Abstract
Serologic monitoring of infectious diseases is important for microbial control in colonies of laboratory mice. Rapid and simple tests that do not require killing animals are valuable for this purpose. In this study, we developed a multiplex immunochromatographic assay (ICA) for detection of antibodies to mouse hepatitis virus (MHV), Sendai virus (also known as hemagglutinating virus of Japan [HVJ]), and Clostridium piliforme (The pathogen that causes Tyzzer disease), which are major infectious diseases in mice. For this assay, an ICA strip was put into a microtube containing 150 μL PBS and either 0.75 μL mouse serum or 1.5 μL whole blood. Binding antibodies were visualized by using protein A-conjugated colloidal gold. Under these conditions, multiplex ICA simultaneously and specifically detected antibodies to multiple antigens. To evaluate the sensitivity and specificity of multiplex ICA, positive serum samples for each infectious disease were used. Sensitivities of the multiplex ICA test for MHV, HVJ, and C. piliforme were 100%, 100%, and 90%, respectively. No nonspecific reaction was observed in any of the 30 positive sera. In addition, 10 samples of uninfected sera did not show any bands except for the control line. These observations indicate high specificity of the multiplex ICA test. Moreover, the multiplex ICA could be applied to diluted blood. These results indicate that the multiplex ICA is appropriate for rapid, simple, and safe serologic testing of laboratory mice.
Abbreviations: HVJ, hemagglutinating virus of Japan (Sendai virus); ICA, immunochromatographic assay; IFA, immunofluorescent antibody assay; IVC, individually ventilated caging; MHV, mouse hepatitis virus; Tyzzer, Clostridium piliforme
Microbiologic monitoring of laboratory animal colonies is important to detect contamination and ensure animals are fit for enrollment in experimental studies. Conventional microbiologic monitoring typically involves using sentinel animals in the mouse rooms to monitor for the presence of pathogens. Analysis of these mice may require the euthanasia of the sentinel. Moreover, the use of individually ventilated cages (IVC) complicates the necessary horizontal transmission of pathogens from experimental animals to sentinels.4,6,8 To address this problem, experimental animals and sentinels must both be examined.
The authors propose a highly sensitive diagnostic method that uses small amounts of blood that can be collected without euthanasia. By detecting antibodies against pathogens, immunochromatographic assays (ICA) have been used widely to diagnose various infectious diseases.5,14,15,21,25 We previously reported an ICA that is a highly sensitive, rapid and simple diagnostic method for detecting IgG to hantavirus in a minute amount of rat serum.1,2 Therefore, ICA is a promising strategy for simultaneously detecting serum antibodies to various mouse pathogens.
In this study, we developed and evaluated a multiplex ICA for mice that detects serum antibodies to mouse hepatitis virus (MHV), Sendai virus (also known as hemagglutinating virus of Japan [HVJ]), and Clostridium piliforme, which is the causative agent for Tyzzer disease.
Materials and Methods
Control antigens.
Inactivated fractions of purified MHV (S strain) and HVJ (MN strain) virions and purified, inactivated C. piliforme (RJ strain) were provided by the ICLAS Monitoring Center (Central Institute for Experimental Animals, Japan).11,12
Serum and blood.
Serum samples positive for antibodies against MHV, HVJ, and C. piliforme were provided by the ICLAS Monitoring Center. These samples were diagnosed through ELISA and immunofluorescent antibody assay, as previously described.12 The protocol for collecting positive serum samples was approved by the IACUC according to the Regulations for Animal Experimentation of the Central Institute for Experimental Animals, Japan. The regulations were established in compliance with the law regarding the humane treatment and management of animals.
Previously described sera24 that had tested negative by microbiologic monitoring for antibodies against MHV, HVJ, and C. piliforme were used as control samples. These sera were collected from mice euthanized under approval from the Hokkaido University Animal Experimentation Committee.
Whole blood was collected from a single, euthanized sentinel ICR mouse, which had been housed in the Institute for Animal Experimentation (Faculty of Medicine, Hokkaido University, Japan). Husbandry and care procedures followed recommendations in the Guide for the Care and Use of Laboratory Animals.20 The health status of SPF animals was assessed 3 times each year for the following pathogens: Citrobacter rodentium, Corynebacterium kutscheri, Mycoplasma pulmonis, Salmonella spp., Clostridium piliforme, ectromelia virus, lymphocytic choriomeningitis virus, mouse hepatitis virus, Sendai virus, ectoparasites, intestinal protozoa, and pinworm. The sentinel mouse was free of these pathogens.
Multiplex ICA.
Preparation of a colloidal gold conjugate.
Protein A-conjugated colloidal gold was prepared as described previously.2 Briefly, a gold colloid solution (WRGH2, Wine Red Chemical, Tokyo, Japan) was mixed with an equal volume of 10 mM Tris-HCl (pH 8.15). Protein A (Nakarai, Tokyo, Japan) was diluted to 1.5 mg/mL by using 10 mM Tris-HCl (pH 8.15) and then mixed with 2 volumes of the colloidal gold dilution in a siliconized tube. After brief sonication, the mixture was incubated for 20 min at room temperature, after which a 1/3 volume of blocking solution (2.5% casein sodium (Wako, Osaka, Japan) in 10 mM Tris-HCl [pH 9.2]) was added. The mixture was sonicated briefly, incubated for 20 min at room temperature, and centrifuged at 14,000 × g for 15 min. After removal of the supernatant, the pellet was resuspended in blocking solution to the initial volume of the colloidal gold. After brief sonication, the mixture was incubated for 20 min at room temperature and then centrifuged at 14,000 × g for 15 min. Finally, the supernatant was removed, and the pellet was resuspended in 200 μL of blocking solution.
Preparation of a conjugate pad.
A glass-fiber conjugate pad (GFDX103000, Millipore, Billerica, MA) was soaked in 0.5% casein in 20 mM sodium phosphate buffer (pH 7.8) for 20 min. The pad was washed with 3.0% sucrose in double-distilled water and air-dried overnight. The pad was coated with the prepared colloidal gold conjugate at a dose of 200 μL per 30 cm. The pad was air-dried overnight and stored at room temperature.
Preparation of a multiplex ICA strip.
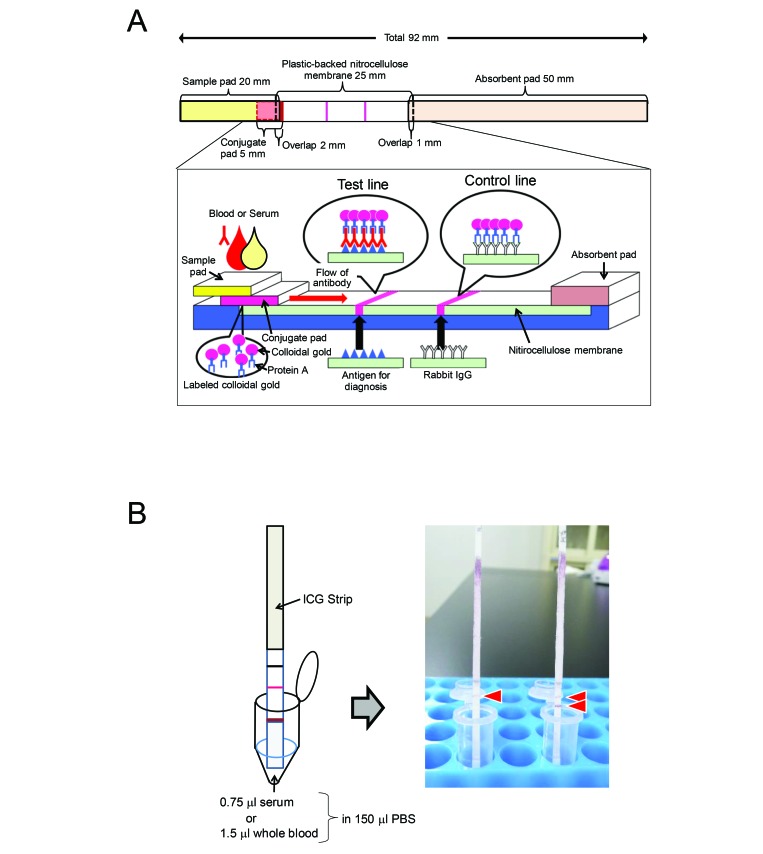
MHV, HVJ, and C. piliforme and rabbit IgG were diluted with 0.05% casein sodium in 10 mM sodium phosphate buffer (pH 7.2) to adjust the concentrations to 0.5 mg/mL for the antigens and to 0.15 mg/mL for the IgG. These reagents were immobilized on nitrocellulose membrane (Hi-Flow Plus 135 Membrane Card, 60 mm × 300 mm, Millipore) by using a pen (Super brush for Copic Sketch and Ciao, Too Marker Products, Shinagawa, Japan) to draw thin lines of reagent at the test line position (antigen) and control line position (rabbit IgG). An ICA strip containing the 3 antigens and the control as separate lines is a multiplex ICA test strip. After the membrane had been dried at room temperature for 15 min, it was soaked in 20 mM sodium phosphate buffer containing 0.5% casein (pH 7.8) for 20 min to block the unsaturated area. Then the membrane was washed twice (5 min each) with double-distilled water, soaked in 3.0% sucrose in double-distilled water, and air-dried overnight. Finally, a sample pad (20 mm × 300 mm; 3-mm Chromatography Paper, Whatman, GE Healthcare BioSciences, Pittsburgh, PA), conjugate pad, nitrocellulose membrane, and absorbent pad (50 mm × 300 mm; catalog no. CFSP20300, Cellulose Fiber Sample Pad, Millipore) were assembled on a membrane card (Millipore). The combined membranes were cut into 2-mm-wide strips by using a paper cutter. The structure of the ICA strip is shown in Figure 1 A. The ICA strip was stored at room temperature in a dry box until use and was used within 3 mo.
Figure 1.
Detection method and scheme of the immunochromatographic test (ICA) strip. (A) Structure of the ICA strip. Antigens and rabbit IgG were placed at the test lines and control line, respectively. The ICA strip consisted of 4 membrane pads: sample pad, conjugate pad, nitrocellulose membrane, and absorbent pad. The conjugate pad contained the protein A–colloidal gold conjugate. (B) Detection method. Serum (0.75 μL) and whole blood (1.5 μL) were diluted with 150 μL of PBS and then placed in a microtube.
Dilution of serum and whole blood for ICA.
A 0.75-μL serum sample was added to 150 μL PBS, making a 1:200 dilution. To prepare a positive serum mixture, 0.75 μL of either 2 or 3 serum samples positive for HVJ, MHV, and C. piliforme added to 150 μL PBS. In addition, 1.5 μL of whole blood was added to the diluted serum to examine the effect of RBC on ICA.
Procedure for ICA.
An ICA strip was put directly into a microtube containing PBS-diluted serum or whole blood (Figure 1 B). After the tube remained still for 15 min, the ICA strip was examined visually. A sample that showed both test and control lines detecting rabbit IgG was regarded as positive, and a sample that showed only the control line was regarded as negative.
Results
Determining the basic conditions for multiplex ICA.
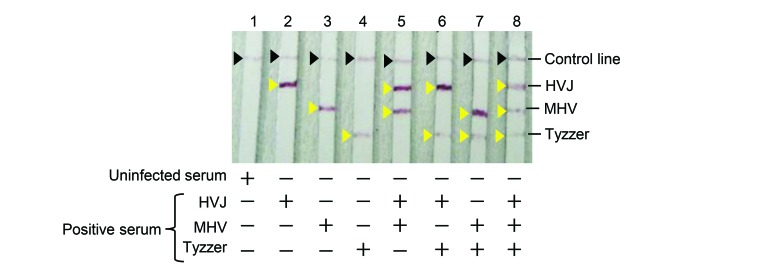
Single ICA tests were performed by using different concentrations of positive serum (1:50, 100, and 200) and antigens (1:1, 10, and 100). Single ICA tests with MHV, HVJ, or C. piliforme antigen at a concentration of 0.5 mg/mL detected antibodies in 1:200-diluted serum (data not shown). In addition, no nonspecific reaction was observed when serum from an uninfected mouse was used under these conditions. Multiplex ICA using all 3 antigens specifically and simultaneously detected antibodies to the corresponding antigens (Figure 2).
Figure 2.
Multiplex ICA using diluted antibody-positive serum. Uninfected (control) serum and each positive serum were diluted with 150 μL PBS. Lanes 1 through 4, 0.75 μL of serum in PBS; lanes 5 through 8, 0.75-μL samples of 2 or 3 antibody-positive sera were added to PBS.
Evaluating the sensitivity and specificity of multiplex ICA.
To evaluate the sensitivity and specificity of multiplex ICA, 10 samples each of antibody-positive sera to MHV, HVJ, and C. piliforme were used. The multiplex ICA strip detected the antigen-specific antibody in all of the HVJ-positive serum samples (Figure 3 A) and all of the MHV-positive serum samples (Figure 3 B). Specific antibody to C. piliforme was detected in all of the C. piliforme -positive serum samples except no. 8 (9 of 10 samples, 90%; Figure 3 C). No nonspecific reaction was observed in any of the 30 antibody-positive sera. Moreover, 10 samples of uninfected sera did not show any bands except for the control line (Figure 3 D). These observations indicate the high specificity of multiplex ICA.
Figure 3.
Evaluation of the sensitivity and specificity of multiplex ICA. Antibody-positive sera to each infectious disease—(A) HVJ, (B) MHV, and (C) Tyzzer—that were diagnosed through ELSA and immunofluorescent antibody assay were used (10 samples per infectious disease). (D) In addition, 10 uninfected sera were used as negative serum samples.
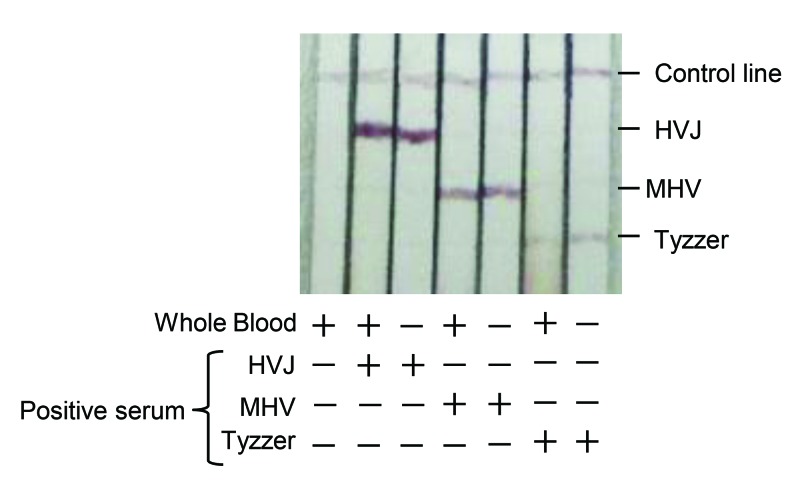
Evaluating multiplex ICA using whole blood.
To investigate the influence of RBC on multiplex ICA, we compared the ICA results for diluted antibody-positive serum with those from diluted antibody-positive sera mixed with whole blood. Multiplex ICA test specifically detected antibodies to the HVJ, MHV, and C. piliforme antigens in the presence of whole blood (Figure 4). These observations suggest that multiplex ICA can detect antibodies to MHV, HVJ, and C. piliformeTyzzer in whole blood, without the need to remove RBC from samples.
Figure 4.
Evaluation of multiplex ICA using whole blood. Whole blood (1.5 μL) was added to 150 μL PBS with or without antibody-positive serum (0.75 μL) to HVJ, MHV, and Tyzzer.
Discussion
Our multiplex ICA containing antigens for HVJ, MHV, and C. piliforme specifically and simultaneously detected antibodies to the corresponding antigens in a small amount of serum. In addition, the multiplex ICA presented here showed high levels of specificity and sensitivity among antigen-positive as well as uninfected serum samples. Moreover, our multiplex ICA was applicable to diluted whole blood.
The use of IVC to house rodents has been increasing in recent years. Exposure of sentinels to soiled bedding has traditionally been the most common method for monitoring the microbiologic status of rodents but is becoming difficult. For example, HVJ is a respiratory agent and is not effectively transmitted through soiled-bedding transfer.6 For C. piliforme, the efficacy of soiled-bedding transfer is unknown, due to insufficient data.8 To solve these problems, methods that assess sentinel exposure by surveying the exhaust air of IVC have been reported.4,6,18,19,26 However, if a pathogenic microbe is detected in animal rooms, sentinels as well as experimental animals must be examined to identify sources of infection or sensitive hosts. In most cases, experimental animals need to be evaluated without euthanizing them because the number of maintenance strains is increasing and because few animals of each strain are cohoused. Assessing microbial status without euthanizing animals, including sentinels, is also important from a 3Rs perspective.
As means for diagnosing infectious disease without euthanasia of mice, multiplex microbead immunoassay and PCR-based detection of antigen in feces have been reported.9,13,17,22,23 However, PCR-based methods are not useful in cases in that lack fecal shedding of the antigen, such as HVJ, or when the period of antigen shedding in feces is very short. In these situations, serologic diagnosis is effective. Recently, the use of bioluminescence imaging for in vivo monitoring of infectious diseases has also been reported.3 However, for inhouse evaluation, these methods are impractical because they require an expensive, dedicated analyzer. Multiplex ICA is useful for inhouse monitoring because it simply, simultaneously, and specifically detects antibodies to multiple antigens; all that is required is to place the ICA strip into a microtube containing a small amount of PBS-diluted serum or blood (Figure 1 B).
ELISA has been the main method for serologic diagnosis in laboratory mice. Recently, a multiplex microbead immunoassay has been used for diagnosis of infectious diseases.13,17,22 In the current study, we used antibody-positive serum samples against MHV, HVJ, and C. piliforme that had been diagnosed by ELISA and immunofluorescent antibody assay to evaluate the sensitivity and specificity of our multiplex ICA. The rate of detection of the specific antibody to C. piliforme was 90% (9 of 10; Figure 3 C). These results indicate that ICA is less sensitive than ELISA for C. piliforme. In addition, a multiplex microbead immunoassay reportedly is as sensitive and specific as ELISA for serodetection,13 thus suggesting that ICA also is less sensitive than a multiplex microbead immunoassay for C. piliforme.. In comparison, the rate of detection of specific antibodies to MHV and HVJ was 100% (10 of 10 samples) for both pathogens (Figure 3 A and B). However, many more positive serum samples need to be analyzed to precisely evaluate the sensitivity of ICA for MHV and HVJ. Conversely, ICA did not produce any nonspecific reactions in any of the 30 positive sera (Figure 3 A through C). In addition, 10 uninfected control sera did not show any bands except for the internal positive control for each test (Figure 3 D). These results indicate that multiplex ICA has high specificity for MHV, HVJ, and C. piliforme.
In the current study, our multiplex ICA specifically detected antibodies to 3 pathogens: HVJ, MHV, and C. piliforme. However, to use multiplex ICA widely for routine serologic screening, it will need to identify antigens to several more infectious diseases than just these 3 agents. According to recommendations from the Federation of European Laboratory Animal Science Associations (FELASA), health monitoring programs in laboratory animal facilities should include at least 11 pathogens.16 Although additional agents need to be included to adapt ICA for microbiologic monitoring, the format we developed in the current study is an effective, rapid tool for evaluating for HVJ, MHV, and C. piliforme or in case of a disease outbreak due to one of these pathogens.
Dried blood has been used widely for human and veterinary diagnostic tests.7,10 Various microsampling devices for collecting blood have become available commercially. Multiplex ICA specifically detected antibodies to HVJ, MHV, and C. piliforme antigens in the presence of whole blood (Figure 4). These results suggest that samples for multiplex ICA do not need to be free of RBC. Additional studies to determine whether multiplex ICA can be applied to dried-blood samples are needed for achieving practical use of this test modality.
Although multiplex ICA is rapid and simple, the preparation of the test strips is time-consuming and labor-intensive, and many laboratories may lack the necessary reagents and technical skill. Commercial production of test strips might overcome these disadvantages. Nonrefrigerated transport of test strips likely is possible, because they can be kept at room temperature for at least 3 mo. In addition, the cost to prepare ICA strips is low: in the current study, we needed only 15 μg of each antigen, 0.75 mg of protein A, and 0.5 mL of gold colloid solution to generate 100 ICA strips.
In the current study, multiplex ICA using 3 antigens—HVJ, MHV, and C. piliforme —simultaneously and specifically detected antibodies to the corresponding antigens in small quantities of serum or diluted blood. These results show that multiplex ICA has promise for future diagnostic applications in laboratory mice. Future studies involving serologic testing of field samples and incorporating additional infectious agents are needed for achieving practical use of ICA.
Acknowledgments
This work was supported by a Grant-in-Aid for Science Research from the Japan Society for the Promotion of Science (no. 15K07717).
References
- 1.Amada T, Yoshimatsu K, Koma T, Shimizu K, Gamage CD, Shiokawa K, Nishio S, Ahlm C, Arikawa J. 2014. Development of an immunochromatography strip test based on truncated nucleocapsid antigens of three representative hantaviruses. Virol J 11:1–8. 10.1186/1743-422X-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amada T, Yoshimatsu K, Yasuda SP, Shimizu K, Koma T, Hayashimoto N, Gamage CD, Nishio S, Takakura A, Arikawa J. 2013. Rapid, whole blood diagnostic test for detecting anti-hantavirus antibody in rats. J Virol Methods 193:42–49. 10.1016/j.jviromet.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 3.Avci P, Karimi M, Sadasivam M, Antunes-Melo WC, Carrasco E, Hamblin MR. 2017. In-vivo monitoring of infectious diseases in living animals using bioluminescence imaging. Virulence 9:28–63. 10.1080/21505594.2017.1371897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brielmeier M, Mahabir E, Needham JR, Lengger C, Wilhelm P, Schmidt J. 2006. Microbiological monitoring of laboratory mice and biocontainment in individually ventilated cages: a field study. Lab Anim 40:247–260. 10.1258/002367706777611497. [DOI] [PubMed] [Google Scholar]
- 5.Chong YM, Tan XH, Hooi PS, Lee LM, Sam IC, Chan YF. 2019. Evaluation of rapid influenza diagnostic tests for influenza A and B in the tropics. J Med Virol 91:1562–1565. 10.1002/jmv.25495. [DOI] [PubMed] [Google Scholar]
- 6.Compton SR, Homberger FR, Paturzo FX, Clark JM. 2004. Efficacy of three microbiological monitoring methods in a ventilated cage rack. Comp Med 54:382–392. [PubMed] [Google Scholar]
- 7.Curry PS, Ribble C, Sears WC, Orsel K, Hutchins W, Godson D, Lindsay R, Dibernardo A, Campbell M, Kutz SJ. 2014. Blood collected on filter paper for wildlife serology: evaluating storage and temperature challenges of field collections. J Wildl Dis 50:308–321. 10.7589/2012-06-150. [DOI] [PubMed] [Google Scholar]
- 8.de Bruin WC, van de Ven EM, Hooijmans CR. 2016. Efficacy of soiled bedding transfer for transmission of mouse and rat infections to sentinels: a systematic review. PLoS One 11:1–11. 10.1371/journal.pone.0158410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furukawa T, Furumoto K, Fujieda M, Okada E. 2002. Detection by PCR of the Tyzzer's disease organism (Clostridium piliforme) in feces. Exp Anim 51:513–516. 10.1538/expanim.51.513. [DOI] [PubMed] [Google Scholar]
- 10.Geers LM, Cohen D, Wehkamp LM, van Hateren K, Koster RA, Fedorenko OY, Semke AV, Bokhan N, Ivanova SA, Kosterink JGW, Loonen AJM, Touw DJ. 2017. Dried blood spot analysis for therapeutic drug monitoring of clozapine. J Clin Psychiatry 78:e1211–e1218. 10.4088/JCP.16m11164. [DOI] [PubMed] [Google Scholar]
- 11.Goto K, Hayashimoto N, Ishida T, Takakura A, Kagiyama N. 2009. First trial in the developmental phase of the “performance evaluation program” based on the ICLAS animal quality network program: self-assessment of microbiological monitoring methods using test samples supplied by ICLAS. Exp Anim 58:47–52. 10.1538/expanim.58.47. [DOI] [PubMed] [Google Scholar]
- 12.Hayashimoto N, Morita H, Ishida T, Yasuda M, Kameda S, Uchida R, Tanaka M, Ozawa M, Sato A, Takakura A, Itoh T, Kagiyama N. 2013. Current microbiological status of laboratory mice and rats in experimental facilities in Japan. Exp Anim 62:41–48. 10.1538/expanim.62.41. [DOI] [PubMed] [Google Scholar]
- 13.Khan IH, Kendall LV, Ziman M, Wong S, Mendoza S, Fahey J, Griffey SM, Barthold SW, Luciw PA. 2005. Simultaneous serodetection of 10 highly prevalent mouse infectious pathogens in a single reaction by multiplex analysis. Clin Diagn Lab Immunol 12:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q, Wang L, Sun Y, Liu J, Ma F, Yang J, Zhao D, Zhang Y, Luo J, Guo J, Deng R, Zhang G. 2019. Evaluation of an immunochromatographic strip for detection of avian avulavirus 1 (Newcastle disease virus). J Vet Diagn Invest 31:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahinc C, Flori P, Delaunay E, Guillerme C, Charaoui S, Raberin H, Hafid J, L'Ollivier C. 2017. Evaluation of a new immunochromatography technology test (LDBio diagnostics) to detect toxoplasma IgG and IgM: comparison with the routine architect technique. J Clin Microbiol 55:3395–3404. 10.1128/JCM.01106-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mähler (Convenor) M, Berard M, Feinstein R, Gallagher A, Illgen-Wilcke B, Pritchett-Corning K, Raspa M. 2014. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab Anim 48:178–192. 10.1177/0023677213516312. [DOI] [PubMed] [Google Scholar]
- 17.Mani A, Ravindran R, Mannepalli S, Vang D, Luciw PA, Hogarth M, Khan IH, Krishnan VV. 2015. Data mining strategies to improve multiplex microbead immunoassay tolerance in a mouse model of infectious diseases. PLoS One 10:1–19. 10.1371/journal.pone.0116262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller M, Brielmeier M. 2017. Environmental samples make soiled bedding sentinels dispensable for hygienic monitoring of IVC-reared mouse colonies. Lab Anim 52:233–239. 10.1177/0023677217739329. [DOI] [PubMed] [Google Scholar]
- 19.Miller M, Ritter B, Zorn J, Brielmeier M. 2016. Exhaust air dust monitoring is superior to soiled bedding sentinels for the detection of pasteurella pneumotropica in individually ventilated cage systems. J Am Assoc Lab Anim Sci 55:775–781. [PMC free article] [PubMed] [Google Scholar]
- 20.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press [Google Scholar]
- 21.Paulini I, Siqueira-Silva J, Thomaz L, Rocha L, Harsi C, Bellei N, Granato C. 2017. Development of a prototype immunochromatographic test for rapid diagnosis of respiratory adenovirus infection. Braz J Infect Dis 21:500–506. 10.1016/j.bjid.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravindran R, Khan IH, Krishnan VV, Ziman M, Kendall LV, Frasier JM, Bates R, Griffey SM, Fahey JR, Luciw PA. 2010. Validation of multiplex microbead immunoassay for simultaneous serodetection of multiple infectious agents in laboratory mouse. J Immunol Methods 363:51–59. 10.1016/j.jim.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Smith GD, Solenberg PJ, Koenig MC, Brune KA, Fox N. 2002. Use of TaqMan reverse transcriptase-polymerase chain reaction analysis and serologic testing to eliminate an enzootic infection of mouse hepatitis virus. Comp Med 52:456–460. [PubMed] [Google Scholar]
- 24.Tosa N, Yoshimatsu K, Takahashi M, Arikawa J. 2019. Comparison of immune response in mice sensitized to an animal allergen, Can f 1, and to a food allergen, ovalbumin. Biomed Res 40:9–15. 10.2220/biomedres.40.9. [DOI] [PubMed] [Google Scholar]
- 25.Xu R, Zhao DY, Lu K, Hong Y, Li H, Lin JJ, Liu JM, Xu YM, Zhu C-g. 2015. [Development of colloidal gold immunochromatography assay strip for schistosomiasis diagnosis in domestic animals] Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 27:474–478. [Article in Chinese] [PubMed] [Google Scholar]
- 26.Zorn J, Ritter B, Miller M, Kraus M, Northrup E, Brielmeier M. 2017. Murine norovirus detection in the exhaust air of IVCs is more sensitive than serological analysis of soiled bedding sentinels. Lab Anim 51:301–310. 10.1177/0023677216661586. [DOI] [PubMed] [Google Scholar]