Abstract
Background
Primary hepatic neuroendocrine tumor is an extremely rare entity. Only case reports are available in the literature. The aim of the study was to analyze the symptoms, diagnosis, management, and the outcome of patients with primary liver neuroendocrine tumors.
Methods
In the study, a total of eight patients were diagnosed with primary liver neuroendocrine tumors between 2001 and 2017 in our center. Data were analyzed from the records available including the presentation, diagnosis, treatment received, and follow-up.
Results
Of eight patients, five were males and three were females. The age of presentation was between 35 and 70 years. Two patients had pain in the right side of the abdomen, while it was accidentally detected in two patients in routine checkup. One patient presented with carcinoid syndrome, while two had ascites and one patient presented only with loose motions. Of eight patients, two patients with poorly differentiated neuroendocrine tumor died within 1 month of follow-up. Four patients are still being followed up, while 10–12 years of follow-up data are available for the remaining two patients. Four patients underwent surgery, and three patients received Sandostatin LAR for tumor recurrence after procedure. Transarterial chemoembolization (TACE) of the tumor was performed in two patients for whom resection was not possible.
Conclusions
Our data suggest that the prognosis of the tumor seems favorable. Surgical resection is the curative treatment. TACE is a favorable option in unresectable tumors.
Keywords: primary hepatic neuroendocrine tumor, surgical resection, transarterial chemoembolization (TACE)
Abbreviations: CgA, Chromogranin A; CK 7, Cytokeratin 7; CT, Computed tomography; HIAA, Hydroxyindoleacetic acid; MRI, Magnetic resonance imaging; NET, Neuroendocrine tumor; NSE, Neuron-specific enolase; PHNET, Primary hepatic neuroendocrine tumor; TACE, Transarterial chemoembolization; USG, Ultrasonography
Neuroendocrine tumors (NETs) comprise around 1–2% of all gastrointestinal tumors. The liver is the most common site of metastasis of these tumors.1 Primary hepatic neuroendocrine tumor (PHNET) is an extremely rare entity. The first case was reported by Edmonson in 1958,2 and since then, fewer than 150 cases have been reported in the literature. PHNETs comprise approximately 0.3% of all neuroendocrine tumors.3 Symptoms related to PHNET are nonspecific, and patients usually present with abdominal pain.4 Because it is a rare entity, proper algorithm for diagnosis and management has not been described. Diagnosis of PHNET is challenging. Hepatocellular carcinoma is initially diagnosed relying on imaging methods. A diagnosis of PHNET is then made from the histological examination of preoperative liver tumor biopsy samples. The survival of patients with PHNET for more than 10 years is reported as high as 73%.3 Treatment includes surgical resection of the liver, transarterial chemoembolization (TACE) therapy, liver transplantation, radiotherapy, chemotherapy, and administration of somatostatin analogs. Surgical resection is the most common modality for localized disease, whereas TACE is mostly used for poor candidates of surgery.4, 5 We analyzed the previous data from 2001 to 2017 and found 8 patients who had PHNET in our center. Their mode of presentation, treatment received, and outcomes were analyzed and are presented in our study.
Methods
Previous records from our hospital between 2001 and 2017 were analyzed, and patients with PHNET were included in our study. It was a single-center retrospective study. Patients with neuroendocrine tumors at other sites were excluded from the study. In addition, liver metastasis with the primary site other than the liver was excluded. Patients with no follow-up were not included. A minimum follow-up period of 5 years was taken into consideration. However, if a patient died within 5 years of presentation, he/she was included in the study. Their age and mode of presentation were noted. Serum chromogranin A (CgA) levels and urinary hydroxyindoleacetic acid (HIAA) levels were analyzed. All patients were alpha-fetoprotein and carcinoembryonic antigen negative. Imaging findings including those of the octreotide scan if carried out were noted. Different types of treatment received and the overall outcome after the treatment were analyzed. Follow-up findings were also analyzed.
Results
From seventeen years of data, we found only eight patients with PHNET. Of eight patients, five were males and three were females. The characteristics and outcomes of individual patients are summarized in Table 1.
Table 1.
Characteristics and Outcome of Treated Patients.
| Case no. | Age (yrs) | Sex | Symptoms | CT findings | Histopathology with IHC | Nuclear scan | Serum CgA levels (normal: <6 nmol/l) | 24-hr urinary HIAA (normal: 2–8 mg in 24 hrs) | Surgery | Other treatment and outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 35 | M | Recurrent loose motions since 1 month | 8.1 × 7.6 cm mass in the left lobe of the liver with mild marginal enhancement with the small nodule in the right lobe | Well-differentiated neuroendocrine tumor (G1), mitosis<2/10 HPF, +for synaptophysin, CD56, NSE, Ki-67 < 2% | Uptake in segment 2/3 and in segment 5/7 in the gallium DOTATATE scan | 928 nmol/l | 20 mg/24 h | Left hepatectomy with wedge resection of the right lobe nodule | Received Sandostatin LAR injections after operation for recurrence. Thereafter disease free in follow-up until now |
| 2 | 42 | M | Abdominal pain since 15 days | Multiple nodular target lesions in both lobes with IVC compression with 9*7 cm lesion in the left lobe with portal vein thrombosis | Well-differentiated NET (G2), mitosis 4/10 HPF, + for synaptophysin, CgA, Ki-67: 10% | Uptake seen in the periphery of the lesion in the left lobe in the octreotide scan | 60.0 nmol/l | 66 mg/24 h | NA | TACE, followed by Sandostatin LAR injections. Disease free in follow-up until now |
| 3 | 60 | F | Abdominal pain since 2 weeks | CT: hepatomegaly with a hypoechoic mass of 7.2*3.3*3.4 cm | Well-differentiated NET, + for synaptophysin, NSE, CD56 | NA | 138 nmol/l | 6 mg/24 h | NA | TACE, disease free in follow-up until 10 years |
| 4 | 56 | M | Carcinoid syndrome, flushes, diarrhea | CT: large mass in the right lobe of the liver about 11*9*7 cms | Well-differentiated NET, + for CK7, synaptophysin, CgA, CD56 | NA | 859 nmol/l | 190 mg/24 h | Right hepatectomy | Received Sandostatin LAR injections owing to recurrence. Disease free in follow-up until 12 years |
| 5 | 65 | M | Asymptomatic | Routine USG: suggestive of hydatid cysts in the right lobe 8.7*7 and 4.2*4.7 cms CT: large mass in the right lobe 7.3*7 and 5.2 cms |
Well-differentiated NET (G2), mitosis 6/10, + for synaptophysin, CgA, NSE, Ki-67: 15% | Uptake in the octreotide scan in the right lobe lesion | 432 nmol/l | 49 mg/24 h | Surgical resection of the tumor | No other treatment, repeat nuclear scan: no uptake. Disease free in regular follow-up until now |
| 6 | 69 | M | Ascites since 1 month | Large exophytic mass in the liver with peritoneal nodules | Poorly differentiated NET,+ for synaptophysin, CgA, CD56, NSE | NA | <6 nmol/l | 4 mg/24 hrs | NA | Expired within 1 month of presentation |
| 7 | 49 | M | Severe pain in the abdomen since 4 days, ascites | Multiple nodules in both lobes of the liver with a large mass of 8*7*6 cms in the right lobe, moderate ascites | CT-guided biopsy sample s/o poorly differentiated NET, + for synaptophysin, CgA, CK 7 | NA | <6 nmol/l | 8 mg/24 h | NA | Expired within 1 month of presentation |
| 8 | 62 | F | Asymptomatic | Left lobe mass of 4.1*3.6*3.5 cms | CT-guided biopsy: well-differentiated neuroendocrine tumor (G1), + for synaptophysin, Ki-67 < 2% | No uptake in the octreotide scan, repeat scan after 1 year shows increasing uptake | <6 nmol/l After 1 year, 433 nmol/l |
6 mg/24 h, after 1 year, 89 mg in 24 hrs | Surgical resection of the tumor | No other treatment. No increase in size and no uptake in the follow-up scan. Disease free until now. |
CK 7, cytokeratin 7; CgA, chromogranin A; HIAA, hydroxyindoleacetic acid; NSE, neuron-specific enolase; NET, neuroendocrine tumor; TACE, transarterial chemoembolization; USG, ultrasonography; IVC, inferior vena cava; HPF, high power field; NA, not applicable; s/o, suggestive of; IHC, immunohistochemistry.
The average age of our patients was 55 years, with range being 35–69 years. Of eight patients, four patients had single tumor and 4 patients had more than one lesion. Of four patients with multiple lesions, one patient with exophytic mass and peritoneal nodules presented with severe pain in the abdomen with ascites at the time of admission and one patient with large liver lesion with multiple nodules in both the lobes presented with only ascites. Both the patients had poorly differentiated neuroendocrine tumor. However, their mitotic index and Ki-67 index were not mentioned in the data since the World Health Organization (WHO) classification on neuroendocrine neoplasms was validated in 2010. Both the patients died within 1 month of presentation. Interestingly, their serum CgA levels and 24-h urinary HIAA levels were normal. The computed tomography (CT) scan did not show lesions anywhere else except in the liver.
In two patients, PHNET was diagnosed in routine medical examination during the health checkup. One patient's ultrasonography was suggestive of hydatid cyst, and the CT scan was suggestive of a large mass in the right lobe. The octreotide scan showed increased uptake in the right lobe, and the tumor was surgically resected. She was disease free in follow-up of 7 years, after which she never showed up. The other patient diagnosed on routine examination also had a single lesion in the left lobe, but the octreotide scan showed no uptake. On follow-up after 1 year, there was an elevation of CgA levels and uptake on the octreotide scan in the left lobe lesion. Surgical resection of the tumor was performed, and after surgery, she was disease free without any symptoms.
Of the remaining four patients, two had pain in the right hypochondrium, one had only recurrent episodes of loose stools, and one had carcinoid syndrome. All these four patients had well-differentiated neuroendocrine tumor. Surgery was performed in two patients, and TACE was performed in the other two patients. Sandostatin LAR injections were required in three patients after procedure for recurrence. Two patients are still being followed up, and they are disease free. Other two were followed up for more than 10 years with no recurrence.
Discussion
PHNET is an extremely rare entity. We had only 8 patients in the last 17 years diagnosed with PHNET. NETs arise from the neuroectodermal cells. These cells do not routinely migrate to the liver, which explains why PHNETs are so rare.6 There are a number of theories that explain the pathogenesis of PHNETs. Hseuh et al7 proposed the presence of ectopic pancreatic or adrenal tissue resulting in PHNET. Alpert et al8 suggested that argentaffin cells located within the bile duct epithelium are responsible for PHNETs.
PHNET is not gender specific; however, prior systematic reviews of case reports described in the literature show that it is more common in females than males.9 However, in our study, it was found more in males than females. PHNETs can occur at any age, but generally described in young and middle-aged patients. But in our study, we found more in middle-aged and old patients, with a mean age of 55 years. Many patients are found to have PHNETs on health examination.9 In the study, we had 2 patients diagnosed with PHNETs on routine health examination.
Tumor markers such as alpha-fetoprotein, carcinoembryonic antigen, and CA 19.9 levels have almost no diagnostic value in PHNETs.9 The 24-h urinary HIAA level may be effective in diagnosis, with a sensitivity and specificity of 73% and 90%, respectively.10 In the study, five patients had elevated HIAA levels in urine. However, both the patients with poorly differentiated NET had normal levels. Serum CgA levels is also a useful diagnostic marker, with a sensitivity of 87–100% and a specificity of 92%.11, 12 Six patients had elevated levels in the study, of which one patient had increase in levels after one year of diagnosis. Surprisingly, patients with poorly differentiated NET had normal levels of CgA as well. Thus, the significance of HIAA levels and serum CgA levels in such patients may be questionable. Large-scale studies are required for the same.
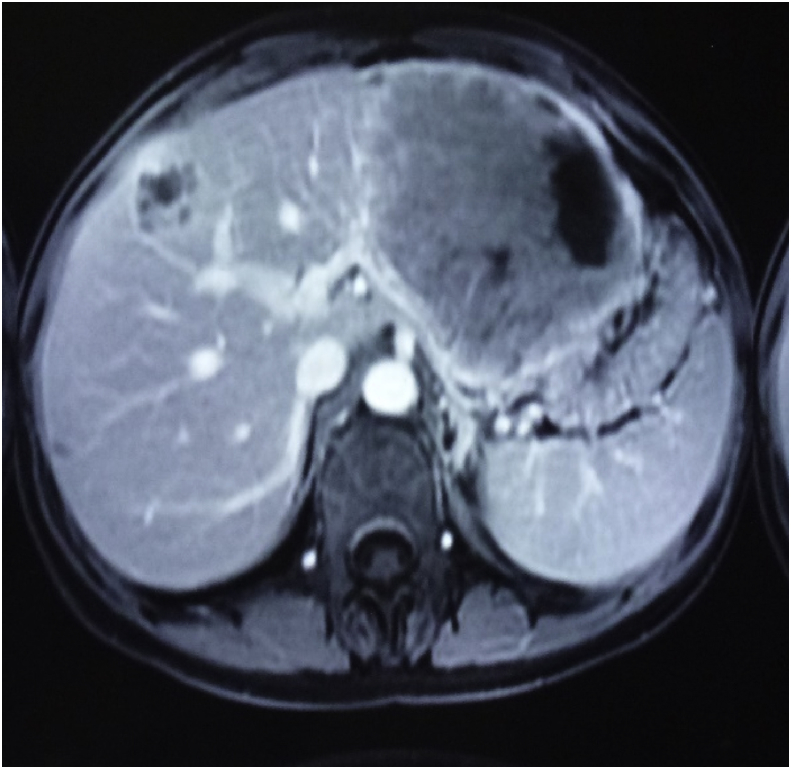
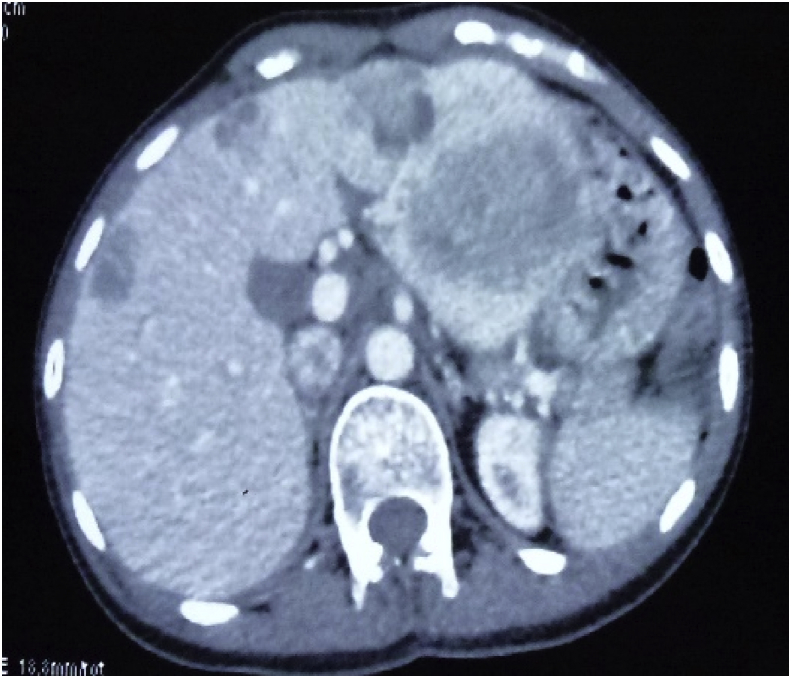
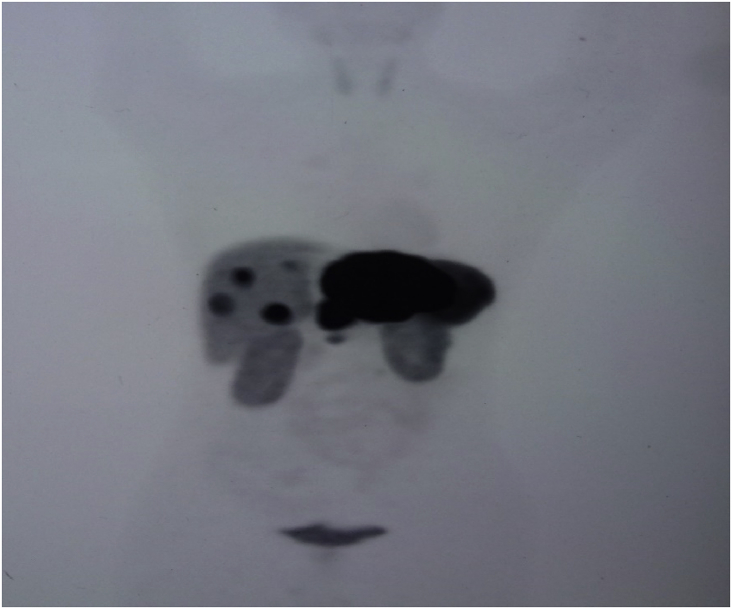
Radiographically, ultrasound, CT, and magnetic resonance imaging (MRI) have low specificity in differentiating PHNET from other hepatic tumors.13 On MRI, both primary tumors and metastases have been reported to appear hypointense on T1-weighted and hyperintense on T2-weighted sequence.14 Hepatic NET metastases also commonly show intense enhancement in the hepatic arterial-dominant phase with washout in portal venous and extracellular phases, reflecting hypervascularity.14 Similar early enhancement has been shown in PHNETs. Figure 1, Figure 2 show the MRI and CT image of one of the patient, the findings of which were consistent with NET in the left lobe of the liver with a small nodule in the right lobe. It was initially diagnosed as liver metastasis, but confirmed as multiple PHNETs after further workup. When PHNET is suspected, Octreoscan is performed using I-111–labeled octreotide. It has a sensitivity and specificity of 90% and 83%, respectively, with a 100% positive predictive value of identifying NET.15 Recently, 68Ga-DOTATATE positron emission tomography (PET)/CT is shown to be superior than Octreoscan in few studies.16 Figure 3 shows the 68Ga-DOTATATE PET/CT picture of one of the patients with multiple PHNETs.
Figure 1.
Axial image of the T1-weighted fat-suppressed post-contrast image shows a large heterogeneously, predominantly peripherally enhancing lesion in the left lobe of the liver, reaching up to the subcapsular region and displacing the adjacent body of the stomach laterally. A similar lesion of a smaller size is seen in the right lobe of the liver.
Figure 2.
Axial image of post-contrast CT study of the abdomen showing multiple round to oval, fairly well-demarcated heterogeneously hypoenhacing lesions scattered in the left and right lobe with few areas of central necrosis. No obvious extrahepatic extension noted. CT.
Figure 3.
68Ga-DOTATATE scan of one of the patients having multiple sites of uptake in both lobes of the liver.
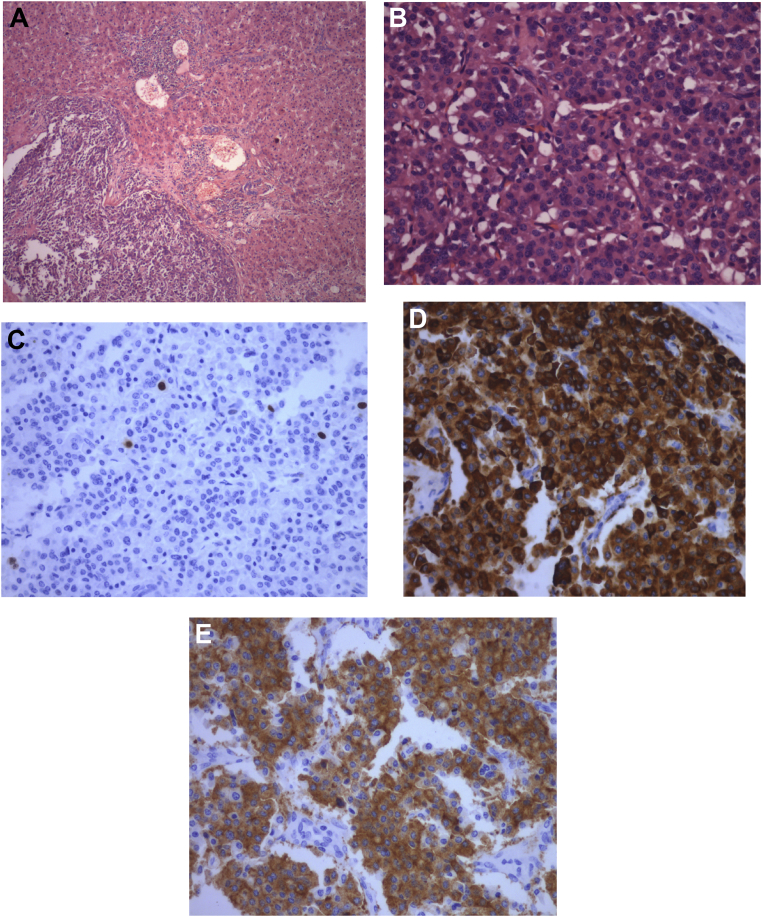
Pathologic diagnosis is the best way to confirm PHNET. Routine hematoxylin and eosin (HE) staining is helpful in the classification of the tumor. Special stains such as Masson's can raise the diagnostic rate to 80% or more.17 In the study, all patients had biopsy-proven neuroendocrine tumor. Immunohistochemistry raises the accuracy rate by detecting markers such as synaptophysin, neuron-specific enolase, CD56, chromogranin, and so on. It also helps in calculating the mitotic index and Ki-67 index. In the study, immunohistochemistry was carried out in all cases, and various markers were detected in the samples that aided in the diagnosis of PHNET. Patients diagnosed after 2010 were classified as per the WHO classification of NETs.18 Two patients were of G1 type, and two were of G2 type. Figure 4A–E shows a well-differentiated NET, with the Ki-67 index of <2% and immunohistochemistry showing cells stained with various markers such as chromogranin and synaptophysin. Although the WHO classification is designed for NET in the more common location such as the small bowel, pancreas, and colon, it has been adopted for the description of PHNETs as well.
Figure 4.
(A) Well-circumscribed tumor composed of small, round cells. They are arranged in trabeculae, cords, and nests along with the hepatic parenchyma. (B) Higher power reveals round to oval cells with central nuclei having salt-and-pepper–like chromatin in a fine vascular network. (C) Tumor cells displaying the Ki-67 index of less than 2%. (D) Tumor cells showing diffuse cytoplasmic positivity for chromogranin. (E) Tumor cells showing diffuse cytoplasmic positivity for synaptophysin.
Surgery with complete resection has been considered as the treatment modality of choice. Up to 85% tumors are resectable.19 The 5-year survival rate is around 74–78%.20 Recurrence rates are as high as 19–20%.3 In the study, four patients underwent surgery, of which tumor excision was performed by hepatectomy in two patients. After surgery, two patients had recurrence and were treated with Sandostatin LAR injections. Somatostatin analogs have shown good efficacy for functional somatostatin receptor (SSTR)-positive NETs. All four patients survived without any tumor recurrence after that.
Other treatment modalities apart from surgery have been less well understood. Liver transplant and TACE have been suggested as alternative modalities in unresectable disease. In the study, TACE was used as the primary mode of treatment in two patients. One patient was a poor candidate for surgery and was not given the fitness. This patient was followed up for 10 years regularly after TACE. She was asymptomatic since then, and repeated Octreoscan did not show any uptake anywhere else in the body. Other patient had multiple lesions on both the lobes with portal vein thrombosis. This patient underwent TACE, followed by Sandostatin LAR injections. Doxorubicin was used during TACE. The survival rate of both the patients was similar to those who underwent surgery. However, no evidence of survival benefit is proven with TACE. A large-scale study is required to prove the role of TACE in resectable disease. Other treatment modalities such as radiotherapy are of questionable value. In the study, two patients had poorly differentiated NET and died within 1 month. Thus, one can assume that the patient presenting with disseminated PHNET can have a poor prognosis.
PHNET is a rare primary liver tumor and presents with nonspecific symptoms. Pathological and immunohistochemical studies are important tools in diagnosing PHNETs. In addition, the possibility of metastatic NETs in the liver should be ruled out first. Serum chromogranin and 24-h urinary HIAA levels should be assessed to aid in the diagnosis. Surgical resection remains the gold standard treatment in resectable disease. TACE can be an effective option in unresectable or medically unfit patients. The recurrence rate being higher, regular follow-up of these patients becomes extremely important. Sandostatin LAR can be tried in recurrence. Overall, the survival rate after treatment of these patients is good.
Conflicts of interest
The authors have none to declare.
References
- 1.Modlin I.M., Sandor A. An analysis of 8305 cases of carcinoid tumours. Cancer. 1997;79:813–829. doi: 10.1002/(sici)1097-0142(19970215)79:4<813::aid-cncr19>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 2.Edmonson H. Tumour of the liver and intrahepatic bile duct. Atlas Tumour Pathol. 1958;7:105–109. [Google Scholar]
- 3.Quartery B. Primary hepatic neuroendocrine tumours: what do we know now? World J Oncol. 2011;2:209–216. doi: 10.4021/wjon341w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin C.W., Lai C.H., Hsu C.C. Primaryhepatic carcinoid tumor: a case report and review of the literature. Cases J. 2009;2:90. doi: 10.1186/1757-1626-2-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akahori T., Sho M., Tanaka T. Significant efficacy of new transcatheterarterialchemoembolization technique for hepatic metastases of pancreaticneuroendocrinetumors. Anticancer Res. 2013;33:3355–3358. [PubMed] [Google Scholar]
- 6.LambrescuIM, Martin S., Cima L., Herlea V., Badiu C., Fica S. Primary hepatic neuroendocrine tumour after 4 years tumour-free follow-up. JGLD. 2015;24:241–244. doi: 10.15403/jgld.2014.1121.242.yrs. [DOI] [PubMed] [Google Scholar]
- 7.Hseuh C., Tsn X.D., Gonzalez-Crussi F. Primary hepatic neuroendocrine carcinoma in a child. Morphologic, immunocytochemical, and molecular biologic studies. Cancer. 1993;71:2660–2665. doi: 10.1002/1097-0142(19930415)71:8<2660::aid-cncr2820710835>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Alpert L.I., Zak F.G., Werthamer S., Bochetto J.F. Cholangiocarcinoma: a clinicopathologic study of five cases with ultrastructural observations. Hum Pathol. 1974;5:709–728. doi: 10.1016/s0046-8177(74)80041-9. [DOI] [PubMed] [Google Scholar]
- 9.Donadon M., Torzilli G., Palmisano A. Liver resection for primary hepatic neuroendocrine tumours: report of three cases and review of the literature. Eur J Surg Oncol. 2006;32:325–328. doi: 10.1016/j.ejso.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Lamberts S.W., Hofland L.J., Nobels F.R. Neuroendocrine tumor markers. Front Neuroendocrinol. 2001;22:309–339. doi: 10.1006/frne.2001.0218. [DOI] [PubMed] [Google Scholar]
- 11.Eriksson B., Oberg K., Stridsberg M. Tumor markers in neuroendocrine tumors. Digestion. 2000;62:33–38. doi: 10.1159/000051853. [DOI] [PubMed] [Google Scholar]
- 12.Stridsberg M., Eriksson B., Oberg K., Janson E.T. A comparison between three commercial kits for chromogranin A measurements. J Endocrinol. 2003;177:337–341. doi: 10.1677/joe.0.1770337. [DOI] [PubMed] [Google Scholar]
- 13.Wang L.X., Liu K., Lin G.W., Jiang T. Primary hepatic neuroendocrine tumours: comparing CT and MRI features with pathology. Cancer Image. 2015;15:13. doi: 10.1186/s40644-015-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Der Hoef M., Crook D.W., Marincek B., Weishaupt D. Primary neuroendocrine tumors of the liver: MRI features in two cases. Abdom Imag. 2004;29:77–81. doi: 10.1007/s00261-003-0064-4. [DOI] [PubMed] [Google Scholar]
- 15.Kellock T., Tuong B., Harris A.C., Yoshida E. Diagnostic imaging of primary hepatic neuroendocrine tumours: a case and discussion of the literature. Case Rep Radiol. 2014;2014:156491. doi: 10.1155/2014/156491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mojtahedi Alireza, Thamake Sanjay, Tworowska Izabela, Ranganathan David, Delpassand Ebrahim S. The value of 68Ga-DOTATATE PET/CT in diagnosis and management of neuroendocrine tumors compared to current FDA approved imaging modalities: a review of literature. Am J Nucl Med Mol Imag. 2014;4:426–434. [PMC free article] [PubMed] [Google Scholar]
- 17.Bastaki W., Mothaffer F., Varro J., Al-Ghanim M., Malak L., Ayyash E., Asfar S. Primary Hepatic carcinoid tumour. Med Princ Pract. 2005;14:288–291. doi: 10.1159/000085753. [DOI] [PubMed] [Google Scholar]
- 18.Klimstra D.S., Arnold R., Capella C. Neuroendocrine neoplasms of thepancreas. In: Bosman F., Carneiro F., Hruban R.H., Theise N., editors. WHO Classification of Tumours of the Digestive System. 4th ed. IARC Press; Lyon: 2010. pp. 322–326. [Google Scholar]
- 19.Iwao M., Nakamuta M., Enjoji M. Primary hepatic carcinoid tumour: case report and review of 53 cases. Med Sci Monit. 2001;7:746–750. [PubMed] [Google Scholar]
- 20.Knox C.D., Anderson C.D., Lamps L.W., Adkins R.B., Pinson C.W. Long-term survival after resection for primary hepaticcarcinoidtumor. Ann Surg Oncol. 2003;10:1171–1175. doi: 10.1245/aso.2003.04.533. [DOI] [PubMed] [Google Scholar]