Abstract
Skin is arguably the largest organ of the body and is continuously subjected to intrinsic, extrinsic, and environmental stresses. Therefore, skin developed elaborate mechanisms to maintain homeostasis, including antioxidant, antiinflammatory, and DNA damage repair capabilities. However, repeated and excessive stresses can overwhelm these systems, causing serious cutaneous damages, including skin carcinogenesis. Phytonutrients present in the diet possess a myriad of health-promoting effects by protecting skin from damaging free radicals as well as by other mechanisms. Although many chemoprotective phytonutrients have been shown to be efficacious individually, a combination of multiple agents could have synergistic response in curtailing or preventing cutaneous damages. Here, we discuss the benefits of natural amalgamation of phytonutrients in select fruits against skin damage including carcinogenesis. However, a majority of these studies have been done in preclinical models. Therefore, clinical studies are needed to determine the human relevance of the available preclinical data, especially in the human population who are at higher risk for skin cancers (e.g., organ transplant patients). In addition, detailed well-structured preclinical animal studies in the models of high-risk skin carcinogenesis could also be useful toward informing the design for human trials.
Introduction
Skin is the body's first line of defense from a variety of external stresses, such as environmental stresses, genetic alterations, and infections, which can impact the integrity of the skin. Therefore, skin has evolved mechanisms ensuring adequate repair and replenishment to counteract these damages. However, cumulative stresses can impair skin's defense systems to result in a variety of cutaneous disorders and diseases. In 2013, over 27% of the population in the United States (nearly 85 million individuals) were seen by a physician for skin diseases [1]. Unfortunately, thousands of deaths were linked to these diagnoses with 60% being from skin cancers including melanoma, nonmelanoma skin cancer (NMSC), and cutaneous lymphomas [1]. Conveniently, there is potential to impact the health of the skin through positive lifestyle enhancement including protection from the sun, not smoking or consuming alcohol, managing stress, and improving dietary habits. In the United States, the USDA dietary guidelines provide key recommendations for a healthy eating pattern in a 2000-calorie-per-day diet by consuming a variety of vegetables, fruits, grains, dairy, proteins, and oils [2]. In this review, we provide insight into the health benefits imparted by whole fruits or extracts of critical components (i.e., stems and seeds) containing phytonutrients, which promote skin health and the reduction of disease. We have also discussed the mechanisms of the protective response of whole fruits such as grapes, berries, pomegranate, apples, and tomatoes in chemical-induced and ultraviolet (UV) radiation–mediated cutaneous damage, including carcinogenesis. These phytonutrient-rich foods afford protection by reducing DNA damage through enhancing repair or acting as a sunscreen, reducing oxidative stress and inflammation, which play into each other to promote tumorigenesis [3,4], and enhancing skin barrier functions. The available data seem to support the concept that whole fruits can promote skin health and impart protection against cutaneous damages, including carcinogenesis.
Skin Architecture
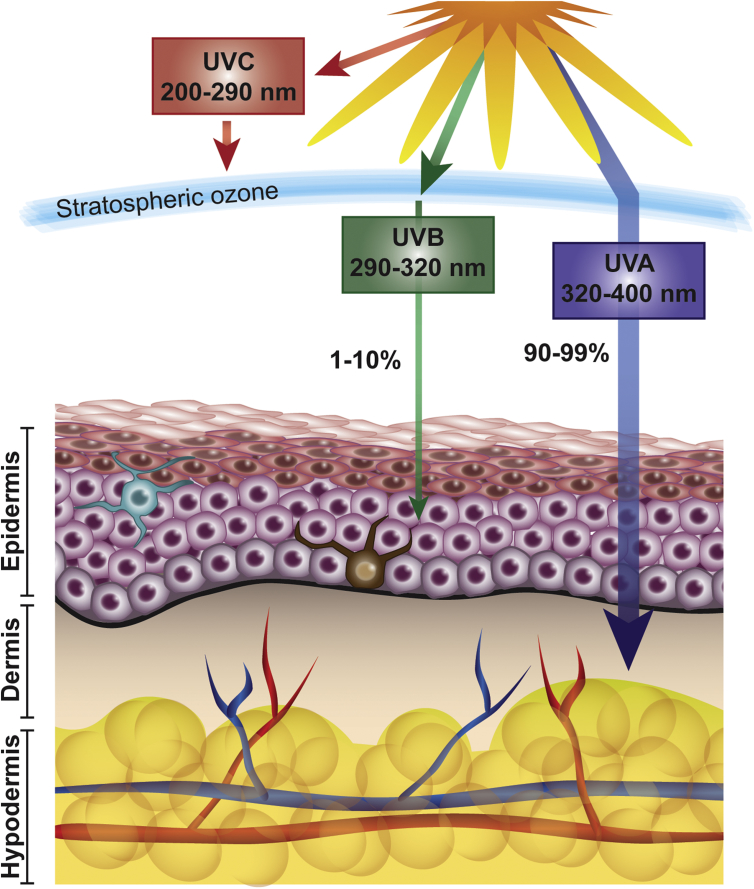
As the largest organ of the body, the skin itself is elaborately organized into three separate layers: hypodermis, dermis, and epidermis [5] (Figure 1). These layers are responsible for protection, absorption, thermal, hormonal, and immune regulations; synthesis of biologically relevant macromolecules; and aesthetics. The hypodermis is mainly composed of adipocytes and connective tissue. The fibroblastic dermis contains hair follicles, glands, blood vessels, and nerve endings and is responsible for flexibility due to the presence of collagen-rich connective tissue and is separated from the outermost epidermis layer by the basal lamina [5,6]. The epidermis is the mechanical, water-proof boundary between the external environment and the milieu intérieur [5]. Approximately 80% of the cells composing the epidermis are keratinocytes that continuously proliferate and differentiate to renew the skin. These cells are organized into functional layers characterized by shape, size, nucleation, keratin expression, and degree of differentiation [7]. The other portion of epidermal cells includes melanocytes responsible for pigment production, sensory Merkel cells, and immune Langerhans cells. All cell types within the dermis and epidermis are responsible for a variety of sophisticated cellular functions and complex crosstalk to maintain a balanced state of homeostasis, thereby providing defense and corrective actions against endogenous and environmental stresses. When cutaneous homeostasis is disrupted because of extensive damage, the deregulation of normal cellular processes can lead to skin disorders including inflammatory diseases and neoplasms [1,8,9].
Figure 1.
Solar ultraviolet radiation and skin: Major wavelengths and their effects on the skin.
Environmental Stresses Associated with Cutaneous Damages
The environmental agents and factors that can damage skin include radiations, heat and cold, chemical irritants including pollutants in the air and water, household cleaners, car emissions, and other compounds. However, solar UV radiation is a major environmental cutaneous offender and is shown to be a complete carcinogen. As outlined in Figure 1, the UV rays that reach our skin is UVA (90–99%), and a small portion is UVB (1–10%), whereas the majority of UVC is absorbed by the stratospheric ozone, therefore not causing measurable skin damages. UVB that penetrates the epidermal layer can be rapidly absorbed by cellular DNA leading to the creation of mutagenic cyclobutane pyrimidine dimers (CPDs) and 6–4 photoproducts [10]. UVA reaches deep in the dermis where absorbed photons promote oxidation reactions, generating reactive oxygen species (ROS) [11] and promoting the production of a key marker of oxidative DNA damage, 8-oxo7,8-dihydroguanyl (8-oxodG), in guanine bases of DNA [12]. Many studies have linked these detrimental UV-induced DNA damages to mutations in key regulatory oncogenes and tumor suppressors seen in skin cancers, such as p53 [13]. The solar UV radiations have many effects on the physiology of the skin, potentially amounting to 104–105 lesions per cell per day [14]. On injury, the upregulation of p53, also known as the “guardian of the genome” [15], causes keratinocytes to arrest the cell cycle and undergo damage response pathways, including nucleotide excision repair (NER), base excision repair (BER), double-strand break repair, and cross-link repair [16]. NER is responsible for repairing lesions from exogenous sources, such as CPDs and 6–4 photoproducts, and BER removes endogenous oxidative-induced base lesions, such as 8-oxodG [16]. However, failure or lack of the early repair mechanisms can push the initiated keratinocytes to a hyperproliferative stage, ultimately leading to neoplastic transformation and cancer progression [17].
Other environmental factors such as diesel fuel exhaust, chemicals, cigarette smoke, and ozone (O3) can also lead to oxidative stress by the creation of ROS. The skin combats ROS by employing antioxidant enzymatic (glutathione peroxidase, superoxide dismutase, catalase) and nonenzymatic (glutathione, α-tocopherol, vitamin E, vitamin C) molecules to maintain redox homeostasis [9]. However, when the accumulation of intrinsic and environmental factors overwhelm the skin's defenses, the detrimental effects of oxidative stress can lead to skin dysfunction. ROS include the superoxide anion (O2−), singlet oxygen (1O2), hydrogen peroxide, and the hydroxyl radical (OH•). The ROS can affect protein structure and function, leading to changes in inflammation, cellular proliferation, and even cell death [9].
Oxidative and Inflammatory Response on Cutaneous Injury
When a cutaneous injury occurs, keratinocytes and fibroblasts secrete proinflammatory cytokines to recruit leukocytes to the site of injury, causing inflammation. Inflammation is a self-limiting process; however, deregulation leading to the persistence of cytokines and chemokines results in chronic inflammation and/or immunosuppression leading to various pathologies and has been suggested as a hallmark of cancer [3,18]. In addition, transient levels of ROS can activate signaling pathways leading to an inflammatory response, which has been linked to the proinflammatory signal transduction pathways, such as MAPK, PI3K/AKT, NF-κB, and AP-1 signaling (reviewed in Ref. [3]).
The MAPK family includes ERK1/2, JNK1/2, and P38 kinases, which are downstream of RAS, activated in the presence of oxidative stress and also well documented for their role in cellular proliferation and cancer [19]. MAPKs also activate many downstream targets including COX-2 and AP-1 [3,20]. AP-1 is a transcription factor responsible for binding Jun and Fos dimers to control keratinocyte proliferation, differentiation, and apoptosis, yet alteration in AP-1 response can lead to tumor development [21]. The increased expression of the RAS protein also plays into PI3K activation and further AKT modulation. NF-κB is responsible for the regulation of many genes involved in the initiation of inflammation, including chemokines and cytokines (IL-1, IL-6, TNF), proinflammatory enzymes (COX-2, iNOS), and genes linked to cellular survival (BCL-2, survivin) and proliferation (cyclins, c-Myc) [22]. Increased inflammation and ROS can feed into each other to enhance downstream biochemical effects leading to accumulation of oxidative damage of proteins, carbohydrates, and lipids within the epidermis and dermis after the skin's antioxidant response has been overpowered [3,4].
Skin Carcinogenesis
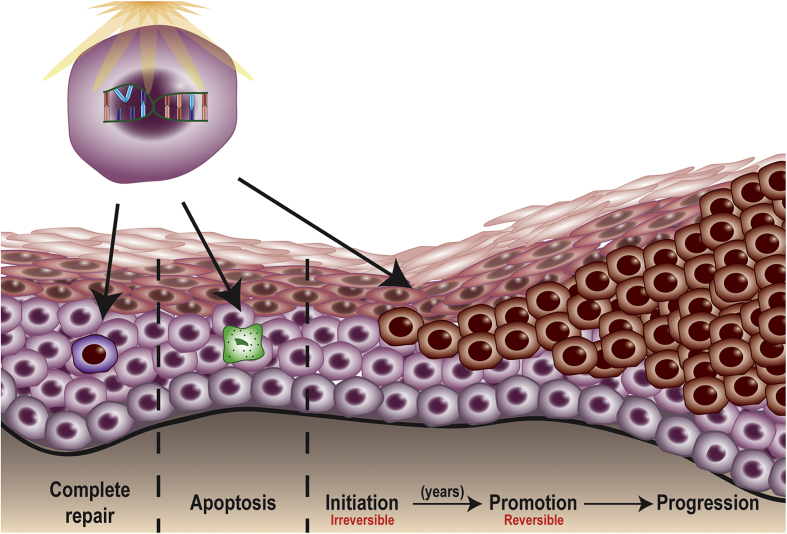
Skin carcinogenesis is a stepwise process with three distinct stages: initiation, promotion, and progression (Figure 2). Initiation can occur through exogenous (chemical, UV radiation) or endogenous (inflammation) factors when the cellular repair capacity is unable to repair DNA damage, being irreversible [23]. CPDs are accountable for the majority of UVB-induced mutations, indicated by the presence of C to T or CC to TT transitions on regulatory genes, such as p53 [24]. UVA-induced mutations occur through AT to CG transversions caused by 8-oxodG [12,24]. Because of the mutation in p53, continued exposure to UVB can lead to clonal expansion (promotion) of initiated mutated cells [25], which appear to have a growth advantage over neighboring normal cells that will go through apoptosis [[25], [26], [27], [28]]. In humans, primary precancerous skin lesions can include actinic keratosis (AK), which harbors a high mutational load when compared with normal skin [29].
Figure 2.
Ultraviolet radiation and skin cancer: Steps involved in skin carcinogenesis.
The most common appearances of NMSC are basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), both of which arise from keratinocytes and are known as keratinocyte carcinomas, and are most prominent in Caucasians [8,12]. Unfortunately, there are no registries to track NMSC incidence; therefore, the available data on predicted incidence are based on epidemiological studies. Rogers et al. estimated 2,191,100 procedures in 1,336,800 individuals in 2012, but this has likely increased over the past years, as predicted by upward trends of the preceding years [30]. Because NMSC development has a long latency, many individuals do not develop lesions until later in life (70–80 years) despite initiating UV signature mutations generally occurring in childhood [30]. NMSC also affects more men than women [31] and is the fifth most costly malignancy to treat in the United States, totaling billions of dollars spent in treatment and care costs on an annual basis [32]. Although not as common, NMSCs can also occur at a younger age [33]. Interestingly, many deaths of individuals diagnosed with NMSC are associated with the development of another cancer type, such as lung, prostate, breast, colon, and melanoma [34]. Therefore, it is critical to determine prevention early on to protect all ages from disease development.
The majority of skin cancer cases diagnosed are BCCs, affecting more than 3 million individuals in the United States on an annual basis [30]. Although not life threatening, BCCs are a slow-growing neoplasm that rarely metastasizes and have nearly 100% survival after diagnosis [35], although, if left untreated, BCC can invade locally [36]. SCC is the second most commonly diagnosed NMSC in the United States with 200,000–400,000 new cases each year, accompanied by 3000 disease-related deaths because of metastatic disease [34,37]. Currently, the risk of SCC metastasis is reported at 4% [38], yet this rate is 2–3 times higher in immunosuppressed individuals, such as organ transplant recipients [39]. Many studies have sought to determine the shift from normal skin to AK, a precancerous lesion, to SCC. Although AK and SCC appear to have a similar profile of dysregulated pathways, it is argued that AK could be responsible for counteracting additional deleterious mutations leading to SCC, therefore the latency of years before progression of the tumor [29].
Although the diagnosis of NMSC can indicate increased susceptibility to subsequent lesions, the goal of current treatments is to completely eradicate the lesion while preserving the structure and function of skin, as well as aesthetics. Current treatments for NMSC include surgical methods such as excision, Mohs micrographic surgery, curettage and electrodesiccation, laser ablation and cryosurgery, and nonsurgical radiation and photodynamic therapies. Generally, surgical procedures are highly effective, with good cosmetic outcomes for treating low-risk lesions [40]; however, unlike excision and Mohs microsurgery, there is a higher risk of reoccurrence after other procedures due to the lack of histological evaluation validating complete tumor excision [41].
Whole Foods in Chemoprevention
Chemoprevention is defined as the use of natural or synthetic agents to prevent, suppress, or reduce the progression of cancer and can be undertaken with single or multiple agents that interfere with numerous pathways in the carcinogenesis process [42]. Many individual phytonutrients have demonstrated beneficial health effects. In our laboratory, previous studies have shown the protective effects of the grape antioxidant, resveratrol, against UVB-mediated carcinogenesis [[43], [44], [45]]. However, as studies have continued to progress toward the utilization of natural products to benefit health, the natural matrix of polyphenols and antioxidants in whole foods has become more and more attractive. This is partially because of wider human acceptance and because individuals can modify their dietary habits, rather than continuously ingesting supplements or using topical ointments/creams.
Fruits and vegetables are well known for their high nutrient content. The prevailing dietary recommendation for fruits in the American 2000 total calorie diet is equivalent to two cups of fruits and vegetables [2]. Overall, it is suggested to ingest whole fruits or 100% fruit juice, therefore preserving the nutrient-dense nature without the possibility of added fats, sugars, and sodium. Whole fruits can be fresh, canned, frozen, or dried [2]. According to the USDA, the most consumed fruits in America are apples, bananas, watermelon, grapes, strawberries, oranges, peaches, cantaloupe, pears, blueberries, raisins, and pineapple [2]. Fruits are packed with phytonutrients that have antioxidant, antiinflammatory, antimutagenic, or anticarcinogenic effects. In the natural amalgamation within whole foods, phytonutrients can synergistically provide health benefits, and many studies have already implicated beneficial effects in several chronic pathological conditions, including cancers (reviewed in Refs. [[46], [47], [48]]). Below, we discuss studies implicating the beneficial effects of whole fruits on skin diseases. Our discussion includes the available in vitro and in vivo data on the potential effects of whole fruit and fruit extracts, against skin diseases and disorders. Because of the amount of available literature, we have limited our discussion on grapes, black raspberries, blackberries, pomegranate, apples, and tomatoes (Table 1).
Table 1.
In Vitro and In Vivo Studies to Assess the Effects of Fruits in Skin and Skin Conditions.
| Chemopreventive Constituents | Experimental Model | Dose of Chemopreventive Agents | Dose of Carcinogen | Reference |
|---|---|---|---|---|
| In Vitro Studies | ||||
| Grape (Burgund Mare) seed extract | HaCaT | 10 or 20 μg/ml | UVB (100 or 200 mJ/cm2) | [59] |
| Black raspberry extract | JB6 C1 41 with AP-1 luciferase reporter | 50 μg/mL, 30 min prior | UVB (2 kJ/m2) | [67,68] |
| Black raspberry extract | JB6 C1 41 with AP-1, or NF-κB, or p53 luciferase reporter | 25 μg/mL, 30 min prior | BPDE (2 μM) | [68] |
| Black raspberry extract | JB6 C1 41 with AP-1, or VEGF luciferase reporter, or PI3K mutant | 25 μg/mL, 30 min prior | BPDE (2 μM) | [69] |
| Blackberry (tropical highland) juice | NHEK | 1:300 or 1:500, 2 h prior, or immediately for 24 h | UVB (25 mJ/cm2) | [72] |
| Blackberry (tropical highland) juice | Human reconstituted skin equivalent | Topical (10 μL), 2 h prior, or immediately for 24 h | UVB (25 mJ/cm2) | [72] |
| Pomegranate fruit extract | NHEK | 10–40 μg/mL, 24 h prior | UVB (40 mJ/cm2) | [78] |
| Pomegranate extract (POMx) | HaCaT | 10–40 μg/mL, 24 h prior | UVB (15 or 30 mJ/cm2) | [79] |
| Pomegranate juice, oil, or extract | EpidermTM FT-200 reconstituted human skin | 1–2 μL, or 5–10 μg/0.1 ml/well, 1 h prior | UVB (60 mJ/cm2) | [80] |
| Apple peel extract | JB6/AP/kB | 1:10 to 1:640, 2 h prior | TPA (20 nmol) or UVB (4 kJ/m2) | [83] |
| In Vivo Studies | ||||
| Grape (Muscat Bailey) stem extract | BALB/c mice | Topical (1 mg in 0.2 ml propylene glycol), daily | UVB (120 mJ/cm2), 3× weekly for 1 month | [58] |
| Grape (Campbell Early) stem extract | C57BL | Topical (50 mg/kg), daily, 1 week prior | UVB (120 mJ/cm2), 3× weekly for 3 weeks | [57] |
| Y-grape juice or ethyl acetate extract | SENCAR | Topical (10 or 20 μL) single dose | TPA (1.7 nmol), single dose | [60] |
| Y-grape juice or ethyl acetate extract | SENCAR | Topical (×2 or ×10), 2× weekly, 30 min prior, or oral, ad libitum (×2 or ×10) | DMBA initiation (2.5 mg/animal) and TPA promotion (1.7 nmol) for 20 weeks | [60] |
| Grape seed proanthocyandins | SKH-1 hairless mice | Oral, ad libitum (0.2% or 0.5% in AIN-76A diet) | UVB (180 mJ/cm2) 3× weekly for 24 weeks | [63] |
| Grape (Burgund Mare) seed extract | SKH-1 hairless mice | Topical, (4 mg/cm2), 30 min prior, single dose | UVB (240 mJ/cm2), single dose | [55] |
| Grape (Burgund Mare) seed extract | SKH-1 hairless mice | Topical (2.5 or 4 mg/cm2), 30 min prior or post | UVB, (240 mJ/cm2), daily for 10 days | [56] |
| Grape (red, green, black) powder | SKH-1 hairless mice | Oral, ad libitum (3% or 5% in AIN-76A diet) | UVB (180 mJ/cm2) 2× weekly for 28 weeks | [64] |
| Grape (red, green, black) powder | SENCAR | Topical (1, 2, or 4 mg), 30 min post | DMBA (100 nmol), 2×/week (4 or 24 weeks) | [61] |
| Powdered grape seed extract | SENCAR | Topical (1, 2, or 4 mg), 30 min post | DMBA (100 nmol), 2×/week for 4 weeks | [61] |
| Grape (red, green, black) powder | SENCAR | Oral (1%, 2%, or 5% in AIN-93GA diet), 2 weeks prior | DMBA (100 nmol), 2× weekly for 12 or 24 weeks | [61] |
| Black raspberry extract | SKH-1 hairless mice | Topical (500 μg), immediately post | UVB (2240 kJ/m2), 3× weekly for 25 weeks | [70] |
| Black raspberry extract | SKH-1 hairless mice | Topical (500 μg), immediately post | UVB (2240 kJ/m2), single dose | [70] |
| Blackberry extract | SKH-1 hairless mice | Topical (10% or 20%), one day prior | UVB (100 mJ/cm2), 3× weekly for 10 weeks | [71] |
| Pomegranate fruit extract | CD-1 | Topical (2 mg), 30 min prior | TPA (3.2 nmol), single dose | [73] |
| Pomegranate fruit extract | CD-1 | Topical (2 mg), prior to TPA | DMBA (50 nmol), TPA (3.2 nmol) 2× weekly for 30 weeks | [73,75] |
| Pomegranate seed oil | CD-1 | Topical (5%), 1 h prior | DMBA (200 nmol), TPA (5 nmol) 2× weekly for 20 weeks | [75] |
| Pomegranate fruit extract | SKH-1 hairless mice | Oral (0.2% in drinking water), 14 days prior | UVB (180 mJ/cm2), single dose | [76] |
| Pomegranate fruit extract | SKH-1 hairless mice | Oral (0.2% in water), 14 days prior up to termination | UVB (180 mJ/cm2), 7 doses on alternating days | [77] |
| Apple peel extract | AP-1 luciferase reporter transgenic mice (C57BL/6 × DBA2) | Oral, ad libitum, in lieu of drinking water and topical, 6× daily | TPA (5 μg) or UVB (10 kJ/m2), single dose on fourth day of APE | [83] |
| Apple peel extract | AP-1-luciferase reporter transgenic mice (C57BL/6 × DBA2) | Oral, ad libitum, in lieu of drinking water, 2 days prior | DMBA (400 nmol), TPA (17 nmol) 14 days later, 2× weekly for 20 weeks | [83] |
| Tangerine tomato powder | SKH-1 hairless mice | Oral, (10% in AIN-93GA diet), 10 weeks prior | UVB (2240 J/m2), single dose at week 10 | [87] |
| Tangerine or red tomato powder | SKH-1 hairless mice | Oral, ad libitum (10% in AIN-93GA diet), through 35 weeks | UVB (2240 J/m2), 3× weekly, weeks 11 through 20 | [88] |
Grape (Vitis vinifera)
Often characterized among one of the world's healthiest foods, grapes have been cultivated for nearly 8000 years, and grape-derived wine has been deemed a highly valued commodity in many ancient cultures [49]. Grapes have been extensively used in many cultures for their medicinal value. The traditional Ayurvedic medicine, originating from the Indian subcontinent, utilized fermented grapes, for example, in the form of “Drakshasava” tonic, against lethargy, weakness, and cardiac disease [50]. Chromatographic evaluation of the Drakshasava indicated the presence of many polyphenols, including resveratrol and pterostilbene [51]. Within the last century, the grape cure was propagated, with some criticism, by a South African dietician Johanna Brandt who promoted grapes as having anticancer effects [52], and the “French paradox” gained popularity. This paradox describes the low heart disease rate of the French population due to the consumption of wine, despite a high-fat diet [53]. Since then, over 1600 compounds have been identified in grapes, including anthocyanins, catechins, resveratrol, lycopene, quercetin, and melatonin, among others [48,54]. Many researchers have moved evaluating key features of the grape including the stem, skin, and seed, while others have explored the benefits of the whole fruit.
Filip et al. determined the protective effects of red grape seed in UVB-induced damages using a prepared extract from the seed of V. vinifera. The extract was assessed for polyphenolic content and topically applied at 4 mg/mouse/cm2 30 min before a single dose of UVB (240 mJ/cm2) on female SKH-1 hairless mouse skin. It was found that the extract attenuated UVB-induced oxidative stress by preventing damages by lipid peroxidation and nitric oxide, while reducing caspase-3 activity [55]. They further evaluated the protective capacity of the topical grape seed extract by extending the study to 10 consecutive days of UVB exposure at 240 mJ/cm2. The extract effectively reduced CPDs, hyperplasia, cytokine release, and oxidative stress while increasing antioxidant response [56]. Utilizing a different portion of the grape, the Jang group has sought to evaluate the efficacy of grape stem extracts in UVB (120 mJ/cm2, thrice weekly for three weeks)-induced cutaneous damages. Using the C57BL strain, male mice were given the extract one week before dosing and throughout the study. The treatment resulted in reduced lipid peroxidation, neutrophil and mast cell infiltrations, and COX-2 expression while retaining the integrity of the skin [57]. In a subsequent study, topical application of an extract from the Muscat Bailey A grape was tested for its potential effect against UVB (120 mJ/cm2, thrice weekly for one month)-mediated damages in male Balb/c mice. It was found that the extract reduced oxidative stress through recovered glutathione peroxidase and superoxide dismutase levels while preserving the integrity of the skin and preventing DNA damage. In addition, the extract reduced inflammatory response as indicated by reduced mast cell and neutrophil infiltration and proinflammatory cytokines [58]. In an in vitro study, spontaneously immortalized human keratinocyte HaCaT cells were irradiated with UVB (25–300 mJ/cm2). A protective effect was observed from Burgund Mare variety red grape extract at concentrations of 10 and 20 μg/mL, increasing viability of the cells while reducing lipid peroxidation, apoptosis, and DNA photoproducts [59].
In an in vivo study, Kobayashi et al. sought to evaluate the protective effects of topical and dietary Y-grape juice and ethyl acetate extract from Vitis coignetiae Pulliat (Y-grape) on 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced edema and 7, 12-dimethylbenzanthracene (DMBA)-TPA carcinogenesis. Six-week-old SENCAR mice were given a single dose of TPA on the inner and outer surfaces of the ear. Both topical and oral treatments appeared to reduce edema in a dose-dependent manner. In a subsequent experiment, mice were subjected to DMBA-TPA carcinogenesis with topical Y-grape juice or oral extract supplementation. All grape treatments imparted significant protection against tumorigenesis, as demonstrated by reduced tumor incidence, tumor number, and COX-2 activity [60]. Hanausek et al. performed a similar study utilizing the DMBA-TPA–treated SENCAR mice. Their experimental protocol involved topical pretreatment with resveratrol, dietary freeze-dried grape powder (GP), or a continuous diet of GP in AIN-93G starting 2 weeks before DMBA and continuing to 12 weeks. All treatments reduced tumor burden as well as COX-2 and DNA damage within the skin [61]. Unfortunately, chemically induced skin carcinogenesis is not very relevant to human situations, as the majority, ∼90% of human NMSCs, are linked to UV radiation as a causative factor [62]. In one photocarcinogenesis study, female SKH-1 mice were subjected to 180 mJ/cm2 UVB three times weekly for 24 weeks while consuming a diet enriched with grape seed proanthocyanidins. The authors found that the proanthocyanidins inhibited UVB-induced inflammatory mediators, including COX-2 and other cytokines in the skin and tumor [63]. In a study from our laboratory, female SKH-1 mice were subjected to 180 mJ/cm2 UVB twice weekly for 28 weeks while consuming diets enriched with freeze-dried GP at concentrations of 0, 3, or 5%. Dietary GP supplementation resulted in a delay in the onset of tumors coupled with a marked reduction of tumor multiplicity and volume. Further mechanistic evaluation of the tumors demonstrated that GP consumption led to enhanced apoptosis and oxidative stress response while reducing proliferative marker and lipid peroxidation [64]. In addition, GP treatment also resulted in an enhanced NER repair capacity in the skin through the reduction of CPDs [64]. Collectively, grapes are one of the most promising whole fruits for health improvement. Currently, there are approximately 200 clinical trials within the United States that focus on grape products for health (Table 2).
Table 2.
Clinical Trials to Assess the Effects of Whole Fruit on Varying Diseases and Health.
| Clinical Trials | |||||
|---|---|---|---|---|---|
| Grape | Pomegranate | Apple | Berry | Tomato | |
| Active, not recruiting | 16 | 4 | 8 | 8 | 6 |
| Completed | 123 | 62 | 132 | 48 | 49 |
| Enrolling by invitation | 1 | 1 | 3 | 0 | 0 |
| Not yet recruiting | 10 | 2 | 9 | 5 | 4 |
| Recruiting | 27 | 4 | 30 | 12 | 7 |
| Unknown status | 21 | 15 | 15 | 6 | 4 |
| Withdrawn | 1 | 2 | 5 | 2 | 3 |
| Terminated | 1 | 0 | 4 | 0 | 1 |
| Total | 200 | 90 | 206 | 81 | 74 |
Plants of the Genus Rubus
Fruits from plants of the genus Rubus, including black raspberry (Rubus occidentalis) and blackberry (Rubus fruticosus), have been associated with modulating many biological activities including antioxidant response and carcinogenesis [65]. The three main classes of polyphenols in these fruits include anthocyanin, ellagitannins, and phenolic monomers, inducing phenolic acids and flavonoids [66]. Huang et al. sought to explore the health-promoting effects of black raspberry, strawberry, and blueberry extracts on UV-irradiated mouse epidermal C1 41 cells. The methanol extract from black raspberries (BRE) (R. occidentalis) appeared to be the most potent inhibitor of UVB-induced NF-κB activation in the cells, as demonstrated by the suppression of IKK activation. The results from this study indicated that MAPKs, and consequently AP-1 activation, were not affected; therefore, the extract acted specifically on NF-κB [67]. A previous study using a similar extract demonstrated a differential inhibition of benzo[a]pyrene diol-epoxide (BPDE)–induced AP-1 and NF-κB in JB6 murine cells, suggesting an inhibition of carcinogen-caused tumor development [68]. A subsequent follow-up study suggested that BRE reduced the expression of AP-1 and VEGF in BPDE-treated epidermal C1 41 cells through inhibition of PI3K/AKT pathway leading to anticarcinogenic effects of black raspberries [69].
Duncan and colleagues examined the in vivo effects of BRE on two SKH-1 hairless mouse models. The first acute damage study subjected SKH-1 mice to one minimal erythemal dose of UVB (2240 J/m2), followed by topical application of 500 μg of BRE, and sacrificed 48 h later. BRE treatment was found to reduce neutrophil activation, edema, and oxidative DNA damage, suggesting the inhibition of many hallmarks of an acute inflammatory response that have been implied in skin cancer development [70]. In the second carcinogenesis model, SKH-1 mice were exposed to 2240 J/m2 dose of UVB thrice weekly for 25 weeks. Each treatment was followed by the topical application of BRE. Here, the chemopreventive effects of BRE were observed though reduced tumor counts, tumor volume, and tumor-infiltrating CD4+ T cells [70]. Collectively, this indicates the antitumor properties of BRE are through alterations of an inflammatory response.
Comparatively, another study used blackberry extract (BBE) to evaluate the beneficial effect on SKH-1 hairless mouse skin [71]. When topically applied the day before each UVB exposure (100 mJ/cm2) alternating every other day for ten weeks, it was found that BBE modulated the MAPK signaling pathway to protect against the UVB-induced damages. The reduction of phosphorylated ERK1/2, p38, and JNK1/2, was accompanied by inhibition of NF-κB nuclear localization and subsequent inflammatory mediators COX-2, iNOS, and prostaglandin E2 [71]. In addition, BBE suppressed CPD formation, as well as oxidative stress and subsequent 8-oxodG formation [71]. Calvo-Castro et al. utilized juice of the highland blackberry (Rubus adenotrichos, BBJ), rich in ellagitannins, to determine protective effects against UVB-exposed human epidermal keratinocytes and a human-reconstituted skin equivalent model. Although BBJ was unable to protect the cells against UVB-induced loss of viability, protection against UVB-mediated baseline guanine oxidation and direct CPD damage was observed in both cell cultures and a 3D skin model, as well as an increase in PARP cleavage [72]. Overall, several studies demonstrated that raspberries and blackberries possess potential against UV-mediated cutaneous damages, including photocarcinogenesis via multiple mechanisms.
Pomegranate (Punica granatum)
Pomegranate is 80% juice and 20% seed, rich in anthocyanins and hydrolyzable tannins [73,74]. Multiple studies have assessed the effects of pomegranate on cutaneous damages and skin carcinogenesis. In one study, female CD-1 mice were given a topical application of 2 mg pomegranate fruit extract (PFE) in acetone, followed by TPA (3.2 nmol/mouse) application 30 min later, followed by sacrifice at specific time points [75]. Topical PFE inhibited the inflammatory response to TPA as shown by the reductions of skin edema, hyperplasia, dermal neutrophils, and ornithine decarboxylase and COX-2. Subsequently, a reduction in the activation of the MAPK pathway was observed. The antitumor effects of PFE were observed against DMBA-initiated and TPA-promoted (2× weekly application) tumorigenesis in CD-1 mice. Not only had PFE extended tumor latency but also at the end of the study, 20% of PFE-mice remained tumor-free, and tumor counts were equivalent to a 64% inhibition [73]. This study supported the chemoprotective effects of pomegranate against tumorigenesis as shown through a prior study, where CD-1 mice underwent the DMBA-TPA protocol and received topical 5% pomegranate seed oil [75].
Furthermore, Gil and colleagues determined the protective effects of PFE in SKH-1 hairless mice exposed to UVB. Two studies provided PFE in the drinking water (0.2% wt/vol) for 14 days before a single dose of UVB [76], or 7 alternate day doses of UVB (180 mJ/cm2) [77]. Oral feeding of PFE reduced cutaneous edema, hyperplasia, infiltration of leukocytes, and COX-2 expression. In addition, apart from enhancing the repair of CPDs and 8-oxodG after a single dose of UVB, PFE treatment increased p53 and p21 expression [76]. As a hallmark of skin carcinogenesis, hyperproliferation of epidermal cells was evaluated by proliferative markers PCNA [76,77], and cyclin D1 [77], demonstrating a reduced proliferative index of PFE. Furthermore, PFE inhibited the UVB-mediated activation of NF-κB as shown by increased accumulation of IκBα to keep NF-κB/p65 tethered to the cytoplasm, and subsequent inhibition of NF-κB and IKKα activation [76,77]. The multiple UVB dose study evaluated upstream regulators of NF-κB in the MAPK family. PFE treatment mediates the decrease in ERK1/2, JNK1/2, and p38 suggested that PFE may modulate the crosstalk of multiple pathways that lead to increased cell survival, inflammation, proliferation, and apoptosis, therefore leading to the photoprotection of skin cells [77]. The beneficial effects of pomegranate were also investigated in vitro. Utilizing PFE and other pomegranate-derived products (juice and oil), multiple studies determined their effects against UVB-mediated damages to NHEK [78], immortalized HaCaT keratinocytes [79], and human reconstituted skin [80]. In addition to the observed reduction of MAPK signaling, proliferation, and DNA damage, PFE was shown to inhibit the phosphorylated activation of c-Jun and c-Fos, and MMP proteins, which are linked to the extracellular matrix and collagen breakdown [79,80]. Taken together, all of these studies demonstrate the protective capacity of the pomegranate fruit against skin damages, including skin cancer.
Apple (Malus pumila)
The second-largest source of fruit phenolics in the United States is apples [81]. Apples have substantially more phenolic content in the skin as compared with the remainder of the fruit. Unfortunately, in the production of canned apples and applesauce, the peel is discarded [82]. The flesh contains catechins, procyanidins, and caffeic acid, whereas the peel contains these all including quercetin glycosides [82]. To determine the chemopreventive effects of fresh apples, Ding et al. determined the effects of apple peel extract (APE) against DMBA-TPA skin tumorigenesis in AP-1 luciferase reporter transgenic mice (C57BL/6 crossed with DBA2). Mice were provided APE in the drinking water ad libitum two days before the initiating dose of DMBA, then throughout the remainder of the study [83]. After 20 weeks of biweekly TPA application, mice were euthanized. Although the authors observed a similar percentage of mice developing tumors, those ingesting the APE had greater than 50% inhibition in the number of papillomas present at the end of the study, accompanied by decreased tumor volume [83]. To determine if this reduction of tumorigenesis is relative to AP-1 activation, the authors performed a separate experiment utilizing the same transgenic mouse strain. In this experiment, the mice were provided ad libitum access to APE in their water and were also given six topical applications of APE over six days. Mice then received a single dose of TPA or UVB irradiation (10 kJ/m2) on the fourth day, and skin biopsies were collected 24 or 72 h later. By 72 h posttreatment, APE significantly inhibited the activation of UVB- and TPA-induced AP-1 activation [83]. Furthermore, in vitro validation in mouse epidermal JB6 cells treated with varying doses of APE and TPA (20 ng/mL) or UVB (4 kJ/m2) demonstrated the same trend of reduced AP-1 activation. The observed effect on AP-1 in JB6 cells was accompanied by the reduction in ERK and JNK in the UVB-treated cells, and ERK in TPA-treated cells. Moreover, APE also appeared to be a potent scavenger for ROS and inhibited TPA-induced transformation of cells. Therefore, this study demonstrated that apple peels could impart skin cancer chemopreventive effects through the reduction of ROS and via modulating MAPK and AP-1 signaling [83]. Similarly, George and Rupasinghe investigated the efficacy of an apple flavonoid fraction, a flavonoid-rich ethanolic extract of the Northern Spy apple cultivar, against various carcinogen-induced toxicity in normal human bronchial epithelial cells. The study demonstrated that apple flavonoids could reduce total ROS generation and protect against carcinogen-induced oxidative DNA damage and facilitate repair mechanisms [84]. However, only limited studies, mostly in vitro, have been conducted in this area. Additional studies in multiple in vivo models and human investigations are warranted.
Tomato (Solanum lycopersicum)
The beneficial health effects of tomatoes have been linked to the presence of carotenoids, which are pigments giving the fruit its color. Lycopene, the primary carotenoid of tomatoes, has been shown to act as a scavenger of ROS [85]. Stahl et al. suggested that the lycopene in tomatoes, combined with other carotenoids and noncarotenoids, imparts a photoprotective effect against UV-dependent skin damage [86]. A study by Kopec et al. utilized the SKH-1 hairless mouse model to comparatively analyze the difference among males and females in photoprotection from UV when on a tomato diet [87]. After 10 weeks of dietary tomato consumption and one UVB exposure (2240 J/m2), female mice had higher levels of skin and blood carotenoids as compared with their male counterparts. Furthermore, tomato-containing diet reduced CPDs and p53 positive epidermal cells. However, the attenuation of UV-induced DNA damage in males was associated with reduced inflammation and CPD levels [87]. In a subsequent study by Cooperstone et al., a tomato diet was given for a 35-week tumorigenesis study in mice. Mice received UVB exposures (2240 J/m2) three times weekly, which resulted in papillomas in male mice at 6–10 weeks and in females at 10–12 weeks. Although there was no difference noted in the female cohort, male mice of the tomato-based diets developed significantly fewer tumors than those on the control diet [88]. Although encouraging, further detailed studies are needed to determine the potential usefulness of whole tomatoes in additional models.
Conclusion
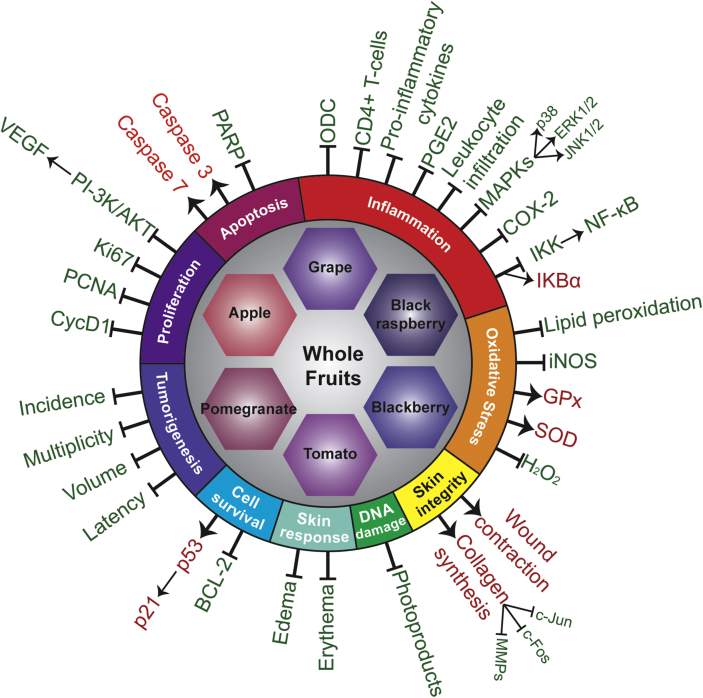
Over the past decades, scientific evidence supporting healthy eating patterns being linked to reduced disease risk has grown monumentally. In this review, we evaluated studies of multiple fruits and their benefits to protect against cutaneous damages, including skin carcinogenesis, suggestively because fruits are packed with phytonutrients that possibly act synergistically to prevent oxidative and inflammatory conditions, such as cancer and aging. Collectively, these studies have demonstrated the beneficial effects of utilizing whole fruits in skin health as they are proapoptotic (for damaged cells and reduce their survival), antiproliferative, and antiinflammatory, while also reducing DNA damage or enhancing DNA repair machinery and reducing oxidative stress (Figure 3). The studies discussed in this review are outlined in Table 1. The idea of utilizing natural agents in food is continuously growing. There are currently over 200 clinical trials for each grape and apple products. The trial numbers are continuing to increase with other fruits as well (Table 2). Currently, increasing life expectancy is continuously enhancing the likelihood of developing age-related diseases, such as cancer. Therefore, we must focus our efforts to understand the underlying protective mechanisms that whole foods can provide to extend our longevity. Indeed, additional studies, including short-term human studies, could be useful in assessing the human relevance of the available preclinical data, based on which further informed dietary guidelines could be implemented for better health. Furthermore, skin cancer chemoprevention studies of whole fruit products are required in the human population who are at higher risk, such as those patients who have compromised immune systems, such as organ transplant patients. In this direction, preclinical studies in models of high-risk skin carcinogenesis could also be useful. In addition, whole fruit products could also be implemented with existing therapeutics for the management of skin disorders, including skin carcinogenesis.
Figure 3.
Molecular mechanisms of whole fruit products involved in the prevention of carcinogenesis process related to skin cancer. Arrows and red text indicate upregulation and/or activation, and lines with a blunt end and green text indicate downregulation and/or inhibition.
Disclosure Statement
The authors have no conflicts of interest to declare.
Acknowledgment
This work was partially supported by funding from the California Table Grape Commission, as well as the National Institutes of Health (grant numbers R01AR059130 and R01CA176748 to NA), and the Department of Veterans Affairs (VA Merit Review Awards I01CX001441 and I01BX004221 and a Research Career Scientist Award IK6BX003780 to NA). We also acknowledge the core facilities supported by the Skin Diseases Research Center (SDRC) Core Grant P30AR066524 from NIH/NIAMS.
References
- 1.Lim H.W., Collins S.A.B., Resneck J.S., Jr., Bolognia J.L., Hodge J.A., Rohrer T.A., Van Beek M.J., Margolis D.J., Sober A.J., Weinstock M.A. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958–972. doi: 10.1016/j.jaad.2016.12.043. e952. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services and U.S. Department of Agriculture . 8th ed. December 2015. 2015–2020 dietary guidelines for Americans.http://health.gov/dietaryguidelines/2015/guidelines/ Available at: [Google Scholar]
- 3.Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pillai S., Oresajo C., Hayward J. Ultraviolet radiation and skin aging: roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation – a review. Int J Cosmet Sci. 2005;27:17–34. doi: 10.1111/j.1467-2494.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 5.Brohem C.A., Cardeal L.B.D., Tiago M., Soengas M.S., Barros S.B.D., Maria-Engler S.S. Artificial skin in perspective: concepts and applications. Pigment Cell Melanoma Res. 2011;24:35–50. doi: 10.1111/j.1755-148X.2010.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumari S., Pasparakis M. Epithelial cell death and inflammation in skin. Curr Top Microbiol Immunol. 2017;403:77–93. doi: 10.1007/82_2015_466. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs E., Raghavan S. Getting under the skin of epidermal morphogenesis. Nat Rev Genet. 2002;3:199–209. doi: 10.1038/nrg758. [DOI] [PubMed] [Google Scholar]
- 8.Apalla Z., Nashan D., Weller R.B., Castellsague X. Skin cancer: epidemiology, disease burden, pathophysiology, diagnosis, and therapeutic approaches. Dermatol Ther (Heidelb) 2017;7:5–19. doi: 10.1007/s13555-016-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bickers D.R., Athar M. Oxidative stress in the pathogenesis of skin disease. J Investig Dermatol. 2006;126:2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 10.Pfeifer G.P. Formation and processing of UV photoproducts: effects of DNA sequence and chromatin environment. Photochem Photobiol. 1997;65:270–283. doi: 10.1111/j.1751-1097.1997.tb08560.x. [DOI] [PubMed] [Google Scholar]
- 11.Barnes J.L., Zubair M., John K., Poirier M.C., Martin F.L. Carcinogens and DNA damage. Biochem Soc Trans. 2018;46:1213–1224. doi: 10.1042/BST20180519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agar N.S., Halliday G.M., Barnetson R.S., Ananthaswamy H.N., Wheeler M., Jones A.M. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: a role for UVA in human skin carcinogenesis. Proc Natl Acad Sci U S A. 2004;101:4954–4959. doi: 10.1073/pnas.0401141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Gruijl F.R., Rebel H. Early events in UV carcinogenesis – DNA damage, target cells and mutant p53 foci. Photochem Photobiol. 2008;84:382–387. doi: 10.1111/j.1751-1097.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 14.Swenberg J.A., Lu K., Moeller B.C., Gao L., Upton P.B., Nakamura J., Starr T.B. Endogenous versus exogenous DNA adducts: their role in carcinogenesis, epidemiology, and risk assessment. Toxicol Sci. 2011;120(Suppl 1):S130–S145. doi: 10.1093/toxsci/kfq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Botchkarev V.A., Flores E.R. p53/p63/p73 in the epidermis in health and disease. Cold Spring Harb Perspect Med. 2014;4 doi: 10.1101/cshperspect.a015248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoeijmakers J.H.J. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 17.Ratushny V., Gober M.D., Hick R., Ridky T.W., Seykora J.T. From keratinocyte to cancer: the pathogenesis and modeling of cutaneous squamous cell carcinoma. J Clin Investig. 2012;122:464–472. doi: 10.1172/JCI57415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Kyriakis J.M., Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 20.Kim A.L., Labasi J.M., Zhu Y., Tang X., McClure K., Gabel C.A., Athar M., Bickers D.R. Role of p38 MAPK in UVB-induced inflammatory responses in the skin of SKH-1 hairless mice. J Investig Dermatol. 2005;124:1318–1325. doi: 10.1111/j.0022-202X.2005.23747.x. [DOI] [PubMed] [Google Scholar]
- 21.Eckert R.L., Adhikary G., Young C.A., Jans R., Crish J.F., Xu W., Rorke E.A. AP1 transcription factors in epidermal differentiation and skin cancer. J Skin Cancer. 2013;2013:537028. doi: 10.1155/2013/537028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell S., Degitz K., Quirling M., Jilg N., Page S., Brand K. Involvement of NF-kappaB signalling in skin physiology and disease. Cell Signal. 2003;15:1–7. doi: 10.1016/s0898-6568(02)00080-3. [DOI] [PubMed] [Google Scholar]
- 23.Pitot H.C., Dragan Y.P. Facts and theories concerning the mechanisms of carcinogenesis. FASEB J. 1991;5:2280–2286. [PubMed] [Google Scholar]
- 24.Benjamin C.L., Ananthaswamy H.N. p53 and the pathogenesis of skin cancer. Toxicol Appl Pharmacol. 2007;224:241–248. doi: 10.1016/j.taap.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rebel H., Mosnier L.O., Berg R.J., Westerman-de Vries A., van Steeg H., van Kranen H.J., de Gruijl F.R. Early p53-positive foci as indicators of tumor risk in ultraviolet-exposed hairless mice: kinetics of induction, effects of DNA repair deficiency, and p53 heterozygosity. Cancer Res. 2001;61:977–983. [PubMed] [Google Scholar]
- 26.Ziegler A., Jonason A.S., Leffell D.J., Simon J.A., Sharma H.W., Kimmelman J., Remington L., Jacks T., Brash D.E. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 27.Mudgil A.V., Segal N., Andriani F., Wang Y., Fusenig N.E., Garlick J.A. Ultraviolet B irradiation induces expansion of intraepithelial tumor cells in a tissue model of early cancer progression. J Investig Dermatol. 2003;121:191–197. doi: 10.1046/j.1523-1747.2003.12320.x. [DOI] [PubMed] [Google Scholar]
- 28.Abel E.L., Angel J.M., Kiguchi K., DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nat Protoc. 2009;4:1350–1362. doi: 10.1038/nprot.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ra S.H., Li X., Binder S. Molecular discrimination of cutaneous squamous cell carcinoma from actinic keratosis and normal skin. Mod Pathol. 2011;24:963–973. doi: 10.1038/modpathol.2011.39. [DOI] [PubMed] [Google Scholar]
- 30.Rogers H.W., Weinstock M.A., Feldman S.R., Coldiron B.M. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081–1086. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 31.Wu S., Han J., Li W.Q., Li T., Qureshi A.A. Basal-cell carcinoma incidence and associated risk factors in U.S. women and men. Am J Epidemiol. 2013;178:890–897. doi: 10.1093/aje/kwt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guy G.P., Jr., Machlin S.R., Ekwueme D.U., Yabroff K.R. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015;48:183–187. doi: 10.1016/j.amepre.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christenson L.J., Borrowman T.A., Vachon C.M., Tollefson M.M., Otley C.C., Weaver A.L., Roenigk R.K. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. J Am Med Assoc. 2005;294:681–690. doi: 10.1001/jama.294.6.681. [DOI] [PubMed] [Google Scholar]
- 34.Rees J.R., Zens M.S., Celaya M.O., Riddle B.L., Karagas M.R., Peacock J.L. Survival after squamous cell and basal cell carcinoma of the skin: a retrospective cohort analysis. Int J Cancer. 2015;137:878–884. doi: 10.1002/ijc.29436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jensen A.O., Lamberg A.L., Jacobsen J.B., Olesen A.B., Sorensen H.T. Non-melanoma skin cancer and ten-year all-cause mortality: a population-based cohort study. Acta Derm Venereol. 2010;90:362–367. doi: 10.2340/00015555-0899. [DOI] [PubMed] [Google Scholar]
- 36.Epstein E.H. Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer. 2008;8:743. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alam M., Armstrong A., Baum C., Bordeaux J.S., Brown M., Busam K.J., Eisen D.B., Iyengar V., Lober C., Margolis D.J. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560–578. doi: 10.1016/j.jaad.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brantsch K.D., Meisner C., Schonfisch B., Trilling B., Wehner-Caroli J., Rocken M., Breuninger H. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. 2008;9:713–720. doi: 10.1016/S1470-2045(08)70178-5. [DOI] [PubMed] [Google Scholar]
- 39.Cooper J.Z., Brown M.D. Special concern about squamous cell carcinoma of the scalp in organ transplant recipients. Arch Dermatol. 2006;142:755–758. doi: 10.1001/archderm.142.6.755. [DOI] [PubMed] [Google Scholar]
- 40.Madan V., Lear J.T., Szeimies R.M. Non-melanoma skin cancer. Lancet. 2010;375:673–685. doi: 10.1016/S0140-6736(09)61196-X. [DOI] [PubMed] [Google Scholar]
- 41.Werlinger K.D., Upton G., Moore A.Y. Recurrence rates of primary nonmelanoma skin cancers treated by surgical excision compared to electrodesiccation-curettage in a private dermatological practice. Dermatol Surg. 2002;28:1138–1142. doi: 10.1046/j.1524-4725.2002.02110.x. discussion 1142. [DOI] [PubMed] [Google Scholar]
- 42.Tsao A.S., Kim E.S., Hong W.K. Chemoprevention of cancer. CA Cancer J Clin. 2004;54:150–180. doi: 10.3322/canjclin.54.3.150. [DOI] [PubMed] [Google Scholar]
- 43.Aziz M.H., Reagan-Shaw S., Wu J., Longley B.J., Ahmad N. Chemoprevention of skin cancer by grape constituent resveratrol: relevance to human disease? FASEB J. 2005;19:1193–1195. doi: 10.1096/fj.04-3582fje. [DOI] [PubMed] [Google Scholar]
- 44.Aziz M.H., Afaq F., Ahmad N. Prevention of ultraviolet-B radiation damage by resveratrol in mouse skin is mediated via modulation in survivin. Photochem Photobiol. 2005;81:25–31. doi: 10.1562/2004-08-13-RA-274. [DOI] [PubMed] [Google Scholar]
- 45.Reagan-Shaw S., Afaq F., Aziz M.H., Ahmad N. Modulations of critical cell cycle regulatory events during chemoprevention of ultraviolet B-mediated responses by resveratrol in SKH-1 hairless mouse skin. Oncogene. 2004;23:5151–5160. doi: 10.1038/sj.onc.1207666. [DOI] [PubMed] [Google Scholar]
- 46.Singh C.K., Liu X., Ahmad N. Resveratrol, in its natural combination in whole grape, for health promotion and disease management. Ann N Y Acad Sci. 2015;1348:150–160. doi: 10.1111/nyas.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Block G., Patterson B., Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 48.Singh C.K., Siddiqui I.A., El-Abd S., Mukhtar H., Ahmad N. Combination chemoprevention with grape antioxidants. Mol Nutr Food Res. 2016;60:1406–1415. doi: 10.1002/mnfr.201500945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGovern P.E. Princeton University Press; Princeton: 2003. Ancient wine: the search for the origins of viniculture. [Google Scholar]
- 50.Sekar S., Mariappan S. Traditionally fermented biomedicines, arishtas and asavas from Ayurveda. Indian J Tradit Knowl. 2008;7:548–556. [Google Scholar]
- 51.Paul B., Masih I., Deopujari J., Charpentier C. Occurrence of resveratrol and pterostilbene in age-old darakchasava, an ayurvedic medicine from India. J Ethnopharmacol. 1999;68:71–76. doi: 10.1016/s0378-8741(99)00044-6. [DOI] [PubMed] [Google Scholar]
- 52.Brandt J. 1928. The grape cure, New York. [Google Scholar]
- 53.Renaud S., de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 54.Pezzuto J.M. Grapes and human health: a perspective. J Agric Food Chem. 2008;56:6777–6784. doi: 10.1021/jf800898p. [DOI] [PubMed] [Google Scholar]
- 55.Filip A., Daicoviciu D., Clichici S., Mocan T., Muresan A., Postescu I.D. Photoprotective effects of two natural products on ultraviolet B-induced oxidative stress and apoptosis in SKH-1 mouse skin. J Med Food. 2011;14:761–766. doi: 10.1089/jmf.2010.0142. [DOI] [PubMed] [Google Scholar]
- 56.Filip A., Daicoviciu D., Clichici S., Bolfa P., Catoi C., Baldea I., Bolojan L., Olteanu D., Muresan A., Postescu I.D. The effects of grape seeds polyphenols on SKH-1 mice skin irradiated with multiple doses of UV-B. J Photochem Photobiol B. 2011;105:133–142. doi: 10.1016/j.jphotobiol.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 57.Che D.N., Xie G.H., Cho B.O., Shin J.Y., Kang H.J., Jang S.I. Protective effects of grape stem extract against UVB-induced damage in C57BL mice skin. J Photochem Photobiol B. 2017;173:551–559. doi: 10.1016/j.jphotobiol.2017.06.042. [DOI] [PubMed] [Google Scholar]
- 58.Cho B.O., Che D.N., Shin J.Y., Kang H.J., Jang S.I. Ameliorative effects of fruit stem extract from Muscat Bailey A against chronic UV-induced skin damage in BALB/c mice. Biomed Pharmacother. 2018;97:1680–1688. doi: 10.1016/j.biopha.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Perde-Schrepler M., Chereches G., Brie I., Tatornir C., Postescu I.D., Soran L., Filip A. Grape seed extract as photochemopreventive agent against UVB-induced skin cancer. J Photochem Photobiol B. 2013;118:16–21. doi: 10.1016/j.jphotobiol.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 60.Arimoto-Kobayashi S., Zhang X., Yuhara Y., Kamiya T., Negishi T., Okamoto G. Chemopreventive effects of the juice of Vitis coignetiae Pulliat on two-stage mouse skin carcinogenesis. Nutr Cancer. 2013;65:440–450. doi: 10.1080/01635581.2013.767916. [DOI] [PubMed] [Google Scholar]
- 61.Hanausek M., Spears E., Walaszek Z., Kowalczyk M.C., Kowalczyk P., Wendel C., Slaga T.J. Inhibition of murine skin carcinogenesis by freeze-dried grape powder and other grape-derived major antioxidants. Nutr Cancer. 2011;63:28–38. doi: 10.1080/01635581.2010.516474. [DOI] [PubMed] [Google Scholar]
- 62.Armstrong B.K., Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 63.Sharma S.D., Katiyar S.K. Dietary grape seed proanthocyanidins inhibit UVB-induced cyclooxygenase-2 expression and other inflammatory mediators in UVB-exposed skin and skin tumors of SKH-1 hairless mice. Pharm Res. 2010;27:1092–1102. doi: 10.1007/s11095-010-0050-9. [DOI] [PubMed] [Google Scholar]
- 64.Singh C.K., Mintie C.A., Ndiaye M.A., Chhabra G., Dakup P.P., Ye T., Yu M., Ahmad N. Chemoprotective effects of dietary grape powder on UVB radiation-mediated skin carcinogenesis in SKH-1 hairless mice. J Investig Dermatol. 2019;139:552–561. doi: 10.1016/j.jid.2018.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bobinaite R., Viskelis P., Venskutonis P.R. Variation of total phenolics, anthocyanins, ellagic acid and radical scavenging capacity in various raspberry (Rubus spp.) cultivars. Food Chem. 2012;132:1495–1501. doi: 10.1016/j.foodchem.2011.11.137. [DOI] [PubMed] [Google Scholar]
- 66.Gouveia-Figueira S.C., Castilho P.C. Phenolic screening by HPLC–DAD–ESI/MSn and antioxidant capacity of leaves, flowers and berries of Rubus grandifolius Lowe. Ind Crops Prod. 2015;73:28–40. [Google Scholar]
- 67.Huang C., Zhang D., Li J., Tong Q., Stoner G.D. Differential inhibition of UV-induced activation of NF kappa B and AP-1 by extracts from black raspberries, strawberries, and blueberries. Nutr Cancer. 2007;58:205–212. doi: 10.1080/01635580701328453. [DOI] [PubMed] [Google Scholar]
- 68.Huang C., Huang Y., Li J., Hu W., Aziz R., Tang M.S., Sun N., Cassady J., Stoner G.D. Inhibition of benzo(a)pyrene diol-epoxide-induced transactivation of activated protein 1 and nuclear factor kappaB by black raspberry extracts. Cancer Res. 2002;62:6857–6863. [PubMed] [Google Scholar]
- 69.Huang C., Li J., Song L., Zhang D., Tong Q., Ding M., Bowman L., Aziz R., Stoner G.D. Black raspberry extracts inhibit benzo(a)pyrene diol-epoxide-induced activator protein 1 activation and VEGF transcription by targeting the phosphotidylinositol 3-kinase/Akt pathway. Cancer Res. 2006;66:581–587. doi: 10.1158/0008-5472.CAN-05-1951. [DOI] [PubMed] [Google Scholar]
- 70.Duncan F.J., Martin J.R., Wulff B.C., Stoner G.D., Tober K.L., Oberyszyn T.M., Kusewitt D.F., Van Buskirk A.M. Topical treatment with black raspberry extract reduces cutaneous UVB-induced carcinogenesis and inflammation. Cancer Prev Res (Phila) 2009;2:665–672. doi: 10.1158/1940-6207.CAPR-08-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Divya S.P., Wang X., Pratheeshkumar P., Son Y.O., Roy R.V., Kim D., Dai J., Hitron J.A., Wang L., Asha P., Shi X., Zhang Z. Blackberry extract inhibits UVB-induced oxidative damage and inflammation through MAP kinases and NF-kappaB signaling pathways in SKH-1 mice skin. Toxicol Appl Pharmacol. 2015;284:92–99. doi: 10.1016/j.taap.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Calvo-Castro L., Syed D.N., Chamcheu J.C., Vilela F.M., Perez A.M., Vaillant F., Rojas M., Mukhtar H. Protective effect of tropical highland blackberry juice (Rubus adenotrichos Schltdl.) against UVB-mediated damage in human epidermal keratinocytes and in a reconstituted skin equivalent model. Photochem Photobiol. 2013;89:1199–1207. doi: 10.1111/php.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Afaq F., Saleem M., Krueger C.G., Reed J.D., Mukhtar H. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-κB pathways and inhibits skin tumorigenesis in CD-1 mice. Int J Cancer. 2005;113:423–433. doi: 10.1002/ijc.20587. [DOI] [PubMed] [Google Scholar]
- 74.Gil M.I., Tomas-Barberan F.A., Hess-Pierce B., Holcroft D.M., Kader A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- 75.Hora J.J., Maydew E.R., Lansky E.P., Dwivedi C. Chemopreventive effects of pomegranate seed oil on skin tumor development in CD1 mice. J Med Food. 2003;6:157–161. doi: 10.1089/10966200360716553. [DOI] [PubMed] [Google Scholar]
- 76.Afaq F., Khan N., Syed D.N., Mukhtar H. Oral feeding of pomegranate fruit extract inhibits early biomarkers of UVB radiation-induced carcinogenesis in SKH-1 hairless mouse epidermis. Photochem Photobiol. 2010;86:1318–1326. doi: 10.1111/j.1751-1097.2010.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khan N., Syed D.N., Pal H.C., Mukhtar H., Afaq F. Pomegranate fruit extract inhibits UVB-induced inflammation and proliferation by modulating NF-kappaB and MAPK signaling pathways in mouse skin. Photochem Photobiol. 2012;88:1126–1134. doi: 10.1111/j.1751-1097.2011.01063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Afaq F., Malik A., Syed D., Maes D., Matsui M.S., Mukhtar H. Pomegranate fruit extract modulates uv-b–mediated phosphorylation of mitogen-activated protein kinases and activation of nuclear factor kappa b in normal human epidermal keratinocytes. Photochem Photobiol. 2005;81:38–45. doi: 10.1562/2004-08-06-RA-264. [DOI] [PubMed] [Google Scholar]
- 79.Zaid M.A., Afaq F., Syed D.N., Dreher M., Mukhtar H. Inhibition of UVB-mediated oxidative stress and markers of photoaging in immortalized HaCaT keratinocytes by pomegranate polyphenol extract POMx. Photochem Photobiol. 2007;83:882–888. doi: 10.1111/j.1751-1097.2007.00157.x. [DOI] [PubMed] [Google Scholar]
- 80.Afaq F., Zaid M.A., Khan N., Dreher M., Mukhtar H. Protective effect of pomegranate-derived products on UVB-mediated damage in human reconstituted skin. Exp Dermatol. 2009;18:553–561. doi: 10.1111/j.1600-0625.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vinson J.A., Su X., Zubik L., Bose P. Phenol antioxidant quantity and quality in foods: fruits. J Agric Food Chem. 2001;49:5315–5321. doi: 10.1021/jf0009293. [DOI] [PubMed] [Google Scholar]
- 82.Wolfe K., Wu X., Liu R.H. Antioxidant activity of apple peels. J Agric Food Chem. 2003;51:609–614. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
- 83.Ding M., Lu Y., Bowman L., Huang C., Leonard S., Wang L., Vallyathan V., Castranova V., Shi X. Inhibition of AP-1 and neoplastic transformation by fresh apple peel extract. J Biol Chem. 2004;279:10670–10676. doi: 10.1074/jbc.M311465200. [DOI] [PubMed] [Google Scholar]
- 84.George V.C., Rupasinghe H.P.V. Apple flavonoids suppress carcinogen-induced DNA damage in normal human bronchial epithelial cells. Oxid Med Cell Longev. 2017;2017:1767198. doi: 10.1155/2017/1767198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Di Mascio P., Kaiser S., Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys. 1989;274:532–538. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- 86.Stahl W., Heinrich U., Aust O., Tronnier H., Sies H. Lycopene-rich products and dietary photoprotection. Photochem Photobiol Sci. 2006;5:238–242. doi: 10.1039/b505312a. [DOI] [PubMed] [Google Scholar]
- 87.Kopec R.E., Schick J., Tober K.L., Riedl K.M., Francis D.M., Young G.S., Schwartz S.J., Oberyszyn T.M. Sex differences in skin carotenoid deposition and acute UVB-induced skin damage in SKH-1 hairless mice after consumption of tangerine tomatoes. Mol Nutr Food Res. 2015;59:2491–2501. doi: 10.1002/mnfr.201500317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cooperstone J.L., Tober K.L., Riedl K.M., Teegarden M.D., Cichon M.J., Francis D.M., Schwartz S.J., Oberyszyn T.M. Tomatoes protect against development of UV-induced keratinocyte carcinoma via metabolomic alterations. Sci Rep. 2017;7:5106. doi: 10.1038/s41598-017-05568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]