Abstract
An increased B-type natriuretic peptide (BNP) level is associated with cardioembolic stroke because of atrial fibrillation. However, data associating the measurement time of BNP and clinical influence of BNP are limited. Herein, we examined the utility of BNP level for prediction of stroke severity when accounting for measurement time. We retrospectively registered 327 patients admitted within 7 days from onset of ischemic stroke. We collected information on patients' background, stroke risk factors, subtype and severity, and outcome at discharge. Measurement of BNP was performed by chemiluminescent enzyme immunoassay. Patients were divided into 3 groups according to the time of BNP measurement from disease onset. Multivariate analyses were performed to evaluate the association of BNP value with outcome after patients were grouped according to BNP measurement time. Of the 327 patients, the numbers of patients whose BNP was measured within 24 h of symptom onset, between 24 and 48 h of symptom onset, and after 48 h of symptom onset were 102, 92, and 133, respectively. Favourable outcome at discharge was negatively correlated with BNP value in patients with a BNP level measured within 24 h of stroke onset. BNP value may be useful for prediction of stroke outcome if measured within 24 h after stroke onset.
Keywords: B-type natriuretic peptide (BNP), Ischemic stroke, Measurement time, Outcome
Highlights
-
•
We investigated the association between earlier BNP value and stroke severity.
-
•
We registered 327 patients admitted to our neurological center within 7 days.
-
•
BNP value was inversely correlated with the outcome when measured within 24 h.
1. Introduction
It is well known that B-type natriuretic peptide (BNP) is secreted from the myocardium at increased intracardiac pressure, especially in heart failure [[1], [2], [3]]. In addition, atrial fibrillation often causes an elevation of BNP [4,5]. Although increased BNP is an independent predictor of short- and long-term mortality in ischemic stroke [[6], [7], [8]], it remains controversial whether an increased BNP level is correlated with poor functional outcome [9,10]. Interestingly, it was also reported that BNP values were positively correlated with brain injury as well as the presence of atrial fibrillation and heart failure [7,11]. Considering the short half-life of BNP, a delay in measurement of BNP after the onset of stroke may influence the precise prediction of stroke outcome. However, few reports have investigated the time of BNP measurement from stroke onset and its relation to the predictive value of BNP level for ischemic stroke outcome.
We hypothesized that earlier BNP measurement may be more precise for the prediction of stroke severity or mortality. Furthermore, we examined the cut-off value of BNP and its reliability depending on measurement time.
2. Materials and methods
We retrospectively registered the stroke patients admitted to our neurological centre within 7 days following the occurrence of ischemic stroke, between 2010 and 2016. Patients were excluded from this study if their BNP value at admission was not examined or their outcome was unknown (Fig. 1). Patients with recurrent stroke were also excluded. We diagnosed patients with ischemic stroke when they had a rapid onset of focal brain disturbance. We confirmed the findings with diffusion weighted imaging or computed tomography after exclusion of haemorrhagic stroke. This study was approved by the ethics committee of Fukuoka University Hospital (IRB No.: 17-09-06).
Fig. 1.
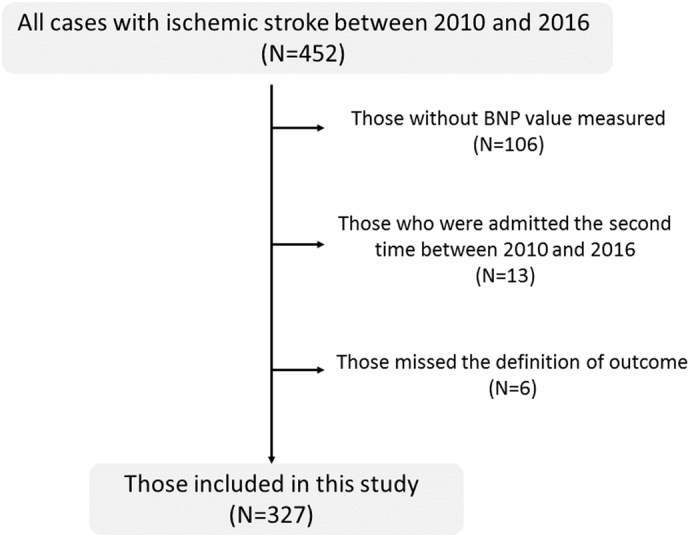
Flow chart of patient selection criteria.
We investigated medical records for stroke risk factors including age, gender, stroke subtype, hypertension, diabetes mellitus, hyperlipidaemia, atrial fibrillation, and current smoking. We also assessed their medical data for the National Institute of Health and Stroke Scale (NIHSS) score, stroke subtype, and outcome at discharge. Stroke subtype was classified using TOAST (the Trial of Org 10172 in Acute Stroke Treatment) criteria [12]. The outcome at discharge was evaluated by the modified Rankin Scale (mRS); a value of 0–1 was considered favourable because the ischemic stroke in these patients was not very severe. The mortality rate was also determined.
2.1. BNP measurement
Whether or not BNP was measured in stroke patients was clinician-dependent. However, BNP was measured when patients were hospitalized, and the measurement time was not clinician-dependent. After whole blood was collected in a tube containing EDTA-2Na and centrifuged, plasma was collected and quickly stored at −80 °C until use. Measurement of BNP levels was promptly performed after blood sampling using a chemiluminescent enzyme immunoassay based on the 2-step sandwich method (Fujirebio Inc., Tokyo, Japan) [13,14]. The 2 monoclonal antibodies against human BNP used were alkaline phosphatase-labelled or particle-bound.
2.2. Statistics
Patients were divided into 3 groups according to the time of measurement BNP: within 24 h of symptom onset (early group), 24–48 h of symptom onset (intermediate group), or after 48 h of symptom onset (late group). We utilized the 24-h and 48-h time points based on a previous study showing that BNP levels at 24 h and 48 h after acute heart failure were predictive of patients outcomes [15]. First, the differences between patients' characteristics were statistically analysed between the early, intermediate, and late groups. To assess the differences of BNP values with respect to patients' outcome and mortality at each BNP measurement time, multivariate logistic regression analysis was used. Independent variables were those that showed a trend toward being significant in univariate analyses (p < .1). If the BNP value was significantly correlated with outcome or mortality, an area under curve analysis was conducted to determine the cut-off value of BNP to predict the outcome of stroke at discharge. All data were analysed using statistical software (SPSS v22.0; IBM, NY, USA). A p-value < .05 was considered to indicate statistical significance.
3. Results
3.1. Baseline characteristics and the time to BNP measurement
We retrospectively registered 327 patients (70.7 ± 12.6 years old; 222 men, 105 women) from a total of 452 patients admitted to our neurological centre within 7 days following the occurrence of ischemic stroke, between 2010 and 2016. There were no significant differences in clinical background and neurological severity and outcome between the 327 patients who were included in this study and the 125 patients who were not (p > .05). Of the 327 patients divided according to the time to measurement of BNP, the numbers of patients in the early, intermediate, and late groups were 102, 92, and 133, respectively. Baseline characteristics of the patients are shown in Table 1. Other than the frequency of hypertension (early group: 80%, intermediate group: 89%, late group: 70%; p = .002), there were no differences between the groups. Furthermore, there was no disparity in stroke subtype or stroke severity depending on the time to measurement.
Table 1.
Baseline characteristics, outcomes and BNP values according to the measurement time of BNP.
| Within 24 h (n = 102) | 24–48 h (n = 92) | After 48 h (n = 133) | P value | |
|---|---|---|---|---|
| Age, years | 74 (65–82) | 74 (64–80) | 71 (62–78) | 0.136 |
| Sex, male (%) | 77 (75%) | 61 (66%) | 84 (63%) | 0.118 |
| Hypertension | 82 (80%) | 82 (89%) | 93 (70%) | 0.002 |
| Diabetes mellitus | 42 (41%) | 29 (32%) | 43 (32%) | 0.274 |
| Dyslipidemia | 43 (42%) | 41 (45%) | 58 (44%) | 0.943 |
| Smoking | 34 (33%) | 28 (30%) | 52 (39%) | 0.383 |
| Atrial fibrillation | 27 (26%) | 28 (30%) | 33 (25%) | 0.846 |
| Ischemic heart disease | 19 (19%) | 13 (14%) | 18 (14%) | 0.534 |
| Chronic heart failure | 3 (2.9%) | 4 (4.3%) | 2 (1.5%) | 0.44 |
| Use of tissue plasminogen activator | 2 (2%) | 4 (4%) | 6 (5%) | 0.504 |
| Main arterial disease | 35 (34%) | 27 (29%) | 28 (21%) | 0.069 |
| TOAST classification | 0.73 | |||
| Cardioembolism | 41 (40%) | 32 (35%) | 48 (36%) | |
| Small vessel occlusion | 22 (22%) | 19 (21%) | 34 (26%) | |
| Large artery atherosclerosis | 15 (15%) | 13 (14%) | 18 (14%) | |
| Other determined etiology | 17 (17%) | 16 (17%) | 15 (11%) | |
| Undetermined | 7 (7%) | 12 (13%) | 16 (12%) | |
| Cardioembolism or others | 41 (40%) | 32 (35%) | 48 (36%) | 0.71 |
| NIHSS score | 3 (1–7) | 3 (1–5) | 2 (1–4) | 0.076 |
| Hospital days | 12 (8–16) | 10 (7–16) | 12 (9–16) | 0.18 |
| Favourable outcome | 53 (52%) | 57 (62%) | 85 (64%) | 0.158 |
| BNP measurement time from the stroke onset, hr. | 15 (8–23) | 38 (31–46) | 90 (69–144) | <0.001 |
| BNP, pg/mL | 65 (30–168) | 91 (32−201) | 52 (17–109) | 0.001 |
BNP; B-type natriuretic peptide, TOAST; the Trial of Org 10,172 in Acute Stroke Treatment, NIHSS; National Institute of Health and Stroke Scale.
The median BNP measurement times were 15 h (early group), 38 h (intermediate group), and 90 h (late group). The BNP value was significantly different depending on the measurement time (early group: 65 pg/mL, intermediate group: 91 pg/mL, late group: 52 pg/mL). BNP was significantly elevated in patients with atrial fibrillation compared with those without it in all groups (p < .001 for all). However, BNP values did not differ depending on newly or already diagnosed atrial fibrillation (170.0 pg/mL vs. 212 pg/mL, respectively; p = .33).
3.2. BNP level and clinical outcomes depending on BNP measurement time
Multivariate analysis indicated that the BNP value was not significantly associated with the outcome at discharge (p = .28). When patients were categorized into the 3 groups, BNP values of patients in the late group were not associated with favourable outcomes at discharge (odds ratio of one-tenth of BNP: 0.98 [0.94–1.01]; p = .12), while univariate study suggested that those in the early (p < .001) and the intermediate (p = .006) groups were inversely correlated with outcome (Table 2). Other than BNP value, age, NIHSS score, and dyslipidaemia were associated with favourable outcome in the intermediate group (p < .1), while age, NIHSS score at admission, atrial fibrillation, main arterial disease, and TOAST classification were associated with favourable outcome in the early group (p < .1). After adjusting for these independent variables, BNP value in the intermediate group was not correlated with favourable outcome, while that in the early group showed a significant association with favourable outcome (Table 2). Furthermore, even when the same multivariate analysis was performed on patients divided into 4 quartiles according to BNP values, the quartile of BNP was still significantly correlated with favourable outcome (odds ratio: 0.49 [0.26–0.93]; p = .028). Area under curve analysis did not allow us to determine the cut-off value of BNP to predict the outcome of stroke at discharge.
Table 2.
Multivariate logistic regression analyses of the association between BNP value and favourable outcome according to BNP measurement time.
| Odds ratio | 95% CI | P | |
|---|---|---|---|
| Early group (within 24 h) | |||
| Age | 0.99 | 0.93–1.05 | 0.71 |
| NIHSS | 0.55 | 0.41–0.74 | <0.001 |
| Atrial fibrillation | 1.87 | 0.24–14.31 | 0.55 |
| Main arterial disease | 0.11 | 0.02–0.48 | 0.004 |
| TOAST | 0.80 | 0.49–1.31 | 0.38 |
| BNP/10 (pg/mL) | 0.89 | 0.80–0.99 | 0.03 |
| Intermediate group (from 24 to 48 h) | |||
| Age | 0.94 | 0.90–0.99 | 0.018 |
| NIHSS | 0.86 | 0.76–0.96 | 0.009 |
| Dislipidemia | 1.90 | 0.68–5.29 | 0.22 |
| BNP/10 (pg/mL) | 1.00 | 0.98–1.01 | 0.47 |
CI; confidence interval, NIHSS; National Institute of Health and Stroke Scale score at admission.
Nine patients died during their hospitalization in this study, with a mortality rate of 2.8%. Of those who died, the BNP value was measured in 7 (77.8%) patients within 24 h of symptom onset. Univariate analysis suggested an association of BNP value with mortality when the BNP value was measured within 24 h (p = .011; odds ratio of BNP/10: 1.02 [1.01-1.04]). However, multivariate logistic regression analyses were not significant (Table 3).
Table 3.
Multivariate logistic regression analysis of the association between BNP value and mortality in the early group.
| Early group (within 24 h) | |||
|---|---|---|---|
| Odds ratio | 95% CI | P | |
| Sex | 0.88 | 0.06–12.9 | 0.92 |
| Hypertension | 0.10 | 0.01–1.26 | 0.08 |
| NIHSS | 1.16 | 1.00–1.33 | 0.045 |
| Atrial fibrillation | 1.09 | 0.06–21.1 | 0.96 |
| Main arterial disease | 9.13 | 0.50–167.5 | 0.14 |
| BNP/10 (pg/mL) | 1.01 | 0.98–1.03 | 0.72 |
4. Discussion
The association of BNP value and stroke outcome remains unclear, although a high BNP value was reported to result from cardioembolic etiology or the occurrence of heart failure. However, a meta-analysis found that BNP levels were associated with all-cause mortality and the time from onset to death [10]. Our results indicate that BNP is inversely correlated with a favourable outcome of stroke if the measurement time is within 24 h of stroke onset, while the BNP level did not provide predictive information for outcome when it was measured after 24 h.
A previous meta-analysis suggested that the BNP value predicts stroke subtype for cardioembolic stroke in cases where BNP was measured within 72 h [16]. Our study is unique in that it indicates the importance of early measurement of BNP for the prediction of outcome after stroke occurrence. Our study showed that a stroke patient whose BNP level increases by 10 pg/mL has an 11% decreased chance of favourable outcome (mRS 0–1) when the measurement of BNP was within 24 h, suggesting that it is a good predictor of patient outcome at discharge. However, further studies are required as we were unable to determine the cut-off value of BNP to predict the outcome of stroke.
Rost et al. reported a possible association of BNP level with stroke outcomes, although the authors did not discuss the influence of measurement time of BNP on outcome [9]. Because the half-life time of BNP is as short as 20 min, the change in BNP level after ischemic stroke may attenuate with time. Our data indicate the importance of measurement time when evaluating BNP level. By contrast, it remains unclear whether the elevation in BNP level is associated with the causes of unfavourable outcome or is the result of the unfavourable outcome. The elevation in BNP level may relate to cardioembolic stroke in patients with atrial fibrillation, which results in severe ischemic stroke [4,5]. The increase of BNP may indicate myocardial injury caused by activation of the renin-angiotensin-aldosterone system and fluid retention, which leads to an unfavourable outcome [17]. Nevertheless, the severity of brain damage may also contribute to an elevation of BNP [7,11]. Clinically, increased serum BNP may predict the severity of ischemic stroke, and also identify a risk for heart failure and requirement for treatment. Moreover, serum BNP levels in patients with sinus rhythm may provide information of the necessity for strict cardiac monitoring to detect paroxysmal atrial fibrillation. BNP measurement in the field of stroke treatment is important, and further studies are required to confirm our findings.
There are several limitations of our study. First, the number of participating patients was small. Second, the measurement of BNP was clinician-dependent, and a number of patients had to be excluded from this study, which may introduce a selection bias. Third, as we did not perform long-term follow-up, we were unable to show an association of BNP value with mRS at 3 months after stroke onset. Fourth, we did not evaluate the association of neurological decline during hospitalization with the BNP value. Fifth, the BNP value in the intermediate group was higher (median: 91 pg/mL) than that in the early group (media: 65 pg/mL). Although, unlike heart failure, BNP values do not always decrease with time in stroke patients, the precise mechanisms of this finding remain unclear. Sixth, we could not determine an accurate cut-off value of BNP for predicting the outcome of stroke, although this may be accounted for, at least in part, by the inclusion of patients with and without atrial fibrillation. Seventh, the lack of information regarding the size and location of the strokes to control for neurological outcomes is a potential bias. Eight, many of the patients enrolled were not treated with thrombolysis. Finally, in our study, the BNP levels of the stroke patients were not significantly correlated with mortality, even if measured within 24 h, which is inconsistent with previous reports [7,18]. This was likely because of the small number of patients in our study who died during hospitalization. Thus, a larger study is needed to confirm the association of BNP with mortality.
5. Conclusion
The BNP value may be a useful marker for the prediction of stroke outcome when it is measured within 24 h after stroke onset.
Funding
None.
Declaration of Competing Interest
None.
Acknowledgments
We thank Ann Turnley, PhD, from Edanz Group (www.edanzediting.com/ac), for editing a draft of this manuscript.
References
- 1.Davis M., Espiner E., Richards G., Billings J., Town I., Neill A., Drennan C., Richards M., Turner J., Yandle T. Plasma brain natriuretic peptide in assessment of acute dyspnoea. Lancet. 1994;343(8895):440–444. doi: 10.1016/s0140-6736(94)92690-5. [DOI] [PubMed] [Google Scholar]
- 2.Maisel A.S., Krishnaswamy P., Nowak R.M., McCord J., Hollander J.E., Duc P., Omland T., Storrow A.B., Abraham W.T., Wu A.H., Clopton P., Steg P.G., Westheim A., Knudsen C.W., Perez A., Kazanegra R., Herrmann H.C., McCullough P.A., Breathing I. Not Properly Multinational Study, Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N. Engl. J. Med. 2002;347(3):161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 3.Wright S.P., Doughty R.N., Pearl A., Gamble G.D., Whalley G.A., Walsh H.J., Gordon G., Bagg W., Oxenham H., Yandle T., Richards M., Sharpe N. Plasma amino-terminal pro-brain natriuretic peptide and accuracy of heart-failure diagnosis in primary care. J. Am. Coll. Cardiol. 2003;42(10):1793–1800. doi: 10.1016/j.jacc.2003.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Schnabel R.B., Larson M.G., Yamamoto J.F., Sullivan L.M., Pencina M.J., Meigs J.B., Tofler G.H., Selhub J., Jacques P.F., Wolf P.A., Magnani J.W., Ellinor P.T., Wang T.J., Levy D., Vasan R.S., Benjamin E.J. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation. 2010;121(2):200–207. doi: 10.1161/CIRCULATIONAHA.109.882241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montaner J., Perea-Gainza M., Delgado P., Ribo M., Chacon P., Rosell A., Quintana M., Palacios M.E., Molina C.A., Alvarez-Sabin J. Etiologic diagnosis of ischemic stroke subtypes with plasma biomarkers. Stroke. 2008;39(8):2280–2287. doi: 10.1161/STROKEAHA.107.505354. [DOI] [PubMed] [Google Scholar]
- 6.Shibazaki K., Kimura K., Iguchi Y., Aoki J., Sakai K., Kobayashi K. Plasma brain natriuretic peptide predicts death during hospitalization in acute ischaemic stroke and transient ischaemic attack patients with atrial fibrillation. Eur. J. Neurol. 2011;18(1):165–169. doi: 10.1111/j.1468-1331.2010.03101.x. [DOI] [PubMed] [Google Scholar]
- 7.Montaner J., Garcia-Berrocoso T., Mendioroz M., Palacios M., Perea-Gainza M., Delgado P., Rosell A., Slevin M., Ribo M., Molina C.A., Alvarez-Sabin J. Brain natriuretic peptide is associated with worsening and mortality in acute stroke patients but adds no prognostic value to clinical predictors of outcome. Cerebrovasc. Dis. 2012;34(3):240–245. doi: 10.1159/000341858. [DOI] [PubMed] [Google Scholar]
- 8.Nigro N., Wildi K., Mueller C., Schuetz P., Mueller B., Fluri F., Christ-Crain M., Katan M. BNP but not s-cTnln is associated with cardioembolic aetiology and predicts short and long term prognosis after cerebrovascular events. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0102704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rost N.S., Biffi A., Cloonan L., Chorba J., Kelly P., Greer D., Ellinor P., Furie K.L. Brain natriuretic peptide predicts functional outcome in ischemic stroke. Stroke. 2012;43(2):441–445. doi: 10.1161/STROKEAHA.111.629212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Berrocoso T., Giralt D., Bustamante A., Etgen T., Jensen J.K., Sharma J.C., Shibazaki K., Saritas A., Chen X., Whiteley W.N., Montaner J. B-type natriuretic peptides and mortality after stroke: a systematic review and meta-analysis. Neurology. 2013;81(23):1976–1985. doi: 10.1212/01.wnl.0000436937.32410.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makikallio A.M., Makikallio T.H., Korpelainen J.T., Vuolteenaho O., Tapanainen J.M., Ylitalo K., Sotaniemi K.A., Huikuri H.V., Myllyla V.V. Natriuretic peptides and mortality after stroke. Stroke. 2005;36(5):1016–1020. doi: 10.1161/01.STR.0000162751.54349.ae. [DOI] [PubMed] [Google Scholar]
- 12.Adams H.P., Jr., Bendixen B.H., Kappelle L.J., Biller J., Love B.B., Gordon D.L., Marsh E.E., III Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 13.Ito Y., Namioka Y. Fundamental evaluation of two BNP assay reagents by chemiluminescent enzyme immunoassay using Lumipulse® F and Lumipulse Presto® II. Jpn. J. Med. Pharm. Sci. 2010;64(6):931–939. [Google Scholar]
- 14.Maruyama K., Uchiyama S., Shiga T., Iijima M., Ishizuka K., Hoshino T., Kitagawa K. Brain natriuretic peptide is a powerful predictor of outcome in stroke patients with atrial fibrillation. Cerebrovasc. Dis. Ext. 2017;7(1):35–43. doi: 10.1159/000457808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noveanu M., Breidthardt T., Potocki M., Reichlin T., Twerenbold R., Uthoff H., Socrates T., Arenja N., Reiter M., Meissner J., Heinisch C., Stalder S., Mueller C. Direct comparison of serial B-type natriuretic peptide and NT-proBNP levels for prediction of short- and long-term outcome in acute decompensated heart failure. Crit. Care. 2011;15(1):R1. doi: 10.1186/cc9398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Llombart V., Antolin-Fontes A., Bustamante A., Giralt D., Rost N.S., Furie K., Shibazaki K., Biteker M., Castillo J., Rodriguez-Yanez M., Fonseca A.C., Watanabe T., Purroy F., Zhixin W., Etgen T., Hosomi N., Jafarian Kerman S.R., Sharma J.C., Knauer C., Santamarina E., Giannakoulas G., Garcia-Berrocoso T., Montaner J. B-type natriuretic peptides help in cardioembolic stroke diagnosis: pooled data meta-analysis. Stroke. 2015;46(5):1187–1195. doi: 10.1161/STROKEAHA.114.008311. [DOI] [PubMed] [Google Scholar]
- 17.Idris I., Hill R., Ross I., Sharma J.C. N-terminal probrain natriuretic peptide predicts 1-year mortality following acute stroke: possible evidence of occult cardiac dysfunction among patients with acute stroke. Age Ageing. 2010;39(6):752–755. doi: 10.1093/ageing/afq098. [DOI] [PubMed] [Google Scholar]
- 18.Shibazaki K., Kimura K., Okada Y., Iguchi Y., Uemura J., Terasawa Y., Aoki J. Plasma brain natriuretic peptide as an independent predictor of in-hospital mortality after acute ischemic stroke. Intern. Med. 2009;48(18):1601–1606. doi: 10.2169/internalmedicine.48.2166. [DOI] [PubMed] [Google Scholar]


