental noise exposure. Eur. Heart J. 2014;35(13):829–836. doi: 10.1093/eurheartj/ehu030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzel T., Sorensen M., Gori T., Schmidt F.P., Rao X., Brook J., Chen L.C., Brook R.D., Rajagopalan S. Environmental stressors and cardio-metabolic disease: part I-epidemiologic evidence supporting a role for noise and air pollution and effects of mitigation strategies. Eur. Heart J. 2017;38(8):550–556. doi: 10.1093/eurheartj/ehw269. [DOI] [PubMed] [Google Scholar]
- NIH . DHHS, PHS; 1985. Guide for the Care and Use of Laboratory Animals. NIH Publication No. 85-23. [Google Scholar]
- Ozougwu J.C., Obimba K.C., Belonwu C.D., Unakalamba C.B. The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. J. Physiol. Pathophysiol. 2013;4(4):46–57. [Google Scholar]
- Park S.E., Park C.Y., Sweeney G. Biomarkers of insulin sensitivity and insulin resistance: past, present and future. Crit. Rev. Clin. Lab. Sci. 2015;52(4):180–190. doi: 10.3109/10408363.2015.1023429. [DOI] [PubMed] [Google Scholar]
- Radziuk J., Pye S. Hepatic glucose uptake, gluconeogenesis and the regulation of glycogen synthesis. Diabetes Metab Res Rev. 2001;17(4):250–272. doi: 10.1002/dmrr.217. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S., Cidlowski J.A. Corticosteroids: mechanisms of action in health and disease. Rheum. Dis. Clin. N. Am. 2016;42(1):15–31. doi: 10.1016/j.rdc.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder M.E., Porte D., Jr., Schwartz R.S., Kahn S.E. Disproportionately elevated proinsulin levels reflect the degree of impaired B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 1998;83(2):604–608. doi: 10.1210/jcem.83.2.4544. [DOI] [PubMed] [Google Scholar]
- Samson J., Sheeladevi R., Ravindran R., Senthilvelan M. Stress response in rat brain after different durations of noise exposure. Neurosci. Res. 2007;57(1):143–147. doi: 10.1016/j.neures.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Schaefer E.J., Tsunoda F., Diffenderfer M., Polisecki E., Thai N., Asztalos B. The measurement of lipids, lipoproteins, apolipoproteins, fatty acids, and sterols, and next generation sequencing for the diagnosis and treatment of lipid disorders. In: Feingold K.R., Anawalt B., Boyce A., Chrousos G., Dungan K., Grossman A., Hershman J.M., Kaltsas G., Koch C., Kopp P., Korbonits M., McLachlan R., Morley J.E., New M., Perreault L., Purnell J., Rebar R., Singer F., Trence D.L., Vinik A., Wilson D.P., editors. Endotext. 2000. South Dartmouth (MA) [PubMed] [Google Scholar]
- Solin A.V., Lyashev Y.D. Stress-induced changes in the liver of rats with different resistance to stress. Bull. Exp. Biol. Med. 2014;157(5):571–573. doi: 10.1007/s10517-014-2617-7. [DOI] [PubMed] [Google Scholar]
- Sorensen M., Andersen Z.J., Nordsborg R.B., Becker T., Tjonneland A., Overvad K., Raaschou-Nielsen O. Long-term exposure to road traffic noise and incident diabetes: a cohort study. Environ. Health Perspect. 2013;121(2):217–222. doi: 10.1289/ehp.1205503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack A.M., Sebastian R.J., Schwartz M.W., Dallman M.F. Glucocorticoids and insulin: reciprocal signals for energy balance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1995;268:R142–R149. doi: 10.1152/ajpregu.1995.268.1.R142. [DOI] [PubMed] [Google Scholar]
- Yuen K.C.J., Chong L.E., Riddle M.C. Influence of glucocorticoids and growth hormone on insulin sensitivity in humans. Diabet. Med. 2013;30:651–663. doi: 10.1111/dme.12184. [DOI] [PubMed] [Google Scholar]