Abstract
Introduction
Women are at increased risk for Alzheimer's disease (AD), but the reason why remains unknown. One hypothesis is that low estrogen levels at menopause increases vulnerability to AD, but this remains unproven.
Methods
We compared neuronal genes upregulated by estrogen in ovariectomized female rhesus macaques with a database of >17,000 diverse gene sets and applied a rare variant burden test to exome sequencing data from 1208 female AD patients with the age of onset < 75 years and 2162 female AD controls.
Results
We found a striking overlap between genes upregulated by estrogen in macaques and genes downregulated in the human postmortem AD brain, and we found that estrogen upregulates the APOE gene and that progesterone acts antagonistically to estrogen genome-wide. We also found that female patients with AD have excess rare mutations in the early menopause gene MCM8.
Discussion
We show with genomic data that the menopausal loss of estrogen could underlie the increased risk for AD in women.
Keywords: Estrogen, Menopause, Mitochondria, APOE, MCM8, ASPM, SORL1, ABCA7, Genetics, Women, HRT, Hormone replacement therapy
1. Introduction
Two-thirds of approximately 5.4 million Americans with Alzheimer's disease (AD) are women [1]. Women are at increased risk for AD, even after adjusting for age and education level [[1], [2], [3]]. Moreover, while APOE is the largest known genetic susceptibility factor for AD [4], women with the risk allele have much higher risk for disease than men [[5], [6], [7]]. Various lines of evidence suggest that the menopause transition underlies the increased vulnerability [[8], [9], [10], [11], [12]]. In vitro studies suggest that estrogen is neuroprotective against various cellular insults [[13], [14], [15], [16], [17], [18]] and can also confer protection from amyloid β (Aβ) toxicity [19,20], whereas postmortem analyses reveal that women with AD have reduced brain estrogen levels [21]. Moreover, early surgical removal of ovaries has been linked to cognitive decline [22] and increased risk for AD-related dementia [23].
Hormone replacement therapy (HRT) can alleviate the adverse symptoms of menopause and has been shown to reduce the risk for dementia [10,11,[24], [25], [26], [27], [28]]. However, the overall outcomes are controversial due to the findings of the Women's Health Initiative Memory Study (WHIMS) which showed that HRT increased the risk for dementia [29,30]. These seemingly contradictory research findings point to significant gaps in our understanding of the effects of estrogen on brain function [8,9]. Many of these knowledge gaps were recently highlighted by the think tank convened by the Women's Alzheimer's Research Initiative [9] and the Society for Women's Health Research Interdisciplinary Network on AD expert panel [8]. These include the need to better understand how estrogen influences risk at the molecular level [8], the need to resolve discrepancies between animal models and human clinical trials [8,9], the need to understand the role of progesterone [9], and the need to better understand how APOE confers female-biased risk [8,9].
A recent hypothesis proposed to explain how menopause increases vulnerability in women suggests that the perimenopause transition is a “bioenergetic transition state” [[31], [32], [33]]. This hypothesis proposes that estrogen promotes glucose metabolism via mitochondria [34] and that the decline in estrogen levels during perimenopause leads to a switch away from glucose toward ketone bodies as a fuel source [35]. Although this hypothesis is supported by a number of animal models [32,34] and brain-imaging studies [31,36], the mechanism by which estrogen acts as the master regulator of glucose uptake and mitochondrial function remains unknown [37].
In this study, we use a unique genomic approach to address some of the recently highlighted research gaps [8,9]. We integrate multiple data sets to gather genomic evidence for AD sex-biased risk factors. Our data identify target genes of estrogen and progesterone action within neurons, shed light on the cause of female-biased risk of APOE, and provide support for the “bioenergetic transition state hypothesis” [[31], [32], [33]] by revealing a central role for mitochondrial function in AD.
2. Methods
2.1. Microarray gene expression data
We downloaded the data set GSE16169 from the Gene Expression Omnibus [38]. We took the mean of the expression values across the two individuals in each treatment and then calculated the estrogen versus placebo fold change by dividing the mean expression values from the estrogen-treated macaques to the mean expression values for the placebo-treated macaques. We calculated the progesterone versus estrogen fold change by dividing the mean expression value of macaques treated with both estrogen and progesterone by the mean expression value of the macaques treated only with estrogen (Please see attached Supplementary Material file for more details.)
2.2. Exome sequencing data
To maximize the power to detect, we only considered white Europeans because they had the largest sample size. We extracted out white Europeans using the self-described race and ethnicity annotation. We then removed samples that were outliers on heterozygosity and outliers on race as determined by principle component analysis, cryptic relatedness, and other quality-control metrics (Supplementary Material). We filtered out all APOE ε4 homozygote individuals and only retained cases with age of onset < 75 years. We then extracted out singletons and filtered for deleterious variants, which we defined as loss of function, nonsense-mediated decay, annotated as ‘HIGH’ impact by SNPEff (version 4.3) [39] or annotated as both missense and probably damaging by Polyphen [40]. We only retained singletons not present in thousand genomes [41] or EXaC [42].
We then developed a rare variant association test similar to what was applied to copy number variations in autism [43], but we modified it for deleterious singleton SNPs. The female comparison involved female cases (n = 1208) versus female controls (n = 2162), the male comparison involved male cases (n = 953) versus male controls (n = 1495), and total comparison involved total cases (n = 2161) versus total controls (n = 3657). We calculated false discovery rate by randomizing the case-control status as described in the study by Pinto et al. [43] 1000 times. The rare variant association test was applied to all protein-coding genes after filtering out genes with less than three samples in either cases or controls with deleterious singletons.
3. Results
3.1. Macaque neuronal gene expression data
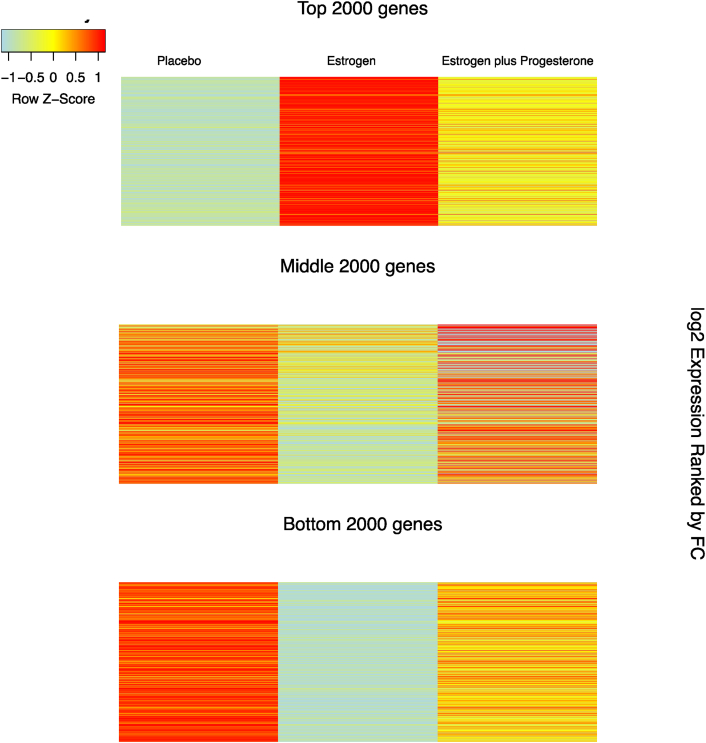
Rhesus macaques have been used to model menopause because they mimic the hormonal changes experienced by women throughout their life course [44]. We obtained microarray gene expression data from laser-captured serotonergic neurons from six female rhesus macaques (with ovaries removed) that were deposited in the Gene Expression Omnibus [38]. In this study, two macaques received estrogen, two received placebo, and two received estrogen with progesterone. We identified estrogen responsive genes by comparing gene expression patterns in macaques receiving estrogen with the gene expression patterns of macaques receiving placebo. We performed an unbiased genome-wide analysis to explore the role of estrogen in neurons, we did this by obtaining expression values from 15,517 genes which we ranked by calculating the fold change (FC) between estrogen and placebo treatments (Fig. 1, Supplementary Table 1).
Fig. 1.
Heat map of estrogen-induced gene expression values of 15,517 genes in macaque neurons. The three columns represent placebo expression, estrogen expression, and estrogen + progesterone expression: genes were ranked by fold change (FC) of estrogen-induced expression versus placebo expression. The three panels correspond to the top 2000, middle 2000, and bottom 2000 genes ranked by FC of estrogen-treated versus placebo-treated. The figure shows progesterone to be antagonistic to estrogen in the top 2000, middle 2000, and bottom 2000 genes.
3.2. Estrogen upregulates genes that are downregulated in AD
To identify any cellular and molecular pathways that may be enriched among estrogen-regulated genes, we compared the genes with greater than two-fold (FC > 2) upregulation (504 genes) with all 17,779 gene sets in the MSigDB [[45], [46], [47]] (a database of annotated gene sets from a variety of biological studies). We performed a hypergeometric ratio test to assess enrichment and found that ranked eighth, with an adjusted P value < 4.8 × 10−12, was a data set corresponding to genes downregulated in the hippocampus of patients with AD [48] (Supplementary Table 2). Among the 1027 genes identified in the AD study by Blalock et al. [48], 84 genes overlapped with the 504 estrogen upregulated genes (FC > 2). To assess the robustness of these results, we performed the same analysis using genes with FC greater than 1.5 (1222 genes) (Supplementary Table 3). This time, the same AD data set from the study by Blalock et al. [48], was ranked first (P value < 2.7 × 10−45), with 226 genes intersecting estrogen upregulated and AD downregulated gene sets (Supplementary Table 4). We compared the overlap of all 4 pairwise comparisons of estrogen upregulated and downregulated genes with Alzheimer's upregulated and downregulated genes and confirmed that the largest overlap was between estrogen upregulated and Alzheimer's downregulated genes (P value < 8.14 × 10−15, Supplementary Fig. 1, Supplementary Table 5). To estimate the false discovery rate, we compared the overlap of 100 randomized sets of 504 genes [45] and found that none of the 100 randomized gene sets had statistically significant (P value < .05) overlap with the AD downregulated gene signature from the study by Blalock et al. [48]. These results reveal that genes upregulated by estrogen are highly enriched for genes that are downregulated in human postmortem AD brains.
3.3. Mitochondrial genes link estrogen to AD risk
To gain insight into underlying mechanisms linking estrogen to AD risk, we compared the 84 genes that intersect estrogen upregulated and AD downregulated genes to pathways in MSigDB [45] and found enrichment for mitochondrial function. Ten pathways were annotated to mitochondrial-related descriptions including oxidative phosphorylation (P value < 2.8 × 10−06), mitochondrial databases (P value < 7.6 × 10−5), and the tricarboxylic acid cycle (P value < .001) (Supplementary Table 6). To confirm that this enrichment is driven by genes downregulated in AD, we compared the overlap of estrogen upregulated genes (FC > 2) with mitochondrial genes, which we obtained from MitoCarta [49] (Supplementary Table 7), and found that the proportion of mitochondrial genes in the 84 intersecting genes was much greater than the proportion of mitochondrial genes in the 504 estrogen upregulated genes (26% vs. 11%, P value < .0007, Supplementary Material, Supplementary Table 7). These results suggest that mitochondrial pathways are an important mechanism linking estrogen loss to AD risk.
3.4. Estrogen upregulates APOE and other synapse genes
We found estrogen upregulates several amyloid and synapse-related genes. Ranked third in estrogen-induced fold change out of the 15,517 genes, with a log2(fold change) of 4.44, was the AD susceptibility gene APOE (Table1). Because a key feature of AD is synapse loss [50,51], we hypothesized that estrogen responsive genes may be enriched for synaptic function. To test this, we compared synaptic genes (obtained from SynaptomeDB [52,53], number of genes = 1644) with the 504 estrogen upregulated genes (FC > 2) (Supplementary Table 4) and found a significant overlap of 140 genes (28%, P value < 2.2e−16), which remained significant even after randomization. We also quantified the overlap between the mitochondrial and synapse enrichment and found that synapse genes (n = 1644) overlapped with mitochondrial genes (n = 988) by 253 genes, which is statistically significant with a hypergeometric ratio test (P value < 6.17e−44). This suggests that estrogen's role in synapse function could at least in part be mediated by the mitochondria.
Table 1.
Top 15 estrogen upregulated genes in macaque neurons
| Log2 (fold change) | Gene | Placebo | Estrogen |
|---|---|---|---|
| 5.475 | CCT7 | 18.388 | 817.972 |
| 4.740 | RPL3 | 169.050 | 4519.695 |
| 4.437 | APOE | 153.891 | 3333.896 |
| 4.377 | EXOSC5 | 12.298 | 255.57 |
| 4.318 | ANXA2 | 22.690 | 452.592 |
| 4.098 | OGN | 55.492 | 950.043 |
| 4.088 | SLC1A6 | 24.018 | 408.522 |
| 3.964 | RPL8 | 121.042 | 1889.565 |
| 3.946 | ARF5 | 29.451 | 454.037 |
| 3.928 | STOML2 | 35.393 | 538.624 |
| 3.823 | ZFP91 | 72.352 | 1024.249 |
| 3.788 | NUDT1 | 19.980 | 276.067 |
| 3.765 | MPZ | 60.006 | 815.877 |
| 3.740 | CRMP1 | 80.719 | 1079.099 |
| 3.722 | RPS10 | 450.882 | 5952.8 |
NOTE. This table shows the log2 fold change for the top 15 genes, with the highest estrogen-induced fold change in the neurons of estrogen-treated versus placebo-treated ovariectomized female rhesus macaques. There were 15,517 genes in total (The full list is in Supplementary Table 1). This table shows that the gene with the highest estrogen-induced expression is the microtubule gene CCT7, and it shows APOE has the third highest increase in expression with a log2 fold change of 4.44.
3.5. Progesterone acts antagonistically to estrogen genome-wide
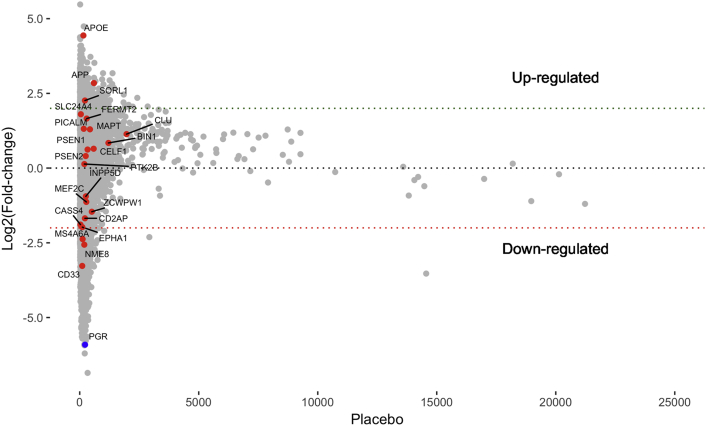
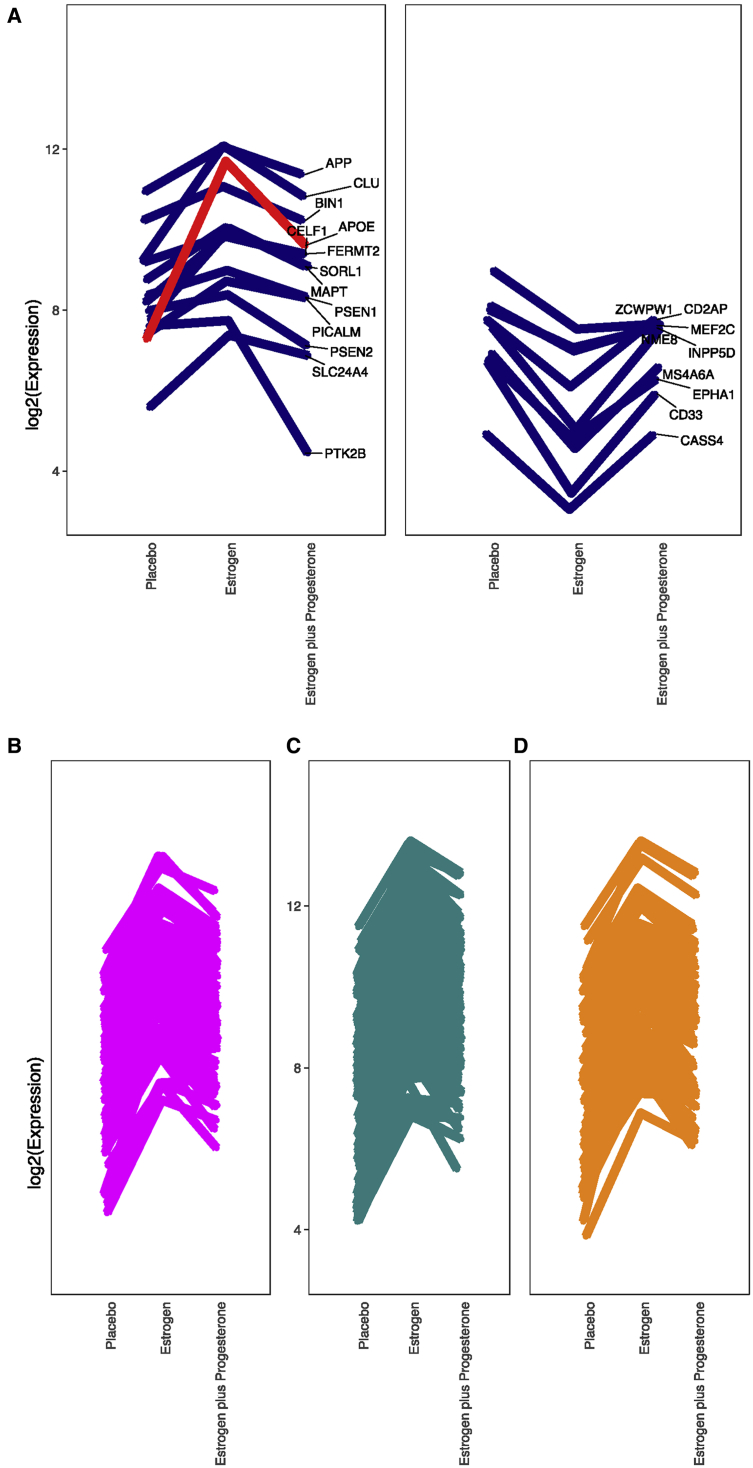
HRT often includes a progesterone component, although not much is known about how progesterone influences gene expression in the brain. Interestingly, we found that estrogen downregulates the progesterone receptor (PGR) by nearly 6-fold (Fig. 2, Supplementary Table 1), and when we compared the expression values of macaques treated with both estrogen and progesterone with macaques treated with estrogen only, we found that adding progesterone antagonized estrogen-induced gene expression on a genome-wide scale (Figure 1, Fig. 3). The mean expression of the 504 estrogen upregulated genes nearly halved after adding progesterone (t-test P value = 6.968e−14), and the mean expression of the estrogen downregulated genes increased by more than two-fold after adding progesterone (t-test P value = .001277). We found that progesterone antagonized the expression of APOE, other known AD risk genes [54] (Supplementary Material), the 84 genes intersecting estrogen upregulated and Alzheimer's downregulated gene sets, and mitochondrial [49] and synapse genes [52,53] (Fig. 3).
Fig. 2.
Fold change of estrogen-induced expression in macaque neurons plotted against placebo expression (We only show genes with placebo expression value < 25,000.). Shown in red are genes previously identified through genome-wide association studies to be associated with AD risk [54] (Supplementary Material). The progesterone receptor (PGR) gene is shown in blue. The plot shows APOE to have the third highest estrogen-induced fold change and PGR to have the fourth largest decrease in expression. It also shows APP and SORL1 to have estrogen-induced log2 fold change of > 2.
Figure 3.
Antagonistic impact of progesterone in various gene sets. (A) GWAs identified AD risk genes [54] (Supplementary Material). (B) 84 genes intersecting estrogen upregulated and Alzheimer downregulated. (C) Synapse genes [52, 53]. (D) Mitochondrial genes [49].
3.6. Rare variant burden test on exome sequencing data
By linking APOE, mitochondrial, and synapse genes to estrogen action in neurons, the macaque experiment provided insight into the mechanism by which estrogen loss at menopause could increase vulnerability to AD in women. To validate our genomic evidence linking menopause to AD risk, we sought to analyze an independent genomic data set. We obtained exome sequencing data from the Alzheimer's disease sequencing project [55]. Our aim was to assess whether any differences in mutation patterns in female AD cases compared with female AD controls could link menopause to increased vulnerability to AD in women. To enrich for AD cases with a strong genetic component, we only considered cases with the age of onset <75 years, which left us with a total of 2161 cases and 3657 controls, in which 1208 were female cases and 2162 were female controls.
To maximize our chances of uncovering genes that disrupt core biology, we focused on ultra-rare mutations known as singletons (mutations only seen once within the data set). These mutations are likely to exert strong biological effects because they have been kept at low frequency by purifying selection. We applied rigorous filtering on these singletons to enrich for causal mutations, which we defined as loss of function, nonsense-mediated decay, annotated as ‘HIGH’ impact by SNPEff (version 4.3) [39] or annotated as both missense and probably damaging by PolyPhen [40] (Please see Supplementary Tables for mutation coordinates). We developed a rare variant collapsing burden test similar to that applied to copy number variations in autism [43] but modified it for deleterious singleton single-nucleotide variants (Supplementary Table 8). Currently, many sequencing studies use the optimal test within the family of sequence kernel association tests [56,57] to identify disease-associated genes; however, we chose to use our rare variant collapsing burden test because simulations have shown that when most variants are likely to be causal, the collapsing burden test outperforms the optimal test within the family of sequence kernel association tests [56,57].
3.7. Validation of our ultra-rare variant association test
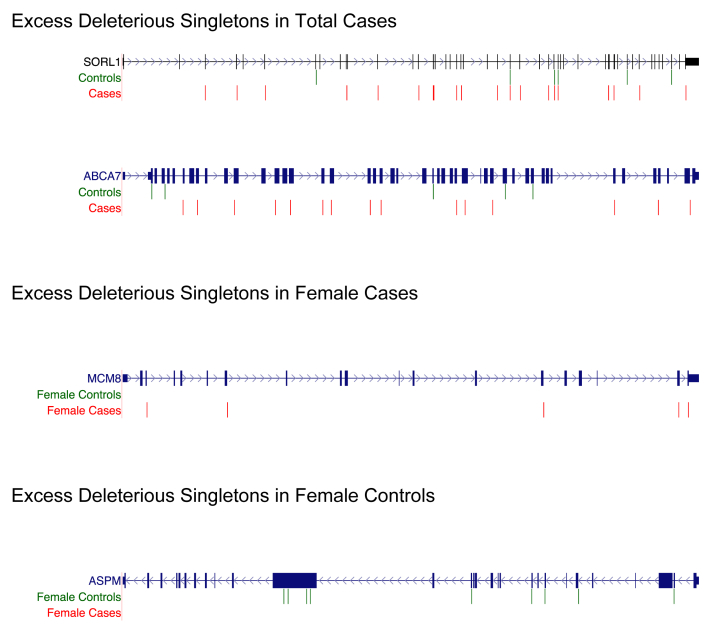
We first aimed to validate our rare variant burden test by performing it on all AD cases (n = 2161) and all AD controls (n = 3657). When we did this, we found our top hit to be SORL1 and our third top hit to be ABCA7 (Fig. 4, Supplementary Table 9). This finding confirmed the validity of our method because both SORL1 and ABCA7 are well-known AD risk loci [54,58,59]. Interestingly, our macaque neuronal gene expression data also identified SORL1 to be highly regulated by estrogen (Fig. 2, Supplementary Table 1).
Fig. 4.
Deleterious singletons identified in SORL1, ABCA7, MCM8, and ASPM. (Genes are not drawn to scale.) We developed a rare variant collapsing burden test similar to what was applied to copy number variations in autism [43] but modified for deleterious singleton SNPs. We compared female cases (n = 1208) with female controls (n = 2162) and compared total cases (n = 2161) with total controls (n = 3657). The genes depicted above have both FDR < .05 and P value < .05. Abbreviations: SNPs, single-nucleotide polymorphism; FDR, false discovery rate. (Please see Supplementary Tables for mutation coordinates).
3.8. Identification of MCM8 as a female-specific risk factor
To identify female-specific risk factors, we performed our rare variant burden test to compare female AD cases (n = 1208) with female AD controls (n = 2162). We identified 50 genes with both false discovery rate and P value < .05 (Table 2, Supplementary Table 10). Of these 50 genes, 45 contained excess mutations in cases and 5 contained excess mutations in controls. The genes with excess mutations in cases included MCM8, which we found to have five missense variants predicted to be damaging by PolyPhen [40] (Supplementary Table 11, Fig. 4). This finding provides robust independent support that menopause could underlie AD vulnerability in women because MCM8 has been strongly associated with primary ovarian insufficiency [60] and early menopause in women [61]. We also found excess mutations in female AD cases in the CCT7 and MCAT genes; which is interesting because our macaque data reveal CCT7 to have the highest estrogen-induced fold change in neurons(Table 1, Supplementary Table 1) and MCAT is a mitochondrial enzyme. To identify potential protective factors, we looked for genes with excess mutations in controls and found the brain size gene ASPM, which had eight missense variants predicted by PolyPhen to be damaging [40] and one stop-gain mutation (Fig. 4, Supplementary Table 12). We also performed our rare variant burden test on male cases (n = 953) and male controls (n = 1495) (Supplementary Table 13) and did not detect statistically significant excess of deleterious singletons in MCM8, ASPM, CCT7, or MCAT.
Table 2.
Genes with excess deleterious singletons in female cases or female controls
| Gene | FDR | Cases | Controls | Type |
|---|---|---|---|---|
| SORL1 | 0 | 13 | 4 | Excess cases |
| DNAH2 | 0.002 | 11 | 3 | Excess cases |
| METTL13 | 0.002 | 6 | 0 | Excess cases |
| INTS7 | 0.003 | 7 | 1 | Excess cases |
| KDM5A | 0.005 | 8 | 2 | Excess cases |
| SMG7 | 0.005 | 5 | 0 | Excess cases |
| OBSCN | 0.007 | 6 | 34 | Excess controls |
| ADSSL1 | 0.007 | 5 | 0 | Excess cases |
| MCM8 | 0.007 | 5 | 0 | Excess cases |
| LCTL | 0.009 | 5 | 0 | Excess cases |
| WDFY4 | 0.009 | 10 | 4 | Excess cases |
| KIF20B | 0.009 | 6 | 1 | Excess cases |
| NLRC5 | 0.01 | 4 | 0 | Excess cases |
| MUC2 | 0.011 | 6 | 1 | Excess cases |
| ZNF780B | 0.011 | 4 | 0 | Excess cases |
| PTPN13 | 0.012 | 0 | 11 | Excess controls |
| MCAT | 0.012 | 4 | 0 | Excess cases |
| RPP40 | 0.012 | 4 | 0 | Excess cases |
| ABCA7 | 0.013 | 7 | 2 | Excess cases |
| CALCR | 0.013 | 4 | 0 | Excess cases |
| CNGA4 | 0.013 | 4 | 0 | Excess cases |
| INTU | 0.013 | 4 | 0 | Excess cases |
| BTRC | 0.014 | 4 | 0 | Excess cases |
| PLEKHH3 | 0.014 | 4 | 0 | Excess cases |
| ELMSAN1 | 0.015 | 4 | 0 | Excess cases |
| ASPM | 0.015 | 0 | 9 | Excess controls |
| TANC2 | 0.016 | 7 | 2 | Excess cases |
| PTPRC | 0.016 | 5 | 1 | Excess cases |
| CTH | 0.017 | 4 | 0 | Excess cases |
| SPHK1 | 0.017 | 4 | 0 | Excess cases |
| AASDH | 0.019 | 4 | 0 | Excess cases |
| SLC39A4 | 0.019 | 5 | 1 | Excess cases |
| NFRKB | 0.019 | 6 | 2 | Excess cases |
| CCT7 | 0.02 | 4 | 0 | Excess cases |
| PGBD4 | 0.02 | 5 | 1 | Excess cases |
| FGA | 0.021 | 4 | 0 | Excess cases |
| KLHL17 | 0.023 | 1 | 13 | Excess controls |
| SCN3A | 0.025 | 5 | 1 | Excess cases |
| RP1L1 | 0.027 | 6 | 2 | Excess cases |
| KDM5B | 0.028 | 5 | 1 | Excess cases |
| KCTD19 | 0.029 | 5 | 1 | Excess cases |
| KIAA1211 | 0.029 | 6 | 2 | Excess cases |
| C7orf57 | 0.03 | 5 | 1 | Excess cases |
| FAT4 | 0.032 | 2 | 17 | Excess controls |
| IQGAP1 | 0.032 | 6 | 2 | Excess cases |
| TTC28 | 0.032 | 10 | 6 | Excess cases |
| FBN2 | 0.032 | 7 | 3 | Excess cases |
| GOLGB1 | 0.033 | 7 | 3 | Excess cases |
| KMT2E | 0.04 | 7 | 3 | Excess cases |
| SIGLEC1 | 0.049 | 7 | 3 | Excess cases |
NOTE. This table shows the significant genes identified from the deleterious singletons association test applied to female cases with the age of onset <75 years (n = 1208) versus female controls (n = 2162). The association test is similar to what was applied to copy number variations in autism [43] but modified for deleterious singleton SNPs. The FDR was calculated by randomizing the case-control status as described in the study by Pinto et al. [43] 1000 times. A total of 50 genes were identified to have both P value < .05 and FDR < .05. After ranking by FDR, and then P value, the most associated gene is SORL1, although MCM8, MCAT, ABCA7, ASPM, and CCT7 genes are also significant. The third and fourth columns show how many deleterious singletons were identified in female cases and female controls. The fifth column highlights whether the gene had excess mutations in female cases or female controls. A total of 45 genes had excess mutations in female cases, and five genes had excess mutations in female controls. ASPM is an example of a gene with excess mutations in female controls (Please see Supplementary Tables for mutation coordinates).
Abbreviations: SNP, single-nucleotide polymorphism; FDR, false discovery rate.
4. Discussion
Here, we use a unique genomic approach to uncover evidence consistent with estrogen loss at menopause underlying increased vulnerability to AD in women. We link estrogen to mitochondrial and synapse function and demonstrate that estrogen upregulates APOE and show that progesterone acts antagonistically to estrogen genome-wide. Finally, we use an independent exome sequencing data set to demonstrate that female AD cases have excess rare, deleterious mutations in the early menopause gene MCM8.
AD is a disorder of the synapse [50,51]. In fact, cognitive decline has been shown to correlate most closely with synapse loss [51]. Our finding that estrogen upregulates synapse genes fits with those of prior imaging studies, which reveal that hippocampal size correlates with estrogen levels throughout the menstrual cycle [62,63]. Our data also reveal a central role for mitochondria. We show that estrogen upregulates mitochondrial genes, and our exome data reveal excess mutations in the mitochondrial enzyme MCAT (Table 2), which is also associated with the reduction of amyloid β production [64]. These findings are consistent with the bioenergetic state transition hypothesis [[31], [32], [33]], which links metabolism deficits in the brains of postmenopausal women [31]. Importantly, our data also reveal a convergence of mitochondrial and synapse genes, which is consistent with the notion that mitochondria could influence synapse growth [65], possibly to help meet synaptic ATP requirements [66].
One of the strengths of our study is that we reveal the target genes of estrogen and progesterone action within neurons. These data reveal both APOE and SORL1 to be highly estrogen responsive (Fig. 2) and, curiously, both APOE and SORL1 are associated with female-specific risk for AD [[5], [6], [7],67]—suggesting that estrogen may interact with genetic mutations to confer sex-biased risk.
We used genomic data spanning multiple data types to cross validate our findings on estrogen loss and AD risk. We found excess deleterious singleton mutations in the ovarian failure [60,[68], [69], [70], [71]] and early menopause [61,[72], [73], [74], [75], [76]] gene MCM8 (Table 2, Supplementary Table 11), providing robust support for our hypothesis that estrogen loss at menopause confers increased vulnerability to AD in women. This finding fits with previous studies that have linked surgical menopause to doubled lifetime risk for dementia [23], increased risk for AD neuropathology [22] and cognitive decline [22].
Our method also provided the opportunity to identify potential protective genetic factors. Our finding of excess rare, deleterious singletons in the brain size gene ASPM [77] (Supplementary Tables 10,12) is interesting because ASPM has also been linked to the human-specific evolution of brain size [78,79] and because ASPM may represent a neural substrate [80] for the “brain reserve” hypothesis [[81], [82], [83], [84]], which reasons that large brain size can protect against cognitive decline [83].
Our finding that progesterone acts antagonistically to estrogen genome-wide is particularly provocative. Cell culture studies have suggested progesterone to be antagonistic [[85], [86], [87]], but our data highlight that the antagonism may be genome-wide. The seemingly contradictory findings from longitudinal studies of HRT use may be resolved by the consideration that progesterone acts antagonistically to estrogen. For instance, a large Finnish study of 230,580 women [88] found HRT containing both estrogen and progesterone seemed to increase risk for dementia, whereas estrogen-only HRT reduced the risk. This is consistent with the recent analyses [89] of two clinical trials, the Kronos Early Estrogen Prevention Study–Cognitive and Affective Study (KEEPS-Cogs) and the Early vs. Late Intervention Trial with Estradiol-Cognitive Endpoints (ELITE-Cog) [89], which found that taking HRT between the ages of 50 and 54 years is not cognitively detrimental, whereas taking HRT between the ages of 65 and 79 years was associated with reductions in global cognition, working memory, and executive function [89].
4.1. Limitations
Our data are valuable for gaining much needed clarity on the role of estrogen in AD risk; however, there are some limitations. First, we relied on macaque gene expression data to understand estrogen response in the human brain—human data would have been better, but no such datasets exist, and the experiments required to get such datasets mean they are unlikely to exist in the future. Macaques are preferable [90] to rodents because nonhuman primates more accurately recapitulate AD-relevant gene expression in the human brain [91]. Another potential limitation is the limited sample size of each treatment, which is why we focused on fold change, as other statistical tests were not feasible. However, although our sample size may be limiting, the APOE gene signal was captured by three probes, and the data were obtained from laser-captured neurons, so are unlikely to be confounded by expression from other cell types. Another potential limitation could be the different sample sizes in the exome data for men compared with women, which may lead to differential power to detect. We focused our analysis on females, which actually had more samples than males, however, not finding an association in males, may be due to decreased power to detect rather than an actual biological difference.
4.2. Future work
Our study is timely as it fits well with recent reports that have linked longer reproductive periods and more months being pregnant with reduced risk for AD [89]. Future work in this field may include stratifying female samples by HRT use to quantify the proportion of risk attributed to genetic mutations versus loss of estrogen and performing genome-wide association studies to uncover any association between common variants in MCM8 and AD risk in women.
4.3. Conclusions
Here, we endeavored to use genomic data to address the knowledge gaps surrounding why women have increased risk for AD, and in doing so, we address the controversy initiated by the Women's Health Initiative Memory Study [29,30]. Our comprehensive, integrative, genomic analyses lead us to conclude that estrogen loss is likely to contribute to AD vulnerability—suggesting that increased risk for AD in women may be attributed to menopause.
Research in context.
-
1.
Systematic review: We searched the literature, recent conference abstracts, YouTube, and review papers by the think tank convened by the Women's Alzheimer's Research Initiative and the Society for Women's Health Research Interdisciplinary Network on Alzheimer's disease (AD) to evaluate the accumulated knowledge related to whether estrogen loss contributes to AD risk in women.
-
2.
Interpretation: Previous findings have been based on observational and randomized control studies. We took a different approach of gene expression and genetic mutation data. Our findings provide quantitative evidence for the role of estrogen in AD risk, with particular emphasis on mitochondrial function.
-
3.
Future directions: Future work can involve stratifying women by HRT use to determine whether the genetic signal is stronger in women without HRT and performing genome-wide association studies to see if common variants in MCM8 confer increased risk for AD.
Acknowledgements
The authors acknowledge the Alzheimer's Disease Sequencing Project for providing the exome sequencing data. The Alzheimer's Disease Sequencing Project (ADSP) comprised two Alzheimer's Disease (AD) genetics consortia and three National Human Genome Research Institute (NHGRI)–funded Large-Scale Sequencing Centers (LSSC). The two AD genetics consortia are the Alzheimer's Disease Genetics Consortium (ADGC) funded by NIA (U01 AG032984) and the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) funded by NIA (R01 AG033193), the National Heart, Lung, and Blood Institute (NHLBI), other National Institute of Health (NIH) institutes, and other foreign governmental and nongovernmental organizations. The discovery phase analysis of sequence data is supported through UF1AG047133 to Drs. Schellenberg, Farrer, Pericak-Vance, Mayeux, and Haines; U01AG049505 to Dr. Seshadri; U01AG049506 to Dr. Boerwinkle; U01AG049507 to Dr. Wijsman; and U01AG049508 to Dr. Goate. The ADGC cohorts include Adult Changes in Thought (ACT), the Alzheimer's Disease Centers (ADC), the Chicago Health and Aging Project (CHAP), the Memory and Aging Project (MAP), Mayo Clinic (MAYO), Mayo Parkinson's Disease controls, University of Miami, the Multi-Institutional Research in Alzheimer's Genetic Epidemiology Study (MIRAGE), the National Cell Repository for Alzheimer's Disease (NCRAD), the National Institute on Aging Late Onset Alzheimer's Disease Family Study (NIA-LOAD), the Religious Orders Study (ROS), the Texas Alzheimer's Research and Care Consortium (TARC), Vanderbilt University/Case Western Reserve University (VAN/CWRU), the Washington Heights-Inwood Columbia Aging Project (WHICAP) and the Washington University Sequencing Project (WUSP), the Columbia University Hispanic-Estudio Familiar de Influencia Genetica de Alzheimer (EFIGA), the University of Toronto (UT), and Genetic Differences (GD). The CHARGE cohorts, with funding provided by 5RC2HL102419 and HL105756, include the following: Atherosclerosis Risk in Communities (ARIC) Study, which is carried out as a collaborative study supported by NHLBI contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C), Austrian Stroke Prevention Study (ASPS), Cardiovascular Health Study (CHS), Erasmus Rucphen Family Study (ERF), Framingham Heart Study (FHS), and Rotterdam Study (RS).
The three LSSCs are the Human Genome Sequencing Center at the Baylor College of Medicine (U54 HG003273), the Broad Institute Genome Center (U54HG003067), and the Washington University Genome Institute (U54HG003079). Biological samples and associated phenotypic data used in primary data analyses were stored at the study investigators' institutions and at the National Cell Repository for Alzheimer's Disease (NCRAD, U24AG021886) at Indiana University funded by NIA. Associated phenotypic data used in primary and secondary data analyses were provided by the study investigators, the NIA-funded Alzheimer's Disease Centers (ADCs), and the National Alzheimer's Coordinating Center (NACC, U01AG016976) and the National Institute on Aging Alzheimer's Disease Data Storage Site (NIAGADS, U24AG041689) at the University of Pennsylvania, funded by NIA, and at the Database for Genotypes and Phenotypes (dbGaP) funded by NIH. Contributors to the genetic analysis data included the study investigators of projects that were individually funded by NIA and other NIH institutes, and by private U.S. organizations or foreign governmental or nongovernmental organizations.
This work was supported by the National Institutes of Health (NIH R01-NIA-AG039521) to B.A.J. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication.
Footnotes
The authors have declared that no conflict of interest exists.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2019.09.004.
Supplementary Data
References
- 1.Andersen K., Launer L.J., Dewey ME, Letenneur L, Ott A, Copeland JR. Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. EURODEM Incidence Research Group. Neurology. 1999;53:1992–1997. doi: 10.1212/wnl.53.9.1992. [DOI] [PubMed] [Google Scholar]
- 2.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seshadri S, Wolf PA, Beiser A, Au R, McNulty K, White R. Lifetime risk of dementia and Alzheimer’s disease. The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49:1498–1504. doi: 10.1212/wnl.49.6.1498. [DOI] [PubMed] [Google Scholar]
- 4.Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farrer L, Cupples A, Haines J, Hyman B, Kukull W, Mayeux R. Effects of Age, Sex, and Ethnicity on the Association between Apolipoprotein E Genotype and Alzheimer Disease. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 6.PM B, Buckwaiter J, Seeman T, Miller C, Poirier J, Schellenberg GD. Evidence for an interaction between apolipoprotein E genotype, gender and Alzheimer disease. Alzheimer Dis Assoc Disord. 1999;13:216–221. doi: 10.1097/00002093-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Payami H, Zareparsi S, Montee KR, Sexton GJ, Kaye JA, Bird TD. Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: a possible clue to the higher incidence of Alzheimer disease in women. Am J Hum Genet. 1996;58:803–811. [PMC free article] [PubMed] [Google Scholar]
- 8.Nebel RA, Aggarwal NT, Barnes LL, Gallagher A, Goldstein JM, Kantarci K. Understanding the impact of sex and gender in Alzheimer’s disease: A call to action. Alzheimers Dement. 2018;14:1171–1183. doi: 10.1016/j.jalz.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snyder HM, Asthana S, Bain L, Brinton R, Craft S, Dubal DB. Sex biology contributions to vulnerability to Alzheimer’s disease: A think tank convened by the Women’s Alzheimer’s Research Initiative. Alzheimers Dement. 2016;12:1186–1196. doi: 10.1016/j.jalz.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waring S, Rocca W, Peterson R, O’Brien P, Tangalos E, Kokmen E. Postmenopausal estrogen replacement therapy and risk of AD: a population-based study. Neurology. 1999;52:965–970. doi: 10.1212/wnl.52.5.965. [DOI] [PubMed] [Google Scholar]
- 11.Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- 12.Paganini-Hill A., Henderson V.W. Estrogen replacement therapy and risk of Alzheimer disease. Arch Intern Med. 1996;156:2213–2217. [PubMed] [Google Scholar]
- 13.McEwen B.S., Woolley C.S. Estradiol and progesterone regulate neuronal structure and synaptic connectivity in adult as well as developing brain. Exp Gerontol. 1994;29:431–436. doi: 10.1016/0531-5565(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 14.Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woolley C.S., McEwen B.S. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galea LA, Spritzer MD, Barker JM, Pawluski JL. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus. 2006;16:225–232. doi: 10.1002/hipo.20154. [DOI] [PubMed] [Google Scholar]
- 18.Inagaki T., Frankfurt M., Luine V. Estrogen-induced memory enhancements are blocked by acute bisphenol A in adult female rats: role of dendritic spines. Endocrinology. 2012;153:3357–3367. doi: 10.1210/en.2012-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marin R, Guerra B, Hernandez-Jimenez JG, Kang XL, Fraser JD, Lopez FJ. Estradiol prevents amyloid-beta peptide-induced cell death in a cholinergic cell line via modulation of a classical estrogen receptor. Neuroscience. 2003;121:917–926. doi: 10.1016/s0306-4522(03)00464-0. [DOI] [PubMed] [Google Scholar]
- 20.Nilsen J, Chen S, Irwin RW, Iwamoto S, Brinton RD. Estrogen protects neuronal cells from amyloid beta-induced apoptosis via regulation of mitochondrial proteins and function. BMC Neurosci. 2006;7:74. doi: 10.1186/1471-2202-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yue X, Lu M, Lancaster T, Cao P, Honda S, Staufenbiel M. Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer’s disease animal model. Proc Natl Acad Sci U S A. 2005;102:19198–19203. doi: 10.1073/pnas.0505203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bove R, Secor E, Chibnik LB, Barnes LL, Schneider JA, Bennett DA. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology. 2014;82:222–229. doi: 10.1212/WNL.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- 24.Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci. 2006;26:10332–10348. doi: 10.1523/JNEUROSCI.3369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LeBlanc ES, Janowsky J, Chan BK, Nelson HD. Hormone replacement therapy and cognition: systematic review and meta-analysis. JAMA. 2001;285:1489–1499. doi: 10.1001/jama.285.11.1489. [DOI] [PubMed] [Google Scholar]
- 26.Henderson VW, Benke KS, Green RC, Cupples LA, Farrer LA, Group MS. Postmenopausal hormone therapy and Alzheimer’s disease risk: interaction with age. J Neurol Neurosurg Psychiatry. 2005;76:103–105. doi: 10.1136/jnnp.2003.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitmer RA, Quesenberry CP, Zhou J, Yaffe K. Timing of hormone therapy and dementia: the critical window theory revisited. Ann Neurol. 2011;69:163–169. doi: 10.1002/ana.22239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shao H, Breitner JC, Whitmer RA, Wang J, Hayden K, Wengreen H. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology. 2012;79:1846–1852. doi: 10.1212/WNL.0b013e318271f823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 30.Craig M.C., Maki P.M., Murphy D.G. The Women's Health Initiative Memory Study: findings and implications for treatment. Lancet Neurol. 2005;4:190–194. doi: 10.1016/S1474-4422(05)01016-1. [DOI] [PubMed] [Google Scholar]
- 31.Mosconi L, Berti V, Guyara-Quinn C, McHugh P, Petrongolo G, Osorio RS. Perimenopause and emergence of an Alzheimer’s bioenergetic phenotype in brain and periphery. PLoS One. 2017;12:e0185926. doi: 10.1371/journal.pone.0185926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brinton RD, Yao J, Yin F, Mack WJ, Cadenas E. Perimenopause as a neurological transition state. Nat Rev Endocrinol. 2015;11:393–405. doi: 10.1038/nrendo.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rettberg J.R., Yao J., Brinton R.D. Estrogen: a master regulator of bioenergetic systems in the brain and body. Front Neuroendocrinol. 2014;35:8–30. doi: 10.1016/j.yfrne.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brinton R.D. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31:529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao J, Hamilton RT, Cadenas E, Brinton RD. Decline in mitochondrial bioenergetics and shift to ketogenic profile in brain during reproductive senescence. Biochim Biophys Acta. 2010;1800:1121–1126. doi: 10.1016/j.bbagen.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosconi L, Berti V, Quinn C, McHugh P, Petrongolo G, Varsavsky I. Sex differences in Alzheimer risk: Brain imaging of endocrine vs chronologic aging. Neurology. 2017;89:1382–1390. doi: 10.1212/WNL.0000000000004425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y., Brinton R.D. Estrogen regulation of mitochondrial respiration is cell type and er subtype specific. Alzheimer's Demen. 2017;13 [Google Scholar]
- 38.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cingolani P, Platts A, Wang le L, Coon M, Nguyen T, Wang L. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adzhubei I., Jordan D.M., Sunyaev S.R. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet. 2013 doi: 10.1002/0471142905.hg0720s76. Chapter 7:Unit7.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Genomes Project C, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bellino F.L., Wise P.M. Nonhuman primate models of menopause workshop. Biol Reprod. 2003;68:10–18. doi: 10.1095/biolreprod.102.005215. [DOI] [PubMed] [Google Scholar]
- 45.MSigDB.
- 46.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liberzon A, Birger C, Thorvaldsdottir H, Ghandi M, Mesirov JP, Tamayo P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1:417–425. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW. Incipient Alzheimer’s disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc Natl Acad Sci U S A. 2004;101:2173–2178. doi: 10.1073/pnas.0308512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calvo S.E., Clauser K.R., Mootha V.K. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 2016;44:D1251–D1257. doi: 10.1093/nar/gkv1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koffie R.M., Hyman B.T., Spires-Jones T.L. Alzheimer's disease: synapses gone cold. Mol Neurodegener. 2011;6:63. doi: 10.1186/1750-1326-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robinson JL, Molina-Porcel L, Corrada MM, Raible K, Lee EB, Lee VM. Perforant path synaptic loss correlates with cognitive impairment and Alzheimer’s disease in the oldest-old. Brain. 2014;137:2578–2587. doi: 10.1093/brain/awu190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Synaptomedb. http://metamoodics.org/SynaptomeDB/index.php
- 53.Pirooznia M, Wang T, Avramopoulos D, Valle D, Thomas G, Huganir RL. SynaptomeDB: an ontology-based knowledgebase for synaptic genes. Bioinformatics. 2012;28:897–899. doi: 10.1093/bioinformatics/bts040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alzheimer's Disease Sequencing Project (ADSP) https://www.niagads.org/adsp/content/about
- 56.Lee S., Wu M.C., Lin X. Optimal tests for rare variant effects in sequencing association studies. Biostatistics. 2012;13:762–775. doi: 10.1093/biostatistics/kxs014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu MC, Lee S, Cai T, Li Y, Boehnke M, Lin X. Rare-variant association testing for sequencing data with the sequence kernel association test. Am J Hum Genet. 2011;89:82–93. doi: 10.1016/j.ajhg.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vardarajan BN, Ghani M, Kahn A, Sheikh S, Sato C, Barral S. Rare coding mutations identified by sequencing of Alzheimer disease genome-wide association studies loci. Ann Neurol. 2015;78:487–498. doi: 10.1002/ana.24466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Le Guennec K, Nicolas G, Quenez O, Charbonnier C, Wallon D, Bellenguez C. ABCA7 rare variants and Alzheimer disease risk. Neurology. 2016;86:2134–2137. doi: 10.1212/WNL.0000000000002627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Desai S, Wood-Trageser M, Matic J, Chipkin J, Jiang H, Bachelot A. MCM8 and MCM9 Nucleotide Variants in Women With Primary Ovarian Insufficiency. J Clin Endocrinol Metab. 2017;102:576–582. doi: 10.1210/jc.2016-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He C, Kraft P, Chen C, Buring JE, Pare G, Hankinson SE. Genome-wide association studies identify loci associated with age at menarche and age at natural menopause. Nat Genet. 2009;41:724–728. doi: 10.1038/ng.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Protopopescu X, Butler T, Pan H, Root J, Altemus M, Polanecsky M. Hippocampal structural changes across the menstrual cycle. Hippocampus. 2008;18:985–988. doi: 10.1002/hipo.20468. [DOI] [PubMed] [Google Scholar]
- 63.Barth C, Steele CJ, Mueller K, Rekkas VP, Arelin K, Pampel A. In-vivo Dynamics of the Human Hippocampus across the Menstrual Cycle. Sci Rep. 2016;6:32833. doi: 10.1038/srep32833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mao P, Manczak M, Calkins MJ, Truong Q, Reddy TP, Reddy AP. Mitochondria-targeted catalase reduces abnormal APP processing, amyloid beta production and BACE1 in a mouse model of Alzheimer’s disease: implications for neuroprotection and lifespan extension. Hum Mol Genet. 2012;21:2973–2990. doi: 10.1093/hmg/dds128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yin F, Yao J, Sancheti H, Feng T, Melcangi RC, Morgan TE. The perimenopausal aging transition in the female rat brain: decline in bioenergetic systems and synaptic plasticity. Neurobiol Aging. 2015;36:2282–2295. doi: 10.1016/j.neurobiolaging.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harris J.J., Jolivet R., Attwell D. Synaptic energy use and supply. Neuron. 2012;75:762–777. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 67.Cellini E, Tedde A, Bagnoli S, Pradella S, Piacentini S, Sorbi S. Implication of sex and SORL1 variants in italian patients with Alzheimer disease. Arch Neurol. 2009;66:1260–1266. doi: 10.1001/archneurol.2009.101. [DOI] [PubMed] [Google Scholar]
- 68.AlAsiri S, Basit S, Wood-Trageser MA, Yatsenko SA, Jeffries EP, Surti U. Exome sequencing reveals MCM8 mutation underlies ovarian failure and chromosomal instability. J Clin Invest. 2015;125:258–262. doi: 10.1172/JCI78473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tenenbaum-Rakover Y, Weinberg-Shukron A, Renbaum P, Lobel O, Eideh H, Gulsuner S. Minichromosome maintenance complex component 8 (MCM8) gene mutations result in primary gonadal failure. J Med Genet. 2015;52:391–399. doi: 10.1136/jmedgenet-2014-102921. [DOI] [PubMed] [Google Scholar]
- 70.Bouali N, Francou B, Bouligand J, Imanci D, Dimassi S, Tosca L. New MCM8 mutation associated with premature ovarian insufficiency and chromosomal instability in a highly consanguineous Tunisian family. Fertil Steril. 2017;108:694–702. doi: 10.1016/j.fertnstert.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 71.Dou X, Guo T, Li G, Zhou L, Qin Y, Chen ZJ. Minichromosome maintenance complex component 8 mutations cause primary ovarian insufficiency. Fertil Steril. 2016;106:1485–1489.e2. doi: 10.1016/j.fertnstert.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 72.Ruth K.S., Murray A. Lessons from Genome-Wide Association Studies in Reproductive Medicine: Menopause. Semin Reprod Med. 2016;34:215–223. doi: 10.1055/s-0036-1585404. [DOI] [PubMed] [Google Scholar]
- 73.Chen C.T., Fernandez-Rhodes L., Brzyski R.G., Carlson C.S., Chen Z., Heiss G. Replication of loci influencing ages at menarche and menopause in Hispanic women: the Women’s Health Initiative SHARe Study. Hum Mol Genet. 2012;21:1419–1432. doi: 10.1093/hmg/ddr570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen CT, Liu CT, Chen GK, Andrews JS, Arnold AM, Dreyfus J. Meta-analysis of loci associated with age at natural menopause in African-American women. Hum Mol Genet. 2014;23:3327–3342. doi: 10.1093/hmg/ddu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stolk L, Zhai G, van Meurs JB, Verbiest MM, Visser JA, Estrada K. Loci at chromosomes 13, 19 and 20 influence age at natural menopause. Nat Genet. 2009;41:645–647. doi: 10.1038/ng.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Day FR, Ruth KS, Thompson DJ, Lunetta KL, Pervjakova N, Chasman DI. Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nat Genet. 2015;47:1294–1303. doi: 10.1038/ng.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bond J, Roberts E, Mochida GH, Hampshire DJ, Scott S, Askham JM. ASPM is a major determinant of cerebral cortical size. Nat Genet. 2002;32:316–320. doi: 10.1038/ng995. [DOI] [PubMed] [Google Scholar]
- 78.Zhang J. Evolution of the human ASPM gene, a major determinant of brain size. Genetics. 2003;165:2063–2070. doi: 10.1093/genetics/165.4.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mekel-Bobrov N, Gilbert SL, Evans PD, Vallender EJ, Anderson JR, Hudson RR. Ongoing adaptive evolution of ASPM, a brain size determinant in Homo sapiens. Science. 2005;309:1720–1722. doi: 10.1126/science.1116815. [DOI] [PubMed] [Google Scholar]
- 80.Steffener J., Stern Y. Exploring the neural basis of cognitive reserve in aging. Biochim Biophys Acta. 2012;1822:467–473. doi: 10.1016/j.bbadis.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Satz P. Brain reserve capacity on symptom onset after brain injury: A formulation and review of evidence for threshold theory. Neuropsychology. 1993;7:273–295. [Google Scholar]
- 82.Whitwell J.L. The protective role of brain size in Alzheimer's disease. Expert Rev Neurother. 2010;10:1799–1801. doi: 10.1586/ern.10.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23:138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- 84.Wolf H, Julin P, Gertz HJ, Winblad B, Wahlund LO. Intracranial volume in mild cognitive impairment, Alzheimer’s disease and vascular dementia: evidence for brain reserve? Int J Geriatr Psychiatry. 2004;19:995–1007. doi: 10.1002/gps.1205. [DOI] [PubMed] [Google Scholar]
- 85.Aguirre C.C., Baudry M. Progesterone reverses 17beta-estradiol-mediated neuroprotection and BDNF induction in cultured hippocampal slices. Eur J Neurosci. 2009;29:447–454. doi: 10.1111/j.1460-9568.2008.06591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yao J, Chen S, Cadenas E, Brinton RD. Estrogen protection against mitochondrial toxin-induced cell death in hippocampal neurons: antagonism by progesterone. Brain Res. 2011;1379:2–10. doi: 10.1016/j.brainres.2010.11.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Woolley C.S., McEwen B.S. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- 88.Imtiaz B, Taipale H, Tanskanen A, Tiihonen M, Kivipelto M, Heikkinen AM. Risk of Alzheimer’s disease among users of postmenopausal hormone therapy: A nationwide case-control study. Maturitas. 2017;98:7–13. doi: 10.1016/j.maturitas.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 89.Conference, AsAI, Pregnancy and Reproductive History May Impact Dementia Risk Plus, the Move to Re-Think the Impact of Hormone Therapy on Cognition. 2018. [Google Scholar]
- 90.Heuer E, Rosen RF, Cintron A, Walker LC. Nonhuman primate models of Alzheimer-like cerebral proteopathy. Curr Pharm Des. 2012;18:1159–1169. doi: 10.2174/138161212799315885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miller J.A., Horvath S., Geschwind D.H. Divergence of human and mouse brain transcriptome highlights Alzheimer disease pathways. Proc Natl Acad Sci U S A. 2010;107:12698–12703. doi: 10.1073/pnas.0914257107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.