Abstract
Objective
Self-monitoring is crucial to raise awareness for own behaviors and emotions, and thus facilitate self-management. The composition of self-monitoring within interventions, however, varies and guidelines are currently unavailable. This review aimed to provide a comprehensive overview of technology-based self-monitoring interventions that intend to improve health in middle-aged and older adults (>45 years).
Methods
Five online databases were systematically searched and articles were independently screened. A narrative synthesis of 26 studies with 21 unique interventions was conducted. Primary focus lay on the composition of self-monitoring within interventions, including technology used, health-aspects monitored, and type of feedback provided. Secondly, the usability of/adherence to the self-monitoring treatment, intervention effects, and their sustainability were examined.
Findings
Studies concentrated on middle-aged adults (mean of 51 years). Mobile technologies seem necessary to ensure flexible self-monitoring in everyday life. Social health aspects were rarely monitored. Mechanisms and the sustainability of intervention effect are understudied.
Conclusion
Digital self-monitoring technologies hold promise for future trials as they seem suitable to understand and support health-related self-management. Key elements including automatic and personal feedback following the blended care principle were highlighted and may guide study designs. Prospectively, research is especially needed to study sustained self-monitoring to support disease prevention and lasting lifestyle changes.
Keywords: Mobile technology, Intervention, Self-monitoring, Momentary assessment, Middle-aged and older adults
Highlights
-
•
Digital self-monitoring interventions for middle-aged and older adults show great diversity.
-
•
Mobile technologies (e.g. smartphones) are needed for flexible self-monitoring.
-
•
Social health aspects (e.g. social networks/company) are rarely monitored.
-
•
Mechanisms and sustainability of intervention effects are understudied.
-
•
More research is needed on prevention and lasting lifestyle changes.
1. Introduction
Self-monitoring is known to be a crucial element of health promotion and disease prevention (Bandura, 1998). Social cognitive theory states that self-monitoring influences a person's motivations and actions as it increases attention towards his/her own behaviors, their occurrence and effects; therefore, the success of self-management depends on the fidelity, consistency and temporal proximity of self-monitoring (Bandura, 1991).
In clinical settings, the monitoring of patients generally occurs periodically. For instance, the average number of medical visits in older Europeans was 7.75 per year per person (Srakar and Rupel, 2016). As the contact is only periodical, individuals tend to be self-responsible during most of their life when it comes to own health-management. Furthermore, self-reports during the visits to doctors happens retrospectively due to the type of questions asked in questionnaires (e.g. “How has your mood been in the past four weeks?”).
More and more innovative eHealth solutions are available for healthcare professionals to support their patients outside of periodical face-to-face sessions and clinicians have overall positive attitudes towards eHealth (Peeters et al., 2016). Digital self-monitoring, for instance, has the potential to address and promote health in an individual's daily life. A benefit of the digital momentary self-monitoring approach is the high ecological validity, as information is gathered in an individual's natural rather than artificial clinical or laboratory environment (Scollon et al., 2003). Furthermore, reporting experiences in the moment in which they occur (e.g. “How enthusiastic are you right now?”) reduces any potential memory bias, which is frequently found in retrospective assessments (Shiffman et al., 2008). Repeated demonstration of self-reports over days or month displays a heterogeneously fluctuating picture of behaviors and experiences (Focht et al., 2004) and allows for the exploration of temporal relationship between variables.
1.1. The experience sampling method and ecological momentary assessment
Diary methods such as the experience sampling method (ESM) (Csikszentmihalyi and Larson, 1987; Csikszentmihalyi and Larson, 2014) or the ecological momentary assessment (EMA) (Stone and S., 1994) offer repeated momentary data-collection situated in daily life and can be utilized in health-related self-monitoring. These approaches provide insight on how individuals think, feel, and behave on a daily basis. At multiple time points, individuals can provide systematic self-reports via (digital) diaries on their behaviors as well as experiences by filling in short questionnaires on smartphone apps or mobile devices. The collection of these reports makes it possible to represent the complex relations between psychological, physiological, and social functioning, and current context (e.g. location, activities, and social company). These momentary self-reports are commonly prompted by the technology through, for example, auditory signals (beeps) at predefined time-intervals (time-sampling) but can, in some cases, also be self-initiated by the individual when a particular event occurs (event-sampling) (Palmier-Claus et al., 2011).
In the present study, the term ‘self-monitoring’ includes all forms of active ambulatory assessments and therefore represents an intersection between both ESM and EMA. Active self-monitoring requires the person to reflect on and evaluate the situation and, thus, needs to be distinguished from passive self-monitoring, where (physical) functioning is recorded automatically (e.g. fitness or smart watches).
1.2. Self-monitoring within interventions
ESM and EMA have traditionally been used to describe and understand disease patterns. Beyond that, researchers and clinicians have integrated real-life data collections in recent decades in intervention approaches with the aim of improving certain aspects of health and modify behavior. Reviews have described the approach as effective to improve health, for example, in serious mental illnesses (Myin-Germeys et al., 2016; Versluis et al., 2016) or alcohol use disorders (Beckjord and Shifmann, 2014; Morgenstern et al., 2014). Heron and Smyth (2010) emphasize that digital self-monitoring via mobile technologies such as smartphone apps or hand-held computers seem to be particularly promising in providing psychosocial and health behavior treatment. A digital approach is furthermore cost-effective and can provide support in situations when it is most needed (Simons et al., 2017; Verhagen et al., 2016).
At the moment, the composition of the self-monitoring in interventions varies strongly between studies. Components seem to differ such as the technology used, intervention duration, follow-up period, amount and intensity of self-monitoring per day, health aspects monitored, and additional features such as personal or automatic feedback. When wishing to work with a self-monitoring approach to support a person's treatment, general guidelines are unavailable and this diversity complicates the clinicians' decision-making process on how to best structure the intervention.
To our knowledge, no recent review is available focusing on digital self-monitoring and its use for health promotion in middle-aged and older adults. Cain et al. (2009) assessed the feasibility and application of ecological momentary assessments in psychological and behavioral research on aging over ten years ago. More recent reviews focus on technological solutions for specific health issues in older adults such as chronic conditions (Guo and Albright, 2018), or physical activity (Jonkman et al., 2018), while the composition of self-monitoring in interventions has not been prioritized yet. In the current aging world, it is particularly important to find the best way to treat and prevent diseases in these populations as they experience a variety of health issues (He et al., 2016; Hong, 2013).
1.3. Review objectives
This systematic review aims to provide a comprehensive overview of digital self-monitoring interventions that intend to improve health in middle-aged and older adults.
The primary focus lies on describing (1) the composition of ESM/EMA self-monitoring interventions. This evaluation includes the technology used for the active self-monitoring, (physical, emotional, social) components monitored, intensity and duration of ESM/EMA, if and what kind of feedback was provided, and which other intervention elements were part of the setup. The second focus lies on describing (2) the usability, adherence to the treatment, the intervention effects, and their sustainability. Identified key elements will be discussed and are expected to inspire and guide future digital self-monitoring interventions. Possibilities and challenges will be debated to illustrate digital self-monitoring as a means to promote a lasting healthy lifestyle and thus contribute to prevention of diseases in middle-aged and older adults.
2. Methods
This systematic review was registered on PROSPERO under the registration number CRD42018100649.
2.1. Search strategy
In April 2018, the bibliographic databases PubMed, PsycINFO, CINAHL, Web of Science, and Cochrane Library were systematically searched to identify studies reporting on intervention approaches using digital self-monitoring (ESM/EMA) with the intention to improve health. Therefore, the following terms were used when searching the database: ‘intervention’, ‘ESM’/‘EMA’, ‘technology’, and ‘health’. Appendix A presents the complete search strategy. To find indexed as well as non-indexed articles, MeSH terms, Thesaurus terms, and also non-MeSH terms were included. The resulting reference lists were reviewed to identify additional relevant articles (e.g. through back citation) for potential inclusion. Manuscripts published between 2007 and April 2018 were included in this review (see Section 2.3 for details).
2.2. Study selection
The identified citations were imported into EndNote and de-duplicated. The reviewers (SB, RvK, FD) read the abstracts and full-texts independently. Every abstract and full-text was read twice. SB and RvK performed the abstract scanning, while SB and FD read the full-text manuscripts. If consensus whether to in-/exclude a study into the review could not be reached between SB and RvK/FD, MdV was consulted as a third reviewer to make the final decision on in-/exclusion of a study.
2.3. Inclusion criteria
A study was included if it met the following criteria: (1) applying ESM/EMA defined as active real-life data collection of at least two out of three health aspects (physical, emotional, and social) with self-monitoring on at least three days in one week; (2) using technology for the self-monitoring; (3) aiming to promote health within an intervention; (4) in middle-aged or older adults. ‘Middle-age’ was here defined as populations with a mean age of 45 years and older (rounded up from 44.5 years).
Self-monitoring of at least two health aspects was chosen to ensure that health was seen as a complex, multi-dimensional construct (Huber et al., 2011; Lehman et al., 2017). Sampling for at least three days was selected to gain a comprehensive view on an individual's daily life, increase generalizability in terms of days, and observe variability of response (Stone and Shiffman, 2002). The third criterion (promote health) got defined broadly as the main focus lay on the composition of the self-monitoring within an intervention, while the intervention outcomes and the effectiveness were secondary. The age cut-off was made to focus this review on middle-aged and older adults, as technologies such as smartphones are already more prevalent in individuals younger than 45 years (Ryan and Lewis, 2017) and health app use has been reported in younger ages (Chen et al., 2017). Studies before 2007 were excluded, as 2007 can be considered to be a turning point in technological development: the mobile phone with smart functions was introduced to the market (Cuthbertson et al., 2015). Finally, only studies written in English were included.
2.4. Data extraction
Data extraction was inspired by the PRISMA guideline (Liberati et al., 2009). Information relating to the general study characteristics was extracted, including the country of data collection, sample size, presence/absence of a control group, and population characteristics (age, gender, health status/diagnosis).
With respect to the primary review focus (composition of self-monitoring), data extraction included the type of technology used for the self-monitoring, information on the (theoretical) approaches the intervention was based on, biological/physical, psychological/emotional, and social aspects of health monitored, details on how often per day monitoring took place (intensity), and for how many days/weeks/months the self-monitoring was performed (duration). Additionally, if, how, and what kind of feedback was given to the participants was deemed of relevance.
The information extracted for the secondary review focus was on the use of/adherence to the self-monitoring, overall effectiveness of the intervention (positive/negative/no effect on chosen outcome measures), the length of the follow-up period, sustainability of the effects (follow-ups), and the topic of ‘prevention’.
2.5. Data synthesis
Due to the heterogeneity of the interventions (population, aim, design, duration, outcome measures, follow-up), a meta-analysis was statistically not appropriate and could thus not be performed. Therefore, a narrative synthesis was conducted. This textual approach summarizes and explains the findings of the synthesis of included studies within a systematic review (Morton et al., 2015; Popay et al., 2006).
3. Results
3.1. Reviewing process
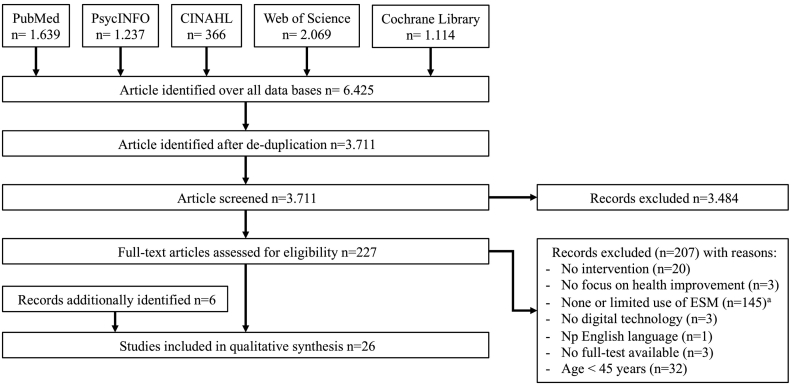
A total of 6425 references were identified through the search strategy. After de-duplication, 3711 hits remained for the abstract screening. 227 articles were included in the full-text screening for eligibility and 20 manuscripts met the inclusion criteria. Reasons for exclusion during the full-text assessment (n = 207) can be seen in Fig. 1. Six additional references were identified via cross-referencing. In total, n = 26 studies with 21 unique interventions (see Appendix A in Supplemental Material) were included (see Reference list in Supplementary Material of included studies).
Fig. 1.
Flow-chart of the review process from data extraction to qualitative synthesis.
Note: a‘None or limited use of ESM’: ESM as outcome only (n = 21), <2 aspects of health monitored (n = 105), ESM on <3 consecutive days (n = 19).
3.2. General study characteristics
3.2.1. Country of data collection
In total, 13 of the studies took place in the United States[1, 2, 4, 8/12, 9, 11, 13, 14, 18, 19, 20, 21/22, 24]. Three studies were conducted in the Netherlands[5, 10/15, 25/26], two in Norway[7, 16/17] and one each in Germany[23], Austria[3], and New Zealand[6].
3.2.2. Sample size and control groups
The average sample size was 94 ranging from ten[7] to 305 participants[20]. 66% of the studies included at least one control group, while 33% were single-armed[3, 6, 7, 8/12, 9, 19, 23].
3.2.3. Population characteristics
The participants' average age (including participants in control groups) across all studies was 51 years, with a maximum age of 89 years[3]. Only two studies had an average age above 65 years of age[23, 25/26]. The study populations show great variability being comprised of people with a substance use disorder[1, 2, 11, 13, 19], schizophrenia/schizoaffective disorders[9], and major depression disorder[10/15]. Studies focused furthermore on mixed outpatients[5], coronary heart disease[3], overweight women[6], underactive adults[14], smoking[8/12, 21/22], people with HIV[1, 2, 11, 24], and chronic pain[16/17, 18, 20]. Other studies targeted caregivers of people with dementia[25/26], and ‘healthy’ individuals[4, 23].
3.2.4. Intervention approaches and elements
All interventions were multi-modal, meaning additional features to the self-monitoring were included. The intervention design consisted of motivational interviewing[1, 2, 11], explicit goal-setting[3, 4, 14, 23], education/skills training[3, 6, 7, 18, 19, 20], group sessions[7, 8], elements from acceptance and commitment therapy (ACT)[5, 6, 16/17], and elements from cognitive behavioral therapy (CBT)[9, 16/17, 18, 19, 20]. Furthermore, studies highlighted regular clinical care or access to a counsellor[3, 4, 7, 8/12], mindfulness training[16/17, 21/22], physical exercise[7], or disease specific approaches (alcohol intervention based on FRAMES[13], pharmacotherapy[8/10]). One intervention aimed at self-regulatory strategies (i.e. goal setting) derived from the social cognition perspective[14], while two other focused on positive affect (PA) as part of the coping process[10/15, 25/26]. One study offered access to a social network via a web-page[20], and one self-management intervention limited the design to self-monitoring in combination with visualized progress tracking[24]. For the full overview of the Intervention elements, see the Supplementary Appendix B.
3.3. Primary review focus: ESM/EMA self-monitoring composition
3.3.1. Self-monitoring Technology
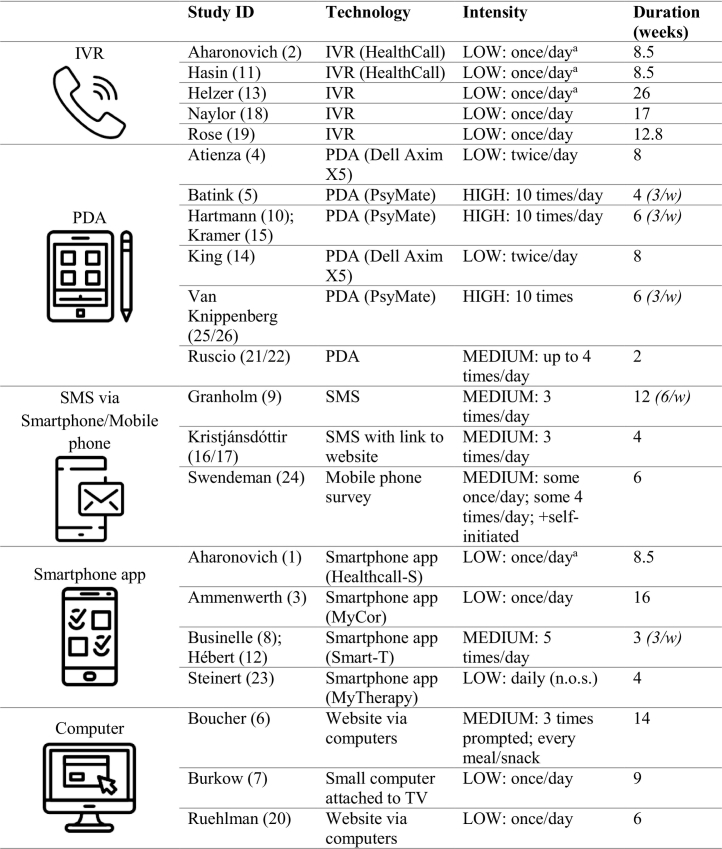
Most technologies were mobile, meaning that participants could enter information at home as well as out of home as the technology could be carried. Different models of personal digital assistants (PDAs) were used: the Dell Axim X5[4, 14,] and the PsyMate[5, 10/15, 25/26]. A PDA is a small mobile device programmed to prompt the participant via a sound signal to complete the digital daily questionnaires. The model of the PDA used in one intervention was not specified[21/22]. Other studies used interactive voice response (IVR), a telephone-based technology that enables the caller to interact with a computer using the telephone keypad as the interface. Three studies did not specify the name for the IVR[13,18,19], while two used an IVR called ‘HealthCall’[2, 11]. Studies also used a mobile phone survey[24], short message services (SMS)[9], and SMS with a link to a website survey[16/17] as the technological self-monitoring approach. Finally, smartphone apps were developed, namely HealthCall-S[1], originated from the IVR HealthCall, MyCor[3], Smart-T[8/12], and MyTherapy[23]. As well as the mobile approach, stationary devices were also used for self-monitoring: in one intervention, a small computer was attached to the participants' TV system[7]. Two studies used websites via computers for daily log-ins[6, 20].
3.3.2. Prompted self-monitoring intensity per day
The intensity of daily self-monitoring ranged from one to ten self-monitoring moments per day. The intensity of the self-monitoring was categorized as ‘low’ with one or two self-monitoring assessments per day, as ‘medium’ with three to five self-monitoring assessments per day, and as ‘high’ with more than five assessments per day.
In total, eleven studies used a low monitoring intensity[1, 2, 3, 4, 7, 11, 13, 14, 18, 19, 20]: In four studies, participants were asked in the beginning of the day to reflect on the previous day[1, 2, 11, 13]. Self-monitoring ones daily on the same day took place in six studies[3, 7, 18, 19, 20]. Two other studies prompted the participants twice daily[4, 14]. Medium intensity was described in six studies: One study had three self-monitoring assessments[16/17], while another study prompted the participants three times and asked to additionally provide information after every food intake[6]. Up to four prompts were sent to the participants by one intervention[21/22]. One study included five daily self-monitoring moments at three days per week[8/12]. Finally, participants answered questions on drug use, sexual behavior, and medication adherence ones per day, plus questions on physical and mental health aspects, and context four times a day[23]. High intensity with ten self-monitoring moments was described by three studies[5, 10/15, 25/26]. All studies with high intensity limited self-monitoring to three days per week. One study did not further specify ‘daily’[23].
3.3.3. Duration
The duration of the self-monitoring ranged from two weeks[21/22] to six months (approximately 26 weeks)[13]. While most studies asked the participants to self-monitor every day during the intervention period, one intervention limited the assessments to six days per week[9], and four other studies focused on three days per week[5, 8/12, 10/15, 25/26]. For details on the duration see Table 1.
Table 1.
Self-monitoring in the interventions: technologies, intensity, and duration.
Note: IVR = interactive voice response; PDA = personal digital assistant; SMS = short message services; (…/w) = number of self-monitoring days per week; aasked to reflect in the morning on previous day.
3.3.4. Health aspects: physical
The Supplementary Appendix C provides an overview of the health aspects that were self-monitored in the interventions All interventions included at least one physical/behavioral/biological health aspects. Participants reported on their location[4, 5, 7, 14, 25/26], ‘activity’[5, 10/15, 20, 25/26], and quality of sleep[5, 25/26]. Additionally, drug/substance use[1, 2, 3, 8/12, 11, 13, 19, 24], smoking urge/cigarette availability[8/12, 24], medication adherence/use[9, 11, 18, 23, 24], HIV-medication adherence[2] or HIV-related health behavior[1] was monitored. The self-monitoring also focused on food and/or water consumption[4, 7, 23], the eating experience (i.e. hunger, fullness)[6], barriers/enabling conditions for healthy food choices[4], and physical activity and well-being[10/15, 11, 14, 23, 24, 25/26]. Participants reported on COPD symptoms[7], pain[16/17, 18, 20], as well as attention bias/cognition[21/22]. Finally, self-monitoring included unspecified ‘more general questions related to health’[13], behavioral factors[14], coping[18], and events[10/15, 25/26].
3.3.5. Health aspects: emotional
All interventions included some form of emotional/psychological or mental health aspect. Self-monitoring questions asked about participants' mood[1, 4, 11, 13, 18, 19, 20], (positive/negative) affect[5, 8/12, 10/15, 25/26], experienced stress[1, 2, 8/12, 18, 24], wellness/subjective well-being[1, 2, 3, 11], depression[16/17, 21/22], feelings and thoughts related to avoidance and catastrophizing[16/17], as well as quality of life[2, 24]. Furthermore, participants reflected on their level of mindfulness[6, 21/22], cognition[5], quality/appraisal of the day[5, 7, 25/26], smoking urge and motivation for cessation[8/12, 19], and coping strategies[9, 18]. Finally, reasons/motivation for drinking or abstinence[11, 13], motivational factors[14], recreational activities (‘time for yourself’)[23], self-esteem, and sense of competence[25/26] were part of the self-monitoring.
3.3.6. Health aspects: social
N = 8 interventions included social aspects, meaning participants provided information if they were alone or with others. The questions focused on sexual interactions and protection[1, 24], social company[5, 10/15, 25/26], socialization[9], or relationships with the partners[13]. Recreational activities reflecting on cultivating social contacts were also included[23]. Thirteen studies did not ask about any social aspects in everyday life (note: some elements might also refer to the other health categories).
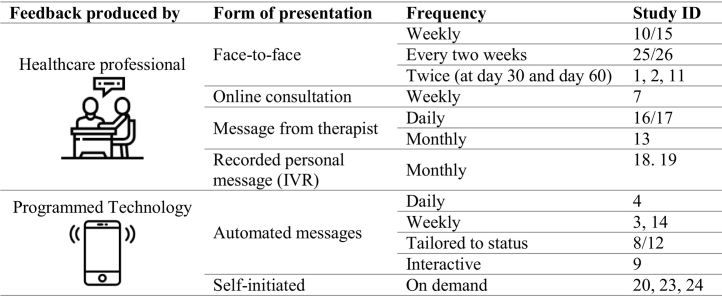
3.3.7. Feedback
The information collected during the self-monitoring was in nearly all studies feedbacked (n = 23) to the participants. Only three study designs did not include any form of feedback[5, 6, 21/22]. The feedback was either produced by a healthcare professional or through a preprogramed technological device/service. Feedback from the healthcare professionals were delivered in face-to-face sessions, online consultations, via e-mail or through voice recorded messages. The technology either send automated messages or could provide visual feedback on demand. In one intervention, healthcare profession provided daily written feedback[16/17]. In most studies, however, healthcare professionals gave feedback on a weekly, bi-monthly, or monthly level. The programmed feedback was available in most studies every day and in two interventions weekly. Table 2 provides details on the characteristics of the feedback.
Table 2.
Characteristics of feedback.
Note: IVR = interactive voice response.
The content of the feedback depended on the self-monitoring items. Therefore, the feedback included drinking behavior or drug use[1, 2, 11, 13], smoking behavior[8/12], or information related to the individually set goals (e.g. steps)[3, 7, 14, 23]. Furthermore, part of the feedback was dietary intake[4], medication adherence, socialization or auditory hallucinations[9], or positive affect in daily contexts[10/15, 25/26]. Interventions included also feedback on general diary content[16/17], coping skills, stress, and pain level[18, 19], or mood/activity boosters and pain soothers[20].
3.4. Secondary review focus: usability, effectiveness, sustainability, and prevention
3.4.1. Usability/compliance/adherence
The terminology varied between studies when reporting on the participants' engagement in self-monitoring including ‘adherence’, ‘use’, ‘compliance’ or ‘engaged’. The lowest average adherence rates were 51% (PDA Dell Axim 5)[4] and 56% (IVR)[19] (low self-monitoring intensity). The highest median use rates were 86/87% (Smart-T; text messages)[8/12, 9] (medium self-monitoring intensity) and 95% (HealthCall-S smartphone app)[1] (low self-monitoring intensity). Most studies reported an adherence rate between 64% and 76%[2, 5, 10/15, 11, 14, 16/17, 21/22, 25/26]. In three studies, no information on the adherence was provided[18, 20, 23]. Finally, one study reported the adherence to self-monitoring without a percentage as ‘most entered data on a daily basis’ (page 7)[7]. A number of studies noticed a decrease of adherence and use over the course of the intervention[3, 4, 6, 13, 19, 24]. One study described furthermore that the web-based visualization focused on survey responses over time were difficult to use and interpret, and therefore rarely used by the participants[24]. One intervention was evaluated in an RCT as well as a process evaluation[25/26]. No other interventions provided process evaluations.
3.4.2. Effectiveness
Details of the effectiveness can be found in the Supplementary Appendix A. 96% of all studies reported health improvements at least on one outcome measure right after the intervention or at follow-up. One study failed to find significant effects on the outcome measures, however, participants reported that the ACT exercise (available on demand in distressing situation to deal with feelings and thoughts in an ACT-consistent manner) and metaphors (illustrated metaphors serving as reminders/cues to reactive previously learned ACT concepts) were useful components[5]. Next to the significant health improvements, n = 11 studies reported that some outcome measures did not show a significant change post-intervention[1, 3, 5, 6, 9, 10/15, 11, 12, 22, 23, 26]. One study found a negative intervention effect with higher alcohol consumption in the self-monitoring IVR group, potentially explained through a confounder effect[13]. The same study highlighted a therapeutic advantage of the feedback compared to self-monitoring only. Findings regarding the added benefit of a feedback on top of the self-monitoring were limited and mixed: studies reported non-significant group differences[10, 25/26], or significant effects of the self-monitoring plus feedback compared to self-monitoring only[15]. Results furthermore stressed the relevance of tailored messages (i.e. with respect to triggers)[12], the advantage of daily self-monitoring compared to bi-weekly to increase awareness and behavioral change[24], and the importance of a personal coach providing face-to-face feedback to stimulate and implement new insights into daily lives[25].
3.4.3. Follow-up and sustainability
Twelve studies did not include or report on follow-up assessments after the main intervention period and therefore no information on the sustainability of the intervention effects was available. Sustained effects were found on at least one outcome measure after two months[26], three months[6]/twelve weeks[8], 14 weeks[20], five months[16], six months[15], eight months[18], and twelve months[2]. In two studies, within-group differences remained, while group-differences disappeared at 11/12-month follow-up[11, 17].
3.4.4. Prevention
A small number of studies included the idea of disease prevention in their intervention. Relapse prevention was part of the six-week inpatient treatment (ACT group therapy) prior to the self-monitoring period (ACT in daily life)[5], as well as the IVR based interventions for chronic pain[18] and alcohol use disorder[19]. Additionally, authors concluded that the intervention prevented increases in functional impairment and symptom levels in women with chronic widespread pain following inpatient rehabilitation[16]. The “Mind, Body, Food” program functioned as a weight gain prevention[6]. Finally, monitoring functioning in caregivers of people with dementia might potentially prevent higher levels of burden in a later stage[26].
4. Discussion
The primary aim of this review was to describe the composition of self-monitoring interventions for middle-aged and older adults aiming to improve health. The reviewed literature resulted in the identification of 26 studies with 21 unique interventions using active ESM/EMA self-monitoring. The strength of this review lies in the inclusion of all active self-monitoring interventions using technology to describe the diversity of intervention designs and thus stimulate new approaches. The chosen definition of self-monitoring aimed to represent the intersection between both ESM and EMA, while the inclusion criteria ensured that health was considered a multi-dimensional construct.
With regards to the general characteristics of the included studies, the interventions focused on a wide range of physical and psychological health issues and were conducted in high-income countries. Studies focused mainly on middle-aged adults as only two studies had an average age above 65 years of age[23, 25/26]. One of these studies provided insufficient information on the design of the four-week program and participant's use of the ‘MyTherapy’ smartphone app (intensity of sampling and compliance unclear)23, which highlights the need for consistent reporting as also suggested by an EMA review in youth (Liao et al., 2016). The other study including older adults26 used the PDA ‘PsyMate’ over six weeks with a high sampling intensity (i.e. 10 times/day) and reported a high compliance rate (78%). This compliance rate is similar to the rate reported in youth (76%) (Heron et al., 2017). Even though younger individuals might be particular amendable to monitor health aspects using technology such as smartphone apps, the willingness seems not to differ across ages, suggesting age itself to not be a barrier per se (Torous et al., 2014). Nevertheless, more research in older adults is needed to determine potential variations from the findings of this review reflecting digital self-monitoring in middle-aged adults.
Digital self-monitoring was also just one element within the multi-modal set-ups. This multi-modality in combination with the diverse study designs (i.e. 2/3 included a control group) limits the expressiveness with respect to the direct effectiveness of digital self-monitoring on health, which was in this review secondary. When reviewing complex interventions, it is recommended to focus on the aspects of complexity that are depict the main research question (Petticrew et al., 2015). In the present review, this focus was on the composition of digital self-monitoring within interventions. The following elements of digital self-monitoring stood out through the narrative synthesis and should be considered in future set-ups:
4.1. Mobile technology
In everyday life, most individuals spend time not only at home, but a variety of places such as at work, public places, or nature. To promote digital self-monitoring in all environments, a mobile technology is essential. Three studies, however, used a stationary device (i.e. computer or computer in combination with TV system)[6, 7, 20], which limits the flexibility of the digital self-monitoring in everyday life resulting in an incomplete view of one's daily functioning. The non-mobile approach could furthermore explain the low self-monitoring adherence in one of the studies: only 10% of the participants used the Eating Awareness Tracker within the ‘Mind, Body, Food’ intervention for at least 12 out of 14 weeks[6].
The studies that used a portable technology chose PDAs, smartphone apps, or mobile phones (surveys, SMS/with link to website). The benefit of smartphone apps for the self-monitoring lies in the fact that users can install apps on his/her own smartphone, which is comfortable as no new device needs to be learned and carried. Additionally, people (users or researchers) do not need to purchase a whole new technology. As individuals spent multiple hours a day on their smartphones (Andrews et al., 2015), the task to repeatedly self-monitor momentary aspects for a couple of minutes seems unproblematic. Contrary, giving the participant a new technological device such as a PDA might increase the excitement and thus adherence. In this review with regard to the adherence, there was no trend identified for one mobile device being superior to another mobile device.
Next to smartphone (apps), IVRs were used through which individuals interacted with a computer by calling it and responding to questions via the keypad of the telephone. All IVR studies instructed the participants to use the IVR once per day and to reflect on the previous day. On one hand, this retrospective approach compared to traditional ESM ‘in-the-moment’ reflections might introduce a slight memory-bias, which could affect the ecological validity (Scollon et al., 2009). On the other hand, the IVR interventions had durations of >8 weeks[2, 11, 18, 19], which was twice as long as some of the non-IVR interventions[5, 8, 16/17, 21/22, 23]. The longest study period described in this review of 6 months also used IVR[13]. This long self-monitoring duration using IVR, however, was also paired with a described reduction of engagement with the self-monitoring in two studies[13, 19] .
Surprisingly, no intervention included wearables or other technological devices to combine active self-monitoring with passive self-monitoring of additional physical information. Recent research reveals that wearables are not only easy to use for older adults, but can also improve aspects of health by, for example, encouraging participants to increase their daily level of physical activity (Alharbi et al., 2019; Grossman et al., 2018). The dichotomy of active and passive self-monitoring used in the present review might furthermore not be applicable to all technologies, as approaches can be combined (Arulnathan et al., 2019) and passively collected data could also lead to awareness for behaviors via feedback and thus health benefits (Fukuoka et al., 2018).
Generally, the choice for one or the other mobile technology seems to be partially influenced by the workplace as the IVR studies, for example, were all conducted or in collaboration with the University of Vermont and Colombia University, while the PDA PsyMate studies origin from Maastricht University. The findings of this review could facilitate researchers and clinicians to expand their horizon and adopt other technological solutions into their institute. This adaptation of new approaches may result in new insights and additional advantages for the target populations. Another issue might lie in the tendency of inventing similar devices/services instead of building on existing knowledge. General guidelines can contribute to less time spent on testing the basics of the technology and more time channeled into designing the modalities that actually improve health and change behaviors.
4.2. Duration, intensity, and reduced engagement
Currently, no guideline for the duration or intensity of digital self-monitoring within interventions exists. Within the studies included in this review, the duration and intensity of the digital self-monitoring varied strongly and no clear trend could be identified. The diversity might be related to the unavailability of guidelines, the variety of health issues targeted by the interventions, and the fact that the health improvements might take unequally long to be reached. A delayed health improvement could be seen, for example, in the study of Van Knippenberg et al. (2018) as some significant changes did not appeared after the intervention, but at two-months follow-up.
When reflecting on the sampling intensity, one benefit of a high sampling intensity (i.e. 10 times/day) is the possibility to identify pattern and fluctuations over the day (Myin-Germeys et al., 2009). Furthermore, the person may be desensitize to the procedure and therefore reduced reactivity to the self-monitoring method through a higher sampling load (Palmier-Claus et al., 2011). Contrarily, a high sampling intensity can be time-consuming and burdensome for the individual in the long-term. In the included studies, there was no visible relation between a high intensity resulting in low adherence. The intensity as well as duration might prospectively be chosen with respect to the individual's preferences. Furthermore, a re-evaluation of the set-up after some weeks or months might be useful, as the health improvement could be reached earlier or later than expected. Flexible adjustment of the intervention in combination with a person-centered approach (Kirschenbaum and Jourdan, 2005) could thus improve optimal functionality.
A number of studies noticed a decrease of adherence over the course of the self-monitoring intervention[3, 6, 13, 19, 24]. This decline in engagement has been noted by other eHealth studies (Davies et al., 2012; Gilliland et al., 2015; Maher et al., 2014; Schoeppe et al., 2016; Yardley et al., 2016). In an EMA review focused on youth, recommendations include offering incentives or integrating measurement bursts (self-monitoring for several days or weeks followed by a break and then continuing) to maintain interest (Heron et al., 2017). Future research needs to investigate ideas on reward systems or gamifications (Deterding, 2012) to motivate sustained engagement resulting potentially in lasting behavioral change. Theoretically, behavioral change maintenance is complex and includes factors related to the individual motive, self-regulation, psychological and physical resources, habits, and environmental and societal influences (Kwasnicka et al., 2016). These factors might guide future developments and intervention designs.
4.3. Self-monitoring of health aspects: a discrepancy between theory and practice
To improve self-management through self-monitoring, health as a complex and dynamic system requires interventions that take all health aspects into account: physical, psychological, and social. Only eight of the 21 interventions asked the participants to reflect on social factors. Social health refers to the view that an individual can manage and maintain a balance between opportunities and limitations in social and environmental challenges and thus experience well-being despite a health issue (Huber et al., 2011). In certain fields, researchers emphasize that more attention needs to be paid to social health to improve participation and well-being (de Vugt and Dröes, 2017; Niederdeppe et al., 2008).
The discrepancy in the reviewed self-monitoring interventions between the theoretical importance of social aspects of health (Lehman et al., 2017) and in practice this aspect being widely neglecting might indicate that the digital self-monitoring interventions need adjustments to optimally support health. In case the interventions included self-monitoring of social aspects, the social information was used in different ways. Two interventions focused one face-to-face feedback session on social interactions and related positive affect[10/15, 26]. This approach could raise awareness for the social network available and the importance to maintain it[25]. Self-monitoring of sexual behavior also increased awareness of the relationship between this behavior and substance use and other triggers[24]. Furthermore, socialization and recreational activities (i.e. cultivate social contact) were main goals that participants could choose to improve[9,23]. The other studies did not include details on how the self-monitored information on social aspects (i.e. sexual interactions and protection, relationship to partner) was used[1, 13]. Conclusively, the evidence on how social aspects can be utilized in self-monitoring interventions is limited and future research is urged to investigate further. Nevertheless, the theoretical importance of interpersonal health aspects is not translated well into practice.
4.4. Feedback: health professional vs. programmed technology
All studies provided some form of feedback, except three[5, 6, 21/22]. While one of them (Batink et al., 2016) did not find a significant change in the self-report outcome measures (i.e. psychological flexibility, symptoms, coping, or quality of life), the results of Boucher et al. (2016) showed significant within-group improvement in intuitive eating, psychological flexibility, and general mental health as well as decrease in binge eating. In this study, however, the success might be more influenced through other intervention elements (teaching ACT-based skills) rather than feedback of self-monitoring information as only 10% of the participants engaged in the whole 14-week self-monitoring[6].
All other interventions reported some of the self-monitoring information back to the individuals. Generally, a trade-off between the frequency and amount of involvement of the healthcare professional could be observed: while automated feedbacks would be used more often, they might also be less personal. The involvement of health professionals such as coaches or psychologists was described as pleasant and useful as described in the process evaluation of one intervention[25] and the qualitative results stated in Burkow et al. (2015). Due to limited resources, however, involvement might not be feasible on a daily level.
Providing feedback can be more challenging than one might think as the question remains if the person can cognitively grasp the information and translate it into a behavioral change (Wilson et al., 2015). Generally, feedback is recommended to be timely and tailored (De Vries et al., 2008). Prospectively in self-monitoring intervention, a combination of both programmed feedback/progress tracking and a weekly or monthly personal conversation might provide the individual with the ideal support. This combined approach is in line with the ‘blended care principle’, which highlights the use of both online modules and session with a personal coach to support self-management (Boots et al., 2017). The personal contact could be provided through written messages[16/17], online consultations[7], face-to-face sessions[1, 2,10/15, 11, 25/26], or even recorded voice messages[18, 19] as illustrated by the reviewed studies.
4.5. Intervention effects, their sustainability, and mechanisms
This systematic review is not intended to fully evaluate the effectiveness and sustainability of effects. Rather, the narrative synthesis provides descriptive conclusions that should not be generalized: almost all intervention reported a significant positive effect on health either by comparing intra- or inter-group differences. As expected, the specific results were diverse. Highlights include improved mood[10, 21, 26], better health-related quality of life[7], healthier eating habits and/or physical activity[3, 4, 6, 14], decreased levels of pain[18, 20], as well as increased medication adherence[9, 23].
One study did not result in significant improvements in the chosen outcome measures (i.e. psychological flexibility, symptoms, avoidant coping)[5]. The primary aim of this project was to assess the feasibility and acceptability of a fully automated mobile ACT intervention in daily life delivered by a PDA (i.e. PsyMate). The participants showed great enthusiasm for participation resulting in twice as many participants as aimed for. Furthermore, the completion rate was high (76%) and the high sampling intensity (10 times/day) did not seem to have interfered. The non-significant results might be explained by the short follow-up period as differences could be visible at a later time point. Additionally, a ceiling effect could have influenced the scores as an intensive inpatient treatment had occurred prior to the intervention[5].
Another study led to negative health outcomes, namely an ‘increase’ in alcohol consumption measured with a retrospective instrument. This outcome might be explained through a measurement confounder[13]. The authors argue that this finding is counterintuitive and a large body of literature supports the beneficial influence of self-monitoring and feedback on alcohol use. The given explanation refers to the observation that individuals commonly underestimate their consumption on the retrospective instrument, but through the self-monitoring their report increased in accuracy, which then resulted in a misleading result. Through another analysis, independent of this confounder, self-monitoring in particular in combination with feedback seems to be beneficial to reduce alcohol consumption[13].
The evaluation of the sustainability of intervention effects was not included in the majority of studies. When follow-ups were included, the periods ranged from two[25/26] to twelve months[2, 11]. Some studies faced thus the fading of significant intervention effects after some weeks or months[11, 17]. Achieving as well as maintaining a behavioral change to improve health is complex and influenced by various internal and external factors. Additional interventions features after the main period might, therefore, be necessary to assess the health status and potentially boost it. Overall, more research is needed as the question how sustainable effects of self-monitoring interventions are with respect to maintained health improvements is understudied. On the level of public health care systems, not only long-term financial solutions are needed, but also the joined development, provision, and monitoring of eHealth approaches including all stakeholders (private sector, beneficiaries, final users) plays an important role in the sustainability (De Rosis and Nuti, 2018).
When trying to explain underlying mechanisms, the Transtheoretical Model of Health Behavior Change claims that individuals go through six ‘stages’ during the process of change namely precontemplation, contemplation, preparation, action, maintenance, and termination (Prochaska and Velicer, 1997). Interventions are therefore suggested to be stage-matching and individualized (West, 2005). Next to self-monitoring, a systematic review linked the following techniques to particularly successful interventions: provision of instructions, relapse prevention, and prompting practice (Dombrowski et al., 2012). Furthermore, the behavior centered design approach urges to create surprise, revalue behavior, and disrupt performance to enable a behavioral change (Aunger and Curtis, 2016). While digital self-monitoring could support this process, the theoretical background might require further investigation. Overall, there are >80 theories on behavior and behavioral change (Davis et al., 2015). Future research might elaborate on the theoretical basis to support the practical recommendations for self-monitoring interventions provided in this review.
4.6. Prevention: possibilities and challenges
The idea to use digital self-monitoring to not only treat already existing health issues, but also prevent problems before they manifest, may (theoretically) sound promising: a person self-monitors him/herself, increases awareness for the own lifestyle, and individuals feel motivated to become or stay healthy. Unfortunately, the mechanisms of disease prevention are more complex.
One difficulty relates to the question how engagement in self-monitoring can be promoted over months and even years to achieve long-lasting health results. Taken the example that a healthier lifestyle can reduce the risk for strokes or dementia (Goldstein et al., 2011; Vos et al., 2017), an individual would need to change and maintain this lifestyle for decades. Innovative solutions with long-term research designs are required to study this process and to gain insight into the motivational aspects. This question aligns with the aspect of sustainability described above. For how long is self-monitoring needed to reach ‘the healthy lifestyle’? How can this lifestyle be maintained? As relatively little faction of health spending gets channeled into preventive compared to curative strategies (Gmeinder et al., 2017), the development and implementation of digital prevention strategies is challenging and in its infancy.
4.7. Limitations and future directions
First, the degree of bias within this review by only including studies published in English and the likely publication bias associated with including only published manuscripts is acknowledged. Second, as the review's focus was on the composition of digital self-monitoring, studies were not included based on the quality of effectiveness. However, the included articles were sources from peer-reviewers journals, signifying that all of them had an academic level and quality. Third, by limiting the time period (2008 to April 2018), this review does not aim to be exhaustive but presents an initial picture of digital self-monitoring interventions. Applying the search strategy once more in September 2019 resulted in 1167 hits for the years 2018–2019 indicating an exponential development. A quick review of the PubMed hits (n = 165) using the same search strategy (Appendix A) resulted in identifying one additional study: In this RCT (Mitchell et al., 2019), participants (n = 171, mean age = 50,6 yrs.) became more physically active by using a website to monitor affect, experienced exertion, and steps (pedometer). The website provided the participants with tips and peer support, but no information on the mobile access of the website was provided. Furthermore, the intervention effects had largely disappeared after twelve-months, which is in line with the presented results (Section 4.5). No elements of this intervention seem to greatly diverge from the findings of this review. The updated search revealed many protocols, therefore we believe that an updated review in a couple of years could be beneficial for the field. With regards to the methodological issues, self-monitoring was defined and operationalized generally orientated on momentary assessments such as ESM and EMA and aiming to find the intersection, but this definition might diverge from other perspectives on ESM/EMA. Finally, even if different populations were included in this review, the results cannot be generalized to other age groups or ethnicities.
In the future, digital self-monitoring interventions as a promising eHealth solution may be used to support people not only in rural areas, but also middle- and low-income countries (Blaya et al., 2010; Sudhahar et al., 2010). Additionally, active self-monitoring could be linked with automatic self-monitoring such as wearables (Myin-Germeys et al., 2018) to evolve the solid status of the current self-monitoring technology further. Even though health professional's acceptance of eHealth interventions and aftercare needs to improve before fully implementing eHealth into practice, which is seen as a complex process, (Hennemann et al., 2017; Peeters et al., 2016), digital phenotyping (Onnela and Rauch, 2016), when used in a responsible and ethical way, may have great advantages in health promotion.
5. Conclusion
Overall, digital self-monitoring technologies seem to be suitable to understand and support health-related self-management and hold promise for future trials. The composition of digital self-monitoring interventions in middle-aged and older adults showed a great diversity, particularly with respect to the duration, sampling intensity, and multi-component design. Nevertheless, several elements stood out and should be considered in future digital self-monitoring interventions: (i) Mobile technology can ensure flexible use in everyday life. (ii) Feedback both automatically and in person may support the individual throughout the intervention. (iii) Social health aspects are partially neglected and need more attention. Research may prospectively investigate the sustainability of intervention effects, ways to promote long-term engagement, possibilities for disease prevention, the most suitable theoretical model for digital self-monitoring, and include more older adults >65 years of age.
Acknowledgments
Acknowledgement
We thank Dr. Stefan Jongen from the Education & Research Support Department, University Library, Maastricht University, for his support during the development of the search strategy. The icons used in Table 1, Table 2 are from www.flaticon.com and made by smashicons (Table 1: IVR, SMS on smartphone; Table 2: programmed technology), prettycons (Table 1: PDA, smartphone app, computer), and eucalyp (Table 2: healthcare professional).
Funding
This research was carried out as part of the H2020 Marie Skłodowska-Curie Actions Innovative Training Network (ITN) action, H2020-MSCA-ITN-2015, under grant agreement number 676265.
Declaration of competing interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.invent.2019.100283.
Contributor Information
Sara Laureen Bartels, Email: sara.bartels@maastrichtuniversity.nl.
Marjolein E. de Vugt, Email: m.devugt@maastrichtuniversity.nl.
Appendix A. Supplementary data
Supplementary material
References
- Alharbi M., Straiton N., Smith S., Neubeck L., Gallagher R. Data management and wearables in older adults: a systematic review. Maturitas. 2019;124:100–110. doi: 10.1016/j.maturitas.2019.03.012. [DOI] [PubMed] [Google Scholar]
- Andrews S., Ellis D.A., Shaw H., Piwek L. Beyond self-report: tools to compare estimated and real-world smartphone use. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0139004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arulnathan A., Vaaheesan S., Denecke K., Fachhochschule B. A mobile application for self-monitoring for patients with heart failure. Stud. Health Technol. Inform. 2019;259:113–116. [PubMed] [Google Scholar]
- Aunger R., Curtis V. Behaviour Centred Design: towards an applied science of behaviour change. Health Psychol. Rev. 2016;10(4):425–446. doi: 10.1080/17437199.2016.1219673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A. Social cognitive theory of self-regulation. Organ. Behav. Hum. Decis. Process. 1991;50(2):248–287. [Google Scholar]
- Bandura A. Health promotion from the perspective of social cognitive theory. Psychol. Health. 1998;13(4):623–649. [Google Scholar]
- Batink T., Bakker J., Vaessen T., Kasanova Z., Collip D., van Os J.…Peeters F. Acceptance and commitment therapy in daily life training: a feasibility study of an mHealth intervention. JMIR mHealth uHealth. 2016;4(3) doi: 10.2196/mhealth.5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckjord E., Shifmann S. Background for real-time monitoring and intervention related to alcohol use. Alcohol Res. 2014:36(1). [PMC free article] [PubMed] [Google Scholar]
- Blaya J.A., Fraser H.S., Holt B. E-health technologies show promise in developing countries. Health Aff. 2010;29(2):244–251. doi: 10.1377/hlthaff.2009.0894. [DOI] [PubMed] [Google Scholar]
- Boots L.M., de Vugt M.E., Smeets C.M., Kempen G.I., Verhey F.R. Implementation of the blended care self-management program for caregivers of people with early-stage dementia (Partner in Balance): process evaluation of a randomized controlled trial. J. Med. Internet Res. 2017;19(12) doi: 10.2196/jmir.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher S., Edwards O., Gray A., Nada-Raja S., Lillis J., Tylka T.L., Horwath C.C. Teaching intuitive eating and acceptance and commitment therapy skills via a web-based intervention: a pilot single-arm intervention study. JMIR Res. Protoc. 2016;5(4) doi: 10.2196/resprot.5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkow T.M., Vognild L.K., Johnsen E., Risberg M.J., Bratvold A., Breivik E.…Hjalmarsen A. Comprehensive pulmonary rehabilitation in home-based online groups: a mixed method pilot study in COPD. BMC Res. Notes. 2015;8(1):766. doi: 10.1186/s13104-015-1713-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain A.E., Depp C.A., Jeste D.V. Ecological momentary assessment in aging research: a critical review. J. Psychiatr. Res. 2009;43:987–996. doi: 10.1016/j.jpsychires.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Lieffers J., Bauman A., Hanning R., Allman-Farinelli M. The use of smartphone health apps and other mobile h ealth (mHealth) technologies in dietetic practice: a three country study. J. Hum. Nutr. Diet. 2017;30(4):439–452. doi: 10.1111/jhn.12446. [DOI] [PubMed] [Google Scholar]
- Csikszentmihalyi M., Larson R. Validity and reliability of the experience sampling method. J. Nverv. Ment. Dis. 1987;175(9):526–536. doi: 10.1097/00005053-198709000-00004. [DOI] [PubMed] [Google Scholar]
- Csikszentmihalyi M., Larson R. Flow and the Foundations of Positive Psychology. Springer; 2014. Validity and reliability of the experience-sampling method; pp. 35–54. [Google Scholar]
- Cuthbertson R., Furseth P.I., Ezell S.J. Innovating in a Service-driven Economy. Springer; 2015. Apple and Nokia: the transformation from products to services; pp. 111–129. [Google Scholar]
- Davies C.A., Spence J.C., Vandelanotte C., Caperchione C.M., Mummery W.K. Meta-analysis of internet-delivered interventions to increase physical activity levels. Int. J. Behav. Nutr. Phys. Act. 2012;9(1):52. doi: 10.1186/1479-5868-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R., Campbell R., Hildon Z., Hobbs L., Michie S. Theories of behaviour and behaviour change across the social and behavioural sciences: a scoping review. Health Psychol. Rev. 2015;9(3):323–344. doi: 10.1080/17437199.2014.941722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosis S., Nuti S. Public strategies for improving eHealth integration and long-term sustainability in public health care systems: findings from an Italian case study. Int. J. Health Plann. Manag. 2018;33(1):e131–e152. doi: 10.1002/hpm.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries H., Kremers S., Smeets T., Brug J., Eijmael K. The effectiveness of tailored feedback and action plans in an intervention addressing multiple health behaviors. Am. J. Health Promot. 2008;22(6):417–424. doi: 10.4278/ajhp.22.6.417. [DOI] [PubMed] [Google Scholar]
- de Vugt M., Dröes R.-M. Towards a Positive Dementia Discourse. Taylor & Francis; 2017. Social health in dementia. [DOI] [PubMed] [Google Scholar]
- Deterding S. Gamification: designing for motivation. Interactions. 2012;19(4):14–17. [Google Scholar]
- Dombrowski S.U., Sniehotta F.F., Avenell A., Johnston M., MacLennan G., Araújo-Soares V. Identifying active ingredients in complex behavioural interventions for obese adults with obesity-related co-morbidities or additional risk factors for co-morbidities: a systematic review. Health Psychol. Rev. 2012;6(1):7–32. [Google Scholar]
- Focht B.C., Gauvin L., Rejeski W.J. The contribution of daily experiences and acute exercise to fluctuations in daily feeling states among older, obese adults with knee osteoarthritis. J. Behav. Med. 2004;27(2):101–121. doi: 10.1023/b:jobm.0000019847.80315.4d. [DOI] [PubMed] [Google Scholar]
- Fukuoka Y., Vittinghoff E., Hooper J. A weight loss intervention using a commercial mobile application in Latino Americans—Adelgaza Trial. Transl. Behav. Med. 2018;8(5):714–723. doi: 10.1093/tbm/ibx039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland J., Sadler R., Clark A., O'Connor C., Milczarek M., Doherty S. Using a smartphone application to promote healthy dietary behaviours and local food consumption. Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/841368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmeinder M., Morgan D., Mueller M. 2017. How Much Do OECD Countries Spend on Prevention? [Google Scholar]
- Goldstein L.B., Bushnell C.D., Adams R.J., Appel L.J., Braun L.T., Chaturvedi S.…Hart R.G. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(2):517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- Grossman J.A., Arigo D., Bachman J.L. Meaningful weight loss in obese postmenopausal women: a pilot study of high-intensity interval training and wearable technology. Menopause. 2018;25(4):465–470. doi: 10.1097/GME.0000000000001013. [DOI] [PubMed] [Google Scholar]
- Guo Y., Albright D. The effectiveness of telehealth on self-management for older adults with a chronic condition: a comprehensive narrative review of the literature. J. Telemed. Telecare. 2018;24(6):392–403. doi: 10.1177/1357633X17706285. [DOI] [PubMed] [Google Scholar]
- He W., Goodkind D., Kowal P.R. United States Census Bureau; Washington, DC: 2016. An Aging World: 2015. [Google Scholar]
- Hennemann S., Beutel M.E., Zwerenz R. Ready for eHealth? Health professionals' acceptance and adoption of eHealth interventions in inpatient routine care. J. Health Commun. 2017;22(3):274–284. doi: 10.1080/10810730.2017.1284286. [DOI] [PubMed] [Google Scholar]
- Heron K.E., Smyth J.M. Ecological momentary interventions: incorporating mobile technology into psychosocial and health behavior treatments. Br. J. Health Psychol. 2010;15(1):1–15. doi: 10.1348/135910709X466063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron K.E., Everhart R.S., McHale S.M., Smyth J.M. Using mobile-technology-based ecological momentary assessment (EMA) methods with youth: a systematic review and recommendations. J. Pediatr. Psychol. 2017;42(10):1087–1107. doi: 10.1093/jpepsy/jsx078. [DOI] [PubMed] [Google Scholar]
- Hong Y.-C. Aging society and environmental health challenges. Environ. Health Perspect. 2013;121(3) doi: 10.1289/ehp.1206334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M., Knottnerus J.A., Green L., van der Horst H., Jadad A.R., Kromhout D.…van der Meer J.W. How should we define health? Bmj. 2011;343:d4163. doi: 10.1136/bmj.d4163. [DOI] [PubMed] [Google Scholar]
- Jonkman N.H., van Schooten K.S., Maier A.B., Pijnappels M. eHealth interventions to promote objectively measured physical activity in community-dwelling older people. Maturitas. 2018;113:32–39. doi: 10.1016/j.maturitas.2018.04.010. [DOI] [PubMed] [Google Scholar]
- Kirschenbaum H., Jourdan A. The current status of Carl Rogers and the person-centered approach. Psychother. Theory Res. Pract. Train. 2005;42(1):37. [Google Scholar]
- Kwasnicka D., Dombrowski S.U., White M., Sniehotta F. Theoretical explanations for maintenance of behaviour change: a systematic review of behaviour theories. Health Psychol. Rev. 2016;10(3):277–296. doi: 10.1080/17437199.2016.1151372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman B.J., David D.M., Gruber J.A. Rethinking the biopsychosocial model of health: understanding health as a dynamic system. Soc. Personal. Psychol. Compass. 2017;11(8) [Google Scholar]
- Liao Y., Skelton K., Dunton G., Bruening M. A systematic review of methods and procedures used in ecological momentary assessments of diet and physical activity research in youth: an adapted STROBE checklist for reporting EMA studies (CREMAS) J. Med. Internet Res. 2016;18(6):e151. doi: 10.2196/jmir.4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.…Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher C.A., Lewis L.K., Ferrar K., Marshall S., De Bourdeaudhuij I., Vandelanotte C. Are health behavior change interventions that use online social networks effective? A systematic review. J. Med. Internet Res. 2014;16(2) doi: 10.2196/jmir.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell B.L., Smith A.E., Rowlands A.V., Fraysse F., Parfitt G., Lewis N.R., Dollman J. Promoting physical activity in rural Australian adults using an online intervention. J. Sci. Med. Sport. 2019;22(1):70–75. doi: 10.1016/j.jsams.2018.07.002. [DOI] [PubMed] [Google Scholar]
- Morgenstern J., Kuerbis A., Muench F. Ecological momentary assessment and alcohol use disorder treatment. Alcohol Research: Current Reviews. 2014;36(1):101–110. [PMC free article] [PubMed] [Google Scholar]
- Morton K., Beauchamp M., Prothero A., Joyce L., Saunders L., Spencer-Bowdage S.…Pedlar C. The effectiveness of motivational interviewing for health behaviour change in primary care settings: a systematic review. Health Psychol. Rev. 2015;9(2):205–223. doi: 10.1080/17437199.2014.882006. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I., Oorschot M., Collip D., Lataster J., Delespaul P., Van Os J. Experience sampling research in psychopathology: opening the black box of daily life. Psychol. Med. 2009;39(9):1533–1547. doi: 10.1017/S0033291708004947. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I., Klippel A., Steinhart H., Reininghaus U. Ecological momentary interventions in psychiatry. Co-Psychiarty. 2016;29(00) doi: 10.1097/YCO.0000000000000255. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I., Kasanova Z., Vaessen T., Vachon H., Kirtley O., Viechtbauer W., Reininghaus U. Experience sampling methodology in mental health research: new insights and technical developments. World Psychiatry. 2018;17(2):123–132. doi: 10.1002/wps.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederdeppe J., Bu Q.L., Borah P., Kindig D.A., Robert S.A. Message design strategies to raise public awareness of social determinants of health and population health disparities. Milbank Q. 2008;86(3):481–513. doi: 10.1111/j.1468-0009.2008.00530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onnela J.-P., Rauch S.L. Harnessing smartphone-based digital phenotyping to enhance behavioral and mental health. Neuropsychopharmacology. 2016;41(7):1691. doi: 10.1038/npp.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmier-Claus J.E., Myin-Germeys I., Barkus E., Bentley L., Udachina A., Delespaul P.…Dunn G. Experience sampling research in individuals with mental illness: reflections and guidance. Acta Psychiatr. Scand. 2011;123(1):12–20. doi: 10.1111/j.1600-0447.2010.01596.x. [DOI] [PubMed] [Google Scholar]
- Peeters J.M., Krijgsman J.W., Brabers A.E., De Jong J.D., Friele R.D. Use and uptake of eHealth in general practice: a cross-sectional survey and focus group study among health care users and general practitioners. JMIR Med. Inform. 2016;4(2) doi: 10.2196/medinform.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petticrew M., Anderson L., Elder R., Grimshaw J., Hopkins D., Hahn R.…Sipe T. Complex interventions and their implications for systematic reviews: a pragmatic approach. Int. J. Nurs. Stud. 2015;52(7):1211–1216. doi: 10.1016/j.ijnurstu.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Popay J., Roberts H., Sowden A., Petticrew M., Arai L., Rodgers M.…Duffy S. 2006. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. A Product From the ESRC Methods Programme Version, 1, b92. [Google Scholar]
- Prochaska J.O., Velicer W.F. The transtheoretical model of health behavior change. Am. J. Health Promot. 1997;12(1):38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- Ryan C.L., Lewis J.M. US Department of Commerce, Economics and Statistics Administration; US: 2017. Computer and Internet Use in the United States: 2015. [Google Scholar]
- Schoeppe S., Alley S., Van Lippevelde W., Bray N.A., Williams S.L., Duncan M.J., Vandelanotte C. Efficacy of interventions that use apps to improve diet, physical activity and sedentary behaviour: a systematic review. Int. J. Behav. Nutr. Phys. Act. 2016;13(1):127. doi: 10.1186/s12966-016-0454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scollon C.N., Kim-Prieto C., Diener E. Experience sampling: promises and pitfalls, strength and weaknesses. J. Happiness Stud. 2003;4(1):5–34. [Google Scholar]
- Scollon C.N., Prieto C.-K., Diener E. Assessing Well-being. Springer; 2009. Experience sampling: promises and pitfalls, strength and weaknesses; pp. 157–180. [Google Scholar]
- Shiffman S., Stone A.A., Hufford M.R. Ecological momentary assessment. Annu. Rev. Clin. Psychol. 2008;4(1):1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Simons C.J.P., Drukker M., Evers S., Van Mastrigt G.A.P.G., Höhn P., Kramer I.…Wichers M. Economic evaluation of an experience sampling method intervention in depression compared with treatment as usual using data from a randomized controlled trial. BMC Psychiatry. 2017 doi: 10.1186/s12888-017-1577-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srakar A., Rupel V.P. Health services utilization in older Europeans: an empirical study. Organizacija. 2016;49(2):127–136. [Google Scholar]
- Stone A.A., S. S. Ecological momentary assessment (EMA) in behavioral medicine. Ann. Behav. Med. 1994;16(3):199–202. [Google Scholar]
- Stone A.A., Shiffman S. Capturing momentary, self-report data: a proposal for reporting guidelines. Ann. Behav. Med. 2002;24(3):236–243. doi: 10.1207/S15324796ABM2403_09. [DOI] [PubMed] [Google Scholar]
- Sudhahar S., Vatsalan D., Wijethilake D., Wickramasinghe Y., Arunathilake S., Chapman K., Seneviratna G. Paper Presented at the 2010 Second International Conference on eHealth, Telemedicine, and Social Medicine. 2010. Enhancing rural healthcare in emerging countries through an eHealth solution. [Google Scholar]
- Torous J., Friedman R., Keshavan M. Smartphone ownership and interest in mobile applications to monitor symptoms of mental health conditions. JMIR mHealth uHealth. 2014;2(1):e2. doi: 10.2196/mhealth.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Knippenberg R.J.M., De Vugt M.E., Ponds R.W., Myin-Germeys I., Verhey F.R.J. An experience sampling method intervention for dementia caregivers: results of a randomized controlled trial. Am. J. Geriatr. Psychiatry. 2018;26(12):1231–1243. doi: 10.1016/j.jagp.2018.06.004. [DOI] [PubMed] [Google Scholar]
- Verhagen S.J.W., Hasmi L., Drukker M., Van Os J., Delespaul P.E.G. Use of the experience sampling method in the context of clinical trials. Evid. Based Ment. Health. 2016;19(3):86–89. doi: 10.1136/ebmental-2016-102418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versluis A., Verkuil B., Spinhoven P., Van der Ploeg M.M., Brosschot J.F. Changing mental health and positive psychological well-being using ecological momentary interventions: a systematic review and meta-analysis. J. Med. Internet Res. 2016;18(6) doi: 10.2196/jmir.5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos S.J., Van Boxtel M.P., Schiepers O.J., Deckers K., De Vugt M., Carrière I.…Ritchie K. Modifiable risk factors for prevention of dementia in midlife, late life and the oldest-old: validation of the LIBRA Index. J. Alzheimers Dis. 2017;58(2):537–547. doi: 10.3233/JAD-161208. [DOI] [PubMed] [Google Scholar]
- West R. Time for a change: putting the Transtheoretical (Stages of Change) Model to rest. Addiction. 2005;100(8):1036–1039. doi: 10.1111/j.1360-0443.2005.01139.x. [DOI] [PubMed] [Google Scholar]
- Wilson G.T., Bhamra T., Lilley D. The considerations and limitations of feedback as a strategy for behaviour change. Int. J. Sustain. Eng. 2015;8(3):186–195. [Google Scholar]
- Yardley L., Spring B.J., Riper H., Morrison L.G., Crane D.H., Curtis K.…Blandford A. Understanding and promoting effective engagement with digital behavior change interventions. Am. J. Prev. Med. 2016;51(5):833–842. doi: 10.1016/j.amepre.2016.06.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material