Abstract
Vertisols occupy approximately 1,200,000 ha in Northern Cameroon. Their richness in smectites allows for the production of “bleaching earths” necessary for refining palm oil, and their effluent is used for leachate treatment. In the present work, two mineral acids (HCl and H2SO4) were compared, and the most efficient acid with the lowest cost was determined for use in industrial applications. Under similar experimental conditions (ratio of acid solution/clay mass = 5/1, temperature = 97 °C, stirring time = 4 h), the quantity of cations (Fe2+, Fe3+, Al3+) solubilised during acid activation, palm oil discolouration rate by each activated sample and the financial cost of 5 L of acid solution that is required for the acid activation of one kilogram of smectite clay were compared. It was found that 2N H2SO4 was more efficient than 1N HCl and 1N H2SO4, considering palm oil bleaching efficiency and cost. The filtrate collected after the acid activation of vertisols was rich in H+ (2.04.10−1M), Fe2+ (2.8.10−3M), Fe3+ (4.2.10−2M) and Al3+ (9.2.10−2M) ions. One gram of smectite clay material produced 9 mL of this filtrate that was used for the treatment of leachate from a controlled landfill. The leachate colour decreased from 4262 to 285 PtCo units, while the corresponding chemical oxygen demand (COD) decreased from 802 to 128 mg/L. Thus, the most effective acid for industrial bleaching earth production from vertisol is 2N H2SO4 acid.
Keywords: Environmental science, Materials chemistry, Smectite, Vertisol, Acid activation, Palm oil, Filtrate, Leachate treatment
Environmental science; Materials chemistry; Smectite; Vertisol; Acid activation; Palm oil; Filtrate; Leachate treatment.
1. Introduction
In the modern world, and especially in tropical countries, palm oil holds an important place among edible oils. For a developing country such as Cameroon, it accounts for most of the production of edible oils, with approximately 1,051 tons exported between 2012 and 2016 (Single Desk of the Cameroon Customs). For its various uses in cooking, cosmetics and soap factories, palm oil must first be refined. Refining involves a major step, fading, which consists in removing β-carotene, the pigment responsible for its red colour by adsorption on the surface of montmorillonites: either natural (Murray, 2000) or activated (Kamal et al., 2011; Komadel, 2016); these materials are called “bleaching earths.” So far, all bleaching earths used in local mills are imported mainly from countries such as China, Germany, France and England. However, several works (Esu and Lombin, 1988; Fasina et al., 2015; Azinwi et al., 2011; Temga et al., 2019), have shown that the vertisols of the different countries of the Sub-Saharan region (North-Cameroon, North-East-Nigeria, South-West-Chad), are rich in smectite clays. In Cameroon, vertisols occur in the north, specifically in the Sudano-Sahelian zone. The detailed characteristics are reported by Brabant (1987), Azinwi et al. (2011), Temga et al. (2019). Vertisols cover a total surface area of 1,200,000 ha. They cover vast areas in the Diamare, Kaele, the Logone and Shari plains and the Benue floodplain. Acid activation can significantly improve their surface properties to enable their use as bleaching earth in vegetable oil refining.
In the activation process, the most commonly used acids are hydrochloric acid (Vicente et al., 1994, 1995a; 1995b, 1996a; 1996b; Suarez et al., 1995; Christidis et al., 1997; Djoufac et al., 2007; Valentin et al., 2007; Pentrak et al., 2009; Frini-Srasra et al., 2010; Sakizci et al., 2011; Usman et al., 2012) and sulfuric acid (Bike et al., 2005; Nde-Aga et al., 2007; Babaki et al., 2008; Nguetnkam et al., 2008, 2011; Steudel et al., 2009; Kamal et al., 2011; Jean Baptiste et al., 2013). Salawudeen et al. (2007) concluded the effectiveness of hydrochloric acid without addressing the financial aspect. Nguetnkam et al. (2008) opted for sulfuric acid without comparing it with hydrochloric acid. From all these previous works, it is apparent that a clear choice (cost of treatment and performance of the material) has not yet been established between these two acids. The production of bleaching earths from vertisols could lead to the release of large quantities of filtrates (washing liquids) composed mainly of hydronium (H+), aluminium (Al3+) and ferric iron (Fe3+) and/or ferrous iron (Fe2+) ions into the environment.
Leachate is a residual liquid derived from the percolation of water in a waste discharge precinct. Its formation can be simplified as water leaching of the soluble and colloidal substances from solid material buried in the ground. During their temporal and spatial evolution, the composition of leachates is controlled by the nature of the original wastes, conditions of biodegradation, geographical situation and nature of site, the mode of exploitation and age of discharge. Globally, they are composed of a mineral and an organic fraction. Regarding their physicochemical and biological composition, leachates constitute the main source of pollution related to discharge with potential ecological and sanitary impacts. In the case of the Banefo landfill, the leachate produced is collected in a decantation basin before it is released into the Mifi River (the main collector of all drainage basins), with COD (carbon oxygen demand) values that can reach as high as 250 mg/L at the exit point. The inhabitants of the nearby villages that have not yet had access to potable water from SNEC (Cameroon National Water Co-operation) are forced to go to the river, in addition to wells, for their water supply. Leachate treatment before its disposal into the river is therefore indispensable in order to avoid bio-ecological and chemical havoc to the environment, living organisms and the riparian population.
Much data has already been documented on the methods of leachate treatment (Ushikoshi et al., 2002; Robinson et al., 2003; Wang et al., 2003; Tjasa et al., 2004). The main goal of all these methods was to separate the pollutant phase from the water or to produce zero effluent. The COD value is an important indicator of global pollution of water or leachate by organic compounds. It is one of the parameters often used to access the pollution taxes to be paid by industries (De Boeck, 2001). The first objective of this work will be to determine the acid (nature and concentration) indicated for economically profitable activation of a smectite-based soil from North Cameroon. The second objective will be to assess the chemical reactivity of the Banefo landfill trap basin and to determine the conditions of the contaminant load by using the filtrate derived from acid activation of vertisols.
2. Materials and methods
2.1. Materials
2.1.1. Clay material and palm oil
The clay materials used in this work were vertisols (Vg) from Garoua (North Cameroon). They were collected 50 m from the Benue River (9°18′N; 13°28′E and altitude 175 m) during the dry season. The morphological characteristics of the raw vertisols in the field are shown in Table 1.
Table 1.
Morphological properties of the North Cameroon vertisols.
| Horizon (depth) | Munsell Colour (dry) |
Structure | Consistency |
Rock fragments | Boundary | Roots | ||
|---|---|---|---|---|---|---|---|---|
| Code | Colour | Dry | Wet | |||||
| A1 (0–30 cm): grey massive blocky horizon | 10YR5/1 | Grey | 3c, abk | h, f | s, p | v | g | c, f |
| B1 (30–100 cm): dark grey massive blocky horizon | 10YR4/1 | Dark grey | 3m, abk | h, f | s, p | n | g | f, f |
| B21 (100–150 cm): dark grey horizon | 10YR4/1 | Dark grey | 3c, abk | h, f | s, p | v | g | - |
| B3g (150–250 cm): very dark grey horizon | 10YR3/1 | Very dark grey | 3c, abk | h, f | s, p | v | - | - |
| Key of properties | |||||||
|---|---|---|---|---|---|---|---|
| Structure |
Consistency |
Rock fragments (%) | Horizon Boundary | Roots | |||
| Size | Type | Grade | Dry | wet | |||
| vf = very fine (G5 mm)f = fine (5–10 mm)m = medium (10–20 mm)c = coarse (20–50mm)vc = very coarse (>50 mm)1 = weak; 2 = moderate; 3 = strong; | g = granular abk = angular blocky sbk = subangular blocky l = lumpy ma = massive |
w = weak (peds barely observable) m = moderate (peds observable) s = strong (peds clearly observable) |
l = loose s = soft h = hard |
s = sticky p = plastic |
n = none (0); v = very few (0–5) c = common (5–15) m = many (15–40) a = abundant (40–80) d = dominant (>80) |
a = abrupt c = clear g = gradual d = diffuse |
f = common; f = few |
The material was grey to very dark grey, massive blocky, hard and firm when dry, sticky and plastic when wet, with no to very few rock fragments and few to common roots at the surface layers. Samples from a 30–100 cm (which was clean and poorer in organic matter content) depth were air-dried in the laboratory to a constant weight before grinding and sieving in a 200 μm sieve. An industrial sample (BC2), which is a commercial bleaching earth from Engelhard (NL) currently used in some oil refineries, was used as reference material.
Palm oil used in this work was from “Société Camerounaise de Palmerais (SOCAPALM),” bought in the Bafoussam local market and preserved at 4 °C in a dark chamber.
2.1.2. Leachates
Leachates were obtained from the Banefo landfill located 5 km from Bafoussam Town (west region of Cameroon) and administered since 2011. This landfill is managed by the Cameroon Hygiene and Sanitation Corporation (HYSACAM) which ensures the collection, treatment and confinement of wastes in the Bafoussam Municipality. This company receives a daily average of 300–400 tons of waste. In the lockers or cavities spread through a surface area of 7 ha, the wastes are compacted, crushed and mixed with earth material before finally been disposed in successive layers to ease their decomposition. A slope of approximately 2% exists from the lower to the upper parts of the collecting tanks in order to shun rainwater and leachate infiltration. This slope, connected by a gutter that surrounds the lockers, plays the role of leachate transportation to the three retention points located at the downslope of the discharge. Further, this seven year-old landfill is intermediate in age (5–10 years) and might thus enclose between 5 and 30% of volatile fatty acids, fulvic acids and humic acids according to Lagier (2000). After a relatively long interruption in the retention basin, the excess leachate flowing out from the third basin flows into the neighbouring river. The leachate produced by the Banefo landfill presents a discharge of 6–60 m3 per day between the rainy and the dry season. In these basins, coarse organic matter decantation and the deposition of some salts take place. The samples analysed in the present study correspond to the leachates sampled behind the sedimentation basin during the rainy season (RSL) and in the middle of the dry season (DSL). Those samples were collected and conserved in the refrigerator at 4 °C before different analyses were then carried out within less than 7 days as recommended by Degremont (1978).
2.2. Experimental methods
2.2.1. Vg chemical and mineralogical analyses
The geochemical analyses were performed by inductively coupled plasma-atomic emission spectrometry (Thermo Fischer ICap 6500 mark) after fusion in LiBO2 and dissolution in nitric acid. The mineralogical analysis of the vertisol samples was performed by X-ray diffraction using a 2080 sigma diffractometer (BRUKER type), which was equipped with a Ni-filter and a Cu anode (quartz mono-chromator, K-alpha 1 wavelength = 1.5418 Å). The semi-quantitative mineral composition was completed by estimating the peak surface area according to Robert (1975).
2.2.2. Acid activation of clay material
In a round-bottom flask, 10 g of dry crushed vertisol powder (Vg) and 50 cm3 of acid solution were added. The mass ratio of acid solution/mass of clay was set at 5/1 because it was the minimum ratio that produced good wettability with a high probability of clay particle abrasion and the formation of fine particles when stirring. This parameter was of great importance when considering the volume of acid solution to be used, and its financial cost. Acid activation is more efficient when the clay particles rub together in a small volume of solution (Komadel and Madejova, 2006). The mixture was then heated in a water bath (97 °C) and agitated under reflux for 4 h throughout the activation process. At the end of the treatment, the mixture was left to settle for 24 h. Subsequently, the supernatant filtrate was recovered for future quantitative determination of iron and aluminium ions. The solid residue was washed several times with distilled water until the traces of the acid completely disappeared. Washing ended when the filtrate no longer reacted with silver nitrate (for hydrochloric acid) or with barium chloride (for sulfuric acid). The activated material was oven-dried at 110 °C before grinding and sieving in a 200 μm diameter sieve.
2.2.3. Acidity evaluation of raw and activated clay material
Acidities of the inactivated clay and of the 2N H2SO4-treated clay were evaluated by adsorption of pyridine followed by Fourier transformed infrared (FTIR) spectroscopy. Pyridine adsorption experiments were performed in a setup under vacuum connected to an IR cell equipped with KBr windows. The powdered clays were pressed into self-supporting wafers of ca. 22 mg for 2 cm2. The samples were heated to 423 K (5 K/min) and kept for 1 h under vacuum (2.10−5 mbar) in order to remove adsorbed water and impurities. After cooling to RT, 1.3 mbar pyridine was contacted at equilibrium on the wafer for 10 min. It was then evacuated for 15 min at 298 K and finally heated to 398 K (2 K/min) and left for 30 min at 398 K. For each sample, the spectra presented were subtracted from spectrum of the solid pre-treated at 423 K and cooled to RT, just before pyridine adsorption. Quantification was carried out using molar absorption coefficients of 2.22 and 1.67 cm μmol−1 reported for the bands at 1440 cm−1 (Lewis) and 1540 cm−1 (Bronsted) (Emeis, 1993; Barzetti et al., 1996).
2.2.4. Palm oil discolouration
It is well known that bleaching earth retains approximately 90% of its weight in oil during filtration. According to Topallar (1998), the best clay/palm oil ratio to use should be 2% in order to minimise the quantity of bleaching earth used during palm oil discolouration. As Vg contains only 25–35% smectite, a clay/palm oil ratio of 4% was used for all the tests. In a round-bottom flask, 25.00 g raw palm oil was added which had been warmed in a water bath (97 ± 2 °C) for 15 min; then, 1.00 g of clay was added, and the suspension was stirred continuously for 1 h. At the end of the discolouration experiment, the suspension was filtered using a filter paper. The effectiveness of the discolouration was evaluated with a UV-light HACH DR/2500 spectrophotometer at a wavelength of 448.5 nm by determining the quantity of residual carotene in the palm oil from the reduction of the absorbance. Each experiment was reproduced twice, and an average was obtained from three measurements. Then, the reduction in colour was calculated as: colour reduction (%) = (crude oil colour-bleached oil colour) x100/crude oil colour.
2.2.5. Filtrate separation and determination of its chemical composition
At the end of the activation, the mixture was allowed to settle for 12 h. The filtrate obtained from the acid activation had a pH < 1 and was yellowish in colour. The first liquid filtrate (F1) was decanted and collected. A volume of distilled water corresponding to the initial volume used during the acid activation was added. The entire mixture was stirred and decanted again, and the filtrate (F2) was finally collected and added to the previously collected first (F1) filtrate. To determine the chemical composition of the filtrate, 10 mL of the filtrate mixture (F1+F2) were neutralised with a solution of limewater (2.3.10−2 M) to pH 7. The precipitate obtained (RHC) was air-dried, and the major elements were analysed by ICP-AES after melting the samples in LiBO2 and dissolving in nitric acid (CRPG Nancy in France).
2.2.6. Quantitative determination of iron and aluminium from the filtrate (F1 + F2)
Fe(II) in the filtrate was first determined by the potentiometric method, which utilizes potassium dichromate in a redox titration with an acidic medium (Skoog et al., 2015). Second, the determination of total Fe was carried out after reduction of Fe(III) into Fe(II) using metallic zinc. Standard solutions of iron (II) and dichromate were used. Aluminium was dosed by colourimetry on a spectrophotometer at a wavelength of λ = 546 nm (Kennedy et al., 1986). A calibration line was plotted in the concentration range of 0–0.03 mg/25 mL. The mass of cations solubilised during different acid treatments was obtained by reporting the determined quantity of each ion to the initial mass of activated clay material. The assays were duplicated, and a third assay was performed in case the difference between the first two assays was greater than 10%.
2.2.7. Mass loss of clay material during acid activation
Standard masses of the clay materials (Vg) were identically activated with 1N HCl, 1N H2SO4 and 2N H2SO4 solutions. At the end of these different treatments, the relative mass losses (Δm/m)×100 (%) were determined by gravimetry. The mass loss (%) recorded during acid activation of the clay material was obtained as the difference between the initial mass of clay material and that of the solid after acid activation. Each experiment was repeated three times, and the average mass was selected.
2.2.8. Leachate chemical analyses
A dry residue was obtained by oven-drying the leachate at 70 °C, and the resulting brown powder was then crushed in a mortar and weighed in a 1/10000 precision balance. Chemical analyses were performed on this dry residue. The major elements were determined by ICP-AES after melting the sample in lithium borate (LiBO2) and dissolution in nitric acid. The results were expressed in the form of their most stable oxides. The trace elements of dry residue were determined by ICP-MS in the same laboratory of CRPG at Nancy (France).
2.2.9. Leachate treatment with filtrate and measurement of some parameters (colour, pH, COD)
In 10 beakers (100 ml capacity), 50 ml of leachates were added. Zero to 10 ml of filtrate (F1 + F2 mixture) was added; then, the entire suspension was stirred for 10 min, and finally, the beaker was allowed to stand for 2 h. To evaluate the treatment efficiency of the leachate, the colour of the supernatant liquid was measured with a spectrophotometer (HACH DR3900) at a wavelength of 455 nm in PtCo units. Distilled water was used as a standard, with a colour of zero PtCo units. A numerical pH-meter (Schott Gerate) was used for pH measurements after being regularly calibrated. The COD of the raw and treated leachate was determined by the colourimetric method under closed reflux (Thermo-reactor ECO8COD vario, model 28822) using a standard solution (183 mg/L) of benzoic acid (Rodier, 1984).
3. Results and discussion
3.1. Mineralogical and chemical analyses
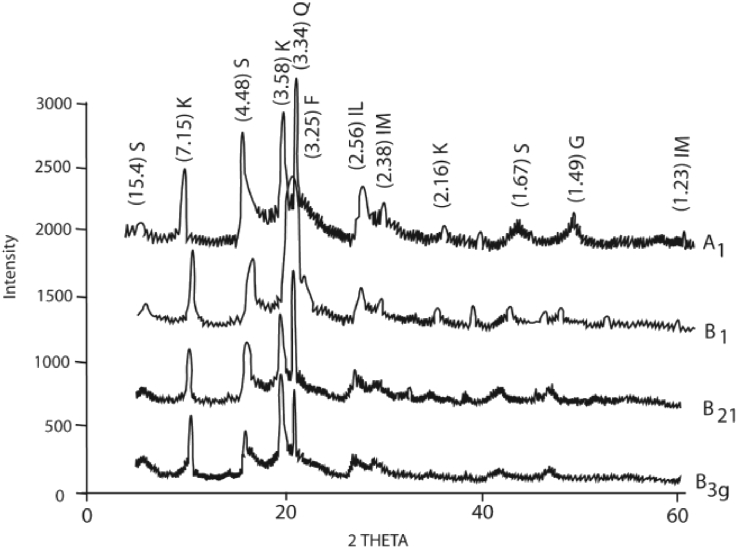
The X-ray diffractograms of the vertisols (Figure 1) revealed that smectite was the most abundant mineral (with characteristic peaks at 15.4 Å, 4.85 Å and 1.67 Å) associated with small amounts of kaolinite (typical peaks at 7.15 Å, 3.85 Å and 2.16 Å) based on peak surface area. These two main minerals are associated with small proportions of illite, feldspar and quartz, while goethite and ilmenite are present in trace amounts. Table 2 lists the chemical composition of the raw material. The high content of Fe2O3 (6.78) indicates that iron compounds are present in Vg and that the smectite is Fe-rich. The Vg sample with a SiO2/Al2O3 ratio of 2.83 indicates the high silica over aluminium ratio typical of smectite mineralogy (Price and Velbel, 2003).
Figure 1.
X-ray diffraction patterns of vertisol horizons from Garoua. (S = smectite; K= Kaolinite; IL = Illite; IM = Ilmenite; F = Feldspar; G = Goethite; Q = Quartz).
Table 2.
Chemical composition (%) of Vg, expressed in terms of oxides.
| Oxide |
SiO2 | Al2O3 | TiO2 | Fe2O3 | K2O | Na2O | MgO | CaO | MnO | P2O5 | L.I. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw sample | |||||||||||
| Vg | 53,46 | 18,88 | 1,02 | 6,78 | 1,85 | 0,85 | 1,06 | 1,16 | 0,08 | 0,09 | 14,64 |
L. I.. = loss on ignition.
3.2. Filtrate recovery and chemical composition
The main objective was to recover the maximum quantity of ions produced by acid activation in a small volume of liquid for further use. When 10 g raw clay material was activated with 50 mL of acid solution, the first filtrate F1 obtained was equal to 40 mL, and the second filtrate F2 was equal to 50 mL F1 + F2 = 90 mL meant that this method allowed the recovery of 9.0 mL of filtrate from 1 g of Vg clay material. The major elements contained into the filtrate (RHC) were compared to those contained in the raw Vg material in Table 3.
Table 3.
Chemical composition of Vg and RHC samples.
| Oxyde | Vg |
RHC |
|---|---|---|
| (%) | ||
| SiO2 | 56,46 | 3,07 |
| Al2O3 | 20,17 | 18,85 |
| Fe2O3 | 6,98 | 17,81 |
| MnO | 0,074 | 0,27 |
| MgO | 1,13 | 2,03 |
| CaO | 1,11 | 21,57 |
| Na2O | 0,84 | 0,11 |
| K2O | 1,95 | 0,38 |
| TiO2 | 1,21 | I.d. |
| P2O5 | 0,12 | 0,31 |
| Loss by ignition | 9,05 | 35,62 |
| Total | 99,08 | 100,02 |
The most important variations (%) observed between raw Vg material and RHC precipitate were the following elements: loss on ignition (9–35.62), CaO (1.11–21.57) and SiO2 (56.46–3.07). Thus, 35.62% of losses on ignition could be attributed to the physiosorbed water fixed by RHC. Further, 21.57% of CaO was due to the use of limewater for neutralisation and precipitation of metals into the filtrate. Only 3.07% of SiO2 was present in the filtrate compared to 56.46% in the raw clay material; this finding confirmed the weak dissolution of the tetrahedral layers of clay minerals of Vg. Al2O3 at 18.85% and Fe2O3 at 17.81% from RHC were the most abundant metals present in the filtrate in ionic form as Fe2+, Fe3+ and Al3+. With a pH < 1, the filtrate resulting from the acidic activation also contained H+ ions.
3.3. Quantification of acidity
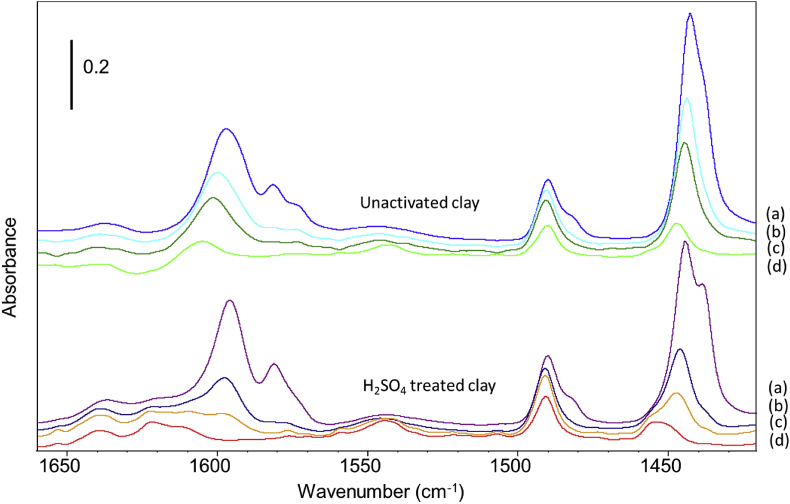
Before the acidity measurement, the samples were initially pre-heated to 423 K. The IR spectra of the samples upon initial heating showed that this pretreatment only induced the removal of physiosorbed water. The adsorption of a base, pyridine, was carried out for the evaluation of the surface acidity of the solids. The spectra after pyridine adsorption are presented in Figure 2. After introduction of pyridine in the cell, physiosorbed pyridine can be observed from the bands ca. 1580-1590 cm−1. Weakly adsorbed pyridine desorbed under vacuum. In addition, bands attributed to pyridine adsorbed on Lewis and Bronsted acid sites were observed. Lewis sites were characterised by the bands at 1444 and 1599 cm−1 initially, and Bronsted acid sites were characterised notably by the band at ca. 1544 cm−1. Generally, for both samples, bands attributed to pyridine adsorbed on Lewis sites progressively decreased upon evacuation and heat treatment, while at the same time, the bands attributed to Bronsted acid sites became more visible. Interestingly, the bands due to Lewis sites evolved differently on the two samples. On the initial clay, the bands at 1444 and 1599 cm−1 only shifted ca. 5 cm−1 upwards upon evacuation at 398 K. By contrast, for the sulfuric acid-treated clay, the remaining bands were finally located at 1455 and 1611 cm−1, thus indicating a higher strength of the Lewis sites. On the acid-treated sample, Bronsted sites, clearly evidenced by the band at 1544 cm−1, were associated with a doublet at 1639 and 1622 cm−1. The quantification of acid sites also highlights some differences between the two samples. Table 4 reports the quantification of Lewis and Bronsted acid sites by the bands at 1444 and 1544 cm−1 respectively.
Figure 2.
IR spectra of the initial clay (top) and the sulfuric acid-treated clay (bottom) after pyridine adsorption. (a): with pyridine in the cell; (b) after evacuation at 298 K; (c) after evacuation at ca. 348 K; (d) evacuated at 398 K. Spectra are subtracted with the spectrum of the solid before adsorption.
Table 4.
Quantification of acidity measured by pyridine adsorption.
| T (K) | Lewis (μmol/g) | Bronsted (μmol/g) | Total (μmol/g) | B/L | % Bronsted | |
|---|---|---|---|---|---|---|
| Clay | 298 | 40.5 | 3.8 | 44.3 | 0.09 | 8.6 |
| 344 | 30.0 | 4.1 | 34.1 | 0.14 | 11.9 | |
| 398 | 9.8 | 5.7 | 15.5 | 0.58 | 36.9 | |
| H2SO4-Clay | 298 | 25.6 | 7.8 | 33.4 | 0.30 | 23.3 |
| 352 | 13.9 | 10.7 | 24.6 | 0.77 | 43.4 | |
| 398 | 5.5 | 9.6 | 15.1 | 1.75 | 63.6 |
The acid-treated clay showed a lower number of acidic sites due to a decrease in the number of Lewis sites. This finding is probably due to a slight degradation of the structure as also indicated by the mass losses induced by cation solubilisation (see section 3.2.). By contrast, it is noteworthy that the number of Bronsted sites significantly increased on the acid-treated sample. At higher temperature, Bronsted acidic sites become predominant over Lewis sites. Therefore, the activation of the initial clay by sulfuric acid has a direct influence on the surface properties, it includes the decrease in the amount of Lewis sites while increasing their overall strength, and it increases the number of Bronsted acid sites.
3.4. Comparison of the two acids: HCl and H2SO4
3.4.1. Amounts of dissolved metal cations from the activated samples
Mass losses demonstrated the degree of solubilisation of the minerals from the clay material with various acid treatments. Data from Table 5 showed that hydrochloric acid was more efficient than sulfuric acid at the same normality (1N).
Table 5.
Mass loss and mass of solubilized cations during acid activation.
| Clay material: Vg | Ions | HCl (1N) | H2SO4 (1N) | H2SO4 (2N) |
|---|---|---|---|---|
| Mass loss (%) recorded during acid activation | / | 12,13 | 10,30 | 15,23 |
| Solubilized mass of ions (in mg/g of clay) | Fe2+ | 2,13 | 1,21 | 1,57 |
| Fe3+ | 14,61 | 11,52 | 23,40 | |
| Total (Fe2++Fe3+) | 16,74 | 12,73 | 24,97 | |
| Al3+ | 17,64 | 14,38 | 24,84 |
The main cations that passed into the solutions were Al3+, Fe3+ and Fe2+. The solubilised amounts from these main cations (Table 5) confirmed the superiority of hydrochloric acid performance during the treatment of the material. The process of acid activation includes the dissolution of acid soluble minerals (amorphous compounds) frequently present in association with vertisols. Protons replace the naturally occurring exchangeable cations (Ca2+, Na+, K+, Mg2+), penetrate the smectite layers and attack the structural OH groups. Dehydroxylation is connected with fractional release of the central atoms from the octahedral site as well as removal of Al from the tetrahedral sheets (Pentráka et al., 2018). PentrákaThe amount of solubilised ions depends both on the nature and the concentration of the acid. These results are consistent with the chemical compositions of the material used. On the other hand, when the concentration of sulfuric acid increased from 1 to 2 N, the numbers of solubilised ions also increased, which generally shows that the solubilisation of cations increases with the concentration of the acid. These observations are consistent with the results of previous studies (Vicente et al., 1996a, 1996b). With the same normality (1 N) and at constant temperature (97 °C), the activity of hydrochloric acid is greater than that of sulfuric acid. The same observations were made by Salawudeen et al. (2007) on Nigerian clay.
3.4.2. Palm oil discolouration
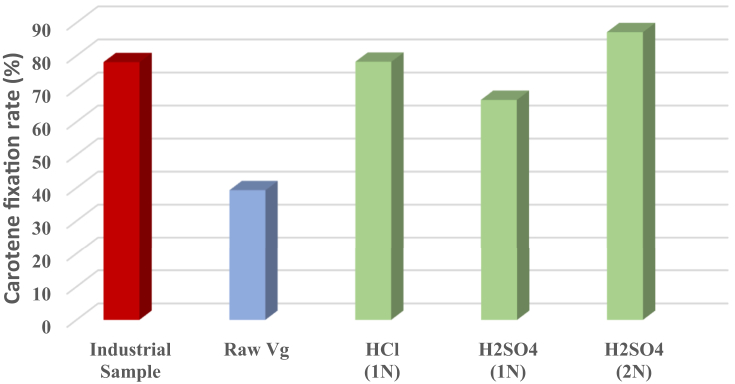
The effectiveness of activated clays obtained was tested on the discolouration of a palm oil sample. Thus, the performances of Vg treated with the acid solutions HCl 1N, H2SO4 1N and H2SO4 2N were evaluated and compared with those of the same raw material, and with those of a reference sample used in the industry (BC2). The results (Figure 3) showed that whatever acid was used, activation significantly improved the bleaching capacities of activated clays. Acid activated local clays attained better levels (the Vg sample reached a value of 78.19% with 1N HCl and 87.14% with 2N H2SO4) than industrial sample (78.07%). Sample of raw clay material showed weak bleaching ability. Clay materials treated with hydrochloric acid (1N) performed better than those treated with sulfuric acid (1N); a value of 78.19% was noted for 1N HCl compared with 66.63% for 1N H2SO4. Thus, from a strictly scientific viewpoint, at equal concentrations (1N), the most effective acid for producing bleaching earth from the studied clay material is hydrochloric acid. When the concentration of the H2SO4 solution was doubled, the material fixed more β-carotene, with a value of 87.14%. Increasing acid concentration caused significant solubilisation of the metal cations, which gave the activated material a greater bleaching capacity. According to Chitnis and Sharma (1997), acid activation promotes catalytic activity by increasing the number of Bronsted and potential Lewis acid sites. Sarier and Guler (1989) found that adsorption of carotenoids can be catalysed by the Bronsted and Lewis acidity. Carotene attaches to the clay surface in the form of carbonium ions either by forming coordination bonds with Lewis sites or by forming hydrogen bonds with Bronsted sites in the activated clay mineral. In view of the evolution of the amounts of Bronsted and Lewis sites after acid treatment (see above section 3.3), the increased amount of Bronsted acid sites may play a predominant role on the adsorption of the carotene.
Figure 3.
Palm oil discolouration with raw and activated Vg.
3.4.3. Cost of different treatments
Obviously, the cost of the acidic solutions necessary to activate a kilogram of clay material must be taken into account. The cost of treatment was considered here by the sole expense related to the purchase of the acids. The ratio of acid solution volume/mass of clay was set at 5/1. Scientific and financial data were taken from the catalogue of an internationally recognised chemical supplier (www.fishersci.fr.) (Web page 1). These results are presented in Table 6.
Table 6.
Scientific and financial information for the preparation of acid solutions.
| Reagent certified for analysis | Hydrochloric acid | Sulfuric acid | |
|---|---|---|---|
| Purity | 37% | 95% | |
| Density | 1.18 | 1.83 | |
| Molar mass (g/mol) | 36.5 | 98.078 | |
| 25L in plastic container: cost in euros | 361 | 410 | |
| Normality | 11.96 | 35.45 | |
| Volume of acid solution necessary for the production of 1 kg bleaching clay | 5L (HCl,1N) | 5L (H2SO4, 1N) | 5L (H2SO4, 2N) |
| Volume of pure acid (mL) | 418 | 141 | 282 |
| Cost of pure acid (euros) | 6.03592 | 2.31 | 4.62 |
2N sulfuric acid solution (87.14% fixed carotene) was more efficient than 1N hydrochloric acid solution (66.63% fixed carotene) with Vg. Five litres of 2N sulfuric acid solution (4.62 €) cost less than five litres of 1N hydrochloric acid solution (6.03592 €). The selection of 2N H2SO4 acid solutions for acid activation of Vg clay material was therefore obvious. Even if the percentage of montmorillonite varied from one sample to another, these results could be generalised to vertisols of the neighbouring countries of Sub-Saharan Africa (Nigeria, Chad, Sudan, Niger, etc.) (FAO-UNESCO-ISRIC, 1990).
3.5. Characterisation of the leachates
3.5.1. Physico-chemical characteristics of leachates
The main physico-chemical characteristics of the leachates are shown in Table 7.
Table 7.
Physico-chemical characteristics of leachates.
| Sample | RSL | DSL |
|---|---|---|
| pH | 8.25 | 8.89 |
| Colour (PtCo units) | Brown (1372) | Dark brown (4262) |
| Density | 1.026 | 1.028 |
| Dry Résidue (g/L) | 4.6 | 6.5 |
| DCO (mg/L) | 117 | 802 |
The leachates were light brown to dark brown in colour (Munsell code). They were basic and did not contain heavy metals susceptible to precipitating as hydroxides according to Nazir et al. (2015). The COD values fluctuated between 117 and 802 mg/L from RSL to DSL; this finding is evidence of the alternating dry and humid seasonal influences on the concentration or the dilution of the leachate.
3.5.2. Chemical analyses of the leachates
Considering the elimination of some volatile organic compounds during the collection of the dry residue by evaporation, the chemical composition of the leachate was mainly represented by the mineral fraction. Table 8 reveals that among the dosed elements, K2O was the most abundant (32.06%), followed by Na2O (9.08%).
Table 8.
Leachate chemical composition, expressed as mass content of the air-dried material.
| oxide | SiO2 | Al2O3 | TiO2 | Fe2O3 | K2O | Na2O | MgO | CaO | MnO | P2O5 | L.I. | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DSL (ppm) | 0.28 | 0.05 | 0.005 | 0.09 | 32.06 | 9.08 | 0.97 | 0.81 | 0.0060 | 0.13 | n.d. | 43.48 |
L.I. = loss on ignition n.d. = not determinated.
The concentrations of the other elements were close to 1% (0.97% and 0.81% respectively for MgO and CaO). The high concentration of alkaline elements (Na and K) followed by alkali-earth elements (Mg, Ca) confirmed the high conductivity of the leachate samples. The low Si, Al and Fe contents was clear evidence of the organic origin of the chemical composition of the leachate. These results also showed that the dosed mineral elements of DSL represented 43.83% of the global leachate volume; the remaining 56.52% volume was lost on ignition or was the sum of dissolved organic matter and the water content. Even though this fraction had not been dosed, it certainly corresponds to the humidity and the organic fraction of the leachate. The humic substances were composed of an aromatic polycyclic nucleus on which lateral chains such as phenolic acids, carbohydrates and polypeptides are linked (Chamayou, 1989). The humic substances are negatively charged through the ionisation of their carboxyl and phenyl groups; they can thus be considered as anionic polyelectrolytes with unknown structures, although they globally have high molecular weights and can be considered dissolved colloids (Senesi et al., 1997).
Table 9 presents the results of trace elements analyses (in ppm) of the leachate sample. The most abundant trace element was rubidium (384 μg/g of DSL), which is an alkaline element in the same column as potassium in the periodic table. Rubidium was followed by strontium (41.5 μg/g), an alkali-earth element in the same column as Ca and Mg in the periodic table; it is one of the most electropositive and alkaline known elements.
Table 9.
Leachate trace elements expressed in ppm.
| Element | As | Ba | Be | Bi | Cd | Ce | Co | Cr | Cs | Cu | Dy | Er |
| DSL (ppm) | <i.d. | 14.5 | <i.d. | 0.18 | <i.d. | 0.519 | 10.6 | 28.9 | 0.37 | 26.6 | 0.023 | 0.015 |
| Element | Eu | Ga | Gd | Ge | Hf | Ho | In | La | Lu | Mo | Nb | Nd |
| DSL (ppm) | 0.007 | 0.19 | 0.021 | 0.05 | 0.06 | 0.005 | <i.d. | 0.186 | <i.d. | 2.21 | 0.10 | 0.157 |
| Element | Ni | Pb | Pr | Rb | Sb | Sm | Sn | Sr | Ta | Tb | Th | Tm |
| DSL (ppm) | 23.1 | 3.02 | 0.044 | 384 | 0.59 | 0.032 | 3.43 | 41.5 | <i.d. | <i.d. | 0.07 | <i.d. |
| Element | U | V | W | Y | Yb | Zn | Zr |
| DSL (ppm) | 0.27 | 14.3 | 0.28 | <i.d. | 0.018 | 17.1 | 3.85 |
It is the 16th most abundant element in the earth's crust and is moderately toxic if ingested. It has no known biological role but has a slight stimulatory effect on metabolism, probably because it is quite similar to potassium. The two elements co-exist in the material and soils, although potassium is much more abundant. Plants absorb Rb faster when stressed by potassium deficiency. No negative environmental effects of Rb have so far been reported.
Considering that sample RSL contains 4.38 g/L dry residue, the balanced concentrations of the trace elements could be calculated (for this sample) and compared with standard norms of water quality for aquatic life according to the European Community Council Directive of the 18th of July 1978 (Rodier, 1984). Rb with 1766 μg/L of leachate was less harmful to the environment, as was Sr with 190.9 μg/L of leachate and Ba with 66.7 μg/L leachate. The 122.36 μg/L of leachate obtained for Cu in RSL was above the internationally recommended 40 μg/L for aquatic life; the value of 78.66 μg/L obtained for Zn in RSL is also higher than the internationally recommended 30 μg/L. It should, however, be noted that in the rainy season, those elements are diluted when they come in contact with river water. In the dry season, they are first concentrated in DSL before they are then diluted by river water and can attain concentrations that are dangerous to aquatic life. The cadmium in DSL was below the detection limits of the apparatus used, that is, < 0.15 μg/L. Pb content (13.89 μg/L) was also below the recommended values (50 μg/L).
The concentrations of the other dosed major and trace elements did not show the presence of particularly dangerous chemical elements to the environment. It should be noted that the wastes stocked at the Banefo landfill reflect the nature of the chemical compounds produced by local inhabitants and the slightly industrialised Bafoussam Town. Those results could not be expected in a large industrial town with many chemical transformation industries.
3.5.3. Chemical reactivity of the leachate
Leachate reacted with acidic solutions (Table 10) and with solutions of iron III and aluminium (III) salts (10−2 M).
Table 10.
Chemical reactivity of dry season leachate (DSL).
| Chemicals | Bases |
Acids |
Salts |
|||
|---|---|---|---|---|---|---|
| NH3 | NaOH | H2SO4 | HCL | FeCl36H2O | Al2(SO4)318H2O | |
| Coagulation and floculation | No reaction | No reaction | reaction | reaction | reaction | Rapid reaction |
| Other réaction | No reaction | No reaction | effervescence | effervescence | effervescence | effervescence |
The leachates did not react with usual bases such as NH3 and NaOH, because they were also naturally basic. Leachates instead reacted with acids and coagulating salts. However, the highest reactivity was obtained with aluminium sulfate (Al2(SO4)3.18H2O), which favoured both coagulation and flocculation of the colloids. The effervescence that followed the formation of the flocs was due to CO2 liberation. The following reactions could be expected:
-
(1)
Reaction of the acids with the bicarbonates contained in the leachate
| H+ +HCO3- → CO2 + H2O. |
-
(2)
Reaction of aluminium sulfate with the bicarbonates contained in leachate
| Al2(SO4)318H2O + 6 HCO3- → 3SO42- + 2 Al(OH)3 + 18 H2O + 6CO2↑. |
The effervescence caused by CO2 liberation was more meaningful in an aluminium sulfate medium than in the presence of FeCl3.
3.5.4. Treatment of leachate with the filtrate resulting from the acidic activation of Vg
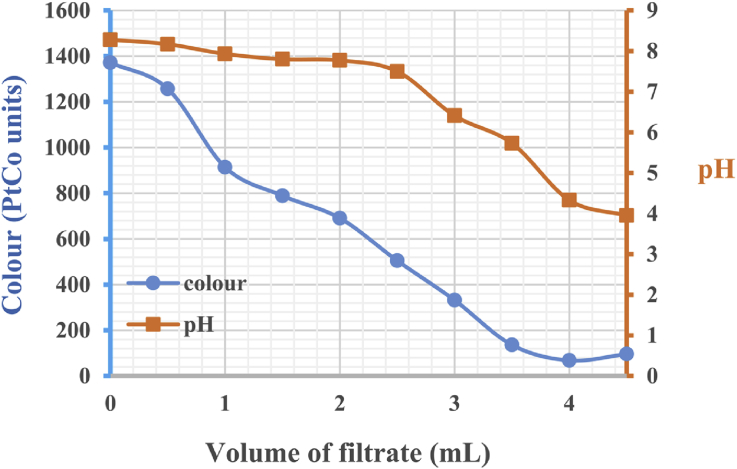
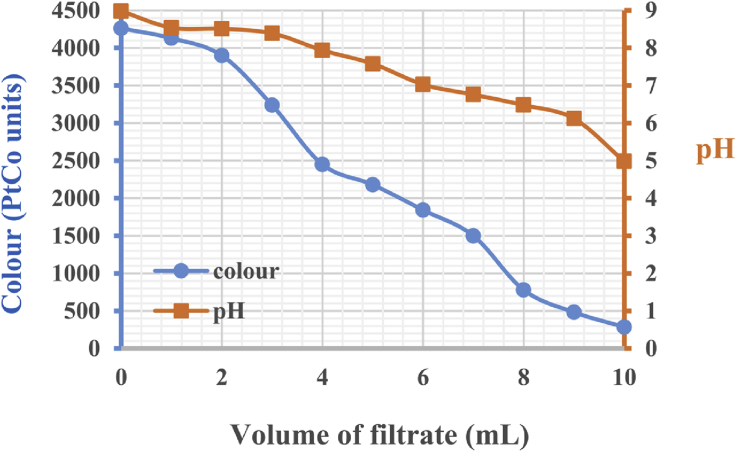
The filtrate derived from vertisol acid activation had the characteristics of a colour value of 161 PtCo units and pH = 0.69. The filtrate reacted with both leachate samples (Figure 4 and Figure 5). Colour and pH decreased as filtrate was added. This decrease attained a maximum at a pH of approximately 5. For 50 mL of RSL, 4 mL of filtrate made it possible to obtain a colour equal to 68 PtCo units, i.e., a discolouration of approximately 95%. Thus, 50 mL DSL required 10 mL of filtrate for maximum discolouration at 285 PtCo units, thus corresponding to 93%. As soon as the maximum discolouration was reached, further filtrate additions caused a decrease in pH and an intensification of the colour of the mixture due to the filtrate colouration. Leachate treatment was confirmed by recorded decreases in COD: the COD of the RSL sample decreased from 117 mg/L to zero and that of the DSL sample decreased from 802 mg/L to 128 mg/L. According to Dai et al. (2011), the revised COD discharge limits from landfill leachate varies in several countries: 100 mg/L in China, 200 mg/L in Germany, and 120 mg/L in France. These COD reductions obtained by coagulation-flocculation using the acidic activation filtrate of vertisols lowered the final CODs (zero and 128 mg/L) to acceptable values in countries such as Germany and France.
Figure 4.
Colour and pH evolution of RSL, according to the volume of filtrate added.
Figure 5.
Colour and pH evolution of DSL, according to the volume of filtrate added.
4. Conclusion
A smectite soil clay was studied for the acid activation and production of bleaching earths. Thus, 2N H2SO4 showed higher performance compared to 1N HCl and 1N H2SO4 considering both palm oil bleaching efficiency and cost. The filtrate obtained from the acid activation of the vertisol sample (Vg) was rich in H+ (2.04.10−1M), Fe2+ (2.8.10−3M), Fe3+ (4.2.10−2M) and Al3+ (9.2.10−2M) ions. One gram of clay material produced 9 mL of this filtrate that was successfully used in the treatment of leachate produced in the Banefo dumping site in the Bafoussam Municipality (West Cameroon). The COD reductions obtained by coagulation-flocculation using the acidic activation filtrate of vertisol lowered the final CODs (zero and 128 mg/L) within acceptable limits. Thus, the most effective acid to be chosen for the industrial bleaching earth production from the vertisol is 2N H2SO4. It has been shown that the activation of the initial clay by sulfuric acid both decreases the number of Lewis sites while increasing their overall strength and increases the number of Bronsted sites, which appears favourable for enhanced discolouration of palm oil.
Declarations
Author contribution statement
Emmanuel Djoufac Woumfo: Conceived and designed the experiments; Wrote the paper.
Hermann Soh Nde, Azinwi Primus: Performed the experiments; Wrote the paper.
Guillaume Clet: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Julien Vieillard, Tsaffo Marlène: Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Azinwi T.P., Djoufac Woumfo E., Bitom D., Njopwouo D. Petrological, physico-chemical and mechanical characterization of the topomorphic vertisols from the Sudano-Sahelian region of north Cameroon. Open Geol. J. 2011;5:33–55. [Google Scholar]
- Babaki H., Salem A., Jafarizad A. Kinetic model for the isothermal activation of bentonite by sulfuric acid. Mater. Chem. Phys. 2008;108:263–268. [Google Scholar]
- Barzetti T., Selli E., Moscotti D., Forni L. Pyridine and ammonia as Probes for FTIR analysis of solid acid catalysts. J. Chem. Soc. Faraday. Trans. 1996;92(8):1401–1407. [Google Scholar]
- Bike, Mbah J.B., Kamga R., Nguetnkam J.P., Fanni J. Adsorption of pigments and free fatty acids from shea butter on activated Cameroonian clays. Eur. J. Lipid Sci. Technol. 2005;107:387–394. [Google Scholar]
- Brabant J. IBSRAM Proceedings. Vol. 6. 1987. Selection of sites for vertisol network: distinction between types of vertisols; pp. 65–70. [Google Scholar]
- Chamayou H., Legros J.P. Presses universitaires de France; Paris: 1989. Les bases physiques et minéralogiques de la science du sol; p. 593. [Google Scholar]
- Chitnis S.R., Sharma M.M. Industrial applications of acid-treated clays as catalysts. React. Funct. Polym. 1997;32:93–115. [Google Scholar]
- Christidis G.E., Scott P.W., Dunham A.C. Acid activation and bleaching capacity of bentonites from the islands of Milos and Chios, Aegean, Greece. Appl. Clay Sci. 1997;12:329–347. [Google Scholar]
- Dai J.G., Song Q.W., Zhang Y., Qin Q. Directions for development of landfill leachate treatment technologies under the new standard in China. J. Environ. Eng. Technol. 2011;1:270–274. [Google Scholar]
- De Boeck, Larcier S.A. University Brussels; 2001. Chemistry of the Environment Air, Water, SOIL, Waste, Editions Deboeck; p. 476. [Google Scholar]
- Degrémont . eighth ed. GauthierVilars; 1978. Water Technical Memento; p. 1175. [Google Scholar]
- Djoufac W.E., Kamga R., Figueras F., Njopwouo D. Acid activation and bleaching capacity of some Cameroonian smectite soil clays. Appl. Clay Sci. 2007;37:149–156. [Google Scholar]
- Emeis C.A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts. J. Catal. 1993;141:347–354. [Google Scholar]
- Esu I.E., Lombin G. Characteristics and management problems of vertisols in the Nigerian Savannah. In: Jutzi S.C., Haque I., McIntire J., Shares Jes, editors. Management of Vertisols in Sub-saharan Africa, ILCA-Addis Abbaba, Ethiopia. 1988. pp. 293–307. [Google Scholar]
- FAO-UNESCO-ISRIC . FAO; Rome: 1990. Soil Map of the World. Revised Legend, World Soil Resources Report. [Google Scholar]
- Fasina A.S., Raji A., Oluwatosin G.A., Omoju O.J., Oluwadare D.A. Properties, genesis, classification, capability and sustainable management of soils from south-western Nigeria. Int. J. Soil Sci. 2015;10:142–152. [Google Scholar]
- Frini-Srasra N., Srasra E. Acid treatment of south Tunisian palygorskite: removal of Cd (II) from aqueous and phosphoric acid solutions. Desalination. 2010;250:26–34. [Google Scholar]
- Jean Baptiste B.M., Esther N., Mirela P., Richard K. Adsorption isotherm and kinetics modeling of carotene and free fatty acids adsorption from palm oil onto montmorillonite. Int. J. Biosci. 2013;3(3):15–24. [Google Scholar]
- Kamal K.T., Tagelsir M.S., Musa A.M. Performance of sudanese activated bentonite in bleaching cottonseed oil. J. Bangladesh Chem. Soc. 2011;24(2):191–201. [Google Scholar]
- Kennedy J.A., Powell H.K.J. Colorimetric determination of aluminium (III) with chrome azurol S and the reactivity of hydrolysed aluminium species. Anal. Chim. Acta. 1986;184:329–333. [Google Scholar]
- Komadel P., Madejova J. Elsevier Science; Amsterdam: 2006. Handbook of clay Science; pp. 263–287. [Google Scholar]
- Komadel P. Acid activated clays: materials in continuous demand. Appl. Clay Sci. 2016 [Google Scholar]
- Lagier T. Thèse de doctorat Université de Poitiers; 2000. Etude des macromolécules de lixiviat, caractérisation et comportement vis à vis des métaux; p. 189. [Google Scholar]
- Murray H.H. Traditional and new application for kaolin, smectite, and palygorskite A general over view. Appl. Clay Sci. 2000;17:207–221. [Google Scholar]
- Nazir R., Khan M., Masab M., Rehman H.U., Rauf N.U., Shahab S. Accumulation of Heavy Metals (Ni, Cu, Cd, Cr, Pb, Zn, Fe) in the soil, water and plants and analysis of physico-chemical parameters of soil and water Collected from Tanda Dam kohat”. J. Pharm. Sci. Res. 2015;7(3):89–97. [Google Scholar]
- Nde-Aga B.J., Kamga R., Nguetnkam J.P. Adsorption of palm oil carotene and free fatty acids onto acid activated Cameroonian clays. Appl. Clay Sci. 2007;7(17):2462–2467. [Google Scholar]
- Nguetnkam J.P., Kamga R., Villiéras F., Ekodeck G.E., Razafitianamaharavo A., Yvon J. Alteration of cameroonian clays under acid treatment. Comparison with industrial adsorbents. Appl. Clay Sci. 2011;52:122–132. [Google Scholar]
- Nguetnkam J.P., Kamga R., Villiéras F., Ekodeck G.E., Yvon J. Assessing the bleaching capacity of some Cameroonian clays on vegetable oils. Appl. Clay Sci. 2008;39:113–121. [Google Scholar]
- Price J.R., Velbel M.A. Chemical weathering indices applied to weathering profiles developed on heterogeneous felsic metamorphic parent rocks. Chem. Geol. 2003;202(3):397–416. [Google Scholar]
- Pentrak M., Madejova J., Komadel P. Acid and alkali treatment of kaolins. Clay Miner. 2009;44:507–519. [Google Scholar]
- Pentráka M., Hronský V., Pálková H., Uhlík P., Komadel P., Madejováa J. Alteration of fine fraction of bentonite from Kopernica (Slovakia) under acid treatment: a combined XRD, FTIR. MAS NMR and AES study Appl. Clay Sci. 2018;163:204–213. [Google Scholar]
- Robert M. Principes de déterminations quantitatives des minéraux argileux à l’aide des rayons X. Ann. Agron. 1975;26:363–399. [Google Scholar]
- Robinson H.D., Walsh T., Carville M.S. Advanced leachate treatment at Buckden landfill, Huntingdon, UK. J. Environ. Eng. Sci. Natl. Res. Counc. Can. 2003;2(4):255–264. [Google Scholar]
- Rodier J. Edition Dunod Paris; 1984. L’analyse de l’eau : Eaux naturelles, eaux résiduaires, eaux de mer. [Google Scholar]
- Sakizci M., Erdogan A.B., Yorukogulları E. SO2 adsorption on acid-treated bentonites from Turkey. Clay Miner. 2011;46:73–83. [Google Scholar]
- Salawudeen T.O., Dada E.O., Alagbe S.O. Performance evaluation of acid treated clays for palm oil bleaching. J. Eng. Appl. Sci. 2007;2(11):1677–1680. [Google Scholar]
- Sarier N.C., Guler C. The mechanism of carotene adsorption on activated montmorillonite. J. Am. Oil Chem. Soc. 1989;66:917–923. [Google Scholar]
- Senesi N., Rizzi F.R., Dellino P., Aquafredda P. Fractal humic acids in aqueous suspensions at various concentrations, ionic strengths, and pH values. Colloids Surf., A. 1997;127:57–68. [Google Scholar]
- Skoog D.A., West D.M., Holler F.J., Crouch S.R. 3ième édition. De Boeck; 2015. Chimie Analytique; p. 1049. [Google Scholar]
- Steudel A., Batenburg L.H., Fischer H.R., Weidler P.G., Emmerich K. Alteration of non-swelling clay minerals and magadiite by acid activation. Appl. Clay Sci. 2009;44:95–104. [Google Scholar]
- Suarez B.M., Gonzalez L.V.F., Rodriguez M.A.V., Pozas J.M.M. Acid activation of a palygorskite with HCl: development of physico-chemical, textural and surface properties. Appl. Clay Sci. 1995;10(3):247–258. [Google Scholar]
- Temga J.P., Azinwi Tamfuh P., Basga Djakba S., Zo'o Zame P., Gouban H., Abossolo M., Nguetnkam J.P., Bitom D.L. Characteristics, classification and genesis of vertisols under seasonally contrasted climate in the Lake Chad Basin, Central Africa. J. Afr. Earth Sci. 2019;150:176–193. [Google Scholar]
- Tjasa B., Nevenka F., Danijel V. Sustainable reclamation of landfill sites. Manag. Environ. Qual. Int. J. 2004;15(1):55–61. [Google Scholar]
- Topallar U. Bleaching kinetics of sunflowerseed oil. JAOCS (J. Am. Oil Chem. Soc.) 1998;75(4):531–533. [Google Scholar]
- Ushikoshi K., Kobayashi T., Uematsu K., Toji A., Kojima D., Matsumoto K. Leachate treatment by the reverse osmosis system. Desalination. 2002;150:121–129. [Google Scholar]
- Usman M.A., Ekwueme V.I., Alaje T.O., Mohammed A.O. Characterization, acid activation, and bleaching performance of Ibeshe clay, lagos, Nigeria. Int Sch. Res. Netw. 2012;2012:1–6. [Google Scholar]
- Valentin J.L., Lopez-Manchado M.A., Rodriguez A., Posadas P., Ibarra L. Novel anhydrous unfolded structure by heating of acid pre-treated sepiolite. Appl. Clay Sci. 2007;36:245–255. [Google Scholar]
- Vicente M.A., Lopez Gonzalez J.D., Banares Munoz M.A. Preparation of microporous solids by acid treatment of a saponite. Microporous Mater. 1995;4:251–264. [Google Scholar]
- Vicente M.A., Lopez Gonzalez J.D., Banares-Munoz M.A. Influence of the free silica generated during acid activation of a sepiolite on adsorbent and textural properties of the resulting solids. J. Mater. Chem. 1995;5:127–132. [Google Scholar]
- Vicente M.A., Suarez Barrios M., Lopez Gonzalez J.D., Banares-Munoz M.A. Characterization, surface area, and porosity analyses of the solids obtained by acid leaching of a saponite. Langmuir. 1996;12(2):566–572. [Google Scholar]
- Vicente M.A., Suarez Barrios M., Lopez-Gonzalez J.D., Banares-Munoz M.A. Acid activation of a ferrous saponite (griffithite): physicochemical characterization and surface area of the products obtained. Clay Miner. 1994;42:724–730. [Google Scholar]
- Vicente M.A., Suarez M., Banares-Munoz M.A., Lopez-Gonzalez J.D. Comparative FTIR study of the removal of octahedral cations and structural modifications during acid treatment of several silicates. Spectrochim. Acta, Part A. 1996;52:1685–1694. [Google Scholar]
- Wang F., Daniel W.S., Gamal M.E. Application of advanced oxidation methods for landfill leachate treatment–A review. Rev. Genie Sci. Environ. 2003;2(6):413–427. [Google Scholar]
- Web page 1,Web page 1 :https://www.fishersci.fr/fr/fr/home.html