Abstract
The isocitrate dehydrogenase (IDH1/2) mutations are frequent genetic abnormalities in the majority of WHO grade II/III glioma and secondary GBM. IDH1-mutated (IDH1Mut) glioma exhibits distinctive patterns in cancer biology and metabolism. In the present study, we showed that bone morphogenetic proteins (BMP4) are significantly upregulated in IDH1Mut glioma. Further, we demonstrated that cancer-associated BMP4 is secreted to tumor microenvironment, which enhances the tumor migration and invasion through an autocrine manner. Mechanistically, BMP4 activates its receptor and concomitant SMAD1/5/8 signaling, which potentiates Wnt/β-catenin signaling by enhancing Frizzled receptor expression. LDN-193189, a selective BMP receptor inhibitor, prolonged the overall survival of mice bearing IDH1-mutated intracranial xenografts by limiting BMP/catenin signaling. These findings demonstrate the pivotal role of BMP4 on tumor aggressiveness in IDH1Mut gliomas, suggesting a possible therapeutic strategy for this type of malignancy.
Introduction
Glioma is the most aggressive neoplastic disease in the central nervous system, which accounts for 80% of malignant primary brain tumors [1]. According to the histological features, glioma can be classified into World Health Organization (WHO) grade I–IV [2]. Recent advances showed that up to 80% of WHO II/III astrocytoma, oligodendroglioma, and secondary glioblastoma carry mutations in isocitrate dehydrogenase 1 and 2 (IDH1/2) [3]. The identification of IDH mutations in glioma revealed a novel disease cluster that share distinctive cancer biology, metabolism signature, and therapeutic vulnerability [3], [4], [5]. Although several pioneered studies reported that patients with IDHMut are commonly associated with prolonged overall survival (OS) with better chemosensitivities in comparison with IDH wild-type (IDHWT) glioma patients, effective cure for this glioma molecular subtype remains limited [3], [6], [7].
Bone morphogenetic proteins (BMPs) are a group of growth factors that originally discovered by their involvement in the formation of bone and cartilage [8]. BMPs are members of the transforming growth factor beta (TGF-β) family and recognize type I and II dual kinase receptors [9]. Type I BMP receptors (BMPRs) consist of activin receptor–like kinase (ALK)-1, -2, -3 (BMPRIA) and -6 (BMPRIB). Type II BMPR includes BMP type II receptor (BMPRII), activin type II receptor (ActRII), and ActRIIB [10]. The activated BMPRI initiates the intracellular signaling by phosphorylating the receptor-regulatory Smad1/5, which interacts with the common mediator Smad4 and translocates into nucleus [10]. Besides their function in bone development, BMPs are recently identified participating key signaling pathways in cancer biology, such as cell proliferation, differentiation, and motility [11]. BMP4 has been reported to be overexpressed in melanoma, colorectal cancer, and hepatocellular carcinoma and promotes the tumor invasion and metastasis [12], [13], [14]. However, little is known about the expression and function of BMP4 in IDH1Mut gliomas.
In the present study, we investigated the BMP4 expression in low-grade glioma (LGG). We identified a significant differential expression of BMP4 between IDH1WT and IDH1Mut cases. Further, we investigated the role of BMP4 on tumor aggressiveness in IDH1Mut gliomas through patient-derived brain tumor–initiating cells (BTIC). Moreover, we demonstrate the cross-link between BMP/Smad signaling and canonical Wnt/β-catenin pathway. As a further validation, we evaluated the therapeutic effect of BMP4 blockade in preclinical xenograft model.
Materials and Methods
Cell Culture and Reagents
Glioma-initiating cells, GSC827, and GSC923 cell lines were derived from patients' glioblastoma samples following the protocol of National Cancer Institute Institutional Review Board (NCI 02C-0140). IDH1 R132H mutant TS603 cell line was kindly gifted from Dr. Timothy A. Chan's lab in Memorial Sloan Kettering Cancer Center. GSC827, GSC923, and TS603 were cultured in Neurobasal (Invitrogen) supplemented with N2, B27, EGF (20 ng/ml), bFGF (20 ng/ml), and 1% antibiotics (100 U/ml penicillin and 10 μg/ml streptomycin) as described before [15]. These GSCs were monolayer cultured following the protocol as described before [16]. To activate the BMP-Smads signaling, cells were treated with BMP4 (50 ng/ml, Thermo Fisher) for 30 min. To block the effect of BMP4, BMPR1A inhibitor LDN-193189 (200 nM, Sigma Aldrich) or BMP4 neutralizing antibody (NA, 8 μg/ml, R&D MAB757) was used to treat the cells.
Human Glioma Specimens
Forty-six human glioma specimens were from Department of Neurosurgery, Beijing Tiantan Hospital affiliated to Capital Medical University and collected by Prof. Fusheng Liu's laboratory during 2015–2017 in Beijing Neurosurgical Institute (Supplementary Table 1). Three experienced neuropathologists confirmed the histological diagnoses. The sample collection was approved by the Institutional Review Board of Beijing Tiantan Hospital affiliated to Capital Medical University.
Establishment of Intracranial IDH1Mut Tumor Models
Following the protocols as described before, six-week-old BALB/c-nu mice were injected with 5 × 105 TS603 cells localizing to the caudate nucleus of mice brain [17]. One week after injection, mice were randomly divided into two groups. The treatment group mice (LDN, n = 9) were intraperitoneally injected with LDN-193189 (3 mg/kg, MedChem Express) every day, and the control group mice (Ctrl, n = 9) were injected with DMSO. Animal studies were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
RNA-Sequencing and Analysis
TS603 cells were treated with DMSO, BMP4 (50 ng/ml), LDN (200 nM), and BMP4 plus LDN for 3 days, respectively. Each treatment was prepared in triplicate. Total RNAs were extracted using PureLink RNA mini kit (Thermo Fisher), and the quality was assessed using RNA600 Pico LabChip kit with bioanalyzer (Agilent Technologies). The sequencing procedure was following the protocols as described before [18]. Data were further analyzed using Ingenuity Pathway Analysis (Qiagen).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 7. Student's t-test was used to analyze the significance of two group results. OS time of the mice was compared using log-rank method. All values are expressed as the mean ± SEM. P < 0.05 was considered statistically significant. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Results
Prompted Expression of BMP4 in IDH1Mut Gliomas
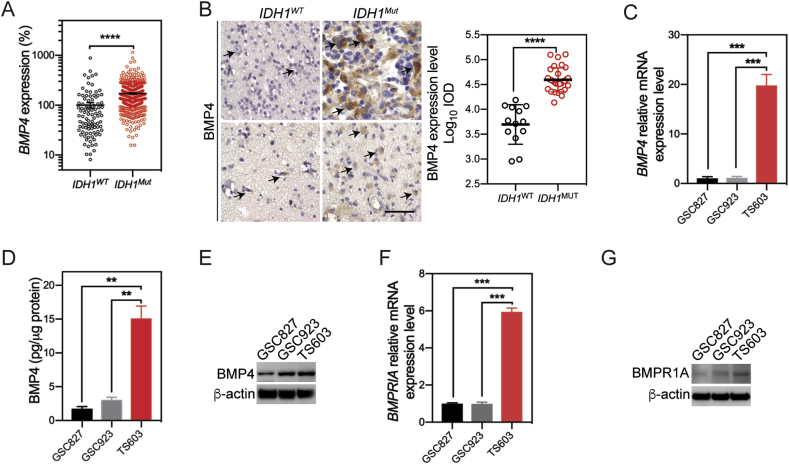
To investigate the differential expressed genes between IDH1WT and IDH1Mut gliomas, we compared the transcriptome profile from 431 cases of IDH1Mut gliomas and 96 cases of IDH1WT gliomas based on the TCGA LGG database. We found out that BMP4 expression is elevated by 72.5% in IDH1Mut gliomas comparing with their wild-type counterparts (Figure 1A). The upregulation of BMP4 was further validated in 43 glioma specimens through immunohistochemistry assay (IDH1WT = 13 cases; IDH1Mut = 30 cases). We showed that BMP4 locates in the cytoplasm of glioma cells, which were elevated by 22.8% in IDH1Mut glioma specimens (Figure 1B). To better understand the expression of BMP4 in IDH1Mut glioma, we quantified the expression of BMP4 in three human BTIC lines (IDH1WT: GSC827, GSC923; IDH1Mut: TS603). We found that mRNA levels of BMP4 were elevated by up to 19.1 folds in IDH1Mut TS603 cells (Figure 1C).
Figure 1.
BMP4 is overexpressed inIDH1Muthuman gliomas and cell lines. (A) In TCGA database, BMP4 is significantly increased in IDH1Mut human gliomas than in IDH1WT gliomas. (B) IHC staining of BMP4 in IDH1WT and IDH1Mut human gliomas. BMP4 expressed in cytoplasm. Scale bar: 50 μm. BMP4 was highly expressed in IDH1Mut human gliomas. (C) In three GICs, BMP4 mRNA levels were higher in TS603 cells (IDH1Mut) than those in GSC827 and GSC923 cells (IDH1WT). (D) In cell medium supernatant, TS603 cells secreted significantly higher BMP4 levels than GSC827 and GSC923 cells. (E) BMP4 expression was detected in three GICs using western blot. BMP4 level was higher in TS603 cells. (F) In three GICs, the mRNA expression of BMPRIA was significantly upregulated in TS603 cells than in GSC827 and GSC923 cells. (G) The expression of BMPRIA was determined increased in TS603 cells than in GSC827 and GSC923 cells. *P < 0.05; **P < 0.01; ****P < 0.0001.
Several pioneered studies showed that cancer-associated BMP4 is secreted into tumor microenvironment, which therefore influences the progression of malignancy [12], [19]. To better illustrate the impact of BMP4 in the microenvironment of IDH1Mut cells, we quantified the levels of BMP4 in cell culture media from BTIC by ELISA. Consistent with the BMP4 expression test, we found the BMP4 secretion was upregulated by 9.1-folds in IDH1Mut TS603, compared with GSC827 or GSC923 (Figure 1D). As a further validation, we collected and concentrated the tissue culture media, and we found through western blotting that the secretion of BMP4 was higher in TS603 (Figure 1E). We also tested the expression level of BMP4 receptors in BTIC. We also investigated the expression of BMP4 and BMPR1A in these cell lines through immunoblotting (Figure 1F and G). These results showed that BMP4 is selectively upregulated in IDH1Mut glioma cells comparing with IDH1WT glioma cells, suggesting novel BMP signaling in IDH1Mut glioma cells.
BMP4 Signaling Enhances Tumor Aggressiveness in IDH1Mut Gliomas
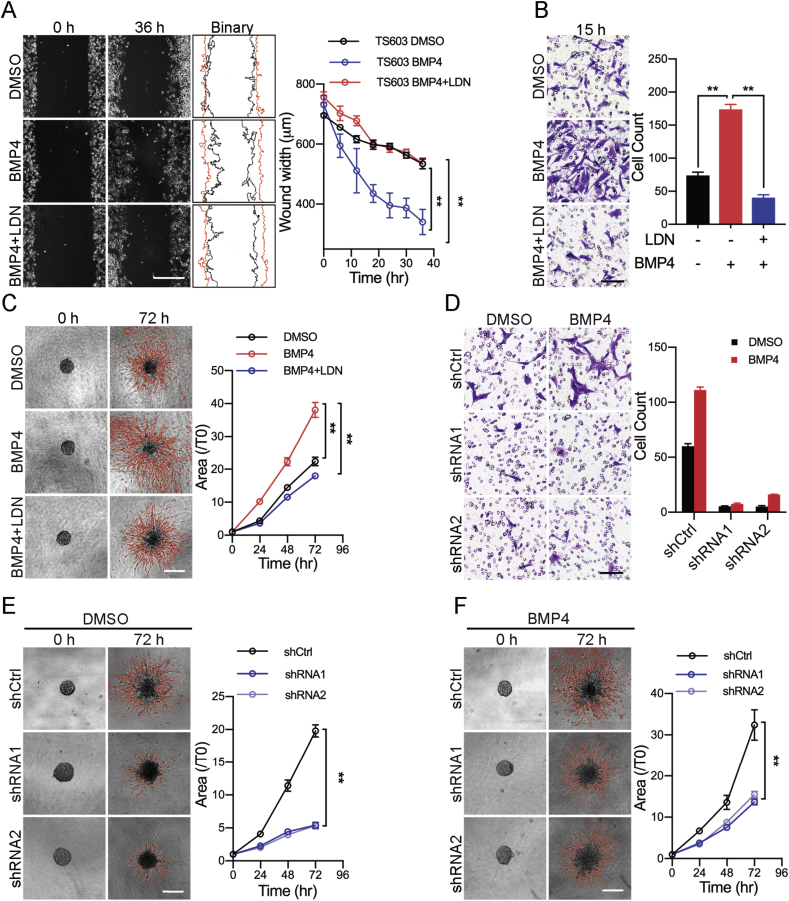
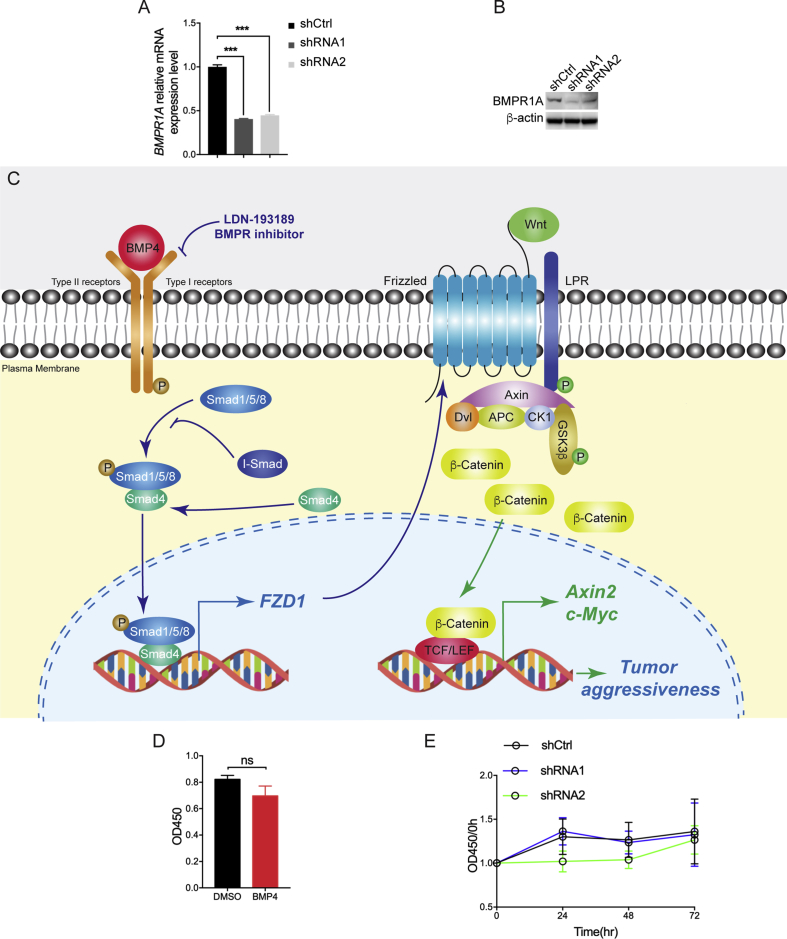
BMP4 has been shown to promote tumor migration and invasion in several types of human malignancies, mainly through its concomitant Smad1/5/8 signaling [13], [20], [21]. To better understand the role of BMP4 in IDH1Mut cells, we firstly analyzed the tumor migration rate in BTIC through wound healing assay. We applied LDN-193189 (LDN), a selective inhibitor for BMP type I receptor ALK2 and ALK3, to block BMP4/Smad signaling. We showed that the presence of BMP4 significantly enhanced cellular migration of TS603 cells, whereas the impact of BMP4 was abolished with LDN treatment (Figure 2A, mean wound width, DMSO = 534.2 ± 9.507 μm, BMP4 = 340.7 ± 24.36 μm, BMP4 + LDN = 535.8 ± 10.5 μm). The increased cellular motility was confirmed by transwell invasion assay. BMP4 increased cellular invasion 2.35 folds for TS603 cells, which was also abolished by LDN treatment (Figure 2B, invaded cell count, DMSO = 74 ± 4, BMP4 = 174 ± 7, BMP4 + LDN = 40 ± 1). As a further validation, we performed three-dimensional tumor spheroids invasion assay to better understand the alteration in glioma invasiveness. Consistently, we found that the presence of BMP4 resulted in aggressive invasion of TS603 cells. The cellular invasion was elevated by 70%, whereas LDN blocked TS603 cells penetration into the extracellular matrix (Figure 2C, area fold change T72h/T0h, DMSO = 22.38 ± 1.266, BMP4 = 38.08 ± 2.253, BMP4 + LDN = 18.01 ± 0.801). To further validate whether BMP4 enhances cellular invasiveness through its receptors, we established TS603 cells that stably express small hairpin RNAs targeting BMPR1A expression (Supplementary Figure A and B). Transwell invasion assay showed that the numbers of invaded cells were significantly decreased in cells with BMPR1A shRNA (Figure 2D). Similarly, three-dimensional tumor spheroids assay also showed the invaded areas of the BMPRIA knockdown cells were significantly decreased (Figure 2E, area fold change ratio T72h/T0h, shCtrl: 19.79 ± 0.9067, shRNA1: 5.429 ± 0.4193, shRNA2: 5.355 ± 0.4895). Moreover, the effect of BMP4 on cellular invasion was attenuated in BMPRIA knockdown cells (Figure 2F, area fold change, shCtrl: 32.41 ± 3.686, shRNA1: 13.71 ± 0.6709, shRNA2: 15.5 ± 0.758). These findings suggest that BMP4 potentiates aggressive phenotype for IDH1Mut glioma cells, whereas BMPR blockade successfully prohibited cancer cells from infiltrating into adjacent extracellular matrix.
Figure 2.
BMP4 signaling enhances tumor aggressiveness inIDH1Mutgliomas. (A) Wound healing assay showed BMP4 treatment promoted the migration ability of TS603 cell. LDN blocked the effect of BMP4. Scale bar: 500 μm. (B) Transwell invasion assay showed that BMP4 treatment increased the invasive ability of TS603 cells. LDN blocked the effect of BMP4. Scale bar: 100 μm. (C) Three-dimensional tumor spheroids invasion assay showed that BMP4 treatment increased the invasive ability of TS603 cells. LDN blocked the effect of BMP4. Scale bar: 500 μm. (D) After BMPRIA was knocked down using shRNAs, the ability of invasion was significantly decreased in transwell invasive assay. Scale bar: 100 μm. (E–F) Knockdown of BMPRIA in TS603 cells decreased the ability of invasion in 3D tumor spheroids invasion assay; BMP4 could no longer promote the invasiveness in shRNAs cells. Scale bar: 500 μm. **P < 0.01; ***P < 0.001.
BMP4-Smad1 Signaling is Activated in an Autocrine Pattern
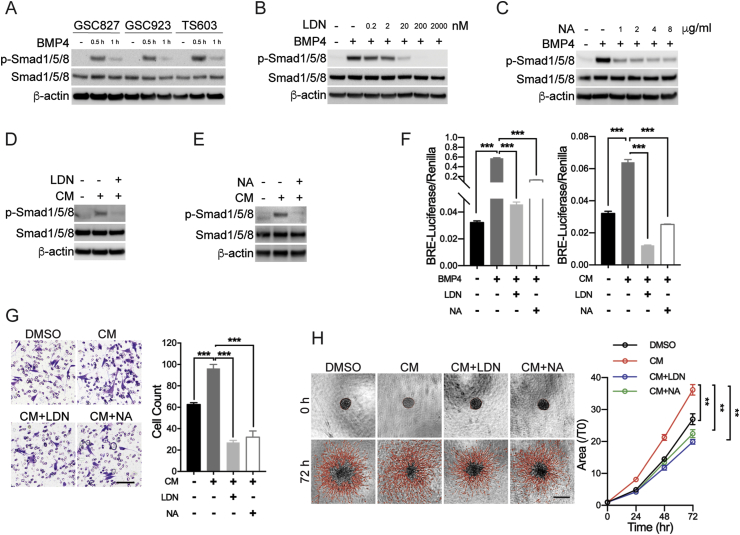
To investigate how BMP4 alters the biology in glioma cells, we firstly determined the phosphorylation of Smad1, the concomitant downstream effector of BMPR signaling. Western blot showed Smad1 was phosphorylated under BMP4 treatment in three BTIC cells (Figure 3A). The phosphorylated Smad1 (p-Smad1) expression in IDH1Mut TS603 cells was more significant, which might be attributed to the higher levels of BMPRIA. Further, the phosphorylation of Smad1 in TS603 cells is abolished by LDN in a dose-dependent manner. The BMP4-induced p-Smad1 expression could be completely inhibited by 200 nM LDN (Figure 3B). Meanwhile, we found that BMP4 NA attenuated the BMP4-induced p-Smad1 expression in a dose-dependent manner (Figure 3C).
Figure 3.
BMP4-Smad1 signaling is activated in an autocrine pattern. (A) p-Smad1 expression was detected to be increased under the treatment of BMP4 for 0.5 h and 1 h, respectively. It was more significant when TS603 cells were treated with BMP4 for 0.5 h. (B) LDN-193189 (LDN) inhibited the BMP4-induced Smad1/5/8 phosphorylation in a dose-dependent manner. We used 200 nM concentration of LDN for the following study. (C) BMP4-neutralizing antibody (NA) inhibited the BMP4-induced Smad1/5/8 phosphorylation in a dose-dependent manner. We use 4 μg/ml concentration of BMP4 NA for the following study. (D) TS603 cells were treated using cell medium supernatant (conditioned medium, CM) in combination with LDN for 30 min; CM induced Smad1/5/8 phosphorylation and LDN blocked the effect of CM on Smad1/5/8 phosphorylation. (E) BMP4 NA attenuated the effect of CM-induced Smad1/5/8 phosphorylation in TS603 cells. (F) BRE-luciferase reporter assay showed BMP4 as well as CM increased the activity of luciferase, and which could be blocked by LDN and NA. (G) Transwell invasion assay showed that CM enhanced the invasiveness of TS603 cells, which could be blocked by LDN and NA. Scale bar: 100 μm. (H) Three-dimensional tumor spheroids invasion assay also showed that CM enhanced the invasiveness of TS603 cells, which could be blocked by LDN and NA. Scale bar: 500 μm. **P < 0.01.
Considering the high secretion level of BMP4 from TS603 cells, it is likely that the secreted BMP4 impact cellular biology through autocrine manner. To test this hypothesis, we collected the conditional media (CM) from TS603 cells and tested whether it could activate BMP/smad1 signaling. We found that CM treatment of TS603 cells induced the expression of p-Smad1, which could be blocked by LDN treatment (Figure 3D). Moreover, BMP4 NA inhibited the CM-induced p-Smad1 expression (Figure 3E). As a further validation, we measured the activation of Smad1 transcriptional activity by an Id1 promoter–derived BMP reporter element (BRE)–luciferase reporter assay [22]. We found out that both of BMP4 and CM significantly increased the activity of BRE-luciferase, and both of conditions were blocked by LDN and BMP4 NA (Figure 3F). These results strongly suggest that TS603 cells activate Smad signaling through BMP4 and BMPR activation.
We further investigated whether BMP4 autocrine supports aggressive phenotype in IDH1Mut TS603 cells. Transwell invasion assay showed that CM significantly increased the invaded cell numbers, whereas LDN and BMP4 NA treatment blocked the effect of CM (Figure 3G, invaded cell count, DMSO = 63 ± 2, CM = 96 ± 3, CM + LDN = 27 ± 1, CM + NA = 32 ± 2). Consistently, CM treatment also increased the cellular penetration into adjacent extracellular matrix, which was also reversible by LDN or NA treatments (Figure 3H, invasion area fold change ratio T72h/T0h, DMSO = 26.98 ± 1.718, CM = 36.19 ± 1.617, CM + LDN = 19.91 ± 0.7548, CM + NA = 22.43 ± 1.29). Together with these results, we demonstrated that the autocrine of BMP4 in IDH1Mut glioma cells promotes tumor aggressiveness, and LDN blockade suppressed the autocrine cycle via inhibiting the BMPRs.
BMP4-Smad1 Signaling Prompts Wnt Pathway via FZD1
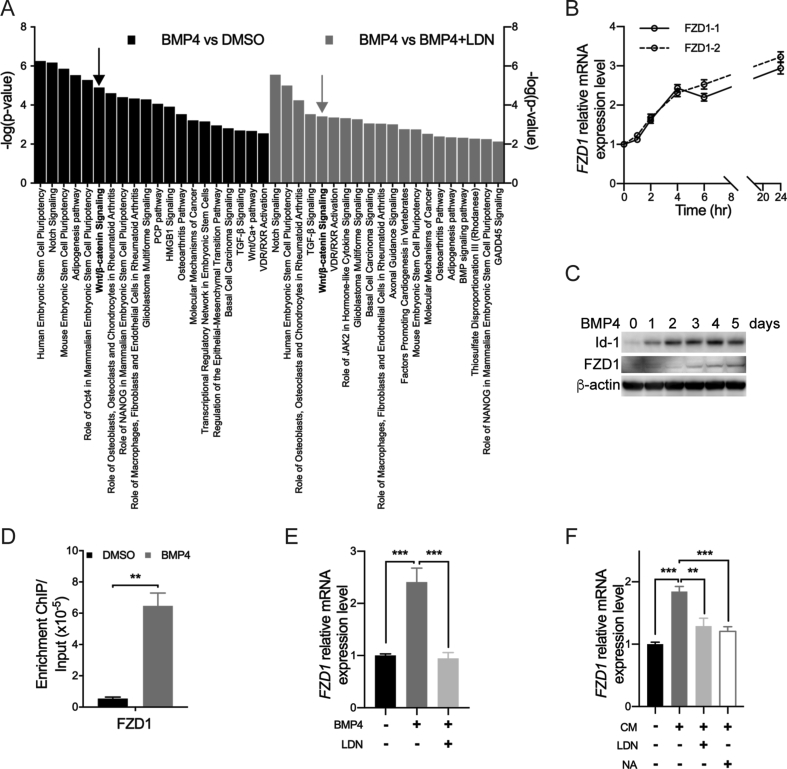
To explore the potential downstream genes or pathways of BMP4-Smad1 signaling that may be related to tumor aggressiveness, RNA-sequencing was performed to compare the expression profile before and after BMP4 treatment in IDH1Mut glioma cells. Bioinformatic analysis showed differentially expressed gene sets involved in the activation of BMP4 signaling (Figure 4A). Among most significantly altered gene sets, we identified Wnt/β-catenin pathway as highly expressed signaling on BMP4 treatment. Within this pathway, Frizzled receptors (FZDs), a family of G protein–coupled receptors, are critical to activate β-catenin. Through quantitative real-time PCR, we showed that the expression of FZD1 continuously increased under the treatment of BMP4 (Figure 4B). Western blot assay confirmed that the expression of FZD1 started to increase after 3 days of BMP4 treatment (Figure 4C). To investigate how BMP signaling activates FZD1 expression, we performed ChIP-qPCR to analyze the Smad1-binding sequences in IDH1Mut TS603 cells. We found that BMP4 treatment significantly increased the affinity of Smad1 to FZD1 promoter region by 11.9-fold, suggesting that FZD1 expression was mediated by Smad1-associated transcriptional activation (Figure 4D). In addition, LDN and NA treatment reduced FZD1 expression in the presence of either BMP4 or CM, suggesting that FZD1 upregulation correlates with BMP4 autocrine (Figure 4E and F).
Figure 4.
FZD1 connects BMP4-Smad1 signaling with Wnt pathway. (A) Top 20 highly activated pathways were listed in comparison of BMP4 vs DMSO group and BMP4 vs BMP4+LDN group from RNA-sequencing results. (B) The mRNA expression of FZD1 was continuously increased under the treatment of BMP4 in a time-dependent manner. (C) Western blot showed that BMP4 treatment increased the expression of Id1 and FZD1. The expression of FZD1 started to increase at day 3. (D) TS603 cells were treated with BMP4 for 72 h. ChIP-qPCR revealed that BMP4-Smad1 signaling directly regulated the expression of FZD1. (E–F) BMP4 and CM treatment of TS603 for 24 h significantly upregulated the expression of FZD1. Both of LDN-193189 and BMP4 neutralizing antibody could block the effect of BMP4-induced FZD1 expression. **P < 0.01; ***P < 0.001.
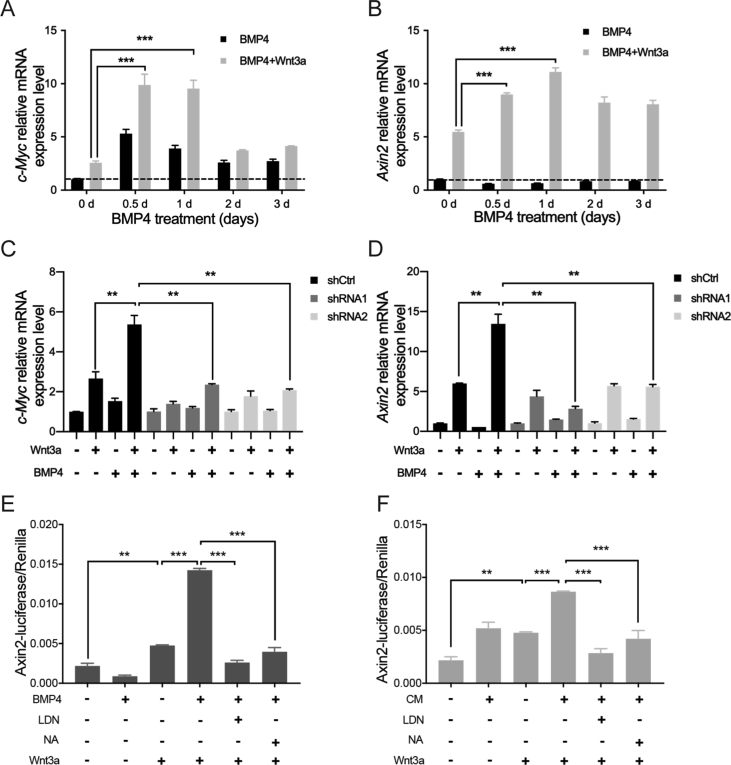
To further verify the crosstalk between BMP/Smad signaling and Wnt/β-catenin pathway, we measured the expressions of downstream genes of Wnt/β-catenin signaling, such as c-Myc and Axin2, by real-time PCR. We found BMP4 enhanced the transcriptional activation of both genes under Wnt3a induction (Figure 5A and B). Moreover, we established TS603 cells with stably expressed shRNA targeting BMPR1A. We found the in these cells, the impact of BMP4 to c-Myc and Axin2 was abolished (Figure 5C and D). These results suggested BMP4 potentiates Wnt/β-catenin signaling, which requires the receptor such as BMPR1A. Consistently, Axin2-luciferase reporter assay also showed that BMP4 or CM treatment significantly increased the activity of Axin2-luciferase comparing with Wnt3a treated alone (Figure 5E and F).
Figure 5.
BMP4-Smad1 signaling promotes the activity of Wnt/β-catenin pathway via upregulating FZD1. (A–B) c-Myc and Axin2, downstream of Wnt/β-catenin pathway, were significantly upregulated under the treatment of BMP4 for 72 h in combination with Wnt3a for 6 h. (C–D) After BMPRIA was knocked down, BMP4 in combination with Wnt3a treatment could no longer significantly induce the expression of c-Myc and Axin2. (E–F) Axin2-luciferase reporter assay showed that the activity of luciferase could be increased under the treatment of BMP4 or CM in combination with Wnt3a. **P < 0.01; ***P < 0.001.
LDN Inhibits Tumor Progression and Prolongs the Overall Survival of Mice with Intracranial IDH1Mut Gliomas
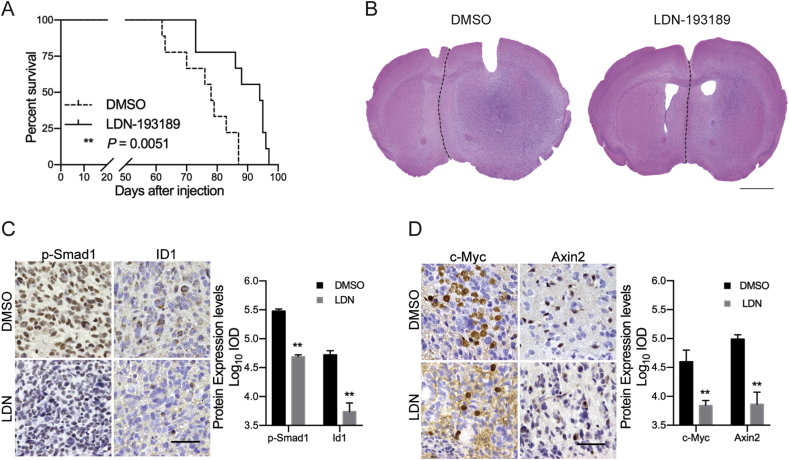
Our above findings showed that LDN inhibits BMP4-Smad1 signaling and hence suppresses tumor aggressiveness in vitro. To clarify whether inhibition of BMPR blockade by LDN could be considered as potential therapeutic approach, we established intracranial IDH1Mut glioma models using TS603 cells and evaluated the therapeutic effect of LDN. OS of the LDN-treated mice were significantly prolonged comparing with the control group (Figure 6A, median survival: DMSO group = 78 days, LDN group = 94 days). H&E staining of the two group mice brain, which were extracted from survival day 87 and day 88, showed that the midline of the brain obviously shifted toward the normal side in DMSO group (Figure 6B). IHC results showed that p-Smad1/5 expression and Id1 expression were significantly downregulated in the LDN-treated mice brains (Figure 6C). Besides, in LDN-treated mice brains, c-Myc and Axin2 expressions were also found to be decreased (Figure 6D). These results suggested that LDN could be an effective inhibitor for BMP4 signaling and hence suppress the tumor progression in IDH1Mut gliomas.
Figure 6.
LDN prolongs the overall survival of mice with intracranialIDH1Mutgliomas. (A) LDN treatment significantly prolonged the overall survival of mice with intracranial IDH1Mut gliomas. Log-rank test. (B) H&E staining of mice brains in DMSO (n = 9) and LDN (n = 9) groups. The medium survival (MS) of DMSO-treated mice was 78 days, and the MS of LDN-treated mice was 94 days. Scale bar: 2 mm. (C) IHC staining of p-Smad1/5 and Id1 in mice brains. p-Smad1/5 localized in nucleus and Id1 localized in both of cytoplasm and nucleus. Scale bar: 50 μm. The expression of p-Smad1/5 and Id1 was decreased under the treatment of LDN. (D) IHC staining of c-Myc and Axin2 in mice brains. c-Myc localized in nucleus and Axin2 localized in cytoplasm. Scale bar: 50 μm. The expression of c-Myc and Axin2 was inhibited under the treatment of LDN. **P < 0.01.
Discussion
In the present study, we found through bioinformatic approaches that BMP4 is upregulated in IDH1Mut gliomas in comparison with IDH1WT gliomas. We demonstrated that BMP4 is overexpressed in patient-derived IDH1Mut glioma specimens and IDH1Mut glioma cells. The BMP4 autocrine loop promotes tumor aggressiveness evidenced by prompted cellular migration and invasion. LDN-193189, a selective inhibitor for BMPRs, blocks the effect of BMP4 and hence inhibits the tumor aggressiveness and prolongs the OS of xenograft mice. Moreover, we discovered that BMP4-Smad1 signaling promotes the activity of Wnt/β-catenin pathway via upregulating the expression of FZD1 (Supplementary Figure A). Our findings highlight a critical role of BMP4 in IDH1Mut glioma aggressiveness and reveal a potential therapeutic approach for IDH1Mut glioma patients.
The Expression of BMP4 in Human Gliomas
In human gliomas, several pioneered studies have investigated through two glioma data sets (CGGA and GSE16011 data sets) and showed that BMP4 is significantly upregulated in IDH1Mut gliomas other than IDH1WT gliomas [23]. Our study confirmed this finding through analyzing TCGA database. We found that BMP4 expression is significantly upregulated in IDH1Mut gliomas as compared with IDHWT counterparts. This result was confirmed using IHC staining of BMP4 in 46 human glioma specimens. In BTICs, the significantly higher levels of BMP4 expression were also recorded in IDH1Mut cells than in IDH1WT cells. Importantly, the secretion level of BMP4 in IDH1Mut cells was found to be much higher than IDH1WT cells, suggesting the tumor cells could be capable to impact microenvironment through BMP4 release (Figure 1). These results showed that BMP4 is upregulated in IDH1Mut glioma specimens and cells. Considering the high incidence of IDH mutation in LGGs, it is crucial to understand the underlying biology signature with BMP signaling.
The Role of BMP4 in IDH1Mut Gliomas
BMP4 has been found exhibiting critical biological functions that are related to cell proliferation, differentiation, apoptosis, migration, invasion, and epithelial–mesenchymal transition (EMT) [24]. In the several types of tumors, BMP4 showed inhibitory effect on cancer cell growth, including myeloma, breast, gastric, and pancreatic cancers [25], [26], [27], [28]. BMP-Smad signaling have been demonstrated to promote reprogramming to pluripotency by inhibiting p16/Ink4a [29].However, in brain tumors, BMP4 shows different biological on tumor cells of different histological and molecular subtypes. BMP4 increases the cell growth of meningioma, whereas inhibits the proliferation of GBM cells [30], [31]. Similarly, in our patient-derived cell models, we did not observe obvious effect on cell proliferation by BMP4 treatment (Supplementary Figure D and E). On the other hand, BMP4 has been reported remarkably to activate cellular migration and invasion in several types of malignancies such as colorectal cancer cells, ovarian cancers, hepatocellular carcinoma, and melanoma and thus promote the aggressiveness of tumor cells [13], [31], [32], [33], [34]. However, only few studies have reported the role of BMP4 on tumor aggressiveness in gliomas, especially in IDH1Mut gliomas. In the present study, we demonstrated that BMP4 promotes the cell migration and invasion in IDH1Mut glioma cells (Figure 2). In addition, when BMPRIA was knocked down using shRNAs, the invasiveness of IDH1Mut gliomas cells were significantly inhibited. These results demonstrated that the overexpression of BMP4 in IDH1Mut gliomas is contributing effects on tumor aggressiveness.
Autocrine BMP4 Signaling in IDH1Mut Gliomas
Several studies have reported that the BMP4 signaling tends to promote tumor growth, pathogenesis, and aggressiveness through autocrine manner [12], [33], [35], [36]. For example, in human ovarian cancer cells, autocrine BMP4 signaling contributes to cancer pathogenesis via regulating ID3 protooncogene expression [36]. In colorectal cancer, inhibition of autocrine BMP4 signaling induces tumor apoptosis through attenuation MAPK activity [12]. In the present study, we found that the secretion of BMP4 in TS603 cells are significantly higher than in GSC827 and GSC923 cells (Figure 1D), which indicates that BMP4 autocrine loop may play a role in the biology of IDH1Mut glioma cells. Furthermore, we showed that tumor-derived BMP4 activated to the phosphorylation of Smad1 and aggressive phenotypes, which can be blocked by LDN or BMP4 NA (Figure 3). These results suggested that the BMP4 autocrine loop from IDH1Mut glioma cells enhances the tumor aggressiveness.
BMP4 Signaling Prompts with Wnt/β-Catenin Pathway
The link between BMP4 and Wnt signaling has been shown in several different types of tumors [24]. Wnt3a activation was discovered to increase BMP4 expression in C4–2B and PC3 prostate cancer cells [37]. β-Catenin was found to specifically promote BMP4 expression and secretion in HCT116 cells [38]. In present study, we performed RNA-sequencing to investigate the downstream effector of BMP4 signaling. Surprisingly, we found that FZD1, which is one of the receptors in Wnt/β-catenin pathway, was upregulated under the treatment of BMP4. Moreover, ChIP-qPCR confirmed that BMP4-induced Smad1 directly binds to the promoter region of FZD1, suggesting the crosstalk is through Smad1-associated gene transcription (Figure 4). This is in accordance with a previous study showing that FZD1 is the downstream of BMP-Smad signaling [39]. Moreover, we also demonstrated that BMP4-induced upregulation of FZD1 promotes the activity of Wnt/β-catenin pathway and hence increases the expression of the downstream genes, such as c-Myc and Axin2 (Figure 5). These results suggested that BMP4-Smad1 signaling promotes the activity of Wnt/β-catenin pathway via upregulating FZD1.
BMP Signaling Inhibitor LDN-193189 as a Potential Therapeutic for IDH1Mut Glioma
LDN is a cell-permeable, highly effective, and selective inhibitor for BMP type I receptors ALK2 and ALK3. It inhibits BMP4-mediated Smad1, Smad5, and Smad8 phosphorylation and exerts substantially weaker effects on activin and TGF-β type I receptors and hence increases the selectivity for BMP signaling [40]. Previous studies reported that LDN suppresses tumor initiating capacity, increases tumor latency, and inhibits EMT and tumor progression or metastasis in various kinds of cancers [41], [42], [43]. Augeri et al. showed that inhibition of BMP downregulates the expression of XIAP and TAK1 leading to tumor cell death [44]. Our results revealed that LDN showed remarkable inhibitory effects on tumor migration and invasion in IDH1Mut glioma cells (Figures 2 and 3) and prolongs OS of mice bearing intracranial IDH1Mut xenografts (Figure 6). We also demonstrated that intraperitoneally injection of LDN suppresses the activity of BMP4-Smad1 signaling and hence the Wnt/β-catenin pathway in vivo. These results suggest that LDN may be an effective molecule for suppressing tumor aggressiveness in IDH1Mut gliomas.
In summary, our results demonstrated that BMP4 is overexpressed in IDH1Mut gliomas and enhances tumor migration and invasion in an autocrine pattern. BMP4 signaling promotes the activity of Wnt/β-catenin pathway via upregulating FZD1. Moreover, LDN, a selective inhibitor for BMP4 signaling, prevents the tumor progression and prolongs the survival of mice with IDH1Mut xenografts.
Funding
This work is supported by Intramural Research Program of the National Institutes of Health.
Conflicts of Interest Statement
The authors declare no potential conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tranon.2019.10.019.
Contributor Information
Fusheng Liu, Email: liufushengs@hotmail.com.
Chunzhang Yang, Email: yangc2@mail.nih.gov.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Weller M., Wick W., Aldape K., Brada M., Berger M., Pfister S.M. Glioma. Nat Rev Dis Primers. 2015;1:15017. doi: 10.1038/nrdp.2015.17. [DOI] [PubMed] [Google Scholar]
- 2.Chhabda S., Carney O., D'Arco F., Jacques T.S., Mankad K. The 2016 world health organization classification of tumours of the central nervous system: what the paediatric neuroradiologist needs to know. Quant Imaging Med Surg. 2016;6:486–489. doi: 10.21037/qims.2016.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan H., Parsons D.W., Jin G., McLendon R., Rasheed B.A., Yuan W. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parsons D.W., Jones S., Zhang X., Lin J.C., Leary R.J., Angenendt P. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waitkus M.S., Diplas B.H., Yan H. Isocitrate dehydrogenase mutations in gliomas. Neuro Oncol. 2016;18:16–26. doi: 10.1093/neuonc/nov136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balss J., Meyer J., Mueller W., Korshunov A., Hartmann C., von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116:597–602. doi: 10.1007/s00401-008-0455-2. [DOI] [PubMed] [Google Scholar]
- 7.Hartmann C., Meyer J., Balss J., Capper D., Mueller W., Christians A. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118:469–474. doi: 10.1007/s00401-009-0561-9. [DOI] [PubMed] [Google Scholar]
- 8.Hogan B.L. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- 9.Katagiri T., Watabe T. Bone morphogenetic proteins. Cold Spring Harb Perspect Biol. 2016;8 doi: 10.1101/cshperspect.a021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyazono K., Kamiya Y., Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 11.Zhao G.Q. Consequences of knocking out BMP signaling in the mouse. Genesis. 2003;35:43–56. doi: 10.1002/gene.10167. [DOI] [PubMed] [Google Scholar]
- 12.Yokoyama Y., Watanabe T., Tamura Y., Hashizume Y., Miyazono K., Ehata S. Autocrine BMP-4 signaling is a therapeutic target in colorectal cancer. Cancer Res. 2017;77:4026–4038. doi: 10.1158/0008-5472.CAN-17-0112. [DOI] [PubMed] [Google Scholar]
- 13.Rothhammer T., Poser I., Soncin F., Bataille F., Moser M., Bosserhoff A.K. Bone morphogenic proteins are overexpressed in malignant melanoma and promote cell invasion and migration. Cancer Res. 2005;65:448–456. [PubMed] [Google Scholar]
- 14.Zeng S., Zhang Y., Ma J., Deng G., Qu Y., Guo C. BMP4 promotes metastasis of hepatocellular carcinoma by an induction of epithelial–mesenchymal transition via upregulating ID2. Cancer Lett. 2017;390:67–76. doi: 10.1016/j.canlet.2016.12.042. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y., Lu Y., Celiku O., Li A., Wu Q., Zhou Y. Targeting IDH1-mutated malignancies with NRF2 blockade. J Natl Cancer Inst. 2019;111(10):1033–1041. doi: 10.1093/jnci/djy230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodin S., Antonsson L., Hovatta O., Tryggvason K. Monolayer culturing and cloning of human pluripotent stem cells on laminin-521-based matrices under xeno-free and chemically defined conditions. Nat Protoc. 2014;9:2354–2368. doi: 10.1038/nprot.2014.159. [DOI] [PubMed] [Google Scholar]
- 17.Lal S., Lacroix M., Tofilon P., Fuller G.N., Sawaya R., Lang F.F. An implantable guide-screw system for brain tumor studies in small animals. J Neurosurg. 2000;92:326–333. doi: 10.3171/jns.2000.92.2.0326. [DOI] [PubMed] [Google Scholar]
- 18.Su Y.T., Chen R., Wang H., Song H., Zhang Q., Chen L.Y. Novel targeting of transcription and metabolism in glioblastoma. Clin Cancer Res. 2018;24:1124–1137. doi: 10.1158/1078-0432.CCR-17-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee Y.C., Cheng C.J., Bilen M.A., Lu J.F., Satcher R.L., Yu-Lee L.Y. BMP4 promotes prostate tumor growth in bone through osteogenesis. Cancer Res. 2011;71:5194–5203. doi: 10.1158/0008-5472.CAN-10-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng L., Lu W., Kulkarni B., Pejovic T., Yan X., Chiang J.H. Analysis of chemotherapy response programs in ovarian cancers by the next-generation sequencing technologies. Gynecol Oncol. 2010;117:159–169. doi: 10.1016/j.ygyno.2010.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivanova T., Zouridis H., Wu Y., Cheng L.L., Tan I.B., Gopalakrishnan V. Integrated epigenomics identifies BMP4 as a modulator of cisplatin sensitivity in gastric cancer. Gut. 2013;62:22–33. doi: 10.1136/gutjnl-2011-301113. [DOI] [PubMed] [Google Scholar]
- 22.Korchynskyi O., ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem. 2002;277:4883–4891. doi: 10.1074/jbc.M111023200. [DOI] [PubMed] [Google Scholar]
- 23.Wu Q., Yao J. BMP4, a new prognostic factor for glioma. World J Surg Oncol. 2013;11:264. doi: 10.1186/1477-7819-11-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kallioniemi A. Bone morphogenetic protein 4 – a fascinating regulator of cancer cell behavior. Cancer Genet. 2012;205:267–277. doi: 10.1016/j.cancergen.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Sneddon J.B., Zhen H.H., Montgomery K., van de Rijn M., Tward A.D., West R. Bone morphogenetic protein antagonist gremlin 1 is widely expressed by cancer-associated stromal cells and can promote tumor cell proliferation. Proc Natl Acad Sci U S A. 2006;103:14842–14847. doi: 10.1073/pnas.0606857103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo D., Huang J., Gong J. Bone morphogenetic protein 4 (BMP4) is required for migration and invasion of breast cancer. Mol Cell Biochem. 2012;363:179–190. doi: 10.1007/s11010-011-1170-1. [DOI] [PubMed] [Google Scholar]
- 27.Hjertner O., Hjorth-Hansen H., Borset M., Seidel C., Waage A., Sundan A. Bone morphogenetic protein-4 inhibits proliferation and induces apoptosis of multiple myeloma cells. Blood. 2001;97:516–522. doi: 10.1182/blood.v97.2.516. [DOI] [PubMed] [Google Scholar]
- 28.Virtanen S., Alarmo E.L., Sandstrom S., Ampuja M., Kallioniemi A. Bone morphogenetic protein-4 and -5 in pancreatic cancer – novel bidirectional players. Exp Cell Res. 2011;317:2136–2146. doi: 10.1016/j.yexcr.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi Y., Hsiao E.C., Sami S., Lancero M., Schlieve C.R., Nguyen T. BMP-SMAD-ID promotes reprogramming to pluripotency by inhibiting p16/INK4A-dependent senescence. Proc Natl Acad Sci U S A. 2016;113:13057–13062. doi: 10.1073/pnas.1603668113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piccirillo S.G., Reynolds B.A., Zanetti N., Lamorte G., Binda E., Broggi G. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 31.Johnson M.D., O'Connell M.J., Vito F., Pilcher W. Bone morphogenetic protein 4 and its receptors are expressed in the leptomeninges and meningiomas and signal via the Smad pathway. J Neuropathol Exp Neurol. 2009;68:1177–1183. doi: 10.1097/NEN.0b013e3181bc6642. [DOI] [PubMed] [Google Scholar]
- 32.Deng H., Ravikumar T.S., Yang W.L. Overexpression of bone morphogenetic protein 4 enhances the invasiveness of Smad4-deficient human colorectal cancer cells. Cancer Lett. 2009;281:220–231. doi: 10.1016/j.canlet.2009.02.046. [DOI] [PubMed] [Google Scholar]
- 33.Theriault B.L., Shepherd T.G., Mujoomdar M.L., Nachtigal M.W. BMP4 induces EMT and Rho GTPase activation in human ovarian cancer cells. Carcinogenesis. 2007;28:1153–1162. doi: 10.1093/carcin/bgm015. [DOI] [PubMed] [Google Scholar]
- 34.Maegdefrau U., Amann T., Winklmeier A., Braig S., Schubert T., Weiss T.S. Bone morphogenetic protein 4 is induced in hepatocellular carcinoma by hypoxia and promotes tumour progression. J Pathol. 2009;218:520–529. doi: 10.1002/path.2563. [DOI] [PubMed] [Google Scholar]
- 35.Grockowiak E., Laperrousaz B., Jeanpierre S., Voeltzel T., Guyot B., Gobert S. Immature CML cells implement a BMP autocrine loop to escape TKI treatment. Blood. 2017;130:2860–2871. doi: 10.1182/blood-2017-08-801019. [DOI] [PubMed] [Google Scholar]
- 36.Shepherd T.G., Theriault B.L., Nachtigal M.W. Autocrine BMP4 signalling regulates ID3 proto-oncogene expression in human ovarian cancer cells. Gene. 2008;414:95–105. doi: 10.1016/j.gene.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Dai J., Hall C.L., Escara-Wilke J., Mizokami A., Keller J.M., Keller E.T. Prostate cancer induces bone metastasis through Wnt-induced bone morphogenetic protein-dependent and independent mechanisms. Cancer Res. 2008;68:5785–5794. doi: 10.1158/0008-5472.CAN-07-6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J.S., Crooks H., Dracheva T., Nishanian T.G., Singh B., Jen J. Oncogenic beta-catenin is required for bone morphogenetic protein 4 expression in human cancer cells. Cancer Res. 2002;62:2744–2748. [PubMed] [Google Scholar]
- 39.Morikawa M., Koinuma D., Tsutsumi S., Vasilaki E., Kanki Y., Heldin C.H. ChIP-seq reveals cell type-specific binding patterns of BMP-specific Smads and a novel binding motif. Nucleic Acids Res. 2011;39:8712–8727. doi: 10.1093/nar/gkr572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu P.B., Deng D.Y., Lai C.S., Hong C.C., Cuny G.D., Bouxsein M.L. BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat Med. 2008;14:1363–1369. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Owens P., Pickup M.W., Novitskiy S.V., Giltnane J.M., Gorska A.E., Hopkins C.R. Inhibition of BMP signaling suppresses metastasis in mammary cancer. Oncogene. 2015;34:2437–2449. doi: 10.1038/onc.2014.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang K., Tang X.D., Guo W., Xu X.L., Ren T.T., Ren C.M. BMPR2–pSMAD1/5 signaling pathway regulates RUNX2 expression and impacts the progression of dedifferentiated chondrosarcoma. Am J Cancer Res. 2016;6:1302–1316. [PMC free article] [PubMed] [Google Scholar]
- 43.Balboni A.L., Hutchinson J.A., DeCastro A.J., Cherukuri P., Liby K., Sporn M.B. DeltaNp63alpha-mediated activation of bone morphogenetic protein signaling governs stem cell activity and plasticity in normal and malignant mammary epithelial cells. Cancer Res. 2013;73:1020–1030. doi: 10.1158/0008-5472.CAN-12-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Augeri D.J., Langenfeld E., Castle M., Gilleran J.A., Langenfeld J. Inhibition of BMP and of TGFbeta receptors downregulates expression of XIAP and TAK1 leading to lung cancer cell death. Mol Cancer. 2016;15:27. doi: 10.1186/s12943-016-0511-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.