Abstract
Nonalcoholic fatty liver disease (NAFLD) has become the most prevalent liver disease worldwide. Despite its high prevalence and rising incidence, there are currently no specific targeted pharmacotherapies approved by the Food and Drug Administration (FDA) for nonalcoholic steatohepatitis (NASH). Current therapies for patients with NAFLD include lifestyle modification. Vitamin E and pioglitazone are recommended for those confirmed to have NASH. However, there are concerns about the long-term safety of both pioglitazone and vitamin E in higher doses. Metformin is essential for managing the abnormal metabolic parameters in patients with NAFLD. Glucagon-like peptide-1 analogue, sodium-dependent glucose cotransporter inhibitors, and peroxisome proliferator-activated receptor agonists have shown benefits in improving metabolic parameters and reducing hepatic lipid accumulation and inflammation. However, the role of these antidiabetic agents in specifically reversing NASH needs to be established. Indeed, statins have been underprescribed in patients with NASH owing to fear of hepatotoxicity despite coronary artery disease being a common cause of death in patients with NAFLD. Statins reduce the risk of cardiovascular morbidity and mortality in patients with NASH and dyslipidemia. However, their use specifically for treatment of NASH needs further evaluation. Optimizing the control of risk factors remains the main strategy for treatment until targeted pharmacotherapies for NASH are available.
Keywords: nonalcoholic fatty liver disease, GLP-1 receptor agonist, SGLT2 inhibitors, PPAR agonist, statins
Abbreviations: Non alcoholic fatty liver disease, NAFLD; Nonalcoholic steatohepatitis, NASH; metabolic syndrome, MetS; type 2 diabetes, T2D; cardiovascular disease, CVD; 5′ adenosine monophosphate-activated protein kinase, AMPK; body mass index, BMI; Alanine Aminotransferase, ALT; Aspartate transaminase, AST; glucagon-like peptide-1 receptor agonist, GLP-1RA; dipeptidyl peptidase-4 inhibitors, DPP-4i; Sodium-dependent glucose cotransporter inhibitor, SGLT-2i; Peroxisome proliferator-activated receptor agonist, PPAR agonist; LFT, liver function test; EASL/EASD/EASO, European Association for the Study of the Liver/European Association for the Study of Diabetes/European Association for the Study of Obesity
Over the years, a strong correlation has been established between metabolic syndrome (MetS) and nonalcoholic fatty liver disease (NAFLD), identifying NAFLD as a hepatic manifestation of MetS.1, 2 The prevalence of NAFLD is found to be two and a half times more in patients with MetS (27%) than without MetS (11%).3 Studies have also reported an increased risk of type 2 diabetes (T2D) and cardiovascular disease (CVD) in patients with NAFLD.4 In patients with T2D and CVD, the prevalence of NAFLD is 75% and 28%, respectively.5, 6 Besides, 28% of patients with NAFLD have hyperlipidemia.7 The pathogenesis of NAFLD is a result of a series of liver insults, known as the ‘multiple-hit’ hypothesis. These include insulin resistance, dietary substrates (carbohydrates and fats), endocrine hormones, gut microflora, and genetic factors.8 In view of the multiple hits responsible for progression of NAFLD to nonalcoholic steatohepatitis (NASH) and NASH-related fibrosis, prevention and management pose a challenge to drug discovery and development. One of the initial measures, which helps modulate the metabolic parameters in NAFLD, is lifestyle modification with diet and exercise.9 A weight reduction of 7–9% has shown to reduce inflammation, and a 10% weight reduction has shown to reduce fibrosis in patients with NASH. However, the lack of success in its implementation and challenge with sustaining weight loss has lead to the need for effective pharmacological agents. In the present review, we have discussed the role of antidiabetic agents and statins in the management of NASH.
Metformin
Originally derived from Galega officinalis, metformin has been the first choice of treatment in patients with T2D.10 As an insulin sensitizer, metformin lowers blood glucose levels by inhibiting hepatic gluconeogenesis, stimulating muscle glucose uptake and decreasing intestinal glucose absorption.11 Metformin induced AMPK (5' adenosine monophosphate activated protein kinase), activation decreases fatty acid synthesis and increases fatty acid oxidation. This contributes to reduction in hepatic fat and improved insulin sensitivity in patients with NAFLD.12 In addition, metformin supplementation has shown to reduce hypertension and hyperlipidemia in patients with NAFLD, thereby reducing cardiovascular morbidity.13
Along with regulation of impaired fasting glucose, hypertension, and dyslipidemia, patients with NAFLD need weight management. Metformin aids in weight loss by decreasing the appetite and sensitizing insulin. A study on the ob/ob mice model of hepatic steatosis showed the role of metformin in normalizing alanine aminotransferase (ALT) levels by decreasing the expression of tumor necrosis factor α.14 In nondiabetic patients with NASH, metformin supplementation significantly improved the body mass index (BMI), insulin sensitivity, ALT levels, and liver volume determined with ultrasonography. However, metformin plus lifestyle intervention did not improve liver histology or aminotransferases, compared with lifestyle intervention alone, independent of dose, treatment duration, or diabetic state.15, 16 Furthermore, in the TONIC trial, involving 173 nondiabetic patients with NASH diagnosed by liver biopsy, metformin supplementation failed to reduce ALT levels and improve liver histology compared with placebo.17 Some studies have also demonstrated that long-term supplementation of metformin may reduce the risk of liver cancer by 62% in patients with T2D.18, 19
Despite improvement in the metabolic parameters, namely, insulin resistance, hypertension, dyslipidemia, and BMI, European Association for the Study of the Liver (EASL) guidelines do not recommend metformin for treatment of NASH in view of lack of histological benefit.20
Incretin-based therapies: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors
Incretins account for up to 70% of insulin response after oral intake of glucose. It is mediated by glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) released from enteroendocrine cells in the gastrointestinal tract after food ingestion. They have a very short half-life as they are degraded by dipeptidyl peptidase-4 (DPP-4). The half-life is prolonged using the DPP-4 inhibitor (DPP-4i), or the levels are increased by using GLP-1 receptor agonists (GLP-1RAs).21
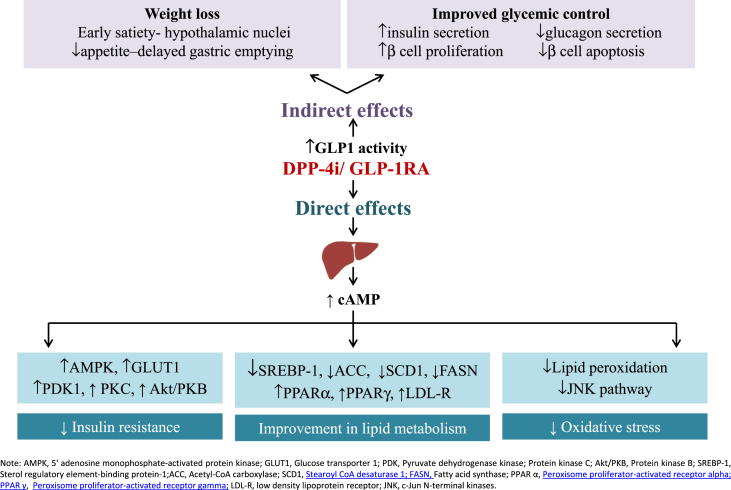
Evidence from studies in mice suggests the presence of hepatic GLP-1 resistance at the GLP-1 receptor level or downstream. Reduced GLP-1R expression and DPP-4 upregulation have been reported in liver tissue of patients with NAFLD. Bernsmeier et al22 showed that GLP-1 secretion is deficient in patients with NAFLD and NASH. GIP secretion is contrarily preserved. Insulin resistance accompanied by hyperglucagonemia is found to be more severe in patients with NASH. It therefore remains unclear whether deficiency in GLP-1 secretion, hepatic GLP-1 resistance, or both the components is involved. These pathophysiologic findings provide evidence for the use of the GLP-1R agonist for the treatment of NAFLD. Incretins have shown to have beneficial effect indirectly by improvement in glycemic control and by inducing weight loss. In addition, they bind to the GLP-1R on hepatocytes to reduce insulin resistance, lipotoxicity, and oxidative stress, as summarized in Figure 1.23
Figure 1.
Direct and Indirect effects of GLP-1RA/DPP-4 i on NAFLD (Adapted and modified from Dhir et al., 201823). GLP-1, glucagon-like peptide-1; DPP-4, dipeptidyl peptidase-4; NAFLD, nonalcoholic fatty liver disease.
Glucagon-like peptide-1 receptor agonist
Several studies have hinted at a potential benefit of GLP-1RA in NAFLD, but until recently evidence is inconclusive. A meta-analysis of six, phase III, randomized controlled trials encompassing the LEAD program was performed by Armstrong et al24 to evaluate the effect of liraglutide on liver parameters compared with active placebo in patients with T2D. Of the 4442 patients analyzed, 50.8% had baseline ALT abnormality. Liraglutide 1.8 mg/day led to significant reductions in ALT (p = 0.003) and hepatic steatosis on CT imaging compared with placebo. However, both effects were lost after adjusting for weight reduction and glycosylated hemoglobin (HbA1c); thus, effects were attributable to changes in weight and glycemic control. In the LEAN study, overweight patients with NASH and diabetes and those with NASH and without diabetes were treated with liraglutide 1.8 mg/day for 48 weeks or placebo. A total of 39% patients in the liraglutide group achieved resolution of steatohepatitis without worsening of fibrosis as against 9% in the placebo group (p = 0.019). In addition, fibrosis progressed in only 9% of patients in the liraglutide group as compared with 36% of patients in the placebo group (p = 0.04).25 In a prospective, single-center study (LIRA–NAFLD), patients with uncontrolled T2D (HbA1C > 7%) were treated with subcutaneous liraglutide 1.2 mg daily on background oral antidiabetic drugs/insulin for 6 months. There was significant reduction in liver fat content in patients treated with liraglutide, and the effect was mainly driven by body weight reduction.26 Eguchi et al reported a reduction in the BMI, visceral fat accumulation, aminotransferases, and hyperglycemia in 19 patients with biopsy-proven NASH treated with liraglutide for 24 weeks. There was also improvement in liver histology in 6 of 10 patients who repeated liver biopsy after 96 weeks of liraglutide treatment.27
In a randomized trial, 30 obese adults with NAFLD were randomized to a supervised program of energy restriction plus moderate-intensity exercise to induce ≥5% weight loss or liraglutide 3 mg daily for 26 weeks, followed by 26 weeks with only advice to prevent weight regain. In the liraglutide group, the participants were only advised to stop eating if they developed satiety, but no specific dietary advice was given. The participants in both groups had significant (p < 0.01) and similar reductions in weight, liver fat fraction, serum ALT, and caspase-cleaved cytokeratin 18 at 26 weeks. Six months after stopping both interventions, these benefits were not sustained in the liraglutide group despite advice on weight maintenance in contrast with the preserved benefits of supervised lifestyle modification. The use of liraglutide for the treatment of NAFLD in overweight and obese adults should be combined with diet and exercise, underscoring the importance of lifestyle modification for the management of NAFLD.28
GLP-1RAs are usually well tolerated, except for a modest increase in gastrointestinal symptoms of nausea and diarrhea. Current evidence does not support the concern of an increased risk of pancreatitis. A meta-analysis showed that the prevalence of pancreatitis and pancreatic cancer in patients on GLP-1RA was not significantly different from that observed in comparator arms.29, 30 The GLP-1RA has been studied in patients with NASH who are obese with/without T2D. Benefits of GLP-1RAs outweigh the risks in patients with NAFLD/NASH and diabetes or those with NAFLD/NASH and without diabetes. Whether the beneficial effects are just related to weight loss or they have direct effects needs further elucidation.31 Although liraglutide has not been further evaluated in phase 3 development, semaglutide, another GLP-1 analogue is under phase 2B trial evaluating its efficacy versus placebo in 372 participants with stage F2–F3 fibrosis and NAFLD activity score (NAS) ≥4 with a score of at least 1 for each of the components (steatosis, ballooning, and lobular inflammation).32
Dipeptidyl peptidase-4 inhibitor
The DPP-4i increases incretin effect by delaying quick inactivation of GLP-1 in plasma. The hepatic expression of DPP-4 is increased significantly in patients with NAFLD. Most of the studies evaluating the DPP-4i in NAFLD have used sitagliptin as it was the earliest molecule widely used in the group. Iwasaki et al33 found that 4 months of treatment with sitagliptin 50 mg/day in 30 patients with NAFLD was associated with significant decrease in aspartate transaminase (AST), ALT, and γ-gamma glutamyl transferase (GGT) levels, in addition to improvement in the glycemic parameters. A study by Yilmaz et al34 showed that treatment with sitagliptin for 1 year was associated with significant reduction in NASH scores and a trend towards improved hepatic steatosis. In contrast, in a study conducted by Fukuhara et al35 in 44 patients with biopsy-proven NAFLD, sitagliptin treatment showed no significant change in liver transaminases despite a reduction in HbA1C levels.
In a study by Cui et al, 50 patients with NAFLD and prediabetes or early stages of diabetes were randomized to sitagliptin 100 mg/day versus placebo and followed up for 24 weeks. Liver fat reduction was not statistically significant as measured by the magnetic resonance imaging (MRI)–based biomarker of proton density fat fraction (PDFF) in several liver segments (p = 0.4).36
The DPP-4i is well tolerated and improves glycemic control; however, current evidence does not support its use in NASH.
Sodium-dependent glucose cotransporter inhibitors
Sodium-dependent glucose cotransporter-2 (SGLT2) is expressed in proximal renal tubules. They are responsible for a majority of glucose reabsorption from the tubular lumen. The sodium-dependent glucose cotransporter-2 inhibitor (SGLT-2i) (gliflozins) inhibits SGLT2 and improves glycemic control by promoting urinary glucose excretion. In addition, it leads to weight loss and blood pressure reduction with a minimal risk of hypoglycemia.37
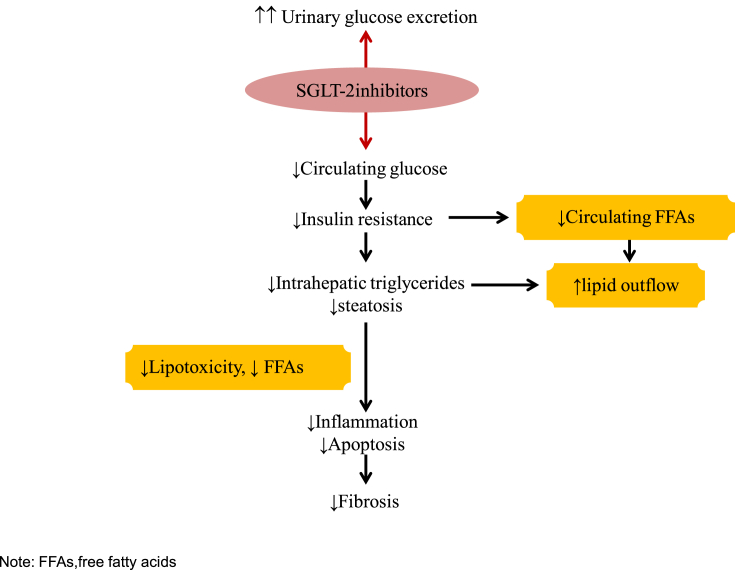
The adipose tissue serves as energy storage reservoirs against calorie overload. Beyond its capacity, the excess calorie accumulates in ectopic nonadipose tissues, such as the liver, skeletal muscle, and pancreas. In these organs, lipotoxicity leads to their metabolic dysfunctions and histopathologic changes. The SGLT-2i reduces de novo lipogenesis by attenuating hyperglycemia and hyperinsulinemia. It promotes fat accumulation in epididymal fat and prevents ectopic fat accumulation in the liver by improving insulin sensitivity in adipose tissue. The fibrosis of white adipose tissue limits its lipid storage capacity via inhibiting adipocyte hypertrophy, which leads to ectopic lipid accumulation in the liver. SGLT-2i lead to the adipose expansion without an increase in inflammation or fibrosis referred to as “healthy adipose expansion.” In mice models of fatty liver, canagliflozin has shown to alter glutathione metabolism favorably to reduce oxidative stress in adipose tissue. The postulated mechanism of action of the SGLT-2i in NAFLD is summarized in Figure 2.38, 39
Figure 2.
Adapted and modified. Mechanism of action of SGLT-2 inhibitors in NAFLD. SGLT2, sodium-dependent glucose cotransporter-2; NAFLD, nonalcoholic fatty liver disease; FFA, free fatty acids.
The benefits of the SGLT-2i in the prevention of NAFLD have been demonstrated in animals.38 Seko et al conducted a retrospective study comparing 24 weeks of treatment with ipragliflozin 50 mg or canagliflozin 300 mg with sitagliptin in patients with T2D and biopsy-proven NAFLD. Both groups had significant improvements in serum AST and ALT levels from baseline; however, weight loss was significant in the SGLT-2i group.39 A retrospective review was conducted by Ohki et al in Japanese patients with NAFLD and T2D who had abnormal ALT levels despite treatment with the GLP-1RA or DPP-4i. Addition of the SGLT-2i as a second-line treatment resulted in significant decrease in ALT (62–38 IU/L; p < 0.01), with 58.3% of patients achieving normalization of ALT levels. It also improved the fibrosis-4 index, from 1.75 to 1.39 (p = 0.04).40
The E-LIFT trial with empagliflozin, a study from India, enrolled 50 patients with T2D and NAFLD who were randomly assigned into two groups for 20 weeks, the empagliflozin group (standard treatment for T2D plus empagliflozin 10 mg daily) and control group (standard treatment without empagliflozin). Change in liver fat was measured by MRI-PDFF. Patients on empagliflozin had significant reduction in liver fat (mean MRI-PDFF difference, 4.0%; p < 0.0001) compared with the control group.41
Two investigator-initiated single-arm open-label studies have used paired liver biopsies to evaluate the effect of the SGLT-2i for the treatment of NASH in patients with T2D. These pilot studies have shown improvement in scores of steatosis, lobular inflammation, ballooning, and fibrosis stage, thus serving as the impetus for larger, randomized, double-blind, placebo-controlled studies on the SGLT-2i for the treatment of NASH.42, 43 In addition, the SGLT-2i improves cardiometabolic parameters such as systolic blood pressure, diastolic blood pressure, fasting blood glucose, and high density lipoprotein (HDL) cholesterol. Improvement in cardiac and renal outcomes is important because CVD is the leading cause of mortality in patients with NAFLD.44, 45 Data evaluating the long-term safety and tolerability of the SGLT-2i are lacking owing to their recent introduction on the market. In 2015, the FDA released warning cautioning prescribers to screen for urinary tract infections in patients on SGLT-2i. In addition, Fournier's gangrene (FG) is a newly identified safety concern in patients receiving SGLT-2i. FG is a newly identified safety concern in patients receiving SGLT-2i.46
Peroxisome proliferator-activated receptor agonist
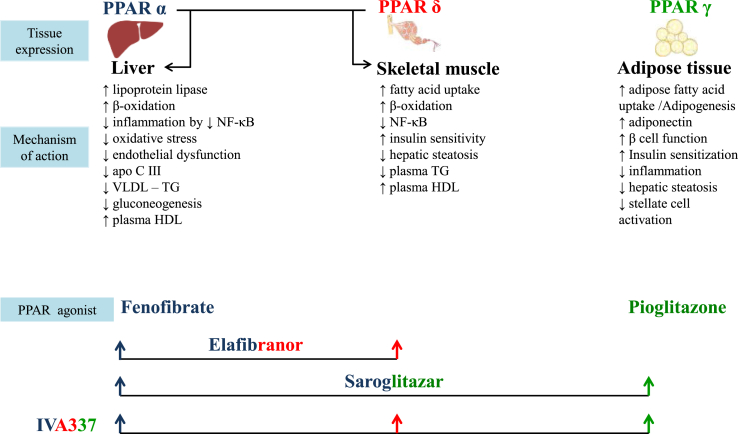
Peroxisome proliferator-activated receptor (PPAR) agonists are ligand-activated nuclear receptors consisting of three isoforms: PPARα, PPARδ, and PPARγ. Each of these isoforms differs from each other with respect to their action (Figure 3). The PPARα agonist fenofibrate has beneficial effects in reducing triglycerides and decreasing synthesis of apolipoproteins. In addition, its anti-inflammatory and antioxidant actions help reduce necroinflammation, apoptosis, and fibrosis. However, data regarding the effect of fenofibrate in NASH are inconclusive.47
Figure 3.
Mechanism of action of the PPAR agonist in NAFLD. PPAR, peroxisome proliferator-activated receptor; NAFLD, nonalcoholic fatty liver disease; NF- kB, nuclear factor kappa-light-chain-enhancer of activated B cells; VLDL, very low density lipoprotein; TG, triglyceride; HDL, high density lipoprotein.
Pioglitazone, a PPARγ agonist, is recommended for patients with T2D. Pioglitazone improves insulin and glucose parameters, increases lipid storage in subcutaneous adipose tissue, increases adiponectin, and reduces lipotoxicity in the liver.48 In the PIVENS trial, nondiabetic patients with NASH were treated with 30 mg pioglitazone. Compared with placebo, pioglitazone reduced liver inflammation but not fibrosis.48 Owing to probability of relapse of NASH after discontinuation, pioglitazone needs to be continued for a longer period. Treatment with pioglitazone for >24 months and at a cumulative dose of >28,000 mg (average daily dose of pioglitazone about 40 mg/day) has been linked to bladder cancer.49 The efficacy of low-dose pioglitazone (7.5–15 mg) in NASH needs further elucidation. In addition to risk of malignancy, pioglitazone has side effects such as weight gain, bone loss, and peripheral edema.50
The coexistence of T2D and dyslipidemia has also led to the development of dual PPAR agonists. The PPARα/γ agonist, saroglitazar, has shown to improve liver histopathology and biochemistry in experimental NASH models.51 Currently, phase 3 randomized controlled trial (RCT) is ongoing in India to assess the effect of saroglitazar versus placebo for 52 weeks in biopsy-proven noncirrhotic NASH. Saroglitazar, unlike pioglitazone, does not lead to weight gain and peripheral edema.52
PPARδ decreases hepatic gluconeogenesis and fatty acid oxidation, improves insulin sensitivity, and inhibits activation of macrophages and Kupffer cells. The dual PPARα/δ agonist, elafibranor, has been shown to improve liver, adipose tissue, and peripheral tissue insulin sensitivity and reduce ALT levels in patients with MetS.53 In a phase 2b randomized double-blind placebo-controlled trial in patients with biopsy-proven noncirrhotic NASH, 19% of study participants with NAS>4 on elafibranor 120 mg/day showed resolution of NASH as compared with 12% on placebo (p = 0.045). Currently, elafibranor 120 mg/day is being evaluated in a phase 3 randomized clinical trial for its effect on liver histology and mortality in patients with NAS >4. Recently, a new-generation pan-PPAR agonist, IVA 337, is found to activate all the three isoforms of PPARα, PPARδ, and PPARγ. In addition to encouraging results in preclinical and clinical studies, its safety profile makes it a promising drug for NASH.54
Statins
Statins reduce cholesterol biosynthesis by inhibiting 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase. In addition, owing to their anti-inflammatory, antioxidant, and antifibrotic effects, they may be beneficial for the treatment of NASH.55 The subclasses of statins function differently with respect to oral absorption, bioavailability, liver extraction, protein binding, and HMG-CoA reductase activity. Atorvastatin, lovastatin, simvastatin, and fluvastatin are lipophilic in nature and are metabolized by the cytochrome P450 system. On the other hand, pravastatin and pitavastatin are hydrophilic and thus are minimally metabolized in the liver, whereas rosuvastatin has an intermediate behavior. Studies have shown that the hydrophobic statins, lovastatin and simvastatin, reach a higher concentration in the liver compared with hydrophilic pravastatin. However, the exact clinical relevance of these differences is uncertain.56
Despite patients with T2D and NAFLD being at a higher risk of CVD compared with patients with diabetes alone, statins are underprescribed in NASH. This is mainly because of the concern regarding their safety in patients with deranged liver function tests (LFTs).56, 57 A comprehensive review of the safety and efficacy of statins in patients with chronic liver disease, including patients with NAFLD, concluded that statins were safe for the management of dyslipidemia in patients with NASH.58
Statins have shown considerable improvement in liver histology in in vivo NASH models. In 3 post hoc analyses of RCT (n = 1,600, n = 1,123, and n = 8864) the use of atorvastatin had beneficial effect in NAFLD/NASH, in terms of reduction in liver enzymes and normalization of liver echogenicity. In the study, the atorvastatin dose of up to 80 mg/day was deemed to be safe.56, 57 In a 1-year pilot study (n = 20), rosuvastatin monotherapy (10 mg/day) lead to complete NASH resolution in biopsy-proven NASH in patients with MetS. In addition, the LFT normalized, and ultrasonography showed absence of steatosis at the end of the study. The beneficial effect of rosuvastatin was attributed to the reduction in systemic inflammation.57, 58In a multicenter, prospective study involving 107 European individuals, statins had a protective effect on NAS (p < 0.001) and the fibrosis stage F2–F4 (p = 0.017). The amelioration of NASH and histological lesions correlated strongly with the dose of statins.56, 57
The recommendations for use of statins in patients with NAFLD are summarized in Table 1.56 The EASL/European Association for the Study of Diabetes/European Association for the Study of Obesity guidelines do not suggest statin administration for the treatment of NAFLD/NASH; however, they suggest statins can be safely prescribed in NAFLD/NASH with dyslipidemia. Further statin therapy is contraindicated for patients with decompensated cirrhosis and acute liver failure.9, 20
Table 1.
Summary of recommendations for statin treatment in patients with NAFLD (Adapted and modified from Pastori et al., 201556).
| Long-term statin treatment and liver toxicity |
|
| Statins for the treatment of dyslipidemia in patients with nonalcoholic fatty liver disease |
|
| Statins for the treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis |
|
LFT, liver function test; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; CVD, cardiovascular disease.
Apart from control of diabetes, antidiabetes drugs may have variable effects on the resolution of NASH (Table 2).59 Optimal control of risk factors with lifestyle modification, antidiabetic drugs, and statins will form an integral part of the management of patients with NAFLD until specific targeted therapies are available to prevent progression of NASH and fibrosis.
Table 2.
Anti diabetic drugs for management of NAFLD (Adapted and modified from Diana et al., 201659).
| Class | Metabolic effect | Drug | Steatosis | Inflammation | Fibrosis |
|---|---|---|---|---|---|
| Biguanides | ↓Hepatic glucose output | Metformin | ↓ | ↔ | ↔ |
| DPP-4 inhibitors | ↑Endogenous level of GLP-1 | Sitagliptin | NA | NA | NA |
| Vildagliptin | |||||
| Linagliptin | |||||
| GLP-1 receptor agonists | ↑Glucose-dependent insulin secretion ↓Gastric emptying and appetite |
Liraglutide | ↓ | ↓ | ↔ |
| SGLT2 inhibitors | ↓Renal glucose reabsorption | Canagliflozin | ↓ | NA | NA |
| Empagliflozin | |||||
| PPAR agonist | PPARα: ↑ insulin sensitivity | Fenofibrate | ↔ | ↓ ALT | ↔ |
| PPARγ: ↑ fatty acid uptake and adipose tissue lipogenesis | Pioglitazone | ↓ ALT | ↔/↓ | ||
| Dual PPARα/γ: ↑insulin sensitivity, ↑β-oxidation | Saroglitazar | ↓ | ?(↓)a | ?(↓)a | |
| Dual PPARα/δ: ↑ insulin sensitivity, ↓ hepatic gluconeogenesis | Elafibranor | ↓ | ?(↓)a | ?(↓)a | |
| Pan-PPARα/β/γ: ↑ insulin sensitivity, ↑ β-oxidation | IVA337 | ↓ | ?(↓)b | ?(↓)b |
DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1; SGLT2, sodium-dependent glucose cotransporter-2; NAFLD, nonalcoholic fatty liver disease; PPAR, peroxisome proliferator-activated receptor; NA, data not available.
↓ - decrease; ↑ - increase; ↔ equivalence; ? – Not known.
Dual PPAR agonists are currently in phase 3 clinical trials.
The pan-PPAR agonist is currently in phase 2b clinical trials.
Conflicts of interest
The authors have none to declare.
References
- 1.Byrne C.D., Targher G. Ectopic fat, insulin resistance, and nonalcoholic fatty liver disease implications for cardiovascular disease. Arterioscler Thromb Vasc Biol. 2014;34:1155–1161. doi: 10.1161/ATVBAHA.114.303034. [DOI] [PubMed] [Google Scholar]
- 2.Dowman J.K., Tomlinson J.W., Newsome P.N. Pathogenesis of non alcoholic fatty liver disease. QJM. 2010;103:71–83. doi: 10.1093/qjmed/hcp158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younossi Z., Anstee Q.M., Marietti M. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 4.Matteoni C.A., Younossi Z.M., Gramlich T., Boparai N., Liu Y.C., McCullough A.J. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 5.Mishra S., Yadav D., Gupta M., Mishra H., Sharma P. Hyperinsulinemia predisposes to NAFLD. Indian J Clin Biochem. 2008;23:130–135. doi: 10.1007/s12291-008-0030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagström H., Nasr P., Ekstedt M. Cardiovascular risk factors in non-alcoholic fatty liver disease. Liver Int. 2018;39:197–204. doi: 10.1111/liv.13973. [DOI] [PubMed] [Google Scholar]
- 7.Shahab O., Biswas R., Paik J., Bush H., Golabi P., Younossi Z.M. Among patients with NAFLD, treatment of dyslipidemia does not reduce cardiovascular mortality. Hepatol Commun. 2018;2:1227–1234. doi: 10.1002/hep4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buzzetti E., Pinzani M., Tsochatzis E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65:1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Chalasani N., Younossi Z., Joel E.L. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American association for the study of liver diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Rangel E., Inzucchi S.E. Metformin: Clinical use in type 2 diabetes. Diabetologia. 2017;60:1586–1593. doi: 10.1007/s00125-017-4336-x. [DOI] [PubMed] [Google Scholar]
- 11.Foretz M., Guigas B., Bertrand L., Pollak M., Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20:953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Madiraju A.K., Erion D.M., Rahimi Y. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510:542–546. doi: 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomopoulos C., Katsimagklis G., Makris T. Metformin and blood pressure lowering: a questioned association. J Hypertens. 2017;35:18–26. doi: 10.1097/HJH.0000000000001146. [DOI] [PubMed] [Google Scholar]
- 14.Loomba R., Lutchman G., Kleiner D.E. Clinical trial:pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29:172–182. doi: 10.1111/j.1365-2036.2008.03869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y., Liu L., Wang B. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Rep. 2012;1:57–64. doi: 10.3892/br.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musso G., Gambino R., Cassader M., Pagano G. A meta- analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- 17.Lavine J.E., Schwimmer J.B., Natta M.L. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2012;305:1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Z.J., Zheng Z.J., Shi R., Su Q., Jiang Q., Kip K.E. Metformin for liver cancer prevention in patients with type 2 diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97:2347–2353. doi: 10.1210/jc.2012-1267. [DOI] [PubMed] [Google Scholar]
- 19.Chen H.P., Shieh J.J., Chang C.C. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 62. 2013:606–615. doi: 10.1136/gutjnl-2011-301708. [DOI] [PubMed] [Google Scholar]
- 20.European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO) EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Baggio L.L., Drucker D.J. Biology of Incretins: GLP-1 and GIP. Gastroenterology. 2007;132:131–157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 22.Bernsmeier C., Meyer-Gerspach A.C., Blaser L.S. Glucose-Induced Glucagon-Like Peptide 1 Secretion Is Deficient in Patients with Non-Alcoholic Fatty Liver Disease. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0087488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhir G., Cusi K. Glucagon like peptide-1 receptor agonists for the management of obesity and non-alcoholic fatty liver disease: a novel therapeutic option. J Investig Med. 2018;66:7–10. doi: 10.1136/jim-2017-000554. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong M.J., Gaunt P., Aithal G.P. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 25.Nauck M., Frid A., Hermansen K. E cacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petit J.-M., Cercueil J.-P., Loffroy R. Effect of liraglutide therapy on liver fat content in patients with inadequately controlled type 2 diabetes: The lira-NAFLD study. J Clin Endocrinol Metab. 2017;102:407–415. doi: 10.1210/jc.2016-2775. [DOI] [PubMed] [Google Scholar]
- 27.Eguchi Y. Pilot study of liraglutide effects in non-alcoholic steatohepatitis and non-alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN-J) Hepatol Res. 2015;54:269–278. doi: 10.1111/hepr.12351. [DOI] [PubMed] [Google Scholar]
- 28.Khoo J., Hsiang J.C., Taneja R. Randomized trial comparing effects of weight loss by liraglutide with lifestyle modification in non-alcoholic fatty liver disease. Liver Int. 2019;39:941–949. doi: 10.1111/liv.14065. [DOI] [PubMed] [Google Scholar]
- 29.Pinto L.C., Rados D.V., Barkan S.S., Leitão C.B., Gross J.L. Dipeptidyl peptidase-4 inhibitors, pancreatic cancer and acute pancreatitis: A meta-analysis with trial sequential analysis. Sci Rep. 2018;8:782. doi: 10.1038/s41598-017-19055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong Y., Lv Q., Li S. Efficacy and safety of glucagon-like peptide-1 receptor agonists in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2017;41:284–295. doi: 10.1016/j.clinre.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Rotman Y., Sanyal A.J. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2017;66:180–190. doi: 10.1136/gutjnl-2016-312431. [DOI] [PubMed] [Google Scholar]
- 32.Connolly J.J., Ooka K., Lim J.K. Future Pharmacotherapy for Non-alcoholic Steatohepatitis (NASH): Review of Phase 2 and 3 Trials. J Clin Transl Hepatol. 2018;6:264–275. doi: 10.14218/JCTH.2017.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwasaki T., Yoneda M., Inamori M. Sitagliptin as a novel treatment agent for non-alcoholic Fatty liver disease patients with type 2 diabetes mellitus. Hepatogastroenterology. 2011;58:2103–2105. doi: 10.5754/hge11263. [DOI] [PubMed] [Google Scholar]
- 34.Yilmaz Y., Yonal O., Deyneli O., Celikel C.A., Kalayci C., Duman D.G. Effects of sitagliptin in diabetic patients with nonalcoholic steatohepatitis. Acta Gastroenterol Belg. 2012;75:240–244. [PubMed] [Google Scholar]
- 35.Fukuhara T., Hyogo H., Ochi H. Efficacy and safety of sitagliptin for the treatment of nonalcoholic fatty liver disease with type 2 diabetes mellitus. Hepatogastroenterology. 2014;61:323–328. [PubMed] [Google Scholar]
- 36.Cui J.Y., Philo L., Nguyen P. Sitagliptin versus placebo in the treatment of non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2016;64:S192–S193. doi: 10.1016/j.jhep.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeFronzo R.A., Norton L., Abdul-Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol. 2017;13:11–26. doi: 10.1038/nrneph.2016.170. [DOI] [PubMed] [Google Scholar]
- 38.Shiba K., Tsuchiya K., Komiya C. Canagliflozin, an SGLT2 inhibitor, attenuates the development of hepatocellular carcinoma in a mouse model of human NASH. Sci Rep. 2018;8:2362. doi: 10.1038/s41598-018-19658-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seko Y., Sumida Y., Tanaka S. Development of hepatocellular carcinoma in Japanese patients with biopsy-proven non-alcoholic fatty liver disease: association between PNPLA3 genotype and hepatocarcinogenesis/fibrosis progression. Hepatol Res. 2017;47:1083–1092. doi: 10.1111/hepr.12840. [DOI] [PubMed] [Google Scholar]
- 40.Ohki T., Isogawa A., Toda N. Effectiveness of ipragliflozin, a sodium-glucose co-transporter 2 inhibitor, as a second-line treatment for non-alcoholic fatty liver disease patients with type 2 diabetes mellitus who do not respond to incretin-based therapies including glucagon-like peptide-1 analogs and dipeptidyl peptidase-4 inhibitors. Clin Drug Investig. 2016;36:313. doi: 10.1007/s40261-016-0383-1. [DOI] [PubMed] [Google Scholar]
- 41.Mohammad S.K., Krishan S., Mishra S.K. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: A randomized controlled trial (E-LIFT trial) Diabetes Care. 2018;41:1801–1808. doi: 10.2337/dc18-0165. [DOI] [PubMed] [Google Scholar]
- 42.Lai L.L., Vethakkan S.R., Nik Mustapha N.R., Mahadeva S., Chan W.K. Empaglifozin for the treatment of nonalcoholic steatohepatitis in patients with type 2 diabetes mellitus. Dig Dis Sci. 2019:1–9. doi: 10.1007/s10620-019-5477-1. [DOI] [PubMed] [Google Scholar]
- 43.Akuta N., Kawamura Y., Watanabe C. Impact of sodium glucose cotransporter 2 inhibitor on histological features and glucose metabolism of non-alcoholic fatty liver disease complicated by diabetes mellitus. Hepatol Res. 2019;49:531–539. doi: 10.1111/hepr.13304. [DOI] [PubMed] [Google Scholar]
- 44.Zinman B., Wanner C., Lachin J.M. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 45.Neal B., Perkovic V., Mahaffey K.W. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;17:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 46.Bersoff-Matcha S.J., Chamberlain C., Cao C., Kortepeter C., Chong W.H. Fournier Gangrene Associated With Sodium–Glucose Cotransporter-2 Inhibitors: A Review of Spontaneous Postmarketing Cases. Ann Intern Med. 2019 doi: 10.7326/M19-0085. [DOI] [PubMed] [Google Scholar]
- 47.Kostapanos M.S., Kei A., Elisaf M.S. Current role of fenofibrate in the prevention and management of non-alcoholic fatty liver disease. World J Hepatol. 2013;5:470–478. doi: 10.4254/wjh.v5.i9.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chalasani N.P., Sanyal A.J., Kowdley K.V. Pioglitazone versus vitamin E versus placebo for the treatment of non-diabetic patients with non-alcoholic steatohepatitis: PIVENS trial design. Contemp Clin Trials. 2008;30:88–96. doi: 10.1016/j.cct.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panikar V. Pioglitazone and bladder cancer: The pros and cons. JAPI. 2012;60:73–74. [PubMed] [Google Scholar]
- 50.Rubenstrunk A., Hanf R., Hum D.W., Fruchart J.C., Staels B. Safety issues and prospects for future generations of PPAR modulators. Biochim Biophys Acta. 2007;1771:1065–1081. doi: 10.1016/j.bbalip.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 51.Jain M.R., Giri S.R., Trivedi C. Saroglitazar, a novel PPARalpha/gamma agonist with predominant PPARalpha activity, shows lipid-lowering and insulin-sensitizing effects in preclinical models. Pharmacol Res Perspect. 2015;3 doi: 10.1002/prp2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pai V., Paneerselvam A., Mukhopadhyay S. A multicenter, prospective, randomized, double-blind study to evaluate the safety and efficacy of Saroglitazar 2 and 4 mg compared to pioglitazone 45 mg in diabetic dyslipidemia (PRESS V) J Diabetes Sci Technol. 2014;8:,132–141. doi: 10.1177/1932296813518680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ratziu V., Harrison S.A., Francque S. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology. 2016;150:1147–1159. doi: 10.1053/j.gastro.2016.01.038. e5. [DOI] [PubMed] [Google Scholar]
- 54.Wettstein G., Poekes L., Oakley F. IVA337, a pan-ppar agonist, reduces non-alcoholic steatohepatitis feature and inhibits the inflammasome in murin models of non-alcoholic steatohepatitis. J Hepatol. 2017;66 S171. [Google Scholar]
- 55.Janicko M., Drazilova S., Pella D., Fedacko J., Jarcuska P. Pleiotropic effects of statins in the diseases of the liver. World J Gastroenterol. 2016;22:6201–6213. doi: 10.3748/wjg.v22.i27.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pastori D., Polimeni L., Baratta F., Pani A., Del Ben M., Angelico F. The efficacy and safety of statins for the treatment of non-alcoholic fatty liver disease. Dig Liver Dis. 2015;47:4–11. doi: 10.1016/j.dld.2014.07.170. [DOI] [PubMed] [Google Scholar]
- 57.Vasilios G.A., Boutari C., Stavropoulos K., Anagnostis P., Imprialos K.P., Doumas Michael, Karagiannis Asterios. Statins: An under-appreciated asset for the prevention and the treatment of NAFLD or NASH and the related cardiovascular risk. Curr Vasc Pharmacol. 2018;16:246. doi: 10.2174/1570161115666170621082910. [DOI] [PubMed] [Google Scholar]
- 58.Tzefos M., Olin J. 3-Hydroxyl-3-methylglutaryl coenzyme a reductase inhibitor use in chronic liver disease: a therapeutic controversy. J Clin Lipodol. 2011;5:450–459. doi: 10.1016/j.jacl.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 59.Barb Diana, Portillo-Sanchez Paola, Cusi Kenneth. Pharmacological management of nonalcoholic fatty liver disease. Metab Clin Exp. 2016;65:1183–1195. doi: 10.1016/j.metabol.2016.04.004. [DOI] [PubMed] [Google Scholar]