Abstract
Introduction
While telomere shortening, a marker of cellular aging, may impact the progression of age-related neurodegenerative diseases, its association with cognition is unclear, particularly in the context of Alzheimer's disease (AD) pathology.
Methods
Telomere, cognitive, and CSF data from 482 participants in the AD Neuroimaging Initiative (148 cognitively normal, 283 mild cognitive impairment, 51 AD) was leveraged to assess telomere length associations with cognition (measured by memory and executive function) and interactions with CSF amyloid-β, tau, and APOE-ε4. Secondary analyses assessed brain volume and thickness outcomes.
Results
Longer telomeres at baseline were associated with faster executive function decline. Amyloid-β and tau interacted with telomere length on cognition, with longer telomeres related to faster decline among biomarker-positive individuals.
Discussion
Telomere associations with cognition shift with AD progression, with longer telomeres related to worse outcomes as pathology increases, highlighting the need for further investigation of telomere length along the AD neuropathological cascade.
Keywords: APOE, Executive function, Cognition, CSF biomarkers, Telomeres
1. Introduction
Telomeres are long, repeated sequences at the end of chromosomes [1], which typically shorten with each cell cycle due to incomplete replication, leading to cellular senescence or cell death [2]. Telomeres serve to prevent genomic instability and chromosome fusion and shortened telomere length is unsurprisingly associated with many age-related diseases [3]. For this reason, telomere length has been leveraged in previous work as a marker of biological aging, a process that may be distinct from chronological aging [4]. Since long telomeres protect cells from cellular senescence and death, it is expected that they would also protect neuronal cells from the oxidative stress and neurodegeneration that are part of the progression of Alzheimer's disease (AD). However, reports of the role of telomere length in AD risk are mixed. Though some studies have observed no association between telomere length and AD [5,6], other studies have found shorter telomeres in participants with AD [[7], [8], [9], [10]] or other types of dementia [11] compared to cognitively normal participants. In contrast, Thomas et al. [7] observed longer telomere length in the hippocampus of individuals who had AD compared to controls. However, these associations varied by age as well as by the cell type and tissue type in which telomere length was measured even within the same study.
In addition to dementia, telomere length has been associated with cognition and AD severity in several studies [8,12,13], although the literature again is not consistent [5,[14], [15], [16]]. Interestingly, one study found a nonlinear association between telomere length and mild cognitive impairment (MCI), where both the shortest and longest telomere-length quintiles were associated with increased risk of amnestic MCI [17]. In a separate study, individuals with MCI who progressed to AD had longer telomeres than those with stable MCI [18]. Together, these findings suggest that the association between telomere length and cognition is complex and may change over the clinical course of the disease.
Our previous work has identified genes involved in telomere maintenance that modify the association between AD biomarkers and downstream neurodegeneration [19,20], highlighting a potential pathway of neuroprotection from the consequences of AD pathology. Specifically, in agnostic scans for genetic variants that modify the association between cerebrospinal fluid (CSF) levels of amyloid-β (Aβ) or tau and neurodegeneration, we identified variants in the protection of telomeres 1 (POT1) gene [20] and the serine palmitoyltransferase long chain base subunit 1 (SPTLC1) gene [19], which regulate telomerase through ceramide synthesis. These associations suggest that telomeres may be particularly relevant to the neurodegenerative response to AD pathology.
Few studies investigating telomere length in the context of AD have assessed associations or interactions with biomarkers of AD pathology [10,18,21]. To date, two studies have observed no association between telomere length and Aβ burden at autopsy or in the CSF [10,18]. Interestingly, Rolyan et al. [21] found that an AD mouse model with shortened telomeres had reduced Aβ pathology and less cognitive impairment compared to AD mice with normal telomeres. More work in larger cohorts assessing interactions between biomarkers of AD neuropathology and telomere length may clarify whether telomere length is relevant to the downstream consequences of neuropathology.
Additionally, APOE-ε4 allele status, a major risk factor for AD, may contribute to the heterogeneity in the association between telomeres and cognition. Wikgren et al. [22] observed that nondemented APOE-ε4 carriers had longer telomeres and a faster rate of attrition compared to noncarriers. Furthermore, longer telomeres were predictive of worse cognitive performance in those APOE-ε4 carriers. The differences in telomere length and rate of shortening between APOE-ε4 carriers and noncarriers is one that bears further study, particularly in the context of AD-related outcomes.
The goal of this project was to assess the association between baseline leukocyte telomere length and cognitive performance, as well as the interaction between biomarkers of AD neuropathology and leukocyte telomere length on cognitive trajectories. Additionally, we examined telomere associations with and biomarker interactions on brain volume outcomes. The outcome of this work will clarify the degree to which baseline telomere length provides protection or susceptibility to the consequences of AD neuropathology.
2. Materials and methods
2.1. Participants
Data were acquired from the AD Neuroimaging Initiative (ADNI), a longitudinal, multisite study launched as a public-private partnership in 2004 led by Principal Investigator Michael W. Weiner, MD. Recruited participants were 55–90 years old and had no significant neuropathological disease, except for AD, and did not have a history of brain lesion, head trauma, or psychoactive medication use. See http://adni.loni.usc.edu/about/ for more details about the ADNI study. For the current analyses, we leveraged data from participants who had baseline telomere length measurement and CSF biomarker acquisition within one year of each other, as well as longitudinal cognition. These criteria left 482 participants for the main analyses. For secondary analyses, we restricted the sample to participants with longitudinal magnetic resonance imaging data, dropping the sample size to 440. Informed consent was obtained from all participants or an authorized representative.
Longitudinal collection of cognitive, imaging, and CSF data began before telomere length measurement. Cognitive or imaging visits more than 180 days before telomere measurement were dropped and the closest visit to telomere measurement was considered baseline for the current analyses. Additionally, any participant who did not have CSF acquisition within one year of telomere measurement was excluded.
2.2. Measurement of telomere length
Genomic DNA from ADNI subjects was extracted from buffy coats or blood at the National Centralized Repository for Alzheimer's Disease and Related Dementias (NCRAD). NCRAD technicians then plated and shipped genomic DNA to the Telomere Biology Core Lab at the University of California, San Francisco. Technicians blinded to demographic and clinical data performed quantitative polymerase chain reaction (qPCR) to determine relative telomere length (i.e., T/S ratio). The current analyses used T/S ratios adjusted for experimental variables, which were then converted into base pairs using the following calculation: telomere base pairs = 3274 + 2413 * (T/S ratio). Only baseline telomere length was used for the current analyses. For more details on telomere length acquisition and quality control, see the ADNI telomere methods document on LONI (http://adni.loni.usc.edu/) and Nudelman et al. [23].
2.3. Cognitive composites
Cognition data was downloaded from the ADNI database on July 12, 2018. Comprehensive neuropsychological evaluations were performed at multiple time points and composite measures of memory and executive function were calculated as reported previously [24,25]. Briefly, the memory composite included item-level data from Rey Auditory Verbal Learning Test, AD Assessment Scale-Cognitive Test, Mini-Mental State Examination, and Logical Memory I and II. The executive function composite included item-level data from Trail Making Test Parts A and B, Digit Span Backward, Digit Symbol, Animal Fluency, Vegetable Fluency, and Clock Drawing Test.
2.4. CSF AD biomarkers
CSF was collected by lumbar puncture. The current analyses used the closest CSF collection to the baseline telomere measurement. Levels of Aβ-42 and total tau (t-tau) were quantified on the multiplex xMAP Luminex platform (Luminex Corp, Austin, TX) and Innogenetics INNO-BIA AlzBio3 (Innogenetics, Ghent, Belgium) immunoassay reagents, as described previously [26]. To interpret results from biomarker interaction analyses, we stratified the analyses by biomarker groups, based on previously defined thresholds of ≤192 pg/mL for Aβ positivity and ≥93 pg/mL for t-tau positivity [27].
2.5. Brain volume and cortical thickness outcomes
Brain volume data was collected at multiple time points as part of ADNI's comprehensive neuroimaging protocol, which has been described elsewhere [28]. The current analyses leveraged volume metrics that we have analyzed in previous work [29] including the hippocampus, inferior lateral ventricles (ILV), and thickness measures [29] from four brain networks that are known to change over the course of aging and AD: the default mode network, frontal lobe, medial temporal cortex, and sensory cortex [30].
2.6. Statistical analyses
Statistical analyses were conducted in R (version 3.5.1, https://www.r-project.org/). Significance was set a priori to α = 0.05. P value corrections were performed using the Bonferroni method for the number of tests in each analysis. We analyzed associations between telomere length and both cross-sectional and longitudinal cognition (executive function and memory), as well as interactions between telomere length and CSF levels of Aβ-42 and t-tau on the cognitive outcomes. Analyses leveraged mixed effects regression models with the intercept and interval (modeled as time in years from baseline) entered as both fixed and random effects. Longitudinal cognitive change was evaluated with an interval-predictor interaction term. Biomarker interaction models were run with continuous CSF measures and models stratified by Aβ and tau positivity were run to interpret observed biomarker interaction effects. All models covaried for sex, age, and diagnosis closest to telomere length acquisition. Secondary analyses were run to assess interactions with APOE-ε4 allele status as well as associations with AD biomarker levels and brain volumes. All brain volume and thickness models covaried for intracranial volume and scanner strength, as well as age, sex, and baseline diagnosis. Associations with both baseline volume or thickness and annual change in volume or thickness were evaluated.
3. Results
Participant characteristics are presented in Table 1. Participants were well-educated and over 50% were MCI. As expected, diagnostic groups differed in baseline cognitive performance, AD biomarker levels, and the prevalence of the APOE-ε4 allele. No group differences in telomere length were observed. In the imaging subset, baseline brain volumes and thicknesses differed by diagnosis, with MCI and AD participants having progressively smaller brain volumes and thickness and larger ILV volumes.
Table 1.
Participant characteristics
| NC | MCI | AD | P value | Total | |
|---|---|---|---|---|---|
| N | 148 | 283 | 51 | 482 | |
| Age at baseline, years | 75.49 ± 6.45 | 72.22 ± 7.41 | 75.39 ± 8.06 | <.01 | 73.56 ± 7.36 |
| Sex, % female | 49% | 46% | 35% | .22 | 46% |
| % Non-Hispanic white | 95% | 96% | 94% | .69 | 95% |
| Education, years | 16.43 ± 2.72 | 16.15 ± 2.61 | 16.04 ± 2.72 | .52 | 16.22 ± 2.65 |
| % APOE-ε4 carriers | 26% | 44% | 75% | <.01 | 42% |
| Memory performance | 1.02 ± 0.60 | 0.28 ± 0.72 | −0.93 ± 0.56 | <.01 | 0.38 ± 0.87 |
| Executive function | 0.85 ± 0.81 | 0.36 ± 0.86 | −0.92 ± 0.89 | <.01 | 0.38 ± 0.98 |
| CSF Aβ-42, pg/mL | 192.33 ± 52.86 | 172.77 ± 50.36 | 142.79 ± 41.23 | <.01 | 175.61 ± 52.18 |
| CSF t-tau, pg/mL | 70.22 ± 32.43 | 90.19 ± 54.89 | 126.92 ± 55.76 | <.01 | 87.87 ± 51.65 |
| Telomere length, bp | 5339.56 ± 376.07 | 5396.59 ± 378.89 | 5434.82 ± 419.15 | .20 | 5383.12 ± 382.92 |
| Imaging outcomes | |||||
| N | 135 | 258 | 47 | 440 | |
| ILV volume, mm3 | 1337.26 ± 962.20 | 1689.84 ± 1272.48 | 3041.30 ± 1531.83 | <.01 | 1726.02 ± 1307.23 |
| Hippocampal volume, mm3 | 7350.61 ± 932.06 | 7057.93 ± 1126.00 | 5325.72 ± 1110.20 | <.01 | 6962.70 ± 1214.52 |
| Default mode network thickness | 55.95 ± 2.94 | 55.91 ± 2.99 | 51.26 ± 3.50 | <.01 | 55.43 ± 3.35 |
| Frontal lobe thickness | 58.64 ± 3.15 | 58.73 ± 2.71 | 56.35 ± 3.57 | <.01 | 58.45 ± 3.03 |
| Medial temporal lobe thickness | 23.45 ± 1.63 | 22.89 ± 2.11 | 20.13 ± 2.29 | <.01 | 22.77 ± 2.20 |
| Sensorimotor cortex thickness | 3443.52 ± 576.90 | 3598.44 ± 524.87 | 3496.50 ± 545.02 | .02 | 3540.02 ± 546.87 |
| Intracranial volume, mm3 | 1494888.74 ± 149356.69 | 1525088.05 ± 153513.36 | 1510446.81 ± 154640.18 | .17 | 1514233.74 ± 152630.51 |
NOTE. Boldface indicates P values less than .05. Values are either mean ± standard deviation or % of total.
Abbreviations: NC, normal cognition; MCI, mild cognitive impairment; AD, Alzheimer's disease; APOE, apolipoprotein E; CSF, cerebrospinal fluid; Aβ-42, β-amyloid-42; bp, base pair; ILV, inferior lateral ventricle.
3.1. Telomere length associations with cognition
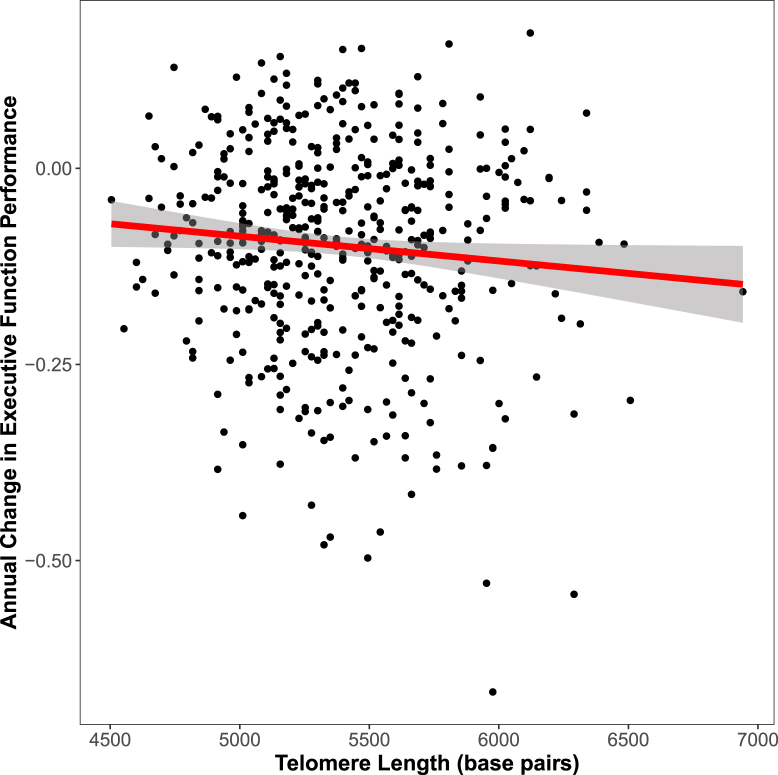
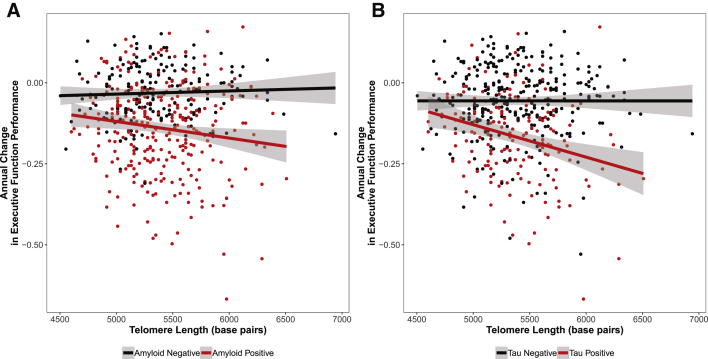
Longer telomeres were associated with faster decline in executive function (Table 2 and Fig. 1). In interaction models, tau interacted with telomere length on baseline executive function and both Aβ and tau interacted with telomere length on change in executive function. In stratified models, longer telomeres were associated with lower baseline executive function among tau-positive individuals and with faster decline in executive function in biomarker-positive individuals (Table 3 and Fig. 2). These associations and interactions passed correction for multiple tests.
Table 2.
Telomere length association with cognition
| Outcome | Model | β | SE | DF | P value |
|---|---|---|---|---|---|
| Executive function | longitudinal | −7.01E-05 | 2.14E-05 | 2042 | .0011* |
| Memory performance | cross-sectional | −1.17E-04 | 9.01E-05 | 478 | .1937 |
| Memory performance | longitudinal | −1.70E-05 | 1.69E-05 | 2066 | .3129 |
| Executive function | cross-sectional | −8.09E-05 | 1.02E-04 | 477 | .4266 |
NOTE. Boldface indicates P values less than .05. Asterisks indicate models that survived Bonferroni correction for four tests.
Abbreviations: β, beta; SE, standard error; DF, degrees of freedom.
Fig. 1.
Telomere length and longitudinal executive function performance. Telomere length is associated with longitudinal executive function decline, shown here with telomere length in base pairs on the x-axis and annual change in executive function on the y-axis.
Table 3.
Telomere length interactions with CSF AD biomarkers on cognition
| Outcome | Model | β |
SE |
DF |
P value |
β |
SE |
DF |
P value |
β |
SE |
DF |
P value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aβ-42 interaction | Aβ-42 positive | Aβ-42 negative | |||||||||||
| Executive function | longitudinal | 1.46E-06 | 3.99E-07 | 2035 | .0003* | −0.0001 | 3.52E-05 | 1168 | .0006* | 5.92E-06 | 2.12E-05 | 866 | .7799 |
| Memory performance | longitudinal | 5.86E-07 | 3.04E-07 | 2059 | .0540 | −3.66E-05 | 2.59E-05 | 1189 | .1583 | 2.12E-05 | 1.62E-05 | 869 | .1903 |
| Executive function | cross-sectional | 3.10E-06 | 1.77E-06 | 474 | .0802 | −6.52E-05 | 0.0001 | 294 | .6287 | 4.96E-05 | 0.0001 | 178 | .7072 |
| Memory performance | cross-sectional | 2.68E-06 |
1.58E-06 |
475 |
.0908 |
−8.48E-05 |
0.0001 |
295 |
.4768 |
−6.01E-05 |
0.0001 |
178 |
.6283 |
| Tau interaction |
Tau positive |
Tau negative |
|||||||||||
| Executive function | longitudinal | −1.79E-06 | 4.44E-07 | 1952 | 5.86E-05* | −0.00021 | 4.86E-05 | 603 | 1.46E-05* | −2.73E-05 | 2.14E-05 | 1348 | .2030 |
| Memory performance | longitudinal | −6.38E-07 | 3.40E-07 | 1976 | .0611 | −7.34E-05 | 3.64E-05 | 616 | .0443 | 6.87E-06 | 1.71E-05 | 1359 | .6881 |
| Executive function | cross-sectional | −4.54E-06 | 1.87E-06 | 455 | .0156 | −0.00021 | 0.0002 | 158 | .3152 | −7.17E-06 | 0.0001 | 295 | .9480 |
| Memory performance | cross-sectional | −1.45E-06 | 1.64E-06 | 456 | .3759 | −0.00011 | 0.0002 | 159 | .5303 | −0.0001 | 9.57E-05 | 295 | .2853 |
NOTE. Boldface indicates P values less than .05. Asterisks indicate models that survived Bonferroni correction for eight tests.
Abbreviations: β, beta; SE, standard error; DF, degrees of freedom; Aβ-42, β-amyloid-42.
Fig. 2.
CSF biomarker interactions with telomere length on longitudinal executive function. Telomere length interacts with both CSF Aβ-42 and tau on change in executive function, such that telomere length is associated with decline in executive function only in biomarker-positive individuals. Annual change in executive function is presented on the y-axis, and telomere length along on the x-axis. Points and lines are colored by CSF amyloid or tau status, with biomarker-positive individuals in red and biomarker-negative individuals in black.
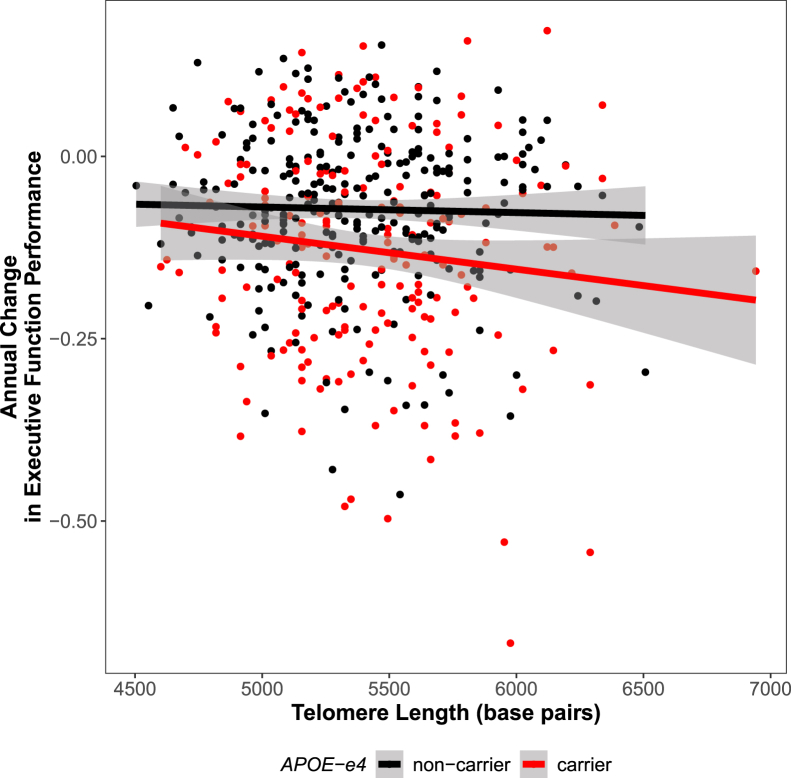
APOE-ε4 allele status also interacted with telomere length significantly to predict executive function trajectories (P = .003; see Fig. 3 and Supplementary Table 1), such that longer telomeres in ε4 carriers are associated with faster decline in executive function performance (P = .001). Both associations survived correction for multiple comparisons. This association appears to be in a dose-dependent fashion with the effect appearing strongest among ε4 homozygotes (see Supplementary Fig. 1).
Fig. 3.
APOE-ε4 carrier status x telomere length interaction on executive function. Telomere length interacts with APOE-ε4 carrier status on change in executive function, such that longer telomeres at baseline are associated with decline in executive function among carriers only. Annual change in executive function is presented on the y-axis, and telomere length on the x-axis. Points and lines are colored by APOE-ε4 carrier status, with carriers in red and noncarriers in black.
3.2. Telomere associations with CSF AD biomarkers and brain volumes
Telomere length was not associated with CSF levels of Aβ or tau (P > .13). Additionally, telomere length was not associated with any of the brain volume outcomes, cross-sectionally or longitudinally (see Supplementary Table 2). However, there was an interaction between Aβ and telomere length on medial temporal lobe thickness that approached our corrected threshold for statistical significance (β = 2 × 10−6, standard error = 1 × 10−6, P = .005), such that longer telomeres were associated with greater decline in medial temporal lobe thickness among biomarker negative individuals (see Supplementary Table 3 and Supplementary Fig. 2).
4. Discussion
The present study provides evidence that the downstream associations of longer baseline telomeres on cognition depends, in part, on the presence or absence of neuropathology. Specifically, longer telomeres at baseline were associated with more rapid cognitive decline in the presence of enhanced biomarkers of AD neuropathology. In contrast, longer telomeres among biomarker-negative individuals displayed null or borderline protective associations with downstream brain aging outcomes. Together, these results suggest that longer telomeres at baseline are associated with worse longitudinal outcomes among people with high levels of baseline pathology, but better longitudinal outcomes among people with lower levels of baseline pathology. Such complex interactions may partially explain the heterogeneity in the telomere literature, as telomere associations appear to change over the course of the neuropathological cascade in AD.
Our primary finding was that longer leukocyte telomeres at baseline were associated with more rapid longitudinal cognitive decline, particularly among individuals with evidence of greater AD pathology. The direction of this association is counterintuitive given that shorter telomeres have previously been linked to cognitive decline in nondemented, aging adults as well as a more severe presentation of AD [8,12,31]. However, another study observed a similar result whereby longer leukocyte telomeres were associated with increased risk of conversion to MCI and AD [18]. Moreover, Liu et al. [32] found that longer peripheral blood cell telomeres were associated with worse Minimum Mental State Examination (MMSE) scores.
There are multiple hypothetical mechanisms that may explain the detrimental associations of longer telomeres among biomarker-positive individuals. One possibility is that telomerase (which maintains telomere length) is upregulated in response to AD pathology and drives a slowing of telomere shortening during the preclinical phases of AD. In support of such a possibility, previous work has demonstrated upregulation of telomerase in response to inflammation [33] and immune activation [34,35], both of which occur in response to AD pathology [36]. In such a scenario telomerase may be actively elongating and maintaining telomeres in response to the inflammatory cascade in AD. In opposition to this hypothesis, there is also evidence that Aβ inhibits telomerase activity [37], suggesting that an upregulation of telomerase's canonical activity may be unlikely. However, as intracellular Aβ may decrease with the development of extracellular plaques [38], telomerase could potentially be more active as the plaques accumulate.
A second possibility is that this detrimental association reflects an epiphenomenon due to a non-telomere-related upregulation of telomerase components. For example, the catalytic subunit of telomerase, TERT, has been shown to protect against both tau- and amyloid-mediated oxidative stress and apoptosis in cultured neurons [39,40]. It may be that longer telomeres reflect an enhanced state of TERT activation that is beneficial early in disease, but becomes detrimental as inflammation accelerates. Regardless of the underlying mechanisms, a more comprehensive assessment of how telomere associations change over the course of the neuropathological cascade in AD is needed.
A comparable interaction was also observed with APOE status, such that longer telomeres in APOE-ε4 homozygotes were associated with faster cognitive decline. This corresponds to another study in which longer telomeres were associated with worse episodic memory performance in ε4 carriers compared to noncarriers [22]. In contrast, Takata et al. [6] found that APOE-ε4 homozygotes with AD had shorter telomeres than heterozygotes and noncarriers, although they did not see an association between telomere length and cognitive decline. The ε4 allele has a proinflammatory effect [41] and a strong association with AD pathology [42], leaving open the possibility that either of the mechanisms discussed above could be driving the APOE interactions observed here.
In contrast to the detrimental associations observed among biomarker positive individuals, we observed a beneficial effect of longer telomeres among biomarker individuals, whereby longer telomeres were associated with a slower rate of cortical thinning in the medial temporal cortex. Comparable associations between telomere length and brain volumes have been reported in the past [43], particularly among nondemented individuals and in subcortical regions of the hippocampus [[43], [44], [45]]. Since the medial temporal lobe is one of the first to exhibit atrophy in the beginning stages of AD [46], the current results suggest that longer telomeres may protect against initial decline before substantial pathology has accumulated. Given that we did not see significant associations between telomere length and brain volume outcomes among biomarker positive individuals, it is possible that the telomere interaction on cognition may result from an alternative pathway of injury in AD. Although neurodegeneration is the primary pathway by which AD pathology drives cognitive impairment [47], there are also alternative pathways such as reductions in cerebral blood flow, white matter degeneration, network connectivity, and glucose metabolism. More studies are needed to truly understand the exact molecular pathways by which longer leukocyte telomeres are associated with worse cognitive outcomes.
This study exhibits multiple strengths including the breadth of cognitive, imaging, and genetic data available in the cohort, the detailed measures of telomere length, and the longitudinal data following telomere measurement. However, the study is not without limitations. One limitation of the present study is that telomeres were measured peripherally, which may not reflect telomeric changes in the brain. Lukens et al. [48] found that shortened leukocyte telomere length correlated with cerebellar telomere length in AD patients, but the correlation remains an open question in the field. A second limitation is that the cohort is primarily well-educated, non-Hispanic white individuals, which limits the ability to generalize the findings to other populations. Additionally, we were unable to control for environmental factors like socioeconomic status or lifestyle which may impact telomere length [49,50]. Finally, the current analyses were underpowered to measure interactions with age or age-stratified models, but that would be an interesting way to assess at what age telomere length is a useful explanatory factor. Larger samples across the spectrum of AD will be needed to fully characterize the complex interactions among age, pathology, and telomeres and how they contribute to cognitive impairment and dementia.
In conclusion, this study has described a unique interaction between telomere length and biomarkers of AD neuropathology, suggesting that telomere associations may depend on the neuropathological context in which they are measured. Additional work focusing on the complex interplay between markers of biological aging, chronological aging, and disease may clarify the context in which telomeres are most relevant to AD risk and progression.
Acknowledgments
This work was supported by the National Institutes of Health [R01 AG059716].
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
The authors would like to acknowledge the members of the Telomere Biology Core Lab at University of California San Francisco, particularly Dr. Jue Lin, where the telomere assay and initial quality control were completed.
Footnotes
The authors have declared that no conflict of interest exists.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2019.11.003.
Supplementary data
References
- 1.Blackburn E.H. Structure and function of telomeres. Nature. 1991;350:569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 2.Victorelli S., Passos J.F. Telomeres and cell senescence - size matters not. EBioMedicine. 2017;21:14–20. doi: 10.1016/j.ebiom.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimura M., Hjelmborg J.V., Gardner J.P., Bathum L., Brimacombe M., Lu X. Telomere length and mortality: a study of leukocytes in elderly Danish twins. Am J Epidemiol. 2008;167:799–806. doi: 10.1093/aje/kwm380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benetos A., Okuda K., Lajemi M., Kimura M., Thomas F., Skurnick J. Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001;37:381–385. doi: 10.1161/01.hyp.37.2.381. [DOI] [PubMed] [Google Scholar]
- 5.Zekry D., Herrmann F.R., Irminger-Finger I., Ortolan L., Genet C., Vitale A.M. Telomere length is not predictive of dementia or MCI conversion in the oldest old. Neurobiol Aging. 2010;31:719–720. doi: 10.1016/j.neurobiolaging.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 6.Takata Y., Kikukawa M., Hanyu H., Koyama S., Shimizu S., Umahara T. Association between ApoE phenotypes and telomere erosion in Alzheimer's disease. J Gerontol A Biol Sci Med Sci. 2012;67:330–335. doi: 10.1093/gerona/glr185. [DOI] [PubMed] [Google Scholar]
- 7.Thomas P., O’Callaghan N.J., Fenech M. Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer's disease. Mech Ageing Dev. 2008;129:183–190. doi: 10.1016/j.mad.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Panossian L.A., Porter V.R., Valenzuela H.F., Zhu X., Reback E., Masterman D. Telomere shortening in T cells correlates with Alzheimer's disease status. Neurobiol Aging. 2003;24:77–84. doi: 10.1016/s0197-4580(02)00043-x. [DOI] [PubMed] [Google Scholar]
- 9.Forero D.A., Gonzalez-Giraldo Y., Lopez-Quintero C., Castro-Vega L.J., Barreto G.E., Perry G. Meta-analysis of telomere length in Alzheimer's disease. J Gerontol A Biol Sci Med Sci. 2016;71:1069–1073. doi: 10.1093/gerona/glw053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franco S., Blasco M.A., Siedlak S.L., Harris P.L., Moreira P.I., Perry G. Telomeres and telomerase in Alzheimer's disease: epiphenomena or a new focus for therapeutic strategy? Alzheimers Dement. 2006;2:164–168. doi: 10.1016/j.jalz.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Honig L.S., Kang M.S., Schupf N., Lee J.H., Mayeux R. Association of shorter leukocyte telomere repeat length with dementia and mortality. Arch Neurol. 2012;69:1332–1339. doi: 10.1001/archneurol.2012.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin-Ruiz C., Dickinson H.O., Keys B., Rowan E., Kenny R.A., Von Zglinicki T. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann Neurol. 2006;60:174–180. doi: 10.1002/ana.20869. [DOI] [PubMed] [Google Scholar]
- 13.Yaffe K., Lindquist K., Kluse M., Cawthon R., Harris T., Hsueh W.C. Telomere length and cognitive function in community-dwelling elders: findings from the Health ABC Study. Neurobiol Aging. 2011;32:2055–2060. doi: 10.1016/j.neurobiolaging.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devore E.E., Prescott J., De Vivo I., Grodstein F. Relative telomere length and cognitive decline in the Nurses' Health Study. Neurosci Lett. 2011;492:15–18. doi: 10.1016/j.neulet.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Insel K.C., Merkle C.J., Hsiao C.P., Vidrine A.N., Montgomery D.W. Biomarkers for cognitive aging part I: telomere length, blood pressure and cognition among individuals with hypertension. Biol Res Nurs. 2012;14:124–132. doi: 10.1177/1099800411406433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mather K.A., Jorm A.F., Anstey K.J., Milburn P.J., Easteal S., Christensen H. Cognitive performance and leukocyte telomere length in two narrow age-range cohorts: a population study. BMC Geriatr. 2010;10:62. doi: 10.1186/1471-2318-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts R.O., Boardman L.A., Cha R.H., Pankratz V.S., Johnson R.A., Druliner B.R. Short and long telomeres increase risk of amnestic mild cognitive impairment. Mech Ageing Dev. 2014;141-142:64–69. doi: 10.1016/j.mad.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moverare-Skrtic S., Johansson P., Mattsson N., Hansson O., Wallin A., Johansson J.O. Leukocyte telomere length (LTL) is reduced in stable mild cognitive impairment but low LTL is not associated with conversion to Alzheimer's disease: a pilot study. Exp Gerontol. 2012;47:179–182. doi: 10.1016/j.exger.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Hohman T.J., Koran M.E.I., Thornton-Wells T.A., Alzheimer's Neuroimaging Initiative Genetic variation modifies risk for neurodegeneration based on biomarker status. Front Aging Neurosci. 2014;6:183. doi: 10.3389/fnagi.2014.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hohman T.J., Koran M.I., Thornton-Wells T.A. Genetic modification of the relationship between phosphorylated tau and neurodegeneration. Alzheimers Dement. 2014;10:637–645. doi: 10.1016/j.jalz.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rolyan H., Scheffold A., Heinrich A., Begus-Nahrmann Y., Langkopf B.H., Holter S.M. Telomere shortening reduces Alzheimer's disease amyloid pathology in mice. Brain. 2011;134:2044–2056. doi: 10.1093/brain/awr133. [DOI] [PubMed] [Google Scholar]
- 22.Wikgren M., Karlsson T., Nilbrink T., Nordfjall K., Hultdin J., Sleegers K. APOE epsilon4 is associated with longer telomeres, and longer telomeres among epsilon4 carriers predicts worse episodic memory. Neurobiol Aging. 2012;33:335–344. doi: 10.1016/j.neurobiolaging.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Nudelman K.N.H., Lin J., Lane K.A., Nho K., Kim S., Faber K.M. Telomere shortening in the Alzheimer's disease neuroimaging initiative cohort. J Alzheimers Dis. 2019;71:33–43. doi: 10.3233/JAD-190010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crane P.K., Carle A., Gibbons L.E., Insel P., Mackin R.S., Gross A. Development and assessment of a composite score for memory in the Alzheimer's Disease Neuroimaging Initiative (ADNI) Brain Imaging Behav. 2012;6:502–516. doi: 10.1007/s11682-012-9186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibbons L.E., Carle A.C., Mackin R.S., Harvey D., Mukherjee S., Insel P. A composite score for executive functioning, validated in Alzheimer's Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav. 2012;6:517–527. doi: 10.1007/s11682-012-9176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw L.M., Vanderstichele H., Knapik-Czajka M., Clark C.M., Aisen P.S., Petersen R.C. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jagust W.J., Landau S.M., Shaw L.M., Trojanowski J.Q., Koeppe R.A., Reiman E.M. Relationships between biomarkers in aging and dementia. Neurology. 2009;73:1193–1199. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jack C.R., Bernstein M.A., Fox N.C., Thompson P., Alexander G., Harvey D. The Alzheimer's disease neuroimaging initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hohman T.J., Tommet D., Marks S., Contreras J., Jones R., Mungas D. Evaluating Alzheimer disease biomarkers as mediators of age-related cognitive decline. Neurobiol Aging. 2017;58:120–128. doi: 10.1016/j.neurobiolaging.2017.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carmichael O., McLaren D.G., Tommet D., Mungas D., Jones R.N. Coevolution of brain structures in amnestic mild cognitive impairment. Neuroimage. 2013;66:449–456. doi: 10.1016/j.neuroimage.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai Z., Yan L.J., Ratka A. Telomere shortening and Alzheimer's disease. Neuromolecular Med. 2013;15:25–48. doi: 10.1007/s12017-012-8207-9. [DOI] [PubMed] [Google Scholar]
- 32.Liu M., Huo Y.R., Wang J., Wang C., Liu S., Liu S. Telomere shortening in Alzheimer's disease patients. Ann Clin Lab Sci. 2016;46:260–265. [PubMed] [Google Scholar]
- 33.Gizard F., Heywood E.B., Findeisen H.M., Zhao Y., Jones K.L., Cudejko C. Telomerase activation in atherosclerosis and induction of telomerase reverse transcriptase expression by inflammatory stimuli in macrophages. Arterioscler Thromb Vasc Biol. 2011;31:245–252. doi: 10.1161/ATVBAHA.110.219808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weng N.P., Levine B.L., June C.H., Hodes R.J. Regulated expression of telomerase activity in human T lymphocyte development and activation. J Exp Med. 1996;183:2471–2479. doi: 10.1084/jem.183.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hathcock K.S., Weng N.P., Merica R., Jenkins M.K., Hodes R. Cutting edge: antigen-dependent regulation of telomerase activity in murine T cells. J Immunol. 1998;160:5702–5706. [PubMed] [Google Scholar]
- 36.Wang M.M., Miao D., Cao X.P., Tan L., Tan L. Innate immune activation in Alzheimer's disease. Ann Transl Med. 2018;6:177. doi: 10.21037/atm.2018.04.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J., Zhao C., Zhao A., Li M., Ren J., Qu X. New insights in amyloid beta interactions with human telomerase. J Am Chem Soc. 2015;137:1213–1219. doi: 10.1021/ja511030s. [DOI] [PubMed] [Google Scholar]
- 38.LaFerla F.M., Green K.N., Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 39.Spilsbury A., Miwa S., Attems J., Saretzki G. The role of telomerase protein TERT in Alzheimer's disease and in tau-related pathology in vitro. J Neurosci. 2015;35:1659–1674. doi: 10.1523/JNEUROSCI.2925-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu H., Fu W., Mattson M.P. The catalytic subunit of telomerase protects neurons against amyloid beta-peptide-induced apoptosis. J Neurochem. 2000;75:117–124. doi: 10.1046/j.1471-4159.2000.0750117.x. [DOI] [PubMed] [Google Scholar]
- 41.Jofre-Monseny L., Minihane A.-M., Rimbach G. Impact of apoE genotype on oxidative stress, inflammation and disease risk. Mol Nutr Food Res. 2008;52:131–145. doi: 10.1002/mnfr.200700322. [DOI] [PubMed] [Google Scholar]
- 42.Hohman T.J., Dumitrescu L., Barnes L.L., Thambisetty M., Beecham G.W., Kunkle B. Sex-specific effects of Apolipoprotein E with cerebrospinal fluid levels of tau. JAMA Neurol. 2018;75:989–998. doi: 10.1001/jamaneurol.2018.0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.King K.S., Kozlitina J., Rosenberg R.N., Peshock R.M., McColl R.W., Garcia C.K. Effect of leukocyte telomere length on total and regional brain volumes in a large population-based cohort. JAMA Neurol. 2014;71:1247–1254. doi: 10.1001/jamaneurol.2014.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wikgren M., Karlsson T., Soderlund H., Nordin A., Roos G., Nilsson L.G. Shorter telomere length is linked to brain atrophy and white matter hyperintensities. Age Ageing. 2014;43:212–217. doi: 10.1093/ageing/aft172. [DOI] [PubMed] [Google Scholar]
- 45.Jacobs E.G., Epel E.S., Lin J., Blackburn E.H., Rasgon N.L. Relationship between leukocyte telomere length, telomerase activity, and hippocampal volume in early aging. JAMA Neurol. 2014;71:921–923. doi: 10.1001/jamaneurol.2014.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jack C.R., Jr., Petersen R.C., Xu Y.C., Waring S.C., O'Brien P.C., Tangalos E.G. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jack C.R., Bennett D.A., Blennow K., Carrillo M.C., Feldman H.H., Frisoni G.B. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–547. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lukens J.N., Van Deerlin V., Clark C.M., Xie S.X., Johnson F.B. Comparisons of telomere lengths in peripheral blood and cerebellum in Alzheimer's disease. Alzheimers Dement. 2009;5:463–469. doi: 10.1016/j.jalz.2009.05.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paul L. Diet, nutrition and telomere length. J Nutr Biochem. 2011;22:895–901. doi: 10.1016/j.jnutbio.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 50.Shiels P.G., McGlynn L.M., MacIntyre A., Johnson P.C., Batty G.D., Burns H. Accelerated telomere attrition is associated with relative household income, diet and inflammation in the pSoBid cohort. PLoS One. 2011;6:e22521. doi: 10.1371/journal.pone.0022521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.