Abstract
Background
Intrahepatic cholangiocarcinoma (iCCA) is usually a fatal malignancy with rising incidence globally. Surgical resection currently remains the only curative treatment. However, as only a minority of iCCA is amenable to resection, new therapeutic modalities are needed. Our aims were to systematically review and perform a meta-analysis on the existing literature regarding the use of ablative therapies for iCCA and to assess their efficacy as a treatment modality by calculating pooled survival results and investigate associations between prognostic factors and survival.
Methods
A comprehensive search of the PubMed database for relevant articles was performed. Studies assessing survival in patients with iCCA undergoing ablation were included. Data were extracted on patient, tumour and treatment characteristics and survival. Random effects meta-analysis was used to pool the data. Galbraith plots were used to investigate heterogeneity; bubble plots were formulated using regression-based meta-analysis.
Results
A total of 10 studies were included in the final analysis, yielding an aggregate of 206 patients (69.5% males, median age: 51.2–72.5) and 320 tumours. Of all patients, 70.4% were recurrent cases of iCCA, and 29.6% were cases of primary iCCA. The median overall survival ranged from 8.7 to 52.4 months. Pooled 1-, 3- and 5-year survival rates were 76% (95% confidence interval: 68–83%), 33% (21–44%) and 16% (7–26%), respectively. No significant association was found between the median age, number of tumours or median tumour size and 1-year survival.
Conclusions
Ablative therapies display promising potential as treatment modalities for iCCA. However, further research is necessary to validate these findings.
Keywords: intrahepatic, cholangiocarcinoma, ablation
Abbreviations: CCA, cholangiocarcinoma; DFS, disease-free survival; eCCA, extrahepatic cholangiocarcinoma; EFS, event-free survival; HBV, hepatitis B virus; HCV, hepatitis C virus; iCCA, intrahepatic cholangiocarcinoma; LT, liver transplantation; MWA, microwave ablation; OS, overall survival; pCCA, perihilar cholangiocarcinoma; PFS, progression-free survival; RFA, radiofrequency ablation; RFS, recurrence-free survival
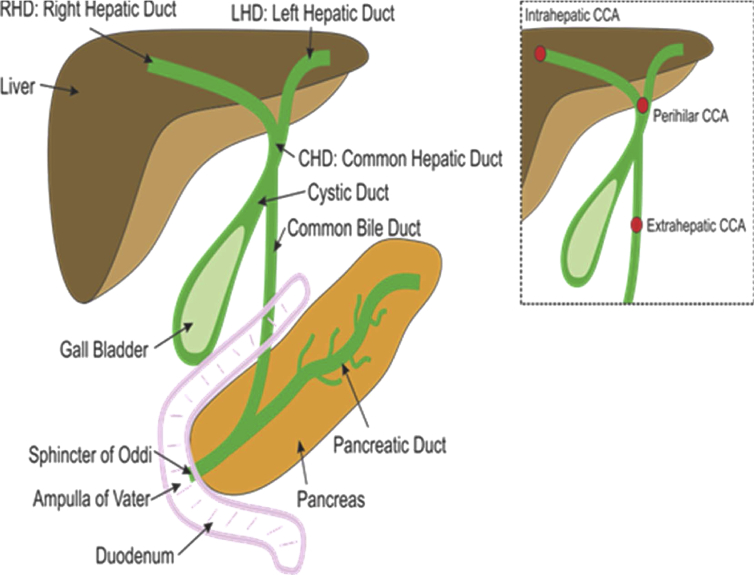
Cholangiocarcinoma (CCA) is the most common biliary tract malignancy, and the subtype intrahepatic cholangiocarcinoma (iCCA) is the second commonest primary hepatic malignancy, accounting for 10–20% of all primary liver cancers globally.1 CCA is classified by its anatomical origin (Figure 1) as iCCA, perihilar CCA (pCCA) or extrahepatic CCA.2 The incidence of CCA varies considerably worldwide, with the age-standardised incidence in Northeast Thailand (80 per 100,000) being significantly higher than in the United States and United Kingdom (1–2 per 100,000).3 iCCA is the least common variant of CCA, representing 10–20% of all CCA diagnoses, but studies have globally reported increased rates of iCCA in the last few decades.1, 4, 5, 6 For example, in the United States, incidence rates rose 165% (0.32–0.85 per 100,000) between the 1970s and 1990s4, 7, 8 The reason for this worldwide increase is currently unclear, and the vast majority of patients diagnosed with iCCA present with advanced disease without an identifiable aetiology.6 Mortality rates of iCCA also display similar trends, with a European Union study reporting both male and female mortality rates increased (0.79–1.1 and 0.55–0.75 per 100,000, respectively) between 2002 and 2007.9
Figure 1.
Illustration of the anatomy of the biliary tree and the different origins of the three types of CCA. CCA: cholangiocarcinoma. Source; Khan SA. Epidemiology of Cholangiocarcinoma. [Lecture] Khon Kaen University. 25–27th April 2016.
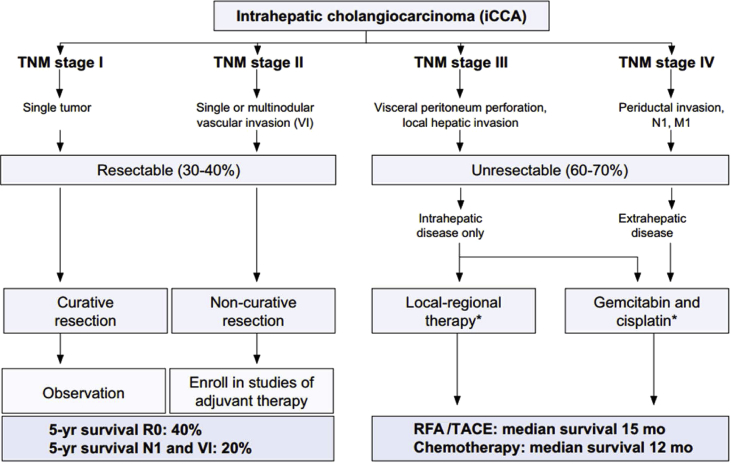
There are a number of treatment modalities for iCCA, but curative methods are limited to surgical resection (Figure 2).8 As iCCA develops, the option of curative treatment diminishes, and supportive therapies become standard practice. Outside the curative option, iCCA has a high mortality rate, with only 5–10% of patients with unresectable disease alive 5 years after initial diagnosis.1 The outcome of surgical resection depends largely on successfully dissected negative surgical margins. Resectability rates are generally between 19% and 74%.10 Survival rates depend on both lymph node status and R0 resection. Studies have shown that after an R0 resection, the 5-year survival was 23–42%, which is a marked improvement compared with the 5-year survival of 0% after an R+ resection.11, 12, 13 Similar trends are found with lymph node status; 5-year survivals in patients with N1 status after resection are 0–9% but can be as high as 43% in N0 graded disease.13, 14
Figure 2.
The suggested guidelines for the management of iCCA, published by Bridgewater et al.8 iCCA: intrahepatic cholangiocarcinoma; RFA: radiofrequency ablation; TACE: transarterial chemoembolization; TNM: tumor, node, metastasis. Source: Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma8.
Liver transplantation (LT) is a topic of controversy in iCCA management. For pCCA, LT is viable, with clear data surrounding selection criteria, neoadjuvant therapy and long-term outcomes.15 Despite this, LT for iCCA is contraindicated by the International Liver Cancer Association (ILCA) owing to a paucity of strong published evidence.8 Without a clearly defined role of LT in iCCA, the mainstay of treatment for unresectable iCCA remains chemotherapy, with combination gemcitabine and cisplatin.16 Even with this combination, the 6-month progressive-free survival has been reported to be at 57.1%, and the median overall survival (OS) is poor, at around 11.7 months.17
Although resection is potentially curative, less than 30% of patients with iCCA are candidates for resection.10, 18, 19, 20 Even after resection with curative intent, studies have reported disappointing 5-year survivals of 23–42%11, 12, 13 and recurrence rates as high as 60–65%.10 Using data from the five largest studies21, 22, 23, 24, 25 in their meta-analysis, Mavros et al26 found that the median OS ranged from only 18–33 months after resection, despite negative surgical margins. There are numerous contraindications for resection in patients with iCCA, including extrahepatic disease, macroscopic vascular invasion, diffuse bilobar involvement and significant comorbidities precluding major surgery. The opportunity for repeated resection is diminished in patients owing to comorbidities or poor functional hepatic reserve.
Given the current treatment limitations, there is a need for novel therapies that can treat the primary cancer or the recurrent disease after intervention. Local regional therapies, such as ablation (radiofrequency ablation [RFA] or microwave ablation [MWA]) and transarterial chemoembolization, were previously only considered for use in the palliative setting. Recently, several studies have shown promise with improving OS and slowing tumour progression.27, 28, 29, 30, 31, 32, 33, 34, 35, 36
The aims of this study were to (a) conduct a systematic review of the published literature on ablative therapies for iCCA; (b) perform a meta-analysis of this literature to investigate the potential for these therapies to be used as a treatment option, rather than a palliative one, by pooling the survival data in the literature for wholly representative and accurate 1-year, 3-year and 5-year survival rates; and (c) investigate the effects of patient and tumour-related factors on survival after ablative therapies for iCCA.
Methods
Study Selection
Electronic systematic searches were performed in the PubMed database with the search strings ‘cholangiocarcinoma’, ‘biliary cancer’, ‘bile duct cancer’ and ‘ablation’ for studies published in any language. In addition, the references of the initially selected studies were analysed to potentially select further studies.
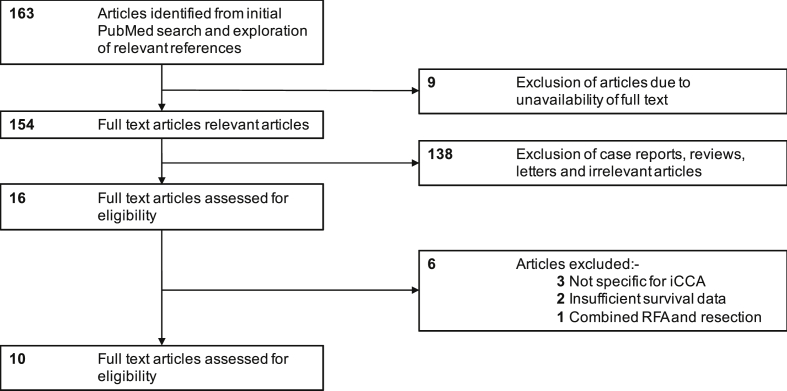
Only studies investigating ablative therapies in humans with iCCA, specifically, were included. Studies with a sample size of more than five and with investigation of survival outcomes after ablation were included. Studies which used RFA, MWA or both were all included. Single-case reports, reviews, letters, conference proceedings and letters were excluded. Studies that incorporated mixed types of CCA and combination therapies of ablation alongside resection were also excluded (Figure 3).
Figure 3.
Flow diagram depicting the selection process of the reviewed studies. iCCA: intrahepatic cholangiocarcinoma; RFA: radiofrequency ablation.
Extraction of Data
Data were extracted from each study regarding patient, tumour and treatment characteristics, as well as survival outcomes. Patient characteristics, where available, included age, gender and the presence of comorbidities, such as cirrhosis, hepatitis B and hepatitis C. Tumour characteristics included the number of tumours, their size(s) and whether they were primary or recurrent cases. Treatment characteristics were the method of ablation performed; RFA or MWA. Finally, survival outcomes included recurrence-free survival (RFS), progression-free survival (PFS), OS and 1-year, 3-year and 5-year survivals.
Data Analysis and Statistical Methods
Percentages and total numbers were used to report categorical variables, such as gender, and median values and ranges were used for reporting the continuous variables from the studies. The meta-analysis was performed using Stata version 13 (TX, 2013). For pooling 1-year, 3-year and 5-year survivals, random effects meta-analysis was used. Forest plots were created to display the survival results from the studies. Statistical heterogeneity between studies was assessed using Galbraith plots and I2, with a P value of P < 0.05 indicating statistically significant heterogeneity. Finally, bubble plots, created using regression-based meta-analysis, were used to look at the relationship between certain factors such as median age and number of tumours against 1-year survival. Statistical significance was set at P < 0.05.
Results
A total of 163 articles were identified from the initial search of the PubMed database. Nine were removed owing to the unavailability of the full text. From the remaining set of 154, 138 articles were excluded mainly as they were either single-case reports or reviews of current therapeutic regimes for iCCA. Of the 16 remaining studies, three were excluded as they reported findings on mixed series of CCA, two were excluded owing to lack of survival data, and one was excluded as it investigated a combined regime of both resection and ablation. The result was a final set of 10 studies, which contained information suitable for a meta-analysis. The majority of these studies originated from Eastern Asia, five from China and two from South Korea. Three studies were based in Europe, two in Italy and one in Austria, with the remaining study from the United States. A resulting aggregate data set of 206 patients with 320 tumours was further analysed. A subgroup analysis was not performed as there were no categorical variables.
Patient Characteristics
Table 1 illustrates both the clinical and pathological characteristics of the patients analysed, as well as their survival outcomes. There was a predominance of men, 121 males (69.5%) compared with 53 females (30.5%), in the studies that provided such data. The median ages of the patients ranged from 51.2 to 72.5 years. Six studies provided data for the presence of comorbidities, resulting in a combined pool of 151 patients. Of these patients, 14 were coinfected with hepatitis B virus, five were coinfected with hepatitis C virus and 27 were diagnosed with cirrhosis.
Table 1.
Summary of the Clinical, Pathological and Survival Data of the Patients in Each Study.
| Article (country) | No. of patients (M/F) | Median age (years) | Primary cases | Cirrhosis/HBV/HCV | No. of tumours | Tumour size (cm) | RFA/MWA | OS (months) | 1/3/5-year survival (%) |
|---|---|---|---|---|---|---|---|---|---|
| Xu et al27 (CN) | 18 (13/5) | 60 | 8/18 | – | 25 | 2.80a | 12/6 | 8.7 | 36.3/30.3/30.3 |
| Fu et al28 (CN) | 17 (9/8) | 54.5 | 7/17 | 5/–/– | 26 | 4.40b | 17/0 | 33.9 | 84.6/43.3/28.9 |
| Kim et al29 (KR) | 20 | 61 | 0/20 | –/6/1 | 29 | 1.50b | 20/0 | 19.5 | 70.0/21/– |
| Yu et al30 (CN) | 15 (11/4) | 60 | 15/15 | –/5/1 | 24 | 3.20a | 0/15 | 10.0 | 60.0/–/– |
| Haidu et al31 (AT) | 17 (12/5) | 62 | 8/17 | – | 26 | 4.20c | 17/0 | 52.4 | 82.2/64.7/47.1 |
| Zhang et al32 (CN) | 77 (58/19) | 51.2 | 0/77 | 22/–/– | 133 | – | –d | 21.3 | 69.8/20.5/– |
| Kim et al33 (KR) | 13 (10/3) | 13/13 | – | 17 | 2.50c | 13/0 | 27.4 | 85.0/51.0/15.0 | |
| Giorgio et al34 (IT) | 10 (5/5) | 70 | 9/10 | –/1/3 | 12 | 3.00 | 10/0 | 19.5c | 100/83.3/83.3 |
| Fu et al35 (CN) | 12 | 0/12 | –/2/– | 19 | 3.20b | 12/0 | 30.0 | 87.5/37.5/– | |
| Butros et al36 (USA) | 7 (3/4) | 65 | 1/7 | – | 9 | 2.30c | 7/0 | 35.0e | 100/60.0/20.0 |
AT: Austria; CN: China; IT: Italy; KR: Korea; USA: United States of America; HBV: hepatitis B virus; HCV: hepatitis C virus; RFA: radiofrequency ablation; MWA: microwave ablation; OS: overall survival.
Mean tumour size: typically values indicated as median values unless otherwise indicated.
Value indicative of the mean/median of largest tumour sizes of patients.
Data not provided ad numerum in the study but calculated using available data.
Number unspecified.
Excludes one patient lost to follow-up.
Tumour Characteristics
The number of tumours per patient in the data set ranged from 1 to 17. The vast majority of patients were single-tumour cases (64.4%). Not all studies provided data on median tumour sizes, and some studies only specified the size of the largest tumour per patient, which limited the true range for the median tumour size. Where possible, we calculated this using the patient data provided. Otherwise, mean tumour size data were used (Table 1), which ranged from 1.50 to 4.40 cm. All studies supplied data on whether patients were primary or recurrent cases of iCCA. In total, there were 61 patients with primary iCCA (29.6%) and 145 with recurrent iCCA (70.4%).
Treatment Characteristics
Information regarding the treatment methods (RFA or MWA) and the survival statistics can also be seen in Table 1. This analysis included studies that used both RFA and MWA techniques. Nine of the ten studies, totalling 129 patients, provided exact numbers for how many patients underwent each method. Overall, RFA was a more commonly practiced technique, used for 108 of 129 patients (83.7%), with MWA being used in the remaining 21 of 129 patients (16.3%). Almost all procedures were performed percutaneously via a form of radiographic guidance. In total, only four patients underwent open RFA in the data set, two from each of the studies carried out by Fu et al,28, 35 and there were no cases of endoscopic RFA. Of the 200 patients for whom such data were provided, 182 underwent ablation with ultrasound (US) guidance (91%), 17 with computed tomography (CT) guidance (8.5%) and 1 with both US + CT (0.5%).
Clinical Outcomes
The median follow-up period ranged from 8.7 to 29.9 months among the nine studies that provided the data. The median OS ranged from 8.7 to 52.4 months, which included the additional data provided by Haidu et al.31 The 1-year, 3-year and 5-year survivals ranged from 36.3–100%, 20.5–83.3% and 15.0–83.3%, respectively. There was disparity among the studies concerning methods of measuring progressive and developmental survival, with three providing data on RFS, four on PFS, two on disease-free survival and three on event-free survival (Table 2).
Table 2.
Summary of the Variable Survival Data of the Studies.
| Study | Recurrence-free survival (months) | Progression-free survival (months) | Event-free survival (months) | Disease-free survival (months) |
|---|---|---|---|---|
| Xu et al27 (CN) | 4.0 | – | – | – |
| Fu et al28 (CN) | 17.0 | – | – | – |
| Kim et al29 (KR) | – | 32.2 | – | – |
| Haidu et al31 (AT) | – | – | – | 24.3b |
| Zhang et al32 (CN) | – | – | – | 6.8 |
| Kim et al33 (KR) | – | 39.8a | 6.1 | – |
| Giorgio et al34 (IT) | – | 14.0c | – | – |
| Fu et al35 (CN) | 21.0 | – | 13.0 | – |
| Butros et al36 (USA) | – | 36.3 | 21.8 | – |
AT: Austria; CN: China; IT: Italy; KR: Korea; USA: United States of America.
Mean value calculation: values are medians unless otherwise stated.
Result from the original study, not the updated data set.
Data not provided ad numerum in the study but calculated using available data.
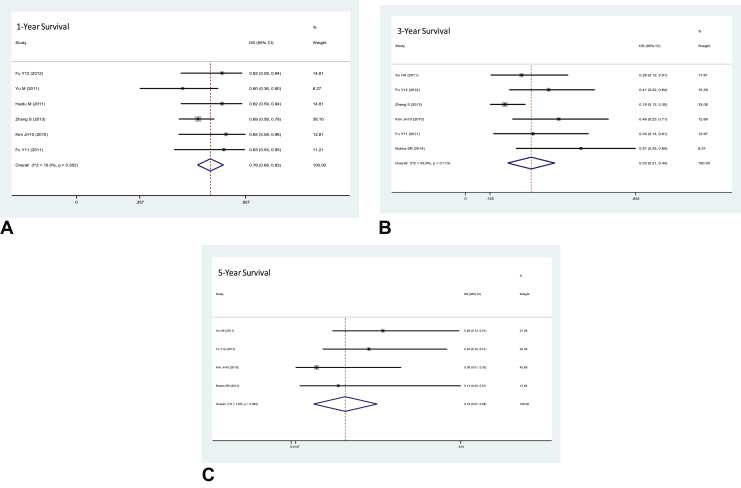
From the initial tests for heterogeneity, high degrees of heterogeneity were apparent in 1-year survival (I2 = 79.64%, P < 0.0001), 3-year survival (I2 = 79.51%, P < 0.0001) and 5-year survival (I2 = 82.25%, P < 0.0001). Galbraith plots were used to discern the most heterogeneous studies, which were removed (Supplementary data). This left six studies for 1-year survival (I2 = 10.0%, P = 0.352) and 3-year survival (I2 = 43.8%, P = 0.113) and four studies for 5-year survival (I2 = 1.8%, P = 0.383) for the calculation of the pooled survival rates (Figure 4). The final pooled survival rates were 76% (95% confidence interval [CI]: 68–83%), 33% (95% CI: 21–44%) and 16% (95% CI: 7–26%) for 1-year, 3-year and 5-year survival, respectively. Publication bias would have been assessed using funnel plots, but there were too few studies.
Figure 4.
Forest plots showing the final pooled proportions of the (A) 1-year (P = 0.352), (B) 3-year (P = 0.113) and (C) 5-year survival rates (P = 0.383). CI: confidence interval; OS, overall survival.
Prognostic Factors
Of the 10 studies, statistical tests were performed in only three studies to compare associations with reduced OS.26, 32, 35 Univariate analysis was performed in these three studies, with Zhang et al32 also incorporating multivariate analysis. Two of the studies27, 32 analysed associations between various factors and OS, as well as RFS, whereas the remaining study35 focused solely on the associations with OS. All three studies reported conflicting results. Xu et al27 found that only the source of the tumour, either primary or recurrent, was significant in affecting OS, with recurrent cases significantly associated with poorer OS. Gender and the number of tumour nodules were both insignificant in reducing OS. In addition, they found that the patient source and number of nodules were both significant in affecting RFS, with gender being insignificant. Fu et al35 reported that the only significant factor in affecting OS was tumour grade, with a poorer grade associated with a worse prognosis. Age, tumour size and number of tumours were found to be insignificant. Finally, Zhang et al32 found that both increased tumour number and reduction of time to recurrence after initial resection were significantly associated with poorer survival. Age, gender and tumour size were not found to be significant.
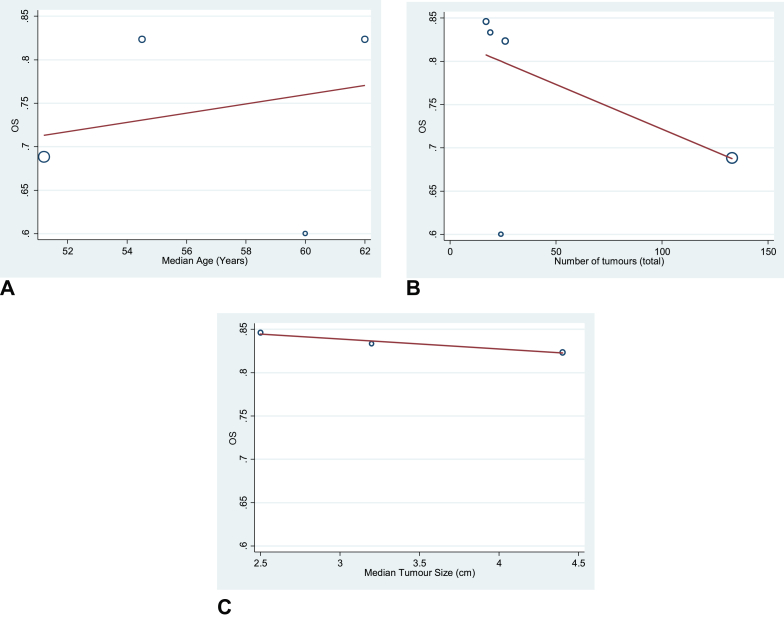
Three factors were analysed to assess their potential prognostic associations with 1-year survival: median patient age, median number of tumours and average tumour size. Owing to the heterogeneity of the original 10 studies, the six studies remaining after removal of the most heterogeneous studies were again used. Bubble plots exhibiting the results of the regression-based meta-analysis are displayed in Figure 5. Although trends were apparent, no statistically significant association was found for either median age (P = 0.713), number of tumours (P = 0.177) or median tumour size (P = 0.897).
Figure 5.
Bubble plots showing associations between 1-year survival (y-axis) against median age (A), number of tumours (B) and median tumour size (C). OS, overall survival.
Discussion
Although iCCA prevalence is relatively low, it carries a dismal prognosis, and its global incidence is rising. Owing to its relative rarity in most parts of the world, few institutions have experience with the disease, and even fewer centres will have extensive experience with ablative therapies in the context of iCCA. Thus, there is a scarcity of data investigating iCCA, more so in studying the benefit of ablative therapies for iCCA. Data that do exist comprise small studies from single institutions. The small sample sizes limit their reliability and create difficulty in establishing associations between prognostic factors and survival, which may explain the heterogeneity exhibited by the studies in this meta-analysis.
Nonetheless, these studies show promise in the potential of ablative therapies to potentially extend survival in patients with iCCA. The 5-year median survival from the studies ranged between 15% and 83.3%, which is higher than that of surgical resection (21–35%), as reported in the meta-analysis by Mavros et al.26 In addition, Zhang et al32 found no significant difference between resection and ablation in OS in patients with iCCA. Although the reports from the literature suggest that ablation may be a suitable primary treatment option alongside resection, our data determined that more research is required. Although the range for the 5-year median survivals was high, the pooled calculation was lower at 16% (95% CI: 7–26%). It is important to note that while the survival value is lower than the 5-year survival of the resection meta-analysis,26 74% of the resections had margin-negative histology (R0).
Percutaneous ablation is minimally invasive, has a low risk of complications and does not require hospital admission. It is cheaper and quicker to perform than resection and reduces hospital admission.37 As with resection, ablative therapies are highly operator dependent38 and have a few documented complications, which include the following: destruction of tissue; thermal injury causing injuries to the diaphragm and bowel and biliary perforation; haemorrhage; infection; seeding of tumour and incomplete ablation.39 Various methods already exist for performing ablation, such as percutaneous, endoscopic and open surgery, and it may be possible that survival rates improve as new techniques and technologies emerge. There are two main methods of performing ablation in the context of iCCA, RFA and MWA. There are potential advantages of MWA over RFA: faster heat generation over a larger volume during heating, less susceptibility to heat sinks and no ground pads,40 which suggests MWA may be a more effective technique for tumour ablation. Xu et al27 included a mixed cohort with 12 patients treated with RFA and six with MWA. The median OS of the RFA cohort was 8 months, compared with the median OS of the MWA cohort being 13.5. Despite this, further testing with univariate analysis showed the difference was not statistically significant. However, both cohort sizes were small, and further research is required to ascertain any difference in the efficacy between these two methods. With growing attention in the field, it is possible that MWA may be used in more studies to assess its role in the treatment of iCCA.
The current ILCA guidelines8 affirm the role of ablative therapies as secondary treatment options in patients for whom resection is not viable. Despite the fact that around 70% of patients have unresectable disease, there is still a paucity of data in investigating the role of ablative therapy. Ablative therapy has the potential to be used in a variety of treatment stages: as a primary treatment option, as adjuvant therapy to surgery, as an alternative therapy in disease recurrence after resection and in its current palliative role in unresectable disease.
We present the largest study of its kind that incorporates both a systematic review and meta-analysis on ablative therapies for iCCA. This study presents a reliable pooled estimate for the 1-year, 3-year and 5-year survival present in the literature. A similar meta-analysis conducted by Han et al41 included patients treated solely with RFA and thus had a significantly smaller sample size of 87 patients. In addition, this is the first study to assess associations between prognostic factors and survival in the setting of ablation in patients with iCCA.
The limitations of the study include discordancy in reporting of the results, that is, some articles expressing results as mean values and others, as median values. Data on patient characteristics, comorbidities, tumour size, location and time gap between primary treatment and ablation for recurrence were inconsistent or missing in the studies. There was a high degree of heterogeneity among the initial set of studies, resulting in the removal of studies before the statistical analyses. The high degree of heterogeneity may be due to the inclusion of various stages of disease, including recurrent disease, patients with inoperable disease due to comorbidities or low chance of survival and primary cases. In addition, the studies reviewed reflect a variety of clinical settings, including Europe, the United States and Asia, which may impact treatment choices. The authors suggest the addition of key characteristic information mentioned previously for future studies that are conducted in this field.
Prognosis remains poor for patients with iCCA, with less than 5% surviving for five years when untreated and as little as 23% surviving for five years when undergoing resection with curative intent. Our study suggests that ablative therapies might prolong survival in patients with iCCA. Although ablation appears promising, further investigation is warranted, including more data with consistent reporting of findings and further information on MWA particularly.
Conflicts of interest
The authors have none to declare.
Funding
This study was funded by grants from AMMF – The Cholangiocarcinoma Charity (www.ammf.org.uk) and from the Trustees of the Imperial College Healthcare Charity. The NIHR Biomedical Facility at Imperial College London provided infrastructure support. The British Department of Health's National Institute for Health Research Biomedical Research Centres (NIHR BRC) funding scheme provided infrastructure support. The authors are also grateful for a charitable donation from Mr and Mrs Barry Winter and to the relatives of Mrs Suzy Dunn towards running costs for this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2019.08.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Shaib Y., El-Serag H.B. The epidemiology of cholangiocarcinoma. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 2.Blechacz B., Komuta M., Roskams T., Gores G.J. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512. doi: 10.1038/nrgastro.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan S.A., Emadossadaty S., Ladep N.G. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56:848–854. doi: 10.1016/j.jhep.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Shaib Y.H., Davila J.A., McGlynn K., El-Serag H.B. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472–477. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 5.Khan S.A., Toledano M.B., Taylor–Robinson S.D. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB. 2008;10:77–82. doi: 10.1080/13651820801992641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blechacz B.R., Gores G.J. Cholangiocarcinoma. Clin Liver Dis. 2008;12:131–150. doi: 10.1016/j.cld.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 8.Bridgewater J., Galle P.R., Khan S.A. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268–1289. doi: 10.1016/j.jhep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Bertuccio P., Bosetti C., Levi F., Decarli A., Negri E., La Vecchia C. A comparison of trends in mortality from primary liver cancer and intrahepatic cholangiocarcinoma in Europe. Ann Oncol. 2013;24:1667–1674. doi: 10.1093/annonc/mds652. [DOI] [PubMed] [Google Scholar]
- 10.Endo I., Gonen M., Yopp A.C. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 11.DeOliveira M.L., Cunningham S.C., Cameron J.L. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang H., Sotiropoulos G.C., Sgourakis G. Operations for intrahepatic cholangiocarcinoma: single-institution experience of 158 patients. J Am Coll Surg. 2009;208:218–228. doi: 10.1016/j.jamcollsurg.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 13.Guglielmi A., Ruzzenente A., Campagnaro T. Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World J Surg. 2009;33:1247–1254. doi: 10.1007/s00268-009-9970-0. [DOI] [PubMed] [Google Scholar]
- 14.Morimoto Y., Tanaka Y., Ito T. Long-term survival and prognostic factors in the surgical treatment for intrahepatic cholangiocarcinoma. J Hepato-Biliary-Pancreatic Sci. 2003;10:432–440. doi: 10.1007/s00534-002-0842-3. [DOI] [PubMed] [Google Scholar]
- 15.Murad S.D., Kim W.R., Harnois D.M. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143 doi: 10.1053/j.gastro.2012.04.008. 98. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valle J.W., Wasan H., Johnson P. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study–The UK ABC-01 Study. Br J Canc. 2009;101:621. doi: 10.1038/sj.bjc.6605211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valle J., Wasan H., Palmer D.H. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 18.Yang J., Yan L. Current status of intrahepatic cholangiocarcinoma. World J Gastroenterol: WJG. 2008;14:6289. doi: 10.3748/wjg.14.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yedibela S., Demir R., Zhang W., Meyer T., Hohenberger W., SchÖnleben F. Surgical treatment of mass-forming intrahepatic cholangiocarcinoma: an 11-year Western single-center experience in 107 patients. Ann Surg Oncol. 2009;16:404. doi: 10.1245/s10434-008-0227-1. [DOI] [PubMed] [Google Scholar]
- 20.Puhalla H., Schuell B., Pokorny H., Kornek G.V., Scheithauer W., Gruenberger T. Treatment and outcome of intrahepatic cholangiocellular carcinoma. Am J Surg. 2005;189:173–177. doi: 10.1016/j.amjsurg.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y., Li J., Xia Y. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188–1195. doi: 10.1200/JCO.2012.41.5984. [DOI] [PubMed] [Google Scholar]
- 22.Ribero D., Pinna A.D., Guglielmi A. Surgical approach for long-term survival of patients with intrahepatic cholangiocarcinoma: a multi-institutional analysis of 434 patients. Arch Surg. 2012;147:1107–1113. doi: 10.1001/archsurg.2012.1962. [DOI] [PubMed] [Google Scholar]
- 23.Farges O., Fuks D., Boleslawski E. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg. 2011;254:824–830. doi: 10.1097/SLA.0b013e318236c21d. [DOI] [PubMed] [Google Scholar]
- 24.de Jong M.C., Nathan H., Sotiropoulos G.C. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29:3140–3145. doi: 10.1200/JCO.2011.35.6519. [DOI] [PubMed] [Google Scholar]
- 25.Jiang W., Zeng Z., Tang Z. A prognostic scoring system based on clinical features of intrahepatic cholangiocarcinoma: the Fudan score. Ann Oncol. 2011;22:1644–1652. doi: 10.1093/annonc/mdq650. [DOI] [PubMed] [Google Scholar]
- 26.Mavros M.N., Economopoulos K.P., Alexiou V.G., Pawlik T.M. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. 2014;149:565–574. doi: 10.1001/jamasurg.2013.5137. [DOI] [PubMed] [Google Scholar]
- 27.Xu H.X., Wang Y., Lu M.D., Liu L.N. Percutaneous ultrasound-guided thermal ablation for intrahepatic cholangiocarcinoma. Br J Radiol. 2012;85:1078–1084. doi: 10.1259/bjr/24563774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu Y., Yang W., Wu W., Yan K., Xing B., Chen M. Radiofrequency ablation for postoperative recurrences of intrahepatic cholangiocarcinoma. Chin J Canc Res. 2011;23:295–300. doi: 10.1007/s11670-011-0295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J.H., Won H.J., Shin Y.M., Kim P.N., Lee S., Hwang S. Radiofrequency ablation for recurrent intrahepatic cholangiocarcinoma after curative resection. Eur J Radiol. 2011;80:e225. doi: 10.1016/j.ejrad.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 30.Yu M., Liang P., Yu X. Sonography-guided percutaneous microwave ablation of intrahepatic primary cholangiocarcinoma. Eur J Radiol. 2011;80:548–552. doi: 10.1016/j.ejrad.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 31.Haidu M., Dobrozemsky G., Schullian P. Stereotactic radiofrequency ablation of unresectable intrahepatic cholangiocarcinomas: a retrospective study. Cardiovasc Interv Radiol. 2012;35:1074–1082. doi: 10.1007/s00270-011-0288-6. [DOI] [PubMed] [Google Scholar]
- 32.Zhang S., Hu P., Wang N. Thermal ablation versus repeated hepatic resection for recurrent intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2013;20:3596–3602. doi: 10.1245/s10434-013-3035-1. [DOI] [PubMed] [Google Scholar]
- 33.Kim J.H., Won H.J., Shin Y.M., Kim K., Kim P.N. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. Am J Roentgenol. 2011;196:W209. doi: 10.2214/AJR.10.4937. [DOI] [PubMed] [Google Scholar]
- 34.Giorgio A., Calisti G., De Stefano G. Radiofrequency ablation for intrahepatic cholangiocarcinoma: retrospective analysis of a single centre experience. Anticancer Res. 2011;31:4575–4580. [PubMed] [Google Scholar]
- 35.Fu Y., Yang W., Wu W., Yan K., Xing B.C., Chen M.H. Radiofrequency ablation in the management of unresectable intrahepatic cholangiocarcinoma. J Vasc Interv Radiol. 2012;23:642–649. doi: 10.1016/j.jvir.2012.01.081. [DOI] [PubMed] [Google Scholar]
- 36.Butros S.R., Shenoy-Bhangle A., Mueller P.R., Arellano R.S. Radiofrequency ablation of intrahepatic cholangiocarcinoma: feasability, local tumor control, and long-term outcome. Clin Imaging. 2014 Aug 31;38:490–494. doi: 10.1016/j.clinimag.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 37.Sutherland Leanne M. Radiofrequency ablation of liver tumors: a systematic review. Archi Surg. 2006;141:181–190. doi: 10.1001/archsurg.141.2.181. 2016. [DOI] [PubMed] [Google Scholar]
- 38.Friedman M., Mikityansky I., Kam A. Radiofrequency ablation of cancer. Cardiovasc Interv Radiol. 2004;27:427–434. doi: 10.1007/s00270-004-0062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nemcek A.A. vol. 23. Thieme Medical Publishers, Inc.; New York, NY 10001, USA: 2006. Complications of radiofrequency ablation of neoplasms; pp. 177–187. (Seminars in Interventional Radiology 2006 Jun). No. 02. 333 Seventh Avenue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brace C. Diagnostic Imaging; 2016. Microwave Ablation Technology Avoids Problems that Plague RFA, Offers Promise for New Applications. [Google Scholar]
- 41.Han K., Ko H.K., Kim K.W., Won H.J., Shin Y.M., Kim P.N. Radiofrequency ablation in the treatment of unresectable intrahepatic cholangiocarcinoma: systematic review and meta-analysis. J Vasc Interv Radiol. 2015;26:943–948. doi: 10.1016/j.jvir.2015.02.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.