Key Points
ELGAN/ELBW neonates are lymphopenic and their T cells have altered homing capacity.
ELGAN/ELBW infants with chorioamnionitis have an altered Treg population.
Abstract
Extremely preterm neonates are particularly susceptible to infections, likely because of severely impaired immune function. However, little is known on the composition of the T cell compartment in early life in this vulnerable population. We conducted a comprehensive phenotypic flow cytometry–based longitudinal analysis of the peripheral conventional T cell compartment of human extremely low gestational age neonates (ELGAN) with extremely low birth weight (ELBW; <1000 g) participating in a randomized placebo-controlled study of probiotic supplementation. PBMCs from ELGAN/ELBW neonates were collected at day 14, day 28, and postmenstrual week 36. Comparisons were made with full-term 14-d-old neonates. Total CD4+ and CD8+ T cell frequencies were markedly lower in the preterm neonates. The reduction was more pronounced among the CD8+ population, resulting in an increased CD4/CD8 ratio. The preterm infants were also more Th2 skewed than the full-term infants. Although the frequency of regulatory T cells seemed normal in the ELGAN/ELBW preterm neonates, their expression of the homing receptors α4β7, CCR4, and CCR9 was altered. Notably, ELGAN/ELBW infants developing necrotizing enterocolitis before day 14 had higher expression of CCR9 in CD4+T cells at day 14. Chorioamnionitis clearly associated with reduced T regulatory cell frequencies and functional characteristics within the preterm group. Finally, probiotic supplementation with Lactobacillus reuteri did not impose any phenotypic changes of the conventional T cell compartment. In conclusion, notable immaturities of the T cell compartment in ELGAN/ELBW neonates may at least partially explain their increased susceptibility to severe immune-mediated morbidities.
Introduction
Preterm birth (delivery before week 37 of gestation) is a major concern for neonatal health worldwide, with a global incidence of ∼15 million cases annually, and is associated with an increased risk of both morbidity and mortality (1). The most vulnerable premature infants are the extremely low gestational age neonates (ELGAN) that are born before gestational week 28. Within this group, the majority of children have extremely low birth weight (ELBW), that is, a birth weight of <1000 g. Although modern neonatal care and management have significantly increased the survival of preterm neonates, still approximately one-fourth of the ELGAN/ELBW infants die in affluent countries, such as Sweden (2). Also, the prevalence of mild to severe disability in infancy is markedly elevated in ELGAN/ELBW infants compared with infants delivered FT (3). The fact that severe infections and immune-associated diseases, such as necrotizing enterocolitis (NEC) and sepsis, are common causes of death in this population is a strong indication that the immune system of these ELGAN/ELBW infants is even more immature compared with full-term (FT) neonates (4), both in quantitative and qualitative aspects.
Immune maturation during this early part of life is complex and involves particular molecular and epigenetic programs that will, at the same time, allow microbial commensal colonization while also developing an efficacious immunity in combating infections. Newborn infants have deficient IFN-γ production and are referred to as Th2 skewed (5). The population of T regulatory cells (Tregs) develops early during gestation, and neonatal T cell immunity in general is prone to tolerance development (6, 7), but the presence of fetal T cells with a memory phenotype (CD45RO+) has also been described (8–10). T cell tissue homing is considered to be key for homeostasis during development and is different in early life compared with adulthood. However, most studies are performed in mice, and data from human neonates, and preterm infants in particular, are very scarce (11, 12). It is also important to remember that most studies of neonatal immune cell characteristics rely on data generated from analyses of cord blood cells, which might not be fully representative of immunity in early life (13, 14).
A recent meta-analysis of prospective randomized controlled trials evaluating whether the use of probiotics can prevent feeding intolerance and NEC in premature infants shows encouraging results, but it was also concluded that there is still insufficient data with regard to the benefits and potential adverse effects in ELBW infants (15). Although several studies have demonstrated that Lactobacillus reuteri modulates the innate and acquired immune responses in humans both in vitro and in vivo (16–21), the influence of L. reuteri supplementation on the phenotypic and functional characteristics and gut-homing properties of T cells of preterm, and particularly ELBW, infants has not been studied.
In this study, we aimed to perform an in-depth investigation of the conventional T cell compartment in ELGAN/ELBW preterm neonates, with the hypothesis that these cells would be highly influenced by extreme preterm birth and its clinical correlates. The study was performed in a longitudinal way at day 14 (D14) and day 28 (D28) after birth and at postmenstrual week (PMW) 36 + 0, and the results were compared with those of T cells in PBMCs from FT neonates isolated 14 d after birth. We investigated naive (NA) and memory CD4+ and CD8+ T cells, T helper subpopulations, and regulatory T cell features as well as tissue-homing characteristics in relation to gestational age, birthweight, L. reuteri supplementation, and clinical outcomes such as sepsis, NEC, and chorioamnionitis. The proactive management of ELGAN preterm infants born before 25 wk of gestation in Sweden made it possible to study the immune system also in ELGAN infants born in gestational weeks 23 and 24. This is unique as previous studies on immune cells lack extensive data on infants born before week 25.
Materials and Methods
Study design and participants
The present study was a part of the prospective, double-blinded, randomized controlled multicenter trial Prophylactic Probiotics to Extremely Low Birth Weight Premature Infants, evaluating the effect of probiotic L. reuteri DSM 17938 on feeding tolerance, growth, severe morbidities, and mortality in ELBW premature infants (http://clinicaltrials.gov, identifier: NCT01603368). The study design has been described in detail elsewhere (22). Briefly, 134 ELBW infants were supplemented either with the probiotic bacterial strain L. reuteri DSM 17938 or placebo. The trial was conducted in 10 neonatal units in Sweden from June 2012 to November 2015 in the regions of Stockholm and Linköping and approved by the Ethics Committee for Human Research in Linköping (Dnr 2012/28‐31, Dnr 2012/433‐32). Infants between gestational week 23 + 0 and 27 + 6 and a birthweight <1000 g were eligible for enrollment within 3 d after delivery. The infants were characterized using comprehensive clinical data, including perinatal data, growth, feeding intolerance, treatment, antibiotics, and mild and severe morbidities, collected daily in a study‐specific case report form until PMW 36 + 0 (Table I). Chorioamnionitis, an acute inflammation of the membranes and chorion of the placenta, was diagnosed by the responsible obstetrician and was based on laboratory inflammatory response and typical clinical manifestations (23). A sepsis diagnosis required positive blood and/or cerebral spinal fluid culture, clinical deterioration, and laboratory inflammatory response. The bacteria detected in positive culture included the following: Gram-negative rods (such as Escherichia coli and Klebisella) and Gram-positive Streptococcus group B, Staphylococcus aureus, and coagulase-negative staphylococci. NEC was staged according to Bell’s criteria (24), and all cases of stage II or greater were recorded. In the cohort, out of 11 cases with NEC (Bell stage II–III), 7 neonates had Bell stage III and were surgical NEC. The active intervention, once-daily L. reuteri DSM 17938 (provided by BioGaia, Stockholm, Sweden), was provided in oil drops. The daily dose was 1.25 × 108 bacteria (0.2 ml drops) (25, 26). The placebo was maltodextrin provided in an identical oil suspension and having a similar smell, taste, and visual appearance as the active product. Intervention was started within 3 d after birth and administered through the gastric tube or by mouth and continued until gestational week 36 + 0. Venous blood was collected on three occasions: D14, D28, and PMW 36 + 0 (see flowchart, Fig. 1). Blood from 29 FT infants (born between week 38 and 42) at 14 d of age was also collected to use as control for the study (Fig. 1). In all figures, the following abbreviations are used: FT D14 (samples from FT infants at D14 after birth), ELBW D14, ELBW D28, and ELBW PMW 36 + 0 (samples from ELGAN/ELBW preterm infants at D14, D28, and PMW 36 +0, respectively).
Table I. Background data of the study participants.
| ELGAN/ELBW | N = 93 |
|---|---|
| Gestational age, wk, mean (range) | 25.3 (23.0–27.9) |
| Birth weight, g, mean (range) | 726 (400–987) |
| Birth weight z-score, mean (range) | −1.0 (−4.7–1.4) |
| Small for gestational age, n (%) | 19 (20) |
| Male, n (%) | 54 (58) |
| Apgar at 5 min, mean (range) | 6.4 (1–10) |
| Infants from multiple pregnancy, n (%) | 36 (39) |
| Antenatal corticosteroids, n (%) | 91 (98) |
| Caesarean section, n (%) | 58 (62) |
| Maternal smoking, n (%) | 8 (9) |
| Preeclampsia, n (%) | 9 (10) |
| Chorioamnionitis, n (%) | 19 (20) |
| Preterm premature rupture of membranes, n (%) | 27 (29) |
| Maternal antibiotics, n (%) | 55 (59) |
| Inclusion site, Stockholm/Linköping, n/n (%/%) | 61/32 (66/34) |
| Antibiotics during first week, n (%) | 92 (99) |
| Antibiotics during second week, n (%) | 77 (83) |
| Days on antibiotics, mean (range) | 28.8 (4–64) |
| Days on mechanical ventilation, mean (range) | 20.8 (0–75) |
| Sepsis, culture proven, n (%) | 35 (38) |
| NEC, Bell stage II–III, n (%) | 11 (12) |
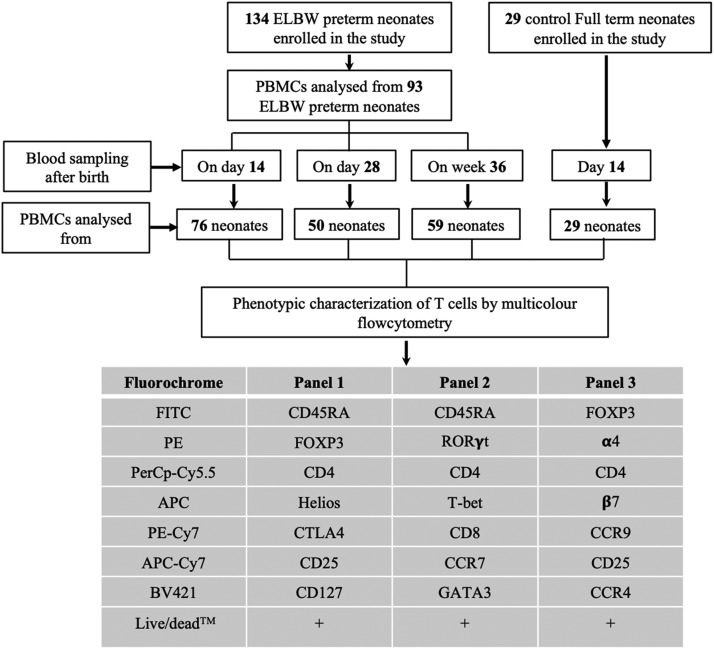
FIGURE 1.
Flow chart showing the number of subjects recruited, time points for blood sampling, number of PBMC samples analyzed by flow cytometry, and the Ab panels used for the experiments. A total of 134 ELBW preterm infants and 29 FT control subjects were recruited for the study. PBMCs were isolated from 76 ELBW infants from D14, 50 ELBW infants from D28, and 59 ELBW infants from PMW 36 + 0 wk. PBMCs from 28 infants were available from all three time points, and PBMCs from 48 neonates were available from at least two time points. FACS panels were designed to characterize conventional T cells, their corresponding subsets, and the homing markers. The number of PBMC samples analyzed by FACS varied in different panels depending on the adequacy of the cells.
Isolation of PBMCs
PBMCs were isolated by Ficoll-Hypaque (GE Healthcare Bio Sciences, Uppsala, Sweden) gradient separation from the FT infants (n = 29 at D14) and from all ELGAN/ELBW infants from whom a sufficient blood volume was obtained (n = 76 at D14, n = 50 at D28, and n = 59 at PMW 36 + 0). Isolated PBMCs were washed three times and resuspended in freezing medium containing 40% RPMI 1640 (HyClone, GE Healthcare Life Sciences, South Logan, UT), 50% FCS (Sigma-Aldrich, St. Louis, MO), and 10% DMSO (Sigma-Aldrich). Cells were gradually frozen in a freezing container and kept in liquid nitrogen until further analysis.
Flow cytometry and Abs
Frozen PBMCs were thawed gently, washed three times, and seeded into the 96-well tissue culture plate (Corning, Kennebunk, ME) at a concentration of 0.5 × 106 cells per well. The cells were rested for 2 h at 37°C with 5% CO2 before staining. Cells were then transferred to V-shaped staining plates and stained with the LIVE/DEAD Fixable Dead Cell Stain Kit–Aqua (Life Technologies, Eugene, OR) according to the manufacturer’s instruction. Blocking of cell surface Fc receptor was done with 10% human serum. Three different FACS panels were used to characterize conventional T cells (Fig. 1). Staining of cell surface markers was performed using the following Abs from BioLegend (San Diego, CA): CD45RA (clone: HI100), CD4 (clone: OKT4), CD25 (clone: BC96), CD127 (clone: A019D5), CD8 (clone: HIT8a), CCR9 (clone: L053E8), CCR4 (clone: L291H4), CCR7 (clone: GO43H7), α4 (clone: VS215), and β7 (clone: FIB504). After surface staining, cells were washed and fixed/permeabilized with the transcription factor buffer set (BioLegend) according to the instruction of the manufacturer. Intracellular blocking was done with 10% human serum. The cells were then stained for intracellular FOXP3 (clone: 150D), CTLA4 (clone: L3D10), Helios (clone: 22F6), T-bet (clone: 4B10), and GATA3 (clone:16E10A23) (all from BioLegend) and RORγt (clone: Q21-559) (from BD Biosciences, San Jose, CA). Stained cells were washed, resuspended in FACS wash buffer, and acquired using FACS Verse instrument and FACS Suite software (BD Biosciences). Data analysis was performed using FlowJo software (Ashland, OR).
Statistical analysis
GraphPad Prism 7 (GraphPad Software, La Jolla, CA) was used for the statistical analyses displayed either as scatter plot with bars or as scatter plots. The results are shown as medians with interquartile ranges in the figures and as medians with the 95% upper and lower confidence intervals in the tables. The nonparametric Kruskal-Wallis test followed by the Dunn multiple comparisons test were performed to compare differences between FT and preterm neonates and also between the different sampling time points within the preterm group. Mann-Whitney U test was used to assess differences between FT and preterm 14-d samples. The Spearman correlation test was used to analyze correlation between variables. Results were considered as significant when p < 0.05, and actual p values are displayed in each figure.
The principal component analyses (PCA) were performed in SPSS V25. All data were checked for normality distribution before statistical testing. Different combinations of cell subset frequencies were reduced to two principle components. The amount of variance in the data explained by one component is mentioned as a percentage on the axis. The total variance in the data that is explained by the two components combined is mentioned in the graphs. The validity of the PCA was checked with the Kaiser-Meyer-Olkin Measure of Sampling Adequacy and the Bartlett Test of Sphericity. The loading of the different cell subset frequencies in the plots was indicated with arrows. The different (age, sepsis, and probiotic supplementation) groups were indicated with different colors, as explained in the figures and figure legends.
Results
The T cell compartments in ELGAN/ELBW preterm infants at 14 d of life are decreased in relative frequencies but show signs of marked activation
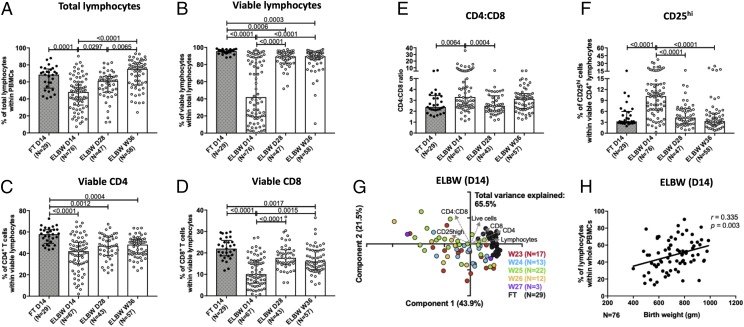
We used multicolor flow cytometry to investigate how the conventional T cell compartment develops after birth in ELGAN/ELBW preterm neonates (in the figures referred to as ELBW) and how it compared with that in FT neonates (in the figures referred to as FT) (Fig. 1, Table I). The gating strategies for all different populations and markers are shown in Supplemental Fig. 1. The percentages of the total and viable lymphocytes were significantly lower in ELGAN/ELBW preterm neonates compared with the FT infants at 14 d of age (Fig. 2A, 2B). Individuals with a lymphocyte viability below 10% were excluded from further analyses. Although the lymphocyte compartment recovered already at D28, the percentage of viable lymphocytes was still lower in the ELGAN/ELBW infants at PMW 36 + 0. This drop in lymphocyte frequency was true for both CD4+ and CD8+ T cells (Fig. 2C, 2D) but most marked among the CD8+ T cells, resulting in an increased CD4/CD8 ratio at D14 in the preterm infants (Fig. 2E). Interestingly, the proportion of CD25hi cells within the CD4+ T cell population was increased at 14 d of age in the preterm infants compared with the FT, but this difference declined with time, and already at D28 of age, the preterm infants had similar CD25 expression as the FT infants (Fig. 2F). Further, gestational age at birth did not seem to influence the composition of the T cell compartment within the preterm infants at 14 d of age (Fig. 2G), but there was a weak but significant correlation between birth weight and the percentages of total lymphocytes at D14 (Fig. 2H).
FIGURE 2.
The frequencies of total and viable lymphocyte populations are decreased in the PBMCs of ELBW preterm infants compared with FT controls. Blood samples from 29 FT infants (D14 after birth) and 134 ELBW preterm infants (D14, D28, and PMW 36 + 0 after birth) were collected, and phenotypic characterization of the T lymphocyte compartment was performed by multicolor flow cytometry. The figure shows the compiled frequencies of (A) total, (B) viable, (C) CD4+, and (D) CD8+ lymphocytes among the PBMCs. The CD4/CD8 ratio is shown in (E), and the frequency of CD25hi cells within the CD4+ population is shown in (F). Bars show medians with interquartile range. Kruskal-Wallis test with Dunn multiple comparison tests was used for group comparisons. PCA comparing the T cell phenotype at D14 of FT neonates (black) with the ELBW neonates born week 23–27 (in color) is shown in (G). The correlation between the percentage of lymphocytes at D14 and birth weight is shown in (H) for the ELBW neonates.
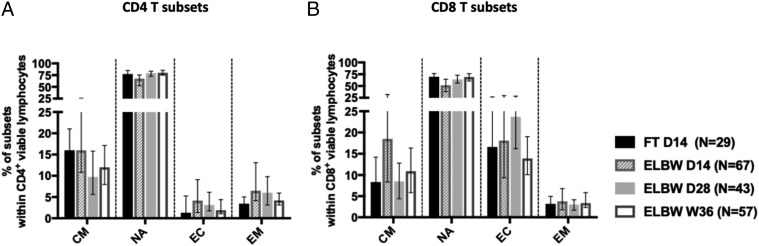
Proportions of NA, effector, and memory subsets of CD4+ and CD8+ T cells are similar in ELGAN/ELBW preterm and FT infants
We next characterized the NA, central memory (CM), effector, and effector memory T cell populations based on the expression of CCR7 and CD45RA on T cells. As expected, the majority of circulating CD4+ and CD8+ T cells from the PBMCs of both FT and preterm children had an NA phenotype (Fig. 3). A substantial portion of the CD4+ T cells also expressed a CM phenotype, whereas the effector and effector memory populations were relatively small (Fig. 3A). For the CD8+ T cells, the effector cells were relatively frequent (Fig. 3B). We did not observe any consistent differences between the FT and preterm infants at any time point.
FIGURE 3.
Proportions of NA, effector (EC), and effector memory (EM) subsets of CD4+ and CD8+ T cells are similar in ELBW preterm and FT infants. The frequencies of CM, NA, EC, and EM cells within (A) CD4+ T cells and (B) CD8+ T cells. Bars show medians with interquartile ranges.
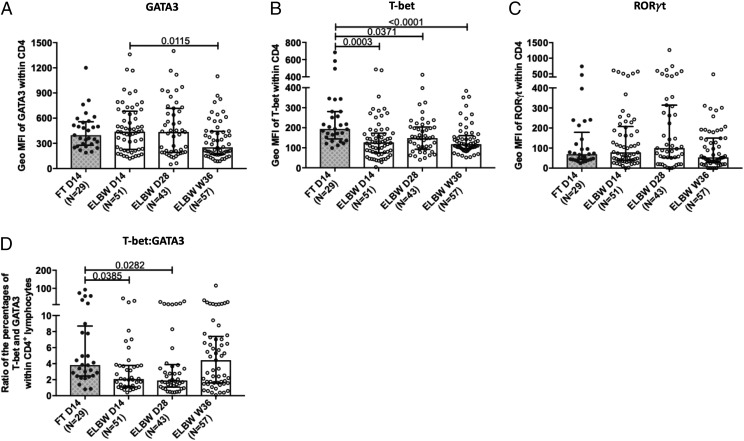
ELGAN/ELBW preterm infants have a reduced T-bet/GATA3 ratio among the CD4+ T cells at 14 and 28 d of age
We continued by investigating the transcription factors T-bet, GATA3, and RORγt that are considered as the master regulators for the generation of Th1, Th2, and Th17 cells, respectively. These transcription factors were possible to detect to the same degree in both FT and ELGAN/ELBW preterm neonates (data not shown). For the CD4+ T cells, the expression level (geometric mean fluorescence intensity) of the Th2-associated transcription factor GATA3 seemed to drop with age in the preterm neonates (Fig. 4A). Notably, there was a significantly lower expression level of the Th1-associated transcription factor T-bet in the preterm infants at all timepoints compared with FT neonates (Fig. 4B). The expression level of RORγt was in general very low (Fig. 4C) and did not differ between the groups. Although the percentage of CD4+ T cells that expressed the individual transcription factors did not differ between the groups (Table II), there was a lower ratio between T-bet– and GATA3-expressing cells in the preterm group at D14 and D28 compared with the FT infants (Fig. 4D). For the CD8+ T cells, there was a similar trend for lower T-bet expression levels in the preterm group (data not shown).
FIGURE 4.
ELBW preterm infants have a reduced T-bet/GATA3 ratio in CD4+ T cells at 14 and 28 d of age. The expression levels of (A) GATA3, (B) T-bet, and (C) RORγt in the CD4+ T cells of control FT neonates at D14 and the ELBW preterm infants at D14, D28, and at PMW 36 + 0. The presented data of geometric mean fluorescence intensity (geo MFI) for transcription factors analyses was done following gating on CD4 cells that are positive for these factors. (D) Ratio between the percentages of T-bet– and GATA3-expressing CD4+ T cells. Bars show medians with interquartile range. Kruskal-Wallis test with Dunn multiple comparison tests was used to make group comparisons.
Table II. Percentage of CD4 T cells expressing different transcription factors.
| Transcription Factors | FT 14D (n = 29) | ELBW 14D (n = 51) | ELBW 28D (n = 43) | ELBW 36W (n = 57) | p Value |
|---|---|---|---|---|---|
| GATA3 | 1.56 (0.6–3.9) | 3.45 (1.7–4.2) | 2.30 (1.2–4.3) | 0.94 (0.7–1.8) | NS |
| T-bet | 8.35 (3.1–13.5) | 7.3 (4.3–10.1) | 4.91 (3.6–8.2) | 3.59 (2.4–5.9) | NS |
| RORγt | 0.36 (0.2–0.8) | 0.67 (0.3–1.3) | 0.97 (0.5–1.7) | 0.92 (0.6–1.2) | NS |
Values are given as percentage of median (95% upper and lower confidence limit).
Analysis of GATA3, T-bet, and RORγt expression within the CD4+ and CD8+ NA, effector, and memory populations (Supplemental Fig. 2 [for CD4+ T cells] and Supplemental Table I [for CD8+ T cells]) revealed high individual variations, and no differences were observed between the ELGAN/ELBW preterm and FT infants.
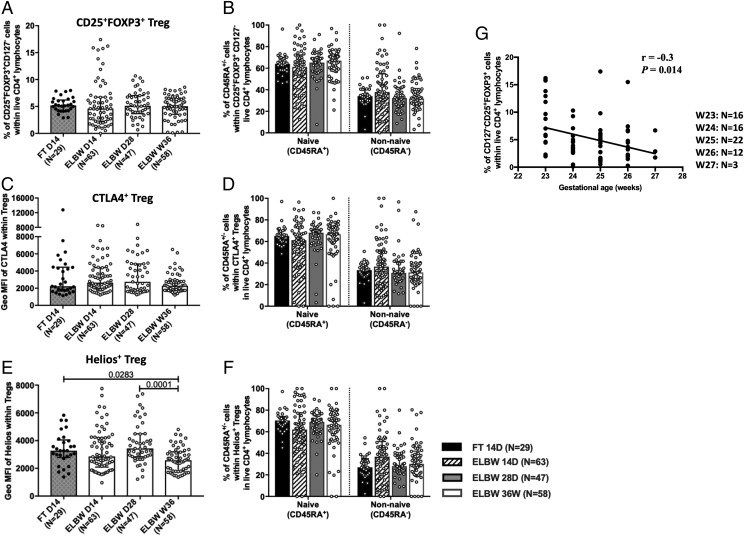
Tregs from ELGAN/ELBW preterm infants express lower levels of the transcription factor Helios, and their frequency is inversely correlated with gestational age
Analysis of the proportion of total Tregs, defined as CD25+FOXP3+CD127− cells among the CD4+ T cell population, revealed a relatively stable frequency of these cells in ELGAN/ELBW preterm infants, which was equivalent to that of the FT infants (Fig. 5A). When extending the analysis to also include CD45RA+ and CD45RA− (NA and non-NA) subpopulations of Tregs, the results were similar (Fig. 5B). The inhibitory receptor CTLA4 was ubiquitously expressed among the Tregs (data not shown), and the level of expression was similar between all groups (Fig. 5C). The CTLA4-expressing Tregs did not differ in frequencies within the NA or non-NA phenotypes (Fig. 5D). The groups had similar frequencies of Tregs that express Helios, a transcription factor suggested to confer a stable phenotype in Tregs (data not shown), but a reduced expression level was observed in the preterm neonates at PMW 36 + 0 compared with the earlier time point and also with the FT infants at D14 (Fig. 5E). However, no differences were observed regarding the NA or non-NA phenotypes of the Helios-expressing cells (Fig. 5F). The total Treg percentage in the 14-d PBMCs from preterm infants inversely correlated with gestational age at birth (Fig. 5G).
FIGURE 5.
Tregs express lower levels of the transcription factor Helios, and their frequency is inversely correlated with gestational age in ELBW preterm infants. Relative proportions of (A) total Tregs (CD25+FOXP3+CD127−) and (B) the frequencies of CD45RA+ and CD45RA− Tregs are shown. (C and D) Expression level of (C) CTLA4 among all Tregs and frequencies of (D) CD45RA+ and CD45RA− cells within CTLA4+ Tregs. (E) The level of Helios expression among Tregs and (F) the frequencies of CD45RA+ and CD45RA− cells within Helios+ Tregs. Bars show medians with interquartile ranges. Kruskal-Wallis test with Dunn multiple comparison tests was used to make group comparisons. (G) Correlation between Treg percentages from ELBW neonates at D14 and the gestational age at birth.
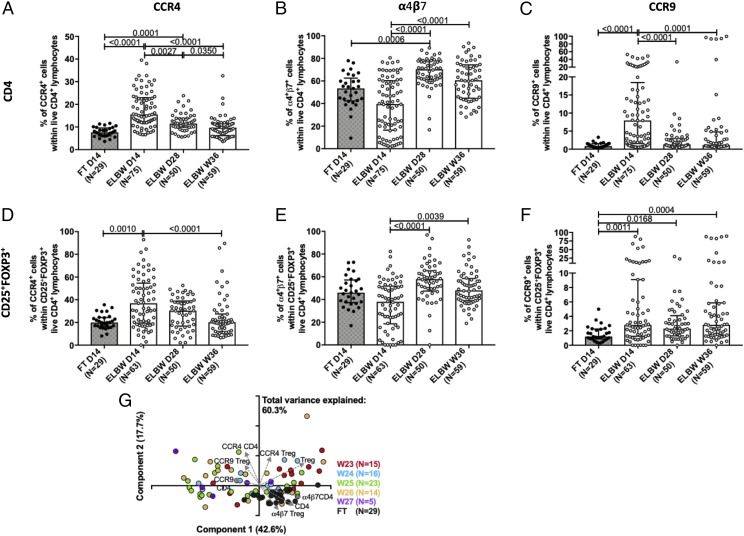
CD4+ T cells and Tregs from 14-d-old ELGAN/ELBW preterm infants have a markedly altered homing capacity
Homing receptors are a requisite for CD4 T cell homing toward various tissues. We therefore examined the expression of the homing receptors CCR4, α4β7, and CCR9. CD4+ T cells from preterm infants showed a significantly altered expression of CCR4 and CCR9 compared with FT neonates at D14 (Fig. 6A–C). The homing receptor expression changed with time, and at PMW 36 + 0, there were no differences in the percentages of CCR4+, α4β7+, and CCR9+ cells between preterm and FT infants. There was a notable difference in how these homing markers were affected by prematurity, as the frequency of α4β7-expressing cells was lower in the preterm infants at D14 (Fig. 6B), whereas the proportions of CCR4- and CCR9-expressing cells were elevated at D14 (Fig. 6A, 6C). A similar trend was observed within the Treg compartment regarding CCR4 and CCR9 cell frequencies (Fig. 6D, 6F), but the proportion of CCR9-expressing Tregs was significantly elevated in preterm infants at all time points compared with FT infants at D14 (Fig. 6F). Notably, gestational age at birth did not influence the proportion of cells expressing homing markers within the CD4+ T cell and Treg populations in the preterm infants at day14 (Fig. 6G).
FIGURE 6.
CD4+ T cells and Tregs from 14-d-old ELGAN/ELBW preterm infants have a markedly altered homing capacity. The frequencies of (A and D) CCR4+, (B and E) α4β7+, and (C and F) CCR9+ within total (A–C) CD4+ T cells and (D–F) Tregs, respectively, are shown. Bars show medians with interquartile ranges. Kruskal-Wallis test with Dunn multiple comparison tests was used for group comparisons. (G) PCA of the influence of gestational age on the frequencies of CCR4-, CCR9- and α4β7-expressing CD4+ T cells and Tregs at D14 in the ELBW (color) neonates. FT infants (black) are shown as a reference.
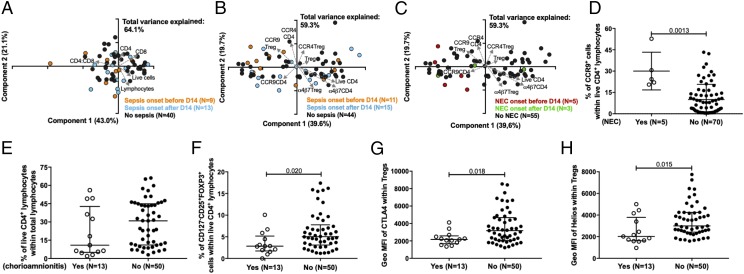
The Treg compartment in ELGAN/ELBW preterm neonates is altered by chorioamnionitis and NEC but is not associated with sepsis
A substantial proportion of the ELGAN/ELBW preterm infants suffered from sepsis (Table I). Therefore, we examined culture-proven sepsis associated with T lymphocyte populations and their homing characteristics at 14 d of age in the preterm infants. They were grouped according to sepsis onset before or after sample collection at 14 d of life. Sepsis did not seem to have any major impact on the overall T cell compartment, nor did differences in the T cell compartment associate with the risk of subsequent sepsis development (Fig. 7A). For the Treg compartment and the respective homing marker expression, sepsis was not a contributing factor to the differences seen within the preterm group (Fig. 7B; compare with Figs. 5, 6). Only 11 ELGAN/ELBW preterm infants developed NEC in our cohort (Table I), and there were cells available from only five and three of those with onset before and after D14, respectively. Still, it is interesting to note that the NEC cases clustered among those individuals with the highest CCR9 expression among the CD4+ T cells (Fig. 7C), which is significantly higher than the non-NEC cases at 14 d of life (Fig. 7D). Importantly, Treg frequencies were very low in the NEC cases (data not shown), which limited further analysis of homing receptors on Tregs within this group.
FIGURE 7.
Chorioamnionitis and NEC affect the Tregs and CD4 T cells, respectively, whereas no association of cause and effect of sepsis is observed on T cell compartment. PCA plot of the proportions of (A) total, CD4+, and CD8+ lymphocytes and their ratio and (B and C) frequencies of CCR4+, CCR9+, and α4β7+ CD4+ T cells and Tregs at D14 from ELBW neonates in sepsis and NEC cases, respectively. The infants were grouped according to if the sepsis/NEC onset was before or after the sample was collected at 14 d of life. (D) Proportions of CCR9+ CD4 T cells in ELBW neonates at D14 of life in NEC cases. Frequencies of (E) total CD4+ T cells, (F) Tregs, (G) CTLA4+, and (H) Helios+ Tregs at D14 for ELGAN/ELBW infants from chorioamnionitis cases. Scatter plots show medians with interquartile ranges. Mann-Whitney U test was used for group comparisons.
We also evaluated the influence of perinatal chorioamnionitis on the T cell compartment in ELGAN/ELBW preterm infants. Although chorioamnionitis did not affect the proportions of CD4+ T cells, this condition was associated with a reduced frequency of Tregs as well as reduced expression of CTLA4 and Helios among Tregs (Fig. 7E–G).
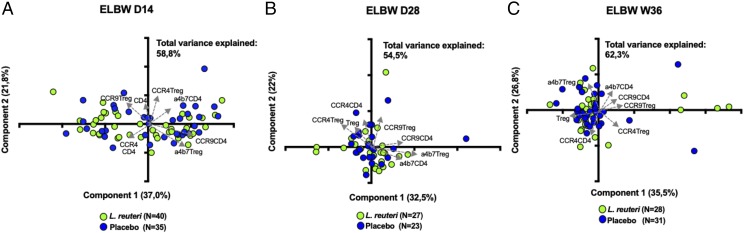
L. reuteri supplementation has no impact on the proportions or functional phenotype of conventional T cells
As L. reuteri has been used in several clinical trials in premature infants, it is of importance to study its effects on the developing immune system. Also, we wanted to investigate whether the L. reuteri supplementation influenced any of the parameters investigated above and whether it could explain the substantial variation sometimes observed within the groups. Data regarding L. reuteri and all investigated parameters are displayed in Supplemental Table II. Notably, L. reuteri supplementation did not alter the viability, relative proportions, or ratios of CD4+ or CD8+ T cells or their subpopulations. Further, the expression of homing receptors within CD4+ T cells or within Tregs was similar in the L. reuteri–supplemented and placebo groups at D14, D28, and PMW 36 + 0 (Fig. 8).
FIGURE 8.
L. reuteri supplementation has no effect on CD4 and Tregs and on their homing properties. PCA plot of the proportions of the frequencies of CCR4+, CCR9+, and α4β7+ CD4+ T cells and Tregs from ELGAN/ELBW neonates supplemented with L. reuteri (green) or placebo (blue) at (A) D14, (B) D28, and (C) PMW 36 + 0.
Discussion
With the aim to get a deeper insight into the maturing T cell compartment in very early life, we made a comprehensive analysis of the conventional T cell compartment in a cohort of premature ELGAN/ELBW infants (Prophylactic Probiotics to Extremely Low Birth Weight Premature Infants trial). Because of the very active management of ELGAN infants in Sweden, we were able to study immune development of a substantial number of infants born as early as gestational weeks 23–25 (approximately two-thirds of our study population).
We followed these children in a longitudinal manner and also investigated the impact of gestational age at birth, birth weight, sepsis, NEC, and chorioamnionitis as well as probiotic supplementation on the T cell phenotypical characteristics. Our findings clearly illustrate that there is an immaturity of several aspects of the T cell compartment that is likely to contribute to the increased risk for infections and NEC.
At 14 d of life, the ELGAN/ELBW preterm neonates showed a marked lymphopenia with a significant decrease in the percentage of total, CD4+, and CD8+ lymphocytes compared with the FT infants, a feature that was also evident up to PMW 36 + 0 (Fig. 2). The reduction was most notable among the CD8+ population, which resulted in an increased CD4/CD8 ratio. Notably, gestational age at birth did not have a major influence on the CD4/CD8 T cell compartment within the ELGAN/ELBW group of infants, whereas the number of lymphocytes in peripheral blood at D14 correlated with birth weight. The decreased proportions of CD4+ and CD8+ T cells are in line with the concept of an impaired T cell compartment and capacity in ELGAN/ELBW infants during the first months of life. This reduction in T cells compared with FT infants could reflect immaturity and a fragile lymphocyte compartment in general, but it could also be due to a suboptimal thymic production of T cells at birth in preterm infants. We did not measure recent thymic emigrants (27) in our study, but a low thymic output has been associated with preterm birth in general (28). It has previously been reported that both CD4+ and CD8+ T cells from premature neonates have a reduced CD31 expression at birth, a feature associated with enhanced effector differentiation, hyperresponsiveness, and respiratory complications after being discharged from the hospital (29, 30). We have not studied functional characteristics of individual lymphocyte populations in our cohort because of limited sample volumes. It is worth noticing, however, that there was a marked increase in the frequency of CD25high CD4+ T cells in ELBW preterm infants at D14, which could not be explained by a higher number of Tregs, indicative of an activated CD4+ T cell compartment in the ELGAN/ELBW group. The higher/increased CD25 expression reported in this study is also in line with what has been reported by others in smaller studies including neonates with a wide prematurity spectrum (31, 32). However, we did not observe any skewing in terms of NA, memory, and effector cell populations, neither within the CD4+ nor the CD8+ compartments (Fig. 3, Table II). Still, the marked reduction in T cell frequencies, in particular the CD8+ T cells, might contribute to the increased risk of infections in these extremely premature neonates.
The transcription factors T-bet, GATA3, and RORγt, are considered as the master regulators for the generation of Th1, Th2, and Th17 cells, respectively. We observed that the preterm infants seemed to be more Th2 skewed than FT infants, as the T-bet expression levels were lower in the preterm group (Fig. 4B), and the ratio of the percentage of T-bet– to GATA3-expressing CD4+ T cells was significantly reduced (Fig. 4D). This is in line with the current dogma of a Th2-biased immunity in early life, which might be another explanation to impaired immunity to infections with intracellular pathogens, as also human neonatal T cells seem to be preprogrammed to a type 2 phenotype in several ways and by different mechanisms (33–35).
Tregs are reported to be increased in frequency in preterm infants, but still little is known about the dynamics during the first weeks of life in ELGAN/ELBW preterm infants (36, 37). Indeed, Treg characteristics also seem to be influenced by prematurity, at least in cord blood (38, 39). In our cohort, the Treg compartment was surprisingly similar to that of FT infants (Fig. 5). Still, the observed inverse correlation between the week of birth and Treg frequencies in the preterm group is in line with previous findings. It is known that fetal Tregs have the capacity to suppress proliferation of activated T cells, suggesting that this arm of immunity is developed early (40, 41). Together with our observation that there were no quantitative differences within the Treg compartment between FT and the ELGAN/ELBW neonates in our study, we could speculate that the Treg compartment in peripheral blood is not significantly influenced by preterm birth, as such.
Protective immunity relies upon T cell differentiation and subsequent migration to target tissues. T cells trafficking to gut and skin depends on the expression of the homing receptors CCR9/α4β7 and CCR4, respectively, and is implicated in the inflammatory recruitment of Tregs in different immunological settings (42, 43). CCR9 signaling also shapes immune responses by inhibiting Treg development (44). We observed that the expression of α4β7, CCR9, and CCR4 was altered within both the total CD4+ T cell and Treg populations (Fig. 6). The markedly elevated CCR9 expression combined with a reduced expression of α4β7 seen in our study could suggest that these receptors are differently regulated during development. An increase of T cells with a skewed CCR9-α4β7 expression in the circulation could therefore just be a mirror of a T cell compartment under development, but it could also be a sign of a reduced gut-homing capacity. The elevated expression of CCR4 (skin-homing) in the preterm group also indicates that although the T cell compartment expresses receptors for tissue homing, they have not as yet reached their tissue targets, which reflects their profound prematurity. It has been shown previously that homing receptors switch from α4β7 to CCR4 on Treg during the first year of life, and in newborn infants, CCR4 is linked to mature phenotype, whereas α4β7 is linked to NA phenotype of Tregs (45).
As most studies on preterm birth and immunity are relatively small, it is difficult to separate the effects of prematurity from the influence of sepsis, chorioamnionitis, NEC, etc., on immune cell characteristics and functions (46–48). Throughout our study, we noted a large variation within all cell populations and markers that we investigated in the ELGAN/ELBW group at D14. We reasoned that this high level of variation could be attributed to certain clinical outcomes, such as sepsis, NEC, and/or chorioamnionitis, but also on the probiotic supplementation that ∼50% of the preterm infants received. Sepsis is known to severely hamper most T cell populations and functions (49). Interestingly, regardless of time point for onset of the disease, sepsis had little impact on the overall T cell compartment, the Treg functional phenotype, or their homing characteristics (Fig. 7). To us, this suggests that the T cell compartment is comparably unresponsive to bacterial infections this early in life and reflects the relative immaturity of the T cell compartment, the enhanced Th2 skewing, and a hampered ability to mount immune responses to infection at these early time points. Only a few children in the cohort developed NEC, and all of them had sepsis, which made it difficult to pursue a reliable statistical analysis with the data on the cause and impact of NEC cases. Still, we observed that all except for one of the NEC cases clustered among the individuals with the highest CCR9 expression among total CD4+ T cells and also among the Tregs (Fig. 7), maybe indicative of a reduced gut-homing capacity of T cells in children that develop NEC. Our observation corroborates with a recent study that has shown increased frequencies of CCR9+ CD4 T cells and Tregs in the circulation of NEC patients (50). One study has shown decreased expression of α4β7 on Treg from lamina propria mononuclear cells of NEC patients (47). However, because of very low frequencies of Tregs in NEC cases in our cohort, it was not possible to further analyze for that homing receptor on Tregs.
In contrast to sepsis, chorioamnionitis was associated with reduced Treg frequencies with downregulated functional characteristics of Tregs at D14 (Fig. 7). This agrees with a previous study conducted on cord blood from preterm infants (41) and could suggest that Tregs had migrated from the circulation to the maternal-fetal interphase to control the inflammatory response associated with chorioamnionitis. However, as the circulating Tregs also had an altered functional phenotype, it is more likely to represent that Tregs are suppressed by this inflammatory condition. Finally, L. reuteri supplementation had no effect on the phenotypic features of the conventional T cell compartment at all, neither at D14, D28, or PMW + 0 (Fig. 8, Supplemental Table II).
To the best of our knowledge, this is the largest reported study investigating the conventional T cell compartment in a longitudinal way during the first months of life in extremely premature infants. Moreover, the strength of our study is that we have collected blood samples from FT controls at 14 d of age, the same age as the ELGAN/ELBW preterm neonates sampled at the first occasion. We also had the power to consider the influence of gestational age at birth, birth weight, sepsis, and chorioamnionitis on all investigated T cell aspects. Although several studies have reported on characteristics and functions of the premature immune system, most existing data stem from the analyses of cord blood, which is not predictive of postnatal immunity (14, 51). Furthermore, other studies using samples collected after birth rely on information from one single time point (52), and many studies are relatively small (30, 31), making it impossible to properly consider variations inferred by sepsis, chorioamnionitis, gestational age, etc. Also, most studies have not focused on extreme prematurity but include a wider spectrum of premature infants (53).
In conclusion, we show that the conventional T cell compartment is markedly affected by prematurity in ELGAN/ELBW infants, particularly during the first weeks of life, but there are also differences that persist for months. At 14 d of life, the ELGAN/ELBW infants are highly lymphopenic, have an increased CD4/CD8 ratio, have features of a Th2-skewed phenotype, and have T cells with an altered homing capacity compared with infants born FT, features that could at least partially explain the increased susceptibility to postnatal infections within this vulnerable group of infants. Chorioamnionitis, rather than sepsis, influenced the Treg compartment, whereas probiotic supplementation had no obvious effects on any of the parameters investigated in this study. The data presented in this study will also contribute to a better understanding of the dynamic development of the immune system during the first months of life.
Supplementary Material
Acknowledgments
We want to thank all participating families and the study nurses Christina Fuxin and Karin Jansmark as well as Anne-Marie Fornander, Camilla Janefjord, and Dr. Veronica Frimmel for valuable assistance.
T.A. and E.S.-E. shared senior authorship.
This work was supported by grants from the Swedish Research Council (Medicine) (Grants 921.2014-7060 to T.A. and 2016-01715_3 to E.S.-E.), the Ekhaga Foundation, the Swedish Society of Medicine, the Research Council for South-East Sweden, ALF grants, Region Östergötland (19-00941-FLR), and BioGaia AB.
The online version of this article contains supplemental material.
- CM
- central memory
- D14
- day 14
- D28
- day 28
- ELBW
- extremely low birth weight
- ELGAN
- extremely low gestational age neonate
- FT
- full-term
- NA
- naive
- NEC
- necrotizing enterocolitis
- PCA
- principal component analysis
- PMW
- postmenstrual week
- Treg
- T regulatory cell.
Disclosures
T.A. has received honoraria for lectures and a grant for the present trial from BioGaia AB. M.J. has also received honoraria for lectures from BioGaia AB. The other authors have no conflicts of interest.
References
- 1.Purisch S. E., Gyamfi-Bannerman C. 2017. Epidemiology of preterm birth. Semin. Perinatol. 41: 387–391. [DOI] [PubMed] [Google Scholar]
- 2.Norman M., Hallberg B., Abrahamsson T., Björklund L. J., Domellöf M., Farooqi A., Foyn Bruun C., Gadsbøll C., Hellström-Westas L., Ingemansson F., et al. 2019. Association between year of birth and 1-year survival among extremely preterm infants in Sweden during 2004-2007 and 2014-2016. JAMA 321: 1188–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manuck T. A., Rice M. M., Bailit J. L., Grobman W. A., Reddy U. M., Wapner R. J., Thorp J. M., Caritis S. N., Prasad M., Tita A. T., et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network 2016. Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. Am. J. Obstet. Gynecol. 215: 103.e1–103.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X., Zhivaki D., Lo-Man R. 2017. Unique aspects of the perinatal immune system. Nat. Rev. Immunol. 17: 495–507. [DOI] [PubMed] [Google Scholar]
- 5.Wilson C. B., Westall J., Johnston L., Lewis D. B., Dower S. K., Alpert A. R. 1986. Decreased production of interferon-gamma by human neonatal cells. Intrinsic and regulatory deficiencies. J. Clin. Invest. 77: 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mold J. E., Venkatasubrahmanyam S., Burt T. D., Michaëlsson J., Rivera J. M., Galkina S. A., Weinberg K., Stoddart C. A., McCune J. M. 2010. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science 330: 1695–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mold J. E., Michaëlsson J., Burt T. D., Muench M. O., Beckerman K. P., Busch M. P., Lee T. H., Nixon D. F., McCune J. M. 2008. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 322: 1562–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhivaki D., Lo-Man R. 2017. In utero development of memory T cells. Semin. Immunopathol. 39: 585–592. [DOI] [PubMed] [Google Scholar]
- 9.Schreurs R. R. C. E., Baumdick M. E., Sagebiel A. F., Kaufmann M., Mokry M., Klarenbeek P. L., Schaltenberg N., Steinert F. L., van Rijn J. M., Drewniak A., et al. 2019. Human fetal TNF-α-cytokine-producing CD4+ effector memory T cells promote intestinal development and mediate inflammation early in life. Immunity 50: 462–476.e8. [DOI] [PubMed] [Google Scholar]
- 10.Li N., van Unen V., Abdelaal T., Guo N., Kasatskaya S. A., Ladell K., McLaren J. E., Egorov E. S., Izraelson M., Chuva de Sousa Lopes S. M., et al. 2019. Memory CD4+ T cells are generated in the human fetal intestine. Nat. Immunol. 20: 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zens K. D., Chen J. K., Guyer R. S., Wu F. L., Cvetkovski F., Miron M., Farber D. L. 2017. Reduced generation of lung tissue-resident memory T cells during infancy. J. Exp. Med. 214: 2915–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crespo M., Martinez D. G., Cerissi A., Rivera-Reyes B., Bernstein H. B., Lederman M. M., Sieg S. F., Luciano A. A. 2012. Neonatal T-cell maturation and homing receptor responses to Toll-like receptor ligands differ from those of adult naive T cells: relationship to prematurity. Pediatr. Res. 71: 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yerkovich S. T., Wikström M. E., Suriyaarachchi D., Prescott S. L., Upham J. W., Holt P. G. 2007. Postnatal development of monocyte cytokine responses to bacterial lipopolysaccharide. Pediatr. Res. 62: 547–552. [DOI] [PubMed] [Google Scholar]
- 14.Olin A., Henckel E., Chen Y., Lakshmikanth T., Pou C., Mikes J., Gustafsson A., Bernhardsson A. K., Zhang C., Bohlin K., Brodin P. 2018. Stereotypic immune system development in newborn children. Cell 174: 1277–1292.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawh S. C., Deshpande S., Jansen S., Reynaert C. J., Jones P. M. 2016. Prevention of necrotizing enterocolitis with probiotics: a systematic review and meta-analysis. PeerJ 4: e2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mu Q., Tavella V. J., Luo X. M. 2018. Role of Lactobacillus reuteri in human health and diseases. Front. Microbiol. 9: 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haileselassie Y., Navis M., Vu N., Qazi K. R., Rethi B., Sverremark-Ekström E. 2016. Postbiotic modulation of retinoic acid imprinted mucosal-like dendritic cells by probiotic Lactobacillus reuteri 17938 in vitro. Front. Immunol. 7: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansson M. A., Björkander S., Mata Forsberg M., Qazi K. R., Salvany Celades M., Bittmann J., Eberl M., Sverremark-Ekström E. 2016. Probiotic lactobacilli modulate Staphylococcus aureus-induced activation of conventional and unconventional T cells and NK cells. Front. Immunol. 7: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haileselassie Y., Navis M., Vu N., Qazi K. R., Rethi B., Sverremark-Ekström E. 2016. Lactobacillus reuteri and Staphylococcus aureus differentially influence the generation of monocyte-derived dendritic cells and subsequent autologous T cell responses. Immun. Inflamm. Dis. 4: 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forsberg A., Abrahamsson T. R., Björkstén B., Jenmalm M. C. 2014. Pre- and postnatal administration of Lactobacillus reuteri decreases TLR2 responses in infants. Clin. Transl. Allergy 4: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forsberg A., Abrahamsson T. R., Björkstén B., Jenmalm M. C. 2013. Pre- and post-natal Lactobacillus reuteri supplementation decreases allergen responsiveness in infancy. Clin. Exp. Allergy 43: 434–442. [DOI] [PubMed] [Google Scholar]
- 22.Wejryd E., Marchini G., Frimmel V., Jonsson B., Abrahamsson T. 2019. Probiotics promoted head growth in extremely low birthweight infants in a double-blind placebo-controlled trial. Acta Paediatr. 108: 62–69. [DOI] [PubMed] [Google Scholar]
- 23.Tita A. T., Andrews W. W. 2010. Diagnosis and management of clinical chorioamnionitis. Clin. Perinatol. 37: 339–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell M. J., Ternberg J. L., Feigin R. D., Keating J. P., Marshall R., Barton L., Brotherton T. 1978. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 187: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oncel M. Y., Sari F. N., Arayici S., Guzoglu N., Erdeve O., Uras N., Oguz S. S., Dilmen U. 2014. Lactobacillus Reuteri for the prevention of necrotising enterocolitis in very low birthweight infants: a randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 99: F110–F115. [DOI] [PubMed] [Google Scholar]
- 26.Indrio F., Riezzo G., Raimondi F., Bisceglia M., Cavallo L., Francavilla R. 2008. The effects of probiotics on feeding tolerance, bowel habits, and gastrointestinal motility in preterm newborns. J. Pediatr. 152: 801–806. [DOI] [PubMed] [Google Scholar]
- 27.Hazenberg M. D., Verschuren M. C., Hamann D., Miedema F., van Dongen J. J. 2001. T cell receptor excision circles as markers for recent thymic emigrants: basic aspects, technical approach, and guidelines for interpretation. J. Mol. Med. (Berl.) 79: 631–640. [DOI] [PubMed] [Google Scholar]
- 28.de Felipe B., Olbrich P., Lucenas J. M., Delgado-Pecellin C., Pavon-Delgado A., Marquez J., Salamanca C., Soler-Palacin P., Gonzalez-Granado L. I., Antolin L. F., et al. 2016. Prospective neonatal screening for severe T- and B-lymphocyte deficiencies in Seville. Pediatr. Allergy Immunol. 27: 70–77. [DOI] [PubMed] [Google Scholar]
- 29.Scheible K. M., Emo J., Laniewski N., Baran A. M., Peterson D. R., Holden-Wiltse J., Bandyopadhyay S., Straw A. G., Huyck H., Ashton J. M., et al. 2018. T cell developmental arrest in former premature infants increases risk of respiratory morbidity later in infancy. JCI Insight. DOI: 10.1172/jci.insight.96724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheible K. M., Emo J., Yang H., Holden-Wiltse J., Straw A., Huyck H., Misra S., Topham D. J., Ryan R. M., Reynolds A. M., et al. 2015. Developmentally determined reduction in CD31 during gestation is associated with CD8+ T cell effector differentiation in preterm infants. Clin. Immunol. 161: 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sava F., Toldi G., Treszl A., Hajdú J., Harmath Á., Tulassay T., Vásárhelyi B. 2016. Expression of lymphocyte activation markers of preterm neonates is associated with perinatal complications. BMC Immunol. 17: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luciano A. A., Yu H., Jackson L. W., Wolfe L. A., Bernstein H. B. 2011. Preterm labor and chorioamnionitis are associated with neonatal T cell activation. PLoS One 6: e16698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Debock I., Flamand V. 2014. Unbalanced neonatal CD4(+) T-cell immunity. Front. Immunol. 5: 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hebel K., Weinert S., Kuropka B., Knolle J., Kosak B., Jorch G., Arens C., Krause E., Braun-Dullaeus R. C., Brunner-Weinzierl M. C. 2014. CD4+ T cells from human neonates and infants are poised spontaneously to run a nonclassical IL-4 program. J. Immunol. 192: 5160–5170. [DOI] [PubMed] [Google Scholar]
- 35.Webster R. B., Rodriguez Y., Klimecki W. T., Vercelli D. 2007. The human IL-13 locus in neonatal CD4+ T cells is refractory to the acquisition of a repressive chromatin architecture. J. Biol. Chem. 282: 700–709. [DOI] [PubMed] [Google Scholar]
- 36.Rennó C., Nadaf M. I., Zago C. A., Carneiro-Sampaio M., Palmeira P. 2016. Healthy preterm newborns show an increased frequency of CD4(+) CD25(high) CD127(low) FOXP3(+) regulatory T cells with a naive phenotype and high expression of gut-homing receptors. Scand. J. Immunol. 83: 445–455. [DOI] [PubMed] [Google Scholar]
- 37.Dirix V., Vermeulen F., Mascart F. 2013. Maturation of CD4+ regulatory T lymphocytes and of cytokine secretions in infants born prematurely. J. Clin. Immunol. 33: 1126–1133. [DOI] [PubMed] [Google Scholar]
- 38.Alroqi F. J., Chatila T. A. 2016. T regulatory cell biology in health and disease. Curr. Allergy Asthma Rep. 16: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukhopadhyay D., Weaver L., Tobin R., Henderson S., Beeram M., Newell-Rogers M. K., Perger L. 2014. Intrauterine growth restriction and prematurity influence regulatory T cell development in newborns. J. Pediatr. Surg. 49: 727–732. [DOI] [PubMed] [Google Scholar]
- 40.Luciano A. A., Arbona-Ramirez I. M., Ruiz R., Llorens-Bonilla B. J., Martinez-Lopez D. G., Funderburg N., Dorsey M. J. 2014. Alterations in regulatory T cell subpopulations seen in preterm infants. PLoS One 9: e95867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rueda C. M., Wells C. B., Gisslen T., Jobe A. H., Kallapur S. G., Chougnet C. A. 2015. Effect of chorioamnionitis on regulatory T cells in moderate/late preterm neonates. Hum. Immunol. 76: 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campbell D. J., Koch M. A. 2011. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat. Rev. Immunol. 11: 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huehn J., Siegmund K., Lehmann J. C., Siewert C., Haubold U., Feuerer M., Debes G. F., Lauber J., Frey O., Przybylski G. K., et al. 2004. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J. Exp. Med. 199: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evans-Marin H. L., Cao A. T., Yao S., Chen F., He C., Liu H., Wu W., Gonzalez M. G., Dann S. M., Cong Y. 2015. Unexpected regulatory role of CCR9 in regulatory T cell development. PLoS One 10: e0134100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grindebacke H., Stenstad H., Quiding-Järbrink M., Waldenström J., Adlerberth I., Wold A. E., Rudin A. 2009. Dynamic development of homing receptor expression and memory cell differentiation of infant CD4+CD25high regulatory T cells. J. Immunol. 183: 4360–4370. [DOI] [PubMed] [Google Scholar]
- 46.Pagel J., Hartz A., Figge J., Gille C., Eschweiler S., Petersen K., Schreiter L., Hammer J., Karsten C. M., Friedrich D., et al. 2016. Regulatory T cell frequencies are increased in preterm infants with clinical early-onset sepsis. Clin. Exp. Immunol. 185: 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weitkamp J. H., Koyama T., Rock M. T., Correa H., Goettel J. A., Matta P., Oswald-Richter K., Rosen M. J., Engelhardt B. G., Moore D. J., Polk D. B. 2013. Necrotising enterocolitis is characterised by disrupted immune regulation and diminished mucosal regulatory (FOXP3)/effector (CD4, CD8) T cell ratios. Gut 62: 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michaëlsson J., Mold J. E., McCune J. M., Nixon D. F. 2006. Regulation of T cell responses in the developing human fetus. J. Immunol. 176: 5741–5748. [DOI] [PubMed] [Google Scholar]
- 49.Jensen I. J., Sjaastad F. V., Griffith T. S., Badovinac V. P., 2018. Sepsis-induced T cell immunoparalysis: the ins and outs of impaired T cell immunity. J. Immunol. 200: 1543–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma F., Li S., Gao X., Zhou J., Zhu X., Wang D., Cai Y., Li F., Yang Q., Gu X., et al. 2019. Interleukin-6-mediated CCR9+ interleukin-17-producing regulatory T cells polarization increases the severity of necrotizing enterocolitis. EBioMedicine 44: 71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Correa-Rocha R., Pérez A., Lorente R., Ferrando-Martínez S., Leal M., Gurbindo D., Muñoz-Fernández M. Á. 2012. Preterm neonates show marked leukopenia and lymphopenia that are associated with increased regulatory T-cell values and diminished IL-7. Pediatr. Res. 71: 590–597. [DOI] [PubMed] [Google Scholar]
- 52.Peoples J. D., Cheung S., Nesin M., Lin H., Tatad A. M., Hoang D., Perlman J. M., Cunningham-Rundles S. 2009. Neonatal cord blood subsets and cytokine response to bacterial antigens. Am. J. Perinatol. 26: 647–657. [DOI] [PubMed] [Google Scholar]
- 53.Melville J. M., Moss T. J. 2013. The immune consequences of preterm birth. Front. Neurosci. 7: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.