Highlights
-
•
A cul-de-sac endometrioid carcinoma adjacent to extraovarian endometriosis was identified during remission of endometrial cancer.
-
•
The origin of the cul-de-sac tumor was malignant transformation of deep infiltrating endometriosis.
-
•
Endometriosis-related cancer was identified in a woman with endometrial cancer during remission.
-
•
Hyperestrogenism due to infertility treatment may contribute to malignant transformation of deep infiltrating endometriosis.
Keywords: Carcinoma, endometrioid; Douglas′ pouch; Endometrial neoplasms; Endometriosis; Fertility preservation; Neoplasms, multiple primary
1. Introduction
Deep infiltrating endometriosis (DIE) is a type of extraovarian endometriosis, which may cause pain-related symptoms of endometriosis, including dysmenorrhea, dyspareunia, and chronic pelvic pain, which may compromise the quality of life of women. Sites of occurrence include the rectovaginal septum, uterosacral ligaments, and utero-ovarian ligaments (Gordts et al., 2017). Malignant transformation occurs in approximately 1% of women with endometriosis and occurs in ovarian endometriomas, where it is called endometriosis-associated ovarian cancer (EAOC). Cases of malignant transformation of DIE have been reported, but the precise prevalence is unclear. Histologically, most EAOCs are endometrioid and clear cell carcinomas (Steed et al., 2004); therefore, DIE-associated cancer may have the same histology.
Fertility-sparing treatment using high dose progestins such as medroxyprogesterone acetate (MPA) becomes increasingly relevant for patients with endometrial cancer who desire fertility. A previous meta-analysis of fertility-sparing treatments for patients with endometrial cancer revealed that the majority of patients achieve an initial remission. However, the recurrence rate is high (Wei et al., 2017). Repeated treatment is effective for intrauterine recurrences after initial treatment because most relapses are localized in the endometrium. Conversely, distant metastasis and/or extrauterine recurrence without local relapse is extremely rare.
We report a case of endometrioid carcinoma arising from malignant transformation of DIE localized in the cul-de-sac in a patient with endometrial cancer who was in remission following fertility-sparing treatment.
Case
A 38-year-old nulliparous woman (height: 158 cm, weight: 48.0 kg, and body mass index: 19.2) was referred to our facility for examination of an endometrial polyp and uterine fibroids. An endometrial biopsy revealed endometrioid carcinoma, which was classified as grade 1 according to the International Federation of Gynecology and Obstetrics (FIGO) classification system, arising from the endometrium. Slight invasion of the myometrium was suspected based on magnetic resonance imaging (MRI), and thus we diagnosed as FIGO Stage IA endometrial cancer and uterine fibroids. The indication of fertility-sparing treatment for early endometrial cancer is limited to grade 1 endometrioid carcinoma without myometrial invasion (Rodolakis et al., 2015); therefore, this case was an exception; the patient had expressed a strong desire to preserve the uterus for future fertility, and was aware that it meant an increased risk of recurrence. Thus, we administered conservative hormonal treatment after receiving an informed consent from the patient. We administered 400 mg oral MPA and 2250 mg of metformin daily for six months, and she achieved remission. Transvaginal ultrasound and endometrial biopsy were performed at two-to-three-month intervals to identify any local recurrence during the follow-up period. After remission, she received fertility treatment, including three instances of ovarian stimulation and transvaginal oocyte retrieval, followed by in vitro fertilization – embryo transfer (IVF-ET) without success. Twenty months after the initial remission, we identified local recurrence in the endometrium. She received daily oral MPA and metformin for further six months, and remission was achieved again. She then conceived with cryopreserved embryo transfer 8 months after the second remission, but spontaneously miscarried.
Forty-one months after the initial remission (15 months after the second remission), we identified a small amount of ascites and a solid tumor with abundant blood flow in the cul-de-sac (Fig. 1). A needle biopsy indicated an adenocarcinoma, and contrast-enhanced computed tomography (CT) and MRI scans revealed that the tumor was localized in the cul-de-sac, and neither retroperitoneal lymph node swelling nor peritoneal dissemination were detected. Although endometrial biopsy was negative for malignancy, we suspected a peritoneal metastasis from the recurrent endometrial cancer. She underwent a hysterectomy, bilateral salpingo-oophorectomy, low anterior resection of the rectum, and retroperitoneal lymphadenectomy. Neither peritoneal dissemination nor pelvic endometriosis were identified at laparotomy, and cytology of the ascites was also negative for malignancy.
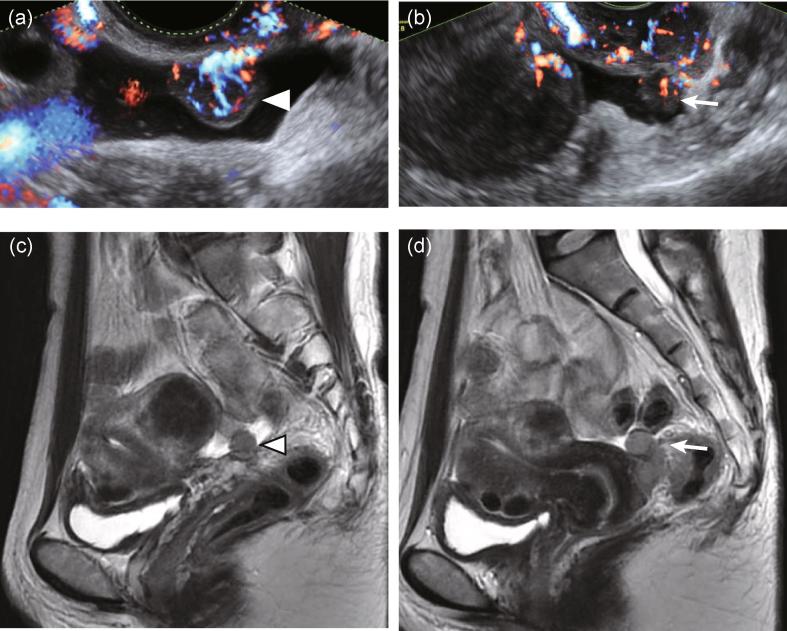
Fig. 1.
Preoperative imaging findings of the cul-de-sac tumors. (a) and (b) Transvaginal ultrasound. (a) Solid tumor attached to the vaginal wall (arrow head) associated with small amount of ascites. Abundant blood flow was evident from the vaginal wall to the tumor. (b) Another solid tumor was observed (arrow) adjacent to the cervix associated with a small amount of ascites. Uterine fibroid was observed on the left. (c) and (d) Magnetic resonance imaging (MRI). (c) T2 weighted sagittal image showing a cul-de-sac tumor (arrow head, same as that of panel (a)) associated with a small amount of ascites and uterine fibroid. (d) T2 weighted sagittal image showing thin endometrium and another cul-de-sac tumor (arrow, same as that of panel (b)).
The cul-de-sac tumor consisted of atypical tubule gland formation with a back-to-back structure lined by columnar cells with rounded nuclei and prominent nucleoli, which was diagnosed as an endometrioid carcinoma that was classified as FIGO grade 1 (Fig. 2). Ectopic endometrial tissue suggesting DIE was broadly observed in the muscularis propria and subserosa of the sigmoid colon and rectum. The glands of endometrioid carcinoma and DIE were adjacent to each other, but no sequential transition nor coexistence in a single tubule was observed. The endometrium of the uterus showed proliferation of the endometrial glands with no obvious atypia. Therefore, we made a diagnosis of complex endometrial hyperplasia without atypia. Neither lymph node metastases nor local recurrence of the endometrial cancer in the endometrium was identified. No significant findings were observed in either ovaries or tubes. We thus concluded that the tumor resulted from malignant transformation in the DIE adjacent to the tumor, and the final diagnosis was FIGO stage II peritoneal cancer. As no standard adjuvant chemotherapy for the endometrioid carcinoma arising from extraovarian endometriosis was established, because of its rarity, we performed adjuvant systemic chemotherapy using six courses of tri-weekly paclitaxel and carboplatin subsequent to the standard adjuvant therapy for ovarian and peritoneal cancer. No recurrence has been recorded in at least two years.
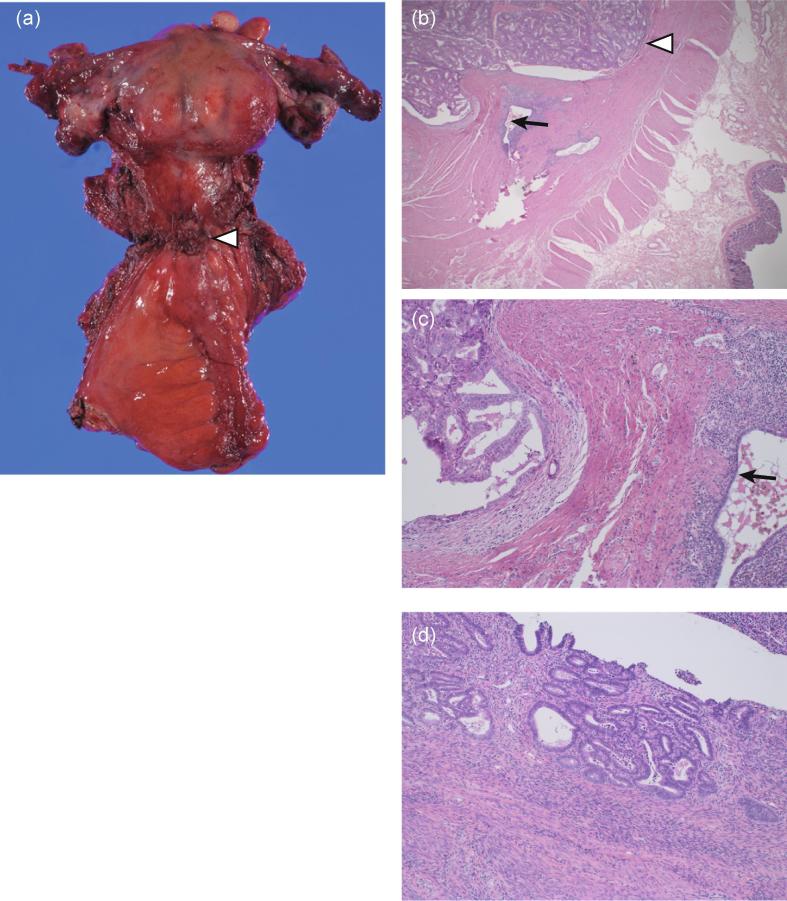
Fig. 2.
Histology of excised specimen. (a) Excised sample consisting of uterus, bilateral adnexa, vaginal wall, and rectosigmoid colon. The uterus contained multiple fibroids. The cul-de-sac tumors (arrow head) were located in the center of the specimen. (b) Endometrioid carcinoma (arrow head) invading the muscularis propria of the sigmoid colon and adjacent DIE (arrow) (H&E staining ×20). (c) Glands contiguous, but no sequential transition observed (H&E staining ×200). (d) Excised endometrium showing complex endometrial hyperplasia without atypia (H&E staining ×200). DIE, deep infiltrating endometriosis.
2. Discussion
We report a case of endometrial carcinoma associated with malignant transformation of DIE localized in the cul-de-sac in a patient with endometrial cancer in remission following fertility-sparing treatment using MPA. The endometrioid carcinomas of the endometrium and cul-de-sac peritoneum were synchronously identified.
Pathological findings suggested that the lesion arose from DIE. The Sampson and Scott criteria, which are used for defining primarily malignant tumors arising from endometriosis, include: (1) the presence of both benign and neoplastic endometrial tissues in the tumor, (2) histological findings compatible with an endometrial origin, (3) the discovery of no other primary tumor sites, and (4) a morphologic demonstration of a continuum between benign and malignant epithelium (Tarumi et al., 2015). According to these criteria, the present case included the following characteristics: (1) neoplastic endometrial tissue was present, but benign endometrial tissue was not present in the tumor, (2) histological findings were compatible with an endometrial origin, (3) primary tumor sites were either DIE or endometrial cancer, and (4) no morphologic demonstration of a continuum between benign and malignant epithelium was identified. For these reasons, it may be debated whether the tumor originated from DIE or represented localized recurrence or metastasis of the endometrial cancer. In fact, the preoperative diagnosis was localized recurrence of the endometrial cancer, which was never confirmed. However, no local relapse of the endometrium was identified in the extracted uterus. If it is considered that the cul-de-sac tumor represented a metastatic site or local recurrence of the endometrial cancer, it is an extremely rare case, because it is unlikely that a metastatic or a recurrent tumor is localized in the cul-de-sac without a local relapse in the endometrium. We have found only one report of an extra endometrial recurrence without local relapse in a patient with endometrial cancer after fertility-sparing treatment; although no significant symptoms were noted, the MRI revealed a 3-cm abnormal focus in the myometrium with a normal endometrial cavity and junctional zone (Hurst et al., 2008).
Although synchronous endometrioid carcinomas arising from the endometrium and DIE are rare, synchronous endometrioid carcinomas of the endometrium and ovary is a well-recognized pathophysiology that occurs in 4.5% of cases, and women with synchronous cancers of the endometrium and ovary have been reported to have a favorable prognosis, and were young, obese, nulliparous, and premenopausal compared with metastasis from one site to another site (Matsuo et al., 2017). In addition, a suspicious case of synchronous endometrioid carcinoma has been reported in a young Japanese woman who received fertility-sparing treatment with MPA for endometrial carcinoma and had a recurrence with an extrauterine lesion, which was peritoneal carcinomatosis (Ushijima et al., 2007). Similar to the current case, there had been no endometrial malignancy three months before the development of the peritoneal lesions. Recently, the application of next generation sequencing to synchronous endometrial and ovarian carcinomas has revealed that these tumors are monoclonal (Schultheis et al., 2016). It is possible that both, the endometrial cancer and endometriosis associated cul-de-sac tumor in the current case are of monoclonal origin.
In the present case, infertility treatment might have contributed to the malignant transformation of the endometriosis. Hyperestrogenism is a risk factor for malignant transformation, particularly for ovarian endometriosis. Moreover, ovarian stimulation for infertility treatment is also a risk for DIE and EAOC. Perimenopause, obesity, and estrogenic stimulation could be causes of such malignant transformation, including DIE and EAOC (Zanetta et al., 2000). In the present case, the woman had received ovarian stimulation for infertility treatment. The resulting hyperestrogenic state might be a risk factor for malignant transformation of endometriosis.
3. Conclusion
Malignant transformation of DIE occurred in a patient with endometrial cancer in remission following fertility-sparing treatment. This case suggests the importance of looking for lesions outside the uterus as well as in the endometrium during follow-up of these patients.
4. Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
References
- Gordts S., Koninckx P., Brosens I. Pathogenesis of deep endometriosis. Fertil. Steril. 2017;108(872–85) doi: 10.1016/j.fertnstert.2017.08.036. [DOI] [PubMed] [Google Scholar]
- Steed H., Chapman W., Laframboise S. Endometriosis-associated ovarian cancer: a clinicopathologic review. J. Obstet. Gynaecol. Can. 2004;26:709–715. doi: 10.1016/s1701-2163(16)30642-9. [DOI] [PubMed] [Google Scholar]
- Wei J., Zhang W., Feng L., Gao W. Comparison of fertility-sparing treatments in patients with early endometrial cancer and atypical complex hyperplasia: a meta-analysis and systematic review. Medicine (Baltimore) 2017;96 doi: 10.1097/MD.0000000000008034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodolakis A., Biliatis I., Morice P., Reed N., Mangler M., Kesic V. European society of gynecological oncology task force for fertility preservation: clinical recommendations for fertility-sparing management in young endometrial cancer patients. Int. J. Gynecol. Cancer. 2015;25:1258–1265. doi: 10.1097/IGC.0000000000000493. [DOI] [PubMed] [Google Scholar]
- Tarumi Y., Mori T., Kusuki I., Ito F., Kitawaki J. Endometrioid adenocarcinoma arising from deep infiltrating endometriosis involving the bladder: a case report and review of the literature. Gynecol. Oncol. Rep. 2015;13:68–70. doi: 10.1016/j.gore.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst S.A., Hartzfeld K.M., Del Priore G. Occult myometrial recurrence after progesterone therapy to preserve fertility in a young patient with endometrial cancer. Fertil. Steril. 2008;89(724):e1–e3. doi: 10.1016/j.fertnstert.2007.03.068. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Machida H., Frimer M., Marcus J.Z., Pejovic T., Roman L.D. Prognosis of women with stage I endometrioid endometrial cancer and synchronous stage I endometrioid ovarian cancer. Gynecol. Oncol. 2017;147:558–564. doi: 10.1016/j.ygyno.2017.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushijima K., Yahata H., Yoshikawa H., Konishi I., Yasugi T., Saito T. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J. Clin. Oncol. 2007;25:2798–2803. doi: 10.1200/JCO.2006.08.8344. [DOI] [PubMed] [Google Scholar]
- Schultheis A.M., Ng C.K., De Filippo M.R., Piscuoglio S., Macedo G.S., Gatius S. Massively parallel sequencing-based clonality analysis of synchronous endometrioid endometrial and ovarian carcinomas. J. Natl Cancer Inst. 2016;108:djv427. doi: 10.1093/jnci/djv427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetta G.M., Webb M.J., Li H., Keeney G.L. Hyperestrogenism: a relevant risk factor for the development of cancer from endometriosis. Gynecol. Oncol. 2000;79:18–22. doi: 10.1006/gyno.2000.5905. [DOI] [PubMed] [Google Scholar]