Alcoholic liver disease (ALD) affects millions of people worldwide and is a condition associated with high morbidity and mortality. Ethanol-induced liver injury encompasses acute liver injury (alcoholic hepatitis) and chronic liver injury (steatohepatitis, fibrosis, and cirrhosis). Currently, few therapeutic options for ALD are available. Corticosteroids remain the gold standard therapy for severe alcoholic hepatitis. New research has sought to understand the role of immune modulation, gut microbiota, caspase inhibitors, granulocyte colony–stimulating factor, interleukin (IL)-1 receptor antagonists, and farnesoid X receptor agonists in altering the pathophysiology and halting the progression of ALD.
The pathophysiology of ALD involves many processes including cellular damage caused by inflammation and oxidative stress and ultimately cellular death. Research groups have proposed that polyphenol epigallocatechin-3-gallate (EGCG) may be a promising therapy for ALD; however, the mechanism is unclear. EGCG is a catechin within the larger group of polyphenols and is a plant extract found in green tea. It has already been investigated because of its ability to mitigate effects of damage caused by lead and amyloid peptides in human neuronal cells, and having antiatherosclerotic, anti-inflammatory, and antioxidant properties.1,2
In the past 2 decades, immune modulation via toll-like receptors (TLRs) has become a topic of interest as a therapeutic target in ALD. In addition to microbial products, such as lipopolysaccharide, TLRs can also recognize endogenous molecules from damaged cells, tissues, extracellular matrix, and danger signals, termed damage-associated molecular patterns. In ALD, ethanol exposure decreases the strength of gastrointestinal junctions, leading to an increase in microbiota and endogenous molecules seen by the liver and thus able to be presented to TLRs. TLR2 is a receptor for gram-positive bacterial cell wall components, such as lipoproteins, and endogenous ligands, high mobility group B-1, serum amyloid A, antiphospholipid antibodies, hyaluronan, and saturated fatty acids. In hepatocytes, TLR2 deficiency has undefined effects with some studies showing TLR2 deficiency may confer resistance to the effects of ethanol exposure.3 TLR3, located in the intracellular endolysosome, can sense microbial-derived nucleic acids and double-stranded RNA. One study has led to the proposed mechanism that TLR3 activation is protective in ALD as TLR3 stimulation leads to Kupffer and stellate cell activation via enhanced production of IL-10, a liver-protective cytokine.4
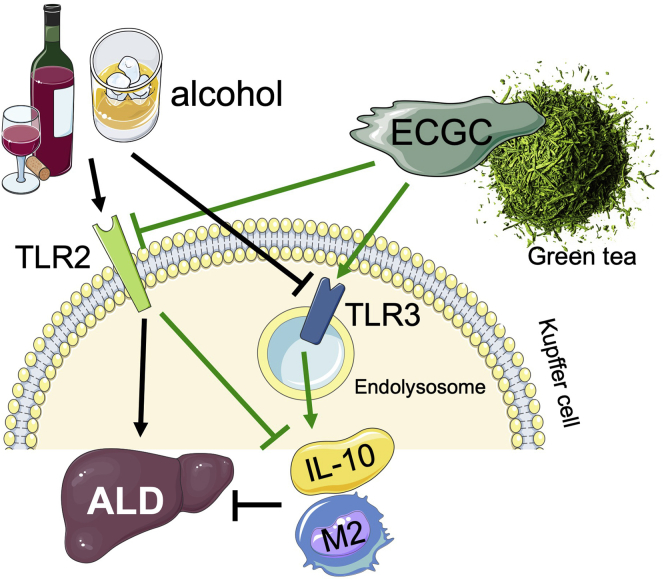
In the current issue of Cellular and Molecular Gastroenterology and Hepatology, Luo et al5 provide additional evidence that in Kupffer cells, EGCG divergently regulates IL-10 expression by interacting with TLR2 and TLR3 receptors (Figure 1).
Figure 1.
ECGC induces IL-10 production and M2 macrophage polarization to suppress ALD development. Alcohol-induced liver injury is mediated through TLR2 but inhibited by TLR3 signaling. ECGC can effectively suppress alcohol-induced liver injury by inducing IL-10 expression and M2 polarization. The ECGC-mediated inhibitory effect on alcohol-induced liver injury is mediated by activating TLR3 and inhibiting TLR2 in Kupffer cells.
This study first provided evidence that ALD injury is decreased with EGCG. In ALD, EGCG decreased all injury and inflammatory markers, such as serum aminotransferase, tumor necrosis factor-α, IL-1β, IL-6, monocyte chemoattractant protein-1, and hepatic nuclear factor (NF)-κB phosphorylation, but surprisingly showed increased anti-inflammatory IL-10 levels.
The study then demonstrated that EGCG could induce IL-10 directly from Kupffer cells. This is novel because it incorporated the use of an in vivo model. EGCG was shown to increase IL-10 transcription and translation. Lipopolysaccharide increased p38 MAPK, ERK, NF-κB p65 phosphorylation, whereas ECGC inhibited p38 MAPK and NF-κB phosphorylation. In addition, EGCG decreased M1 markers, such as inducible nitric oxide synthase, but increased M2 markers, including Arginase-1. This demonstrated that EGCG contributes to Kupffer cell M2 polarization to protect against ALD.
The effect of EGCG on ALD was further investigated in TLR2-/- and TLR3-/- mice. Supplementation of EGCG in TLR2-/- mice yielded decreased liver injury, whereas in TLR3-/- mice, EGCG supplementation revealed increased liver injury. EGCG was posited to work in a protective manner in the absence of TLR2 stimulation and act to have an opposite effect in the absence of TLR3. These experiments were repeated in IL10-/- mice. EGCG supplementation had decreased protection if IL-10 was knocked out, suggesting that IL-10 is at least one mediator in the protective effects induced by EGCG. In mice with present TLR2/3, IL-10 production yielded liver protection. It remains unknown how mechanistically EGCG interacts with TLR2 and TLR3. Although these experiments were conducted in TLR2 and TLR3 knockout Kupffer cells, the mechanism of Kupffer cell dysfunction and its role in ALD additionally warrants further investigation. Other key mediators among TLR2/3, NF-κB, and IL-10 remain to be investigated. This experiment is novel in that it demonstrated in vivo benefits of EGCG, which could be superior to other treatments including resveratrol, silibinin, N-Acetyl Cysteine, and prednisolone. In this manner, the study was critical in demonstrating strong evidence for a proposed mechanism for EGCG action. This study markedly furthers the understanding of EGCG within the context of ALD pathophysiology and provides a new area of investigation in therapies that may benefit patients suffering from ALD.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This work is supported by National Institutes of Health grants R01AA027036, R01DK085252, and R21AA025841.
References
- 1.Ayyalasomayajula N., Ajumeera R., Chellu C.S., Challa S. Mitigative effects of epigallocatechin gallate in terms of diminishing apoptosis and oxidative stress generated by the combination of lead and amyloid peptides in human neuronal cells. J Biochem Mol Toxicol. 2019 doi: 10.1002/jbt.22393. [DOI] [PubMed] [Google Scholar]
- 2.Eng Q.Y., Thanikachalam P.V., Ramamurthy S. Molecular understanding of Epigallocatechin gallate (EGCG) in cardiovascular and metabolic diseases. J Ethnopharmacol. 2018;210:296–310. doi: 10.1016/j.jep.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 3.Roh Y.S., Zhang B., Loomba R., Seki E. TLR2 and TLR9 contribute to alcohol-mediated liver injury through induction of CXCL1 and neutrophil infiltration. Am J Physiol Gastrointest Liver Physiol. 2015;309:G30–G41. doi: 10.1152/ajpgi.00031.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byun J.S., Suh Y.G., Yi H.S., Lee Y.S., Jeong W.I. Activation of toll-like receptor 3 attenuates alcoholic liver injury by stimulating Kupffer cells and stellate cells to produce interleukin-10 in mice. J Hepatol. 2013;58:342–349. doi: 10.1016/j.jhep.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Luo P., Wang F., Wong N.-K., Lv Y., Li X., Li M., Tipoe G.L., So K.-F., Xu A., Chen S., Xiao J., Wang H. Divergent roles of Kupffer cell TLR2/3 signaling in alcoholic liver disease and the protective role of EGCG. Cell Mol Gastroenterol Hepatol. 2020;9:145–160. doi: 10.1016/j.jcmgh.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]