Abstract
Anterior cruciate ligament (ACL) tears are unfortunate but common injuries in the athletic population. The standard of care for ACL tears is a surgical intervention to reconstruct the ACL to restore knee functionality as well as quality of life. In recent years, bone marrow aspirate concentrate (BMAC) has seen increasing use in various orthopaedic settings. This increase can be attributed to the potential beneficial qualities that mesenchymal stem cells, progenitor cells, and growth factors, all of which are present in BMAC, can provide. In this technical note and accompanying video, we describe an anatomic allograft ACL reconstruction infused with BMAC to utilize BMAC's potential benefits.
Anterior cruciate ligament (ACL) tears are one of the most common acute injuries in the active population and are often caused by cutting sports such as American football and basketball.1, 2 With an estimated incidence rate of 60.9 per 100,000 persons,3 untreated ACL tears may lead to decreased postural control, increased rates of meniscal injury, and earlier-onset osteoarthritis.4, 5, 6 Although uncommon, if graft failure after an ACL reconstruction (ACLR) does occur, the results can significantly hamper recovery because revision ACLR surgery has an inferior outcome.1, 2, 7, 8 Thus, the success of the primary ACLR is critical if knee functionality is to be restored and maintained. In addition, allografts are thought to have inferior rates of biological integration compared with autografts.9 Therefore, biological augmentation or the infusion of stem cells has been seen as a potential technique to improve ACLR outcomes.
In the past few years, bone marrow aspirate concentrate (BMAC), which is bone marrow that has been surgically harvested and then centrifuged, has been seen as a potential tool in various orthopaedic applications including combating osteoarthritis and chondral injuries owing to its high concentration of mesenchymal stem cells (MSCs), progenitor cells, and growth factors.10, 11, 12 The purpose of this technical note is to describe an allograft ACLR followed by a BMAC infusion to promote graft incorporation and avoid future revision procedures.
Surgical Technique
Patient Positioning
The patient is placed supine on a standard operating table and anesthetized using general anesthesia. A tourniquet is applied to the proximal thigh to improve visualization and minimize blood loss. The operative leg is treated with preoperative skin preparation solution from the mid thigh to the foot and then draped in the usual sterile fashion.
Graft Preparation
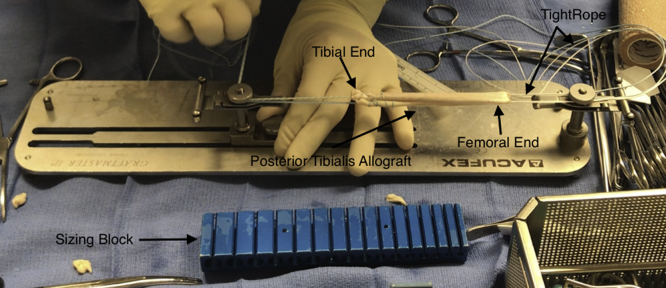
An appropriately sized posterior tibialis allograft is whipstitched with No. 2 FiberWire (Arthrex, Naples, FL) on the back table in the operating room (Fig 1). The allograft is then loaded onto an ACL TightRope RT (Arthrex) and sized with a graft sizing block.
Fig 1.
The posterior tibialis allograft is prepared on the back table for use in anterior cruciate ligament repair in the right knee. The tibial ends are whipstitched, and the graft is then folded in half. The femoral end is loaded with a TightRope, which contains the button that will sit flush with the right lateral femoral cortex.
Diagnostic Arthroscopy
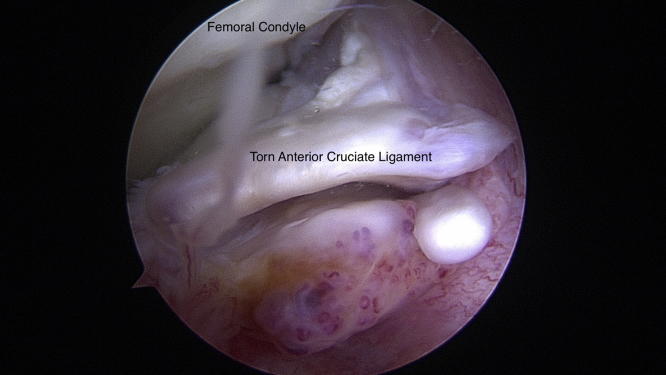
An anterolateral portal is created using a No. 11 blade by making a vertical incision adjacent to the lateral border of the patellar tendon at the level of the joint line. A blunt trocar and arthroscopic sheath are used to dilate the portal. The trocar is removed, and a 30° 4.0-mm arthroscope is inserted in the anterolateral portal to perform a complete diagnostic knee arthroscopy (Fig 2). An anteromedial portal is then created just medial to the patellar tendon at the joint line.
Fig 2.
Intraoperative arthroscopic image of the torn anterior cruciate ligament in the right knee as seen through the anteromedial portal using a 30° 4.0-mm arthroscope (Arthrex). This remaining stump is debrided at the femoral and tibial footprints with a No. 4-0 nonaggressive shaver and electrocautery.
Bone Marrow Aspirate Harvest and Processing
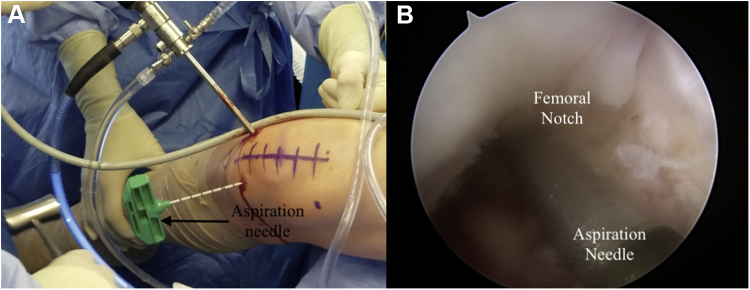
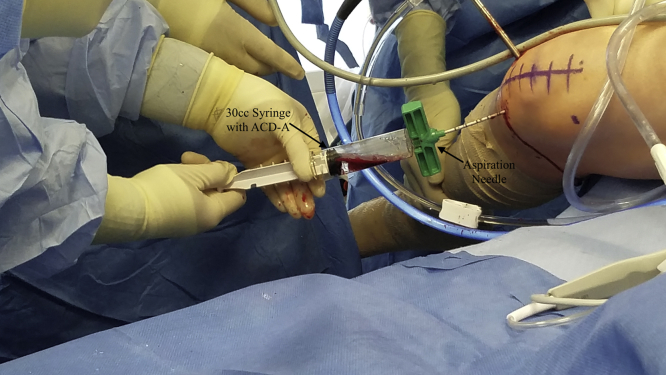
The soft tissue anterior to the intercondylar notch is debrided with a No. 4 shaver and a 3-mm 90° radiofrequency ablation wand to maximize visualization. The knee is hyperflexed, and a guide pin is fired through the anteromedial portal into the intercondylar femoral notch. The guide pin is removed, and an 11-gauge aspiration needle is placed inside the guide hole (Fig 3). The needle is struck with a mallet, and the arthroscopic inflow is stopped before the inner trocar is removed. The needle is then connected to a 30-mL syringe filled with 5 mL of acid-citrate-dextrose formula A, and the plunger is pulled back 10 mL to create negative pressure (Fig 4). This negative pressure is released once blood enters the syringe and is then re-created by locking the plunger at the 30-mL mark to harvest the bone marrow aspirate. Once the harvest is complete, the inner trocar is reinserted, and an outward pronation-supination motion is used to remove the aspiration needle. The harvested marrow is processed by connecting the syringe to the Angel System (Arthrex). This system processes the aspirate to create the final BMAC (Fig 5). Pearls and pitfalls of the technique are summarized in Table 1.
Fig 3.
Advancement of the aspiration needle through the intercondylar femoral notch in the right knee. (A) Intraoperative photograph of arthroscopy-guided aspiration needle advancement, viewed from outside the right knee. (B) Arthroscopic view of aspiration needle advancement viewed from the anterolateral portal in the right knee.
Fig 4.
The 30-mL syringe with 5 mL of acid-citrate-dextrose formula A (ACD-A) is connected to the adaptor of the aspiration needle, which has been placed through the anteromedial portal, and locked at 30 mL of negative pressure to aspirate bone marrow from the intercondylar femoral notch in the right knee.
Fig 5.
Bone marrow aspirate concentrate (BMAC)—the final product after the bone marrow aspirate has been concentrated and processed by the Angel System.
Table 1.
Pearls and Pitfalls of Anterior Cruciate Ligament Reconstruction With Bone Marrow Aspirate Concentrate Injection
| Pearls |
| Release negative pressure and return the plunger back to the 30-mL line once blood begins to flow into the syringe. |
| If the desired yield is not obtained, reinsert the trephine and insert the aspiration needle a few more millimeters further. |
| If needed, use fluoroscopic imaging to confirm the aspiration needle's position. |
| Pitfalls |
| Avoid contact with the articular cartilage of the medial femoral condyle to avoid iatrogenic chondral damage. |
| If inserting the aspiration needle further to increase the yield, avoid going beyond the posterior femoral cortex. |
ACL Technique
A standard allograft ACLR with femoral suspension and tibial screw fixation is performed.
Bone Marrow Aspirate Infusion
Two milliliters of the processed BMAC is suctioned into a syringe. An 18-gauge needle is placed through the anteromedial portal. This solution is infused into various sites along the newly reconstructed ACL (Fig 6). These key steps are demonstrated in Video 1.
Fig 6.
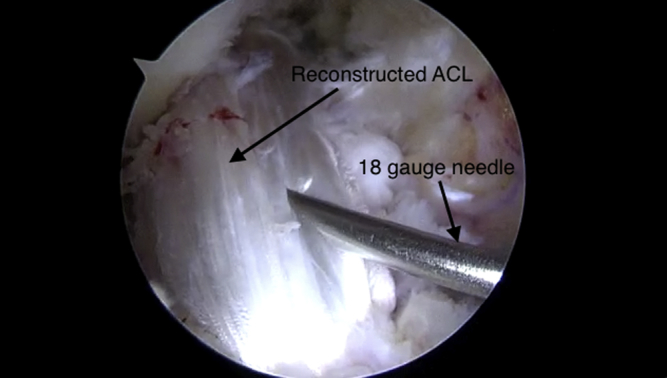
Bone marrow aspirate concentrate injection into the anterior cruciate ligament (ACL) allograft using an 18-gauge needle as viewed from the anterolateral portal in the right knee.
Final Examination and Postoperative Care
Knee stability is assessed through the Lachman and pivot-shift tests. Fluoroscopic imaging of the distal femur and proximal tibia is acquired to confirm that the ACL button sits flush on the lateral femoral cortex and an adequate tibial tunnel trajectory has been achieved, respectively. A No. 11 blade is then used to remove any excess ACL graft to ensure that the final graft lies flush with the tibial cortex tunnel. The incision site is irrigated and closed in the standard fashion. Immediately after the procedure, the knee is placed in a functional brace locked in extension. The brace is worn at all times, and patients are encouraged to achieve 90° of flexion by their first postoperative visit. Patients are allowed to jog 4 months postoperatively and conduct sport-specific drills such as dribbling and shooting baskets 6 months postoperatively. A full return to activity is allowed at the 8- to 10-month mark.
Discussion
The gold-standard treatment for ACL tears in younger patients is an ACLR with autograft because of decreased retear rates.2, 13 However, Kaeding et al.2 noted that the retear rate decreases by 9% each year the patient ages. By the time patients reach their mid thirties, there is no clinical significance between allograft and autograft. With over 100,000 ACLRs performed in the United States each year, proper graft integration is crucial for ensuring optimal ACLR outcomes and minimizing revision rates.9, 14 In older patient populations in whom the clinical significance between allograft and autograft is minimal, the use of an allograft may be preferred because of decreased donor-site morbidity and postoperative pain (Table 2).9 However, one disadvantage, as noted by Osti et al.,13 is that allografts heal and incorporate more slowly relative to autografts.
Table 2.
Advantages and Disadvantages of Allograft Anterior Cruciate Ligament Reconstruction
| Advantages |
| Nonexistent donor-site morbidity |
| Decreases postoperative pain |
| Reduces operative time |
| Disadvantages |
| Possibility of allogeneic disease transfer |
| Higher rate of failure in patients aged < 40 yr |
| Slower biological integration relative to autografts |
The proposed technique hopes to minimize the possible disadvantages of an allograft ACLR while maintaining the advantages an allograft provides by infusing BMAC into the graft. However, this technique may be adapted to autograft ACLRs to harness the potential benefits of BMAC. Today, BMAC is one of the few U.S. Food and Drug Administration–approved methods orthopaedic surgeons have at their disposal for stem cell delivery.11 Although the use of BMAC is still in its preliminary stages, a systematic review of the existing literature by Chahla et al.11 indicates overall positive outcomes when used in various orthopaedic settings ranging from focal chondral lesions to early-stage arthritis. Many of the benefits provided by BMAC are currently attributed to the MSCs, progenitor cells, and growth factors present in BMAC.10, 11 In particular, MSCs have been found to create a microenvironment that facilitates regeneration, stimulates angiogenesis, and reduces scar tissue formation.15, 16 The proposed explanation for this mechanism lies in MSCs' ability to differentiate into various cell lineages including bone, cartilage, and ligament tissues and recruit cells to facilitate the healing process.9, 16, 17
To our knowledge, there have been no studies conducted directly analyzing the impact of BMAC on ACLRs. However, evidence of BMAC's benefits in knee pathology does exist. Kim et al.10 found that BMAC injections significantly improved pain and function in patients with osteoarthritis. Oladeji et al.18 noted that the use of BMAC with osteochondral allograft transfers for articular cartilage lesions improved graft integration. Kenaya et al.19 found that in a rat model with partial ACL tears, BMAC improved ACL healing. In a clinical study assessing partial tears, Centeno et al.20 found that BMAC improved pain and function. In augmenting our reconstruction with BMAC, we hope to utilize the benefits provided by BMAC to potentially improve graft incorporation and thus ACLR success.
One potential disadvantage of our technique is that BMAC from the intercondylar notch may have reduced mesenchymal cell concentrations compared with BMAC from the iliac crest, as found by de Girolamo et al.21 (Table 3). At the same time, our current technique cannot guarantee that BMAC stays infused inside of the reconstructed ACL for long periods. Although data on BMAC use and outcomes exist for various orthopaedic procedures, further research is necessary to analyze the long-term impact of BMAC augmentation of ACLRs. This report outlines our technique of infusing an ACL allograft with BMAC to promote graft incorporation. Further studies with long-term follow-up are necessary to determine what impact BMAC use has on ACLR revision rates.
Table 3.
Advantages and Disadvantages of Harvesting Bone Marrow Aspirate From Intercondylar Notch
| Advantages |
| Donor-site morbidity and the number of surgical sites are minimized as opposed to harvesting from the iliac crest. |
| The time spent under general anesthesia is reduced. |
| The procedure is a single-step procedure. |
| Disadvantages |
| Bone marrow aspirate from the knee may have decreased concentrations of mesenchymal stem cells and growth factors. |
| Donor-site morbidity still exists. |
Footnotes
The authors report the following potential conflicts of interest or sources of funding: J.L.C. is an educational consultant for Arthrex and receives compensation for medical educational lectures and instruction only. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Anterior cruciate ligament reconstruction in right knee with stem cell infusion from bone marrow aspirate concentrate. Viewing from the anterolateral portal, soft tissue is removed from the intercondylar femoral notch of the right knee using a 3-mm 90° radiofrequency ablation wand to maximize visualization. A pilot hole is then created through the anteromedial portal using a guide pin, followed by the insertion of an 11-gauge aspiration needle. The inner trocar (stylus) is removed, and a 30-mL syringe preloaded with 5 mL of acid-citrate-dextrose formula A, an anticoagulant, is connected to the aspiration needle. Negative pressure is created by drawing the syringe back until bone marrow begins to flow, at which point it is released. The syringe is then locked at the 30-mL mark and filled with bone marrow aspirate, which is injected into the Angel System for further processing. The final concentrated aspirate is placed into a sterile cup, drawn into a syringe, and infused into the reconstructed anterior cruciate ligament with an 18-gauge needle.
References
- 1.Borchers J.R., Pedroza A., Kaeding C. Activity level and graft type as risk factors for anterior cruciate ligament graft failure. Am J Sports Med. 2009;37:2362–2367. doi: 10.1177/0363546509340633. [DOI] [PubMed] [Google Scholar]
- 2.Kaeding C.C., Pedroza A.D., Reinke E.K. Risk factors and predictors of subsequent ACL injury in either knee after ACL reconstruction. Am J Sports Med. 2015;43:1583–1590. doi: 10.1177/0363546515578836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkkari J., Pasanen K., Mattila V.M., Kannus P., Rimpela A. The risk for a cruciate ligament injury of the knee in adolescents and young adults: A population-based cohort study of 46 500 people with a 9 year follow-up. Br J Sports Med. 2008;42:422–426. doi: 10.1136/bjsm.2008.046185. [DOI] [PubMed] [Google Scholar]
- 4.Ardern C.L., Sonesson S., Forssblad M., Kvist J. Comparison of patient-reported outcomes among those who chose ACL reconstruction or non-surgical treatment. Scand J Med Sci Sports. 2016;27:535–544. doi: 10.1111/sms.12707. [DOI] [PubMed] [Google Scholar]
- 5.Negahban H., Mazaheri M., Kingma I., van Dieën J.H. A systematic review of postural control during single-leg stance in patients with untreated anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2014;22:1491–1504. doi: 10.1007/s00167-013-2501-4. [DOI] [PubMed] [Google Scholar]
- 6.Chalmers P.N., Mall N.A., Moric M. Does ACL reconstruction alter natural history?: A systematic literature review of long-term outcomes. J Bone Joint Surg Am. 2014;96:292–300. doi: 10.2106/JBJS.L.01713. [DOI] [PubMed] [Google Scholar]
- 7.Chen J.L., Allen C.R., Stephens T.E. Differences in mechanisms of failure, intraoperative findings, and surgical characteristics between single- and multiple-revision ACL reconstructions. Am J Sports Med. 2013;41:1571–1578. doi: 10.1177/0363546513487980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Condello V., Zdanowicz U., Di Matteo B. Allograft tendons are a safe and effective option for revision ACL reconstruction: A clinical review. Knee Surg Sports Traumatol Arthrosc. 2019;27:1771–1781. doi: 10.1007/s00167-018-5147-4. [DOI] [PubMed] [Google Scholar]
- 9.Mehran N., Moutzouros V.B., Bedi A. A review of current graft options for anterior cruciate ligament reconstruction. JBJS Rev. 2015;3:1. doi: 10.2106/JBJS.RVW.O.00009. [DOI] [PubMed] [Google Scholar]
- 10.Kim J.-D., Lee G.W., Jung G.H. Clinical outcome of autologous bone marrow aspirates concentrate (BMAC) injection in degenerative arthritis of the knee. Eur J Orthop Surg Traumatol. 2014;24:1505–1511. doi: 10.1007/s00590-013-1393-9. [DOI] [PubMed] [Google Scholar]
- 11.Chahla J., Dean C.S., Moatshe G., Pascual-Garrido C., Serra Cruz R., LaPrade R.F. Concentrated bone marrow aspirate for the treatment of chondral injuries and osteoarthritis of the knee. Orthop J Sports Med. 2016;4 doi: 10.1177/2325967115625481. 232596711562548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Youn G.M., Woodall B.M., Elena N. Arthroscopic bone marrow aspirate concentrate harvesting from the intercondylar notch of the knee. Arthrosc Tech. 2018;7:e1173–e1176. doi: 10.1016/j.eats.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osti L., Buda M., Osti R., Massari L., Maffulli N. Preoperative planning for ACL revision surgery. Sports Med Arthrosc Rev. 2017;25:19–29. doi: 10.1097/JSA.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 14.Mall N.A., Chalmers P.N., Moric M. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42:2363–2370. doi: 10.1177/0363546514542796. [DOI] [PubMed] [Google Scholar]
- 15.Murrell W.D., Anz A.W., Badsha H., Bennett W.F., Boykin R.E., Caplan A.I. Regenerative treatments to enhance orthopedic surgical outcome. PM R. 2015;7:S41–S52. doi: 10.1016/j.pmrj.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Kiapour A.M., Murray M.M. Basic science of anterior cruciate ligament injury and repair. Bone Joint Res. 2014;3:20–31. doi: 10.1302/2046-3758.32.2000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moatshe G., Morris E.R., Cinque M.E. Biological treatment of the knee with platelet-rich plasma or bone marrow aspirate concentrates. Acta Orthop. 2017;88:670–674. doi: 10.1080/17453674.2017.1368899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oladeji L.O., Stannard J.P., Cook C.R. Effects of autogenous bone marrow aspirate concentrate on radiographic integration of femoral condylar osteochondral allografts. Am J Sports Med. 2017;45:2797–2803. doi: 10.1177/0363546517715725. [DOI] [PubMed] [Google Scholar]
- 19.Kenaya A., Deie M., Adachi N. Intra-articular injection of mesenchymal stromal cells in partially torn anterior cruciate ligaments in a rat model. Arthroscopy. 2007;35:962–979. doi: 10.1016/j.arthro.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Centeno C., Pitts J., Al-Sayegh H., Freeman M. Anterior cruciate ligament tears treated with percutaneous injection of autologous bone marrow nucleated cells: A case series. J Pain Res. 2015;8:437–447. doi: 10.2147/JPR.S86244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Girolamo L., Bertolini G., Cervellin M., Sozzi G., Volpi P. Treatment of chondral defects of the knee with one step matrix-assisted technique enhanced by autologous concentrated bone marrow: In vitro characterisation of mesenchymal stem cells from iliac crest and subchondral bone. Injury. 2010;41:1172–1177. doi: 10.1016/j.injury.2010.09.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Anterior cruciate ligament reconstruction in right knee with stem cell infusion from bone marrow aspirate concentrate. Viewing from the anterolateral portal, soft tissue is removed from the intercondylar femoral notch of the right knee using a 3-mm 90° radiofrequency ablation wand to maximize visualization. A pilot hole is then created through the anteromedial portal using a guide pin, followed by the insertion of an 11-gauge aspiration needle. The inner trocar (stylus) is removed, and a 30-mL syringe preloaded with 5 mL of acid-citrate-dextrose formula A, an anticoagulant, is connected to the aspiration needle. Negative pressure is created by drawing the syringe back until bone marrow begins to flow, at which point it is released. The syringe is then locked at the 30-mL mark and filled with bone marrow aspirate, which is injected into the Angel System for further processing. The final concentrated aspirate is placed into a sterile cup, drawn into a syringe, and infused into the reconstructed anterior cruciate ligament with an 18-gauge needle.