Abstract
NHP are a small, but critical, portion of the animals studied in research laboratories. Many NHP are imported or raised at one facility and subsequently moved to another facility for research purposes. To improve our understanding of the effects of transportation and relocation on the NHP immune system, to minimize potential confounds associated with relocation, and to maximize study validity, we examined the phenotype and function of PBMC in cynomolgus macaques (Macaca fascicularis) that were transported approximately 200 miles by road from one facility to another. We evaluated the phenotype of lymphocyte subsets through flow cytometry, mitogen-specific immune responses of PBMC in vitro, and plasma levels of circulating cytokines before transportation, at approximately 24 h after arrival (day 2), and after 30 d of acclimation. Analyses of blood samples revealed that the CD3+ and CD4+ T-cell counts increased significantly, whereas NK+, NKT, and CD14+CD16+ nonclassical monocyte subsets were decreased significantly on day 2 after relocation compared with baseline. We also noted significantly increased immune cell function as indicated by mitogen-specific proliferative responses and by IFNγ levels on day 2 compared with baseline. After 30 d of acclimation, peripheral blood CD4+ T-cells and monocyte counts were higher than baseline, whereas B-cell numbers were lower. The mitogen-induced responses to LPS and IFNγ production after stimulation with pokeweed mitogen or phytohemagglutinin remained significantly different from baseline. In conclusion, the effects of transportation and relocation on immune parameters in cynomolgus monkeys are significant and do not fully return to baseline values even after 30 d of acclimation.
Abbreviation: PWM, pokeweed mitogen
NHP are used in biomedical research to improve our understanding of behavior and cognitive function, immune function, reproduction, and preclinical studies. The data derived from these studies can be significantly affected by stress, thus necessitating a deeper understanding of how to maintain NHP health and wellbeing in captivity. In particular, NHP typically undergo transportation and relocation at least once in their lifetimes, given that breeding facilities and research facilities are frequently located at different institutions. Transporting and relocating animals is a well-established source of stress that elicits changes and variability in almost all aspects of animal physiology.6,8,40 To prevent relocation stress from confounding experimental results and to promote animal health and wellbeing, a period of acclimation to a new environment allows for a return to physiologic homeostasis. However, empirical evidence from NHP regarding the appropriate acclimation timeframe for the research variable of interest is often lacking. This lack of information can be especially problematic for research involving the immune system given that the effects of stress on the immune system can be long-lasting.11,59
Macaques (Macaca spp.) are the most frequently used genus of NHP in research, and in these animals, transportation and relocation stress has been shown to cause significant changes in the animals. Changes such as increased indicators of behavioral stress in juvenile Macaca fascicularis,23 stress leukograms and increased serum cortisol in juvenile male M. fascicularis,27 altered blood chemistry in male M. fascicularis,41 increased fecal cortisol in M. tonkeana,7 and increased fetal loss in M. nemestrina.have all been reported51 The variation in time required for return to homeostasis in these studies illustrates that the acclimation period can vary markedly depending on the outcome measure of interest. For example, behavioral changes lasted for at least 3 wk after transport and relocation,23 whereas the neutrophil-to-lymphocyte ratio returned to baseline by 1 wk.27 Given the wide variation in behavioral and physiologic responses to acclimation, the appropriate acclimation period prior to study initiation should be based on empirical evidence concerning the ways in which transportation and relocation stress affect the outcome measures of interest in the species studied. Currently, researchers studying immune function outcome measures in macaques are limited in their ability to determine an appropriate acclimation period due to the lack of data on the immunologic effects of transportation and relocation on immune function. To address this gap in knowledge, we measured lymphocyte subsets, selected T-cell functional immune parameters, and plasma cytokine levels in cynomolgus macaques (M. fascicularis) before, at 24 h after, and at 30 d after transportation and relocation to a new facility.
Materials and Methods
Animals.
Experiments were approved by the IACUC of the University of Texas MD Anderson Cancer Center and were carried out according to the principles included in the Guide for the Care and Use of Laboratory Animals, the provisions of the Animal Welfare Act and Public Health Service Animal Welfare Policy, and the policies of the University of Texas MD Anderson Cancer Center.2,24,32,43
Subjects were 10 female cynomolgus macaques (M. fascicularis; age, 8 to10 y) of Vietnamese origin with unknown socialization histories. The animals were moved domestically by ground transportation to the Michale E Keeling Center for Comparative Medicine and Research (University of Texas, Bastrop, TX) in a climate-controlled USDA-approved trailer. Macaques were singly housed during transport and for 30 d after arrival, after which time they were socially housed. Water and a commercial chow (Monkey Chow, Purina, St Louis, MO, or Teklad 7195, Envigo, Huntingdon, United Kingdom) were freely available, and environmental enrichment was provided according to the facility environmental enrichment plan, which includes social, physical, feeding, occupational (twice daily), and sensory enrichment. The macaques were examined by veterinarians before and after transportation and were determined to be healthy.
Collection of samples.
Blood samples (10 mL) were collected by venipuncture of the femoral vein prior to transportation (baseline), at 24 h after arrival (day 2), and at 30 d after arrival (day 30). Blood was collected between 0900 and 1100 in EDTA anticoagulant tubes after animals were anesthetized by using ketamine (10 mg/kg, Vedco, Saint Joseph, MO). Blood sampling volumes were approved by the IACUC and clinical veterinarian, and the macaques remained healthy throughout the study. Blood samples were processed at the Keeling Center after domestic overnight shipment from the original location or within 4 h of collection onsite. PBMC were isolated by density gradient separation as described previously.36 Erythrocytes were removed through osmotic lysis (ACK Lysing Buffer, Life Technologies, Grand Island, NY), and the remaining nucleated cells were washed twice with RPMI supplemented with 10% FBS (Atlanta Biologic, Flowery Branch, GA) before use in immune assays.
Hematologic analyses.
Hematology was performed on EDTA-preserved blood by using an automated analyzer (Siemens Healthcare Diagnostics, Tarrytown, NY). The absolute number of lymphocytes, as obtained from hematologic analysis, was used to convert the frequency of the lymphocyte population obtained from FACS analysis, to calculate the absolute numbers in each of the lymphocyte subset populations.
Flow cytometry.
A series of commercially available human monoclonal antibodies that cross react with NHP mononuclear cells were used in flow cytometric analyses, as described previously.34,55 Briefly, 100 μL of whole blood was added to each polystyrene test tube (12 mm × 75 mm; Falcon, Lincoln Park, NJ) containing a monoclonal antibody against CD3 (CD3−PE, clone SP-34), CD4 (CD4–PerCP, clone L200), CD8 (CD8–FITC, clone SK1), or CD20 (CD20–APC, clone L27; all from BD Biosciences, San Diego, CA) and incubated for 15 min at room temperature in the dark. RBC were lysed with 1× FACS lysing solution (Becton Dickinson) diluted according to the manufacturer's instructions. The samples were washed thoroughly in PBS; cells were pelleted by centrifugation, suspended in 1% paraformaldehyde in PBS buffer, (300 μL), and data acquired on a 4-color flow cytometer (FACSCalibur, BD Biosciences). All samples evaluated in this study were compensated for spectral overlap of fluorochromes by using singly-labeled cells. Lymphocytes were gated on forward scatter compared with side scatter dot plots to analyze CD3+, CD4+, and CD8+ T-cell and CD20+ B-cell subsets by using FlowJo software (Tree Star, Ashland, OR).
For NK, NKT, and monocyte subsets analysis, 100-µL samples of blood were stained with the combination of antiCD3 (CD3–Percp, clone SP-34); antiCD14 (CD14–PE, clone M5E2), and antiCD16 (CD16–APC, clone 3G8; all from BD Pharmingen, San Jose, CA) antibodies, as described earlier. Data from the stained cells were acquired through flow cytometry (FACSCalibur, Becton Dickinson) and analyzed by using FlowJo software (Tree Star).
In vitro stimulation with mitogen.
Freshly prepared PBMC were more than 90% viable, as determined by trypan blue exclusion, and their proliferation was determined by using the standard MTT dye reduction assay, as previously described.31,34,50 Briefly, PBMC (105 per well) were seeded in triplicate in 96-well, flat-bottom plates and stimulated for 72 h with 1 of 4 mitogens: phytohemagglutinin, concanavalin A, LPS, and pokeweed mitogen (PWM; Sigma, St Louis, MO), each at 1 µg/mL final concentration. Culture medium without mitogen served as a negative control. Plates were incubated for 72 h at 37 °C in 5% CO2, after which 10 µL of freshly prepared and filtered MTT dye (5 mg/mL in PBS) was added to each well, and the plate was incubated for an additional 4 h. The medium was then replaced with 100 µL of 4 mM hydrochloric acid in isopropanol (Sigma) and left for 30 min at room temperature for color development, after it was read by an ELISA plate reader by using a 540-nm filter (Victor, PerkinElmer, Shelton, CT). Results were expressed as the optical density after subtraction of the medium-only sample. Data are reported as the mean of 3 replicates. The concentration of mitogen, number of PBMC, and incubation time were standardized in our laboratory as optimal for stimulation of PBMC isolated from healthy animals.
ELISPOT assay for detecting IFNγ-producing cells.
Samples of freshly isolated PBMC each were cultured with phytohemagglutinin, concanavalin A, LPS, or PWM, each at 1 µg/mL final concentration as stimuli to determine the numbers of IFNγ-producing cells. To enumerate and quantify IFNγ-producing cells, we followed the standard ELISPOT protocol (Monkey IFNγ ELISPOT ALP, Millipore, Bedford, MA) as reported earlier.33,63 Briefly, PBMC (105 per well) were seeded in duplicate wells of 96-well plates (polyvinylidene difluoride–backed plates, MAIP S 45, Millipore), which were precoated with primary antiIFNγ antibody and stimulated with the various mitogens. After incubation for 30 to 32 h at 37 °C, the cells were removed, the wells were thoroughly washed with 1× PBS, and spots were developed as described by the manufacturer. Purple-colored spots representing individual IFNγ−secreting cells were counted by an independent agency (ZellNet Consulting, Fort Lee, NJ) by using the KS-ELISPOT automatic system (Carl Zeiss, Thornwood, NY) for quantitative analysis of the number of IFNγ spot-forming cells for 105 input PBMC. Responses were considered positive when the number of spot-forming cells after mitogen treatment was at least 5 spots greater the background control values from cells cultured in medium alone. Data are presented as the number of spot-forming cells per 105 PBMC.
Cytokine multiplex assays.
The plasma concentration of the cytokines IFNγ, IL10, IL13, IL12(p40), IL1β, ILra, TNFα, and MCP1 were measured (NHP Multiplex Cytokine Kit, Millipore) as described previously.34 Briefly, EDTA-preserved plasma samples were centrifuged (900 × g for 10 min), and aliquots were frozen at −80 °C until use. On the day of assay, plasma samples were thawed and precleared by centrifuging at 900 × g for 5 min. The 96-well plates provided in the kit were blocked in assay buffer for 10 min at room temperature and washed, and 25 µL of test or control sample was added to appropriate wells. After 25 µL of beads were added to each well, the plate was incubated on a shaker overnight at 4 °C. The next day, after the plate was washed twice with wash buffer, incubated with detection antibody for 1 h, and incubated for with 25 µL of streptavidin–phycoerythrin for 30 min. All incubation and washing steps were performed on a shaker at room temperature. After the plate was washed twice with wash buffer, 150 µL of sheath fluid was added to each well, and cytokines were measured by acquiring data from beads (Bio-Plex 200 system, Bio-Rad, Hercules, CA). Fluorescence data were analyzed by using Bio-Plex Manager 5.0 software (Bio-Rad). The minimal detectable concentration for each cytokine, calculated by using immunoassay analysis software (Multiplex Analyst, Millipore), was as follows: IFNγ, 2.2 pg/mL; IL10, 6.2 pg/mL; IL13, 5.8 pg/mL; IL12(p40), 1.2 pg/mL; IL1β, 1.2 pg/mL; ILra, 2.4 pg/mL; TNFα, 2.1 pg/mL; and MCP1, 3.1 pg/mL.
Statistical analysis.
Baseline and posttransportation samples were compared statistically by using the Dunnett test for one-way, repeated-measures ANOVA. The Geisser-Greenhouse method was used to correct violations of sphericity in the data. All statistical analyses were conducted by using Prism version 7.01 (GraphPad Software, San Diego, CA).
Results
In this study, we examined the effects of transportation- and relocation-induced stress on leukocyte distribution and immune function of cynomolgus macaques and discuss the significance of stress-induced changes in the immune system.
Influence of transport on key lymphocyte subsets in peripheral blood.
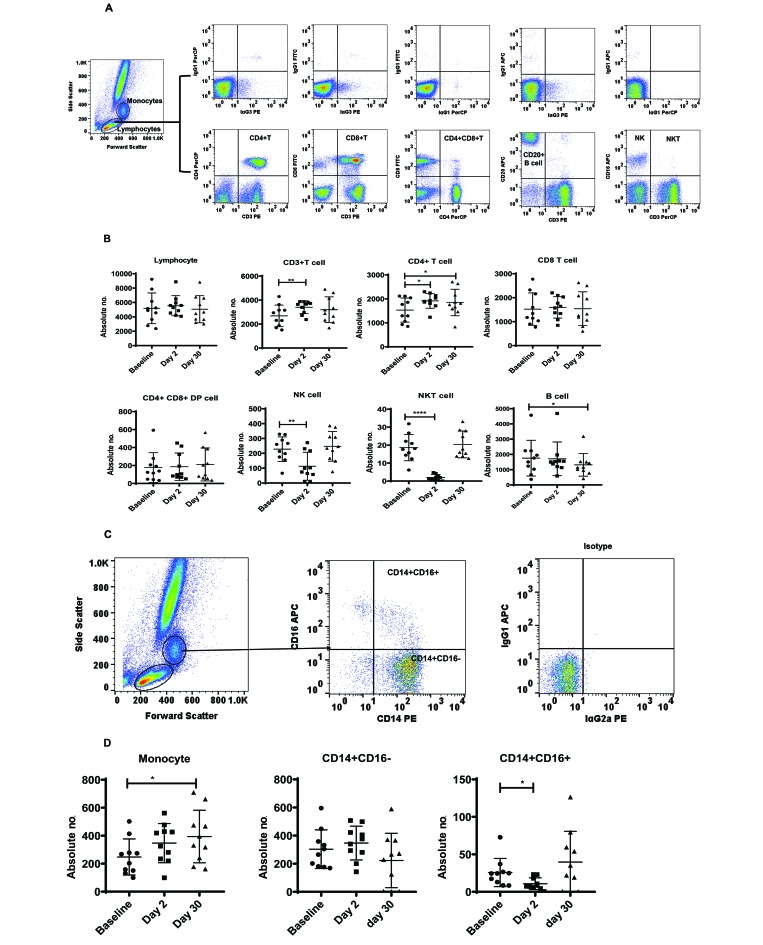
To determine the phenotypic profile of lymphocytes in the peripheral blood, T-cell subsets and B cells from whole blood were analyzed through flow cytometry using the gating strategy shown in (Figure 1 A). We observed a significant increase in the absolute numbers of CD3+ T cells on day 2 (F2,17 = 6.7, P < 0.0001, η2 = 0.74) day 2 and of CD4+ T cells on days 2 and 30 (F2,17 = 6.6, P < 0.05, η2 = 0.74) on day 2 (P < 0.01) and day 30 (P < 0.05) compared with baseline values. In contrast to the increased numbers of T cells, the absolute numbers of NK and NKT cells declined significantly on day 2 compared with baseline (NK cells: (F1.4,12.9 =13.41, P < 0.01, η2 = 0.31; NKT cells: F1.9,17.6 =35.1, P < 0.001, η2 = 0.67; (Figure 1 B). In addition, the absolute number of B cells was significantly decreased on day 30 (F2,17 = 5.37, P < 0.05, η2 = 0.01) compared with baseline (Figure 1 B). In addition, we noted changes in the absolute number of monocytes and monocyte subsets. The absolute number of monocytes was significantly increased at day 30 (F1.3,12 = 7.1, P < 0.05, η2 = 0.787), whereas nonclassical monocytes (that is, CD14+CD16+) were significantly fewer immediately after relocation on day 2 (F2, 17 = 5.37, P < 0.05, η2 = 0.01) compared with baseline (Figure 1 C and D). There were no changes in the absolute numbers of classic monocytes (that is, CD14+CD16−), total lymphocytes, CD8+ T cells, or CD4+CD8+ double-positive T cells at either of the posttransportation time points compared with baseline.
Figure 1.
(A and C) Gating strategy for phenotypic analyses of PBMC from a representative cynomolgus macaque. Lymphocytes and monocytes were first gated based on forward scatter (FCS) compared with side scatter (SSC), and then CD3+, CD4+, CD8+ ,CD4+CD8+, and CD20+ cells and NK and NKT cells were positively identified from the lymphocyte subset. From the monocyte subset, CD14+CD16− and CD14+CD16+ cells were positively identified. The specificity of staining for the various markers was ascertained according to the isotype control antibody staining used for each pair of combination markers, as shown. (B) Phenotype analysis of lymphocytes from cynomolgus macaques. Aliquots of EDTA-treated whole blood were stained with fluorescence-labeled antibodies to CD3+, CD4+, CD8+, and CD20+ to identify lymphocyte subpopulations pre- and posttransportation and relocation. Values on the Y-axis are absolute lymphocyte cells. P values were considered statistically significant at P < 0.05.(D) Phenotype analysis of monocytes from cynomolgus macaques. Aliquots of EDTA-treated whole blood were stained with fluorescence-labeled antibodies to CD14+ and CD16+ to identify monocyte subpopulations. Values on the y-axis are absolute monocyte cells. P values were considered statistically significant at P < 0.05.
Influence of relocation on mitogen-induced proliferation and IFNγ ELISPOT responses.
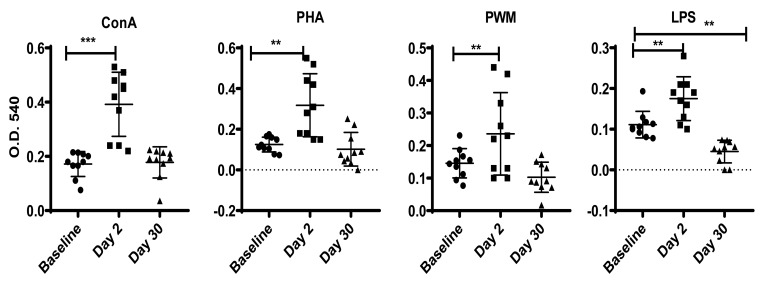
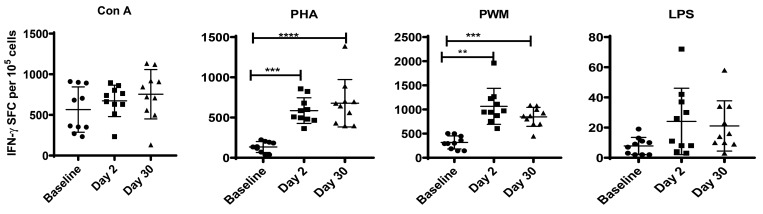
To assess the effects of transportation on the response of the innate immune system to pathogen-associated molecular patterns, we stimulated PBMC with concanavalin A, phytohemagglutinin, PWM, or LPS and assessed proliferation by using the MTT assay and IFNγ-producing cells by ELISpot assay. On day 2 after relocation, there were significant increases in the proliferative responses of PBMC to concanavalin A (F1.7,15 = 25, P < 0.0001, η2 = 2.84), phytohemagglutinin (F1.6,14 = 19, P < 0.0002, η2 = 2.123), PWM (F1.3,12 = 11.1, P < 0.003, η2 = 1.24), and LPS (F1.5,13 = 28.3, P < 0.0001, η2 = 3.14; Figure 2). By day 30, only proliferation responses to LPS differed significantly from baseline (F1.5,13.4 = 28.3, P < 0.001, η2 = 0.66). Specifically, whereas the concanavalin A, phytohemagglutinin, and PWM treated cells returned to baseline levels of proliferation, LPS-treated cells showed a significant decrease on day 30 compared with baseline (Figure 2). A different pattern emerged for IFNγ production in response to mitogen stimulation. We observed significantly increased IFNγ production on days 2 and 30 compared with baseline after stimulation of PBMC with phytohemagglutinin (F2,17 = 28.38, P < 0.0001, η2 = 0.062) and PWM (F1.5,13 = 21, P < 0.0001, η2 = 2.31; Figure 3). No significant changes in IFNγ production were apparent after stimulation with concanavalin A or LPS.
Figure 2.
Proliferative response of PBMC to mitogens from cynomolgus macaques. We used the standard MTT dye reduction assay to determine the proliferative responses of PBMC to various mitogens (ConA, concanavalin A; PHA, phytohemagglutinin). Proliferation responses were measured as optical density (OD) and expressed as percentage viability relative to medium only. P values of less than 0.05 were considered statistically significant using a Dunnett's test for one-way repeated-measures ANOVA.
Figure 3.
IFNγ ELISPOT response of cynomolgus macaques to mitogens. Duplicate wells of the 96-well microtiter plates, precoated with IFNγ antibody, were seeded with 105 PBMC and incubated for 36 h at 37 °C with 5 μg of mitogen. At the end of the incubation period, the wells were washed and stained with biotinylated secondary IFNγ antibody. The total number of spot-forming cells in each of the mitogen-stimulated wells was counted and adjusted to that in control medium. P values less than 0.05 were considered statistically significant (Dunnett test for one-way repeated-measures ANOVA).
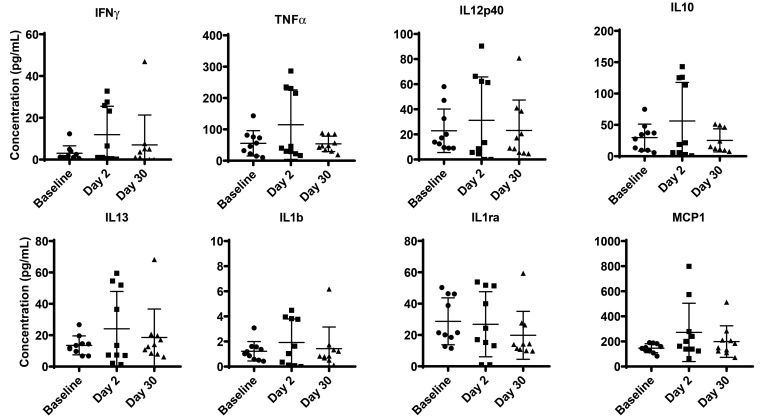
Influence of relocation on circulating cytokines in plasma.
To determine whether transportation and relocation affected circulating cytokine levels in cynomolgus macaques, we measured IFNγ, IL10, IL1ra, IL13, IL1β, MCP1, TNFα, and IL12 p40. No significant differences were detected (Figure 4).
Figure 4.
Plasma cytokine bead assay of cynomolgus macaques. In duplicate wells of a 96-well filter plate, 25 μL of plasma was incubated overnight at 4°C with 25 μL of cytokine-coupled beads followed by washing and staining with biotinylated detection antibody. The minimal detectable concentrations (in pg/mL) for IFNγ (2.2), TNFα (2.1), IL2 (0.7), IL6 (0.3), IL10 (6.2), and IL12(p40) (1.2) were used to determine positive responses. P values of less than 0.05 (Dunnett test for multiple comparison, one-way ANOVA) were considered statistically significant.
Discussion
The most important findings from this study are that, even after 30 d of acclimation, multiple immune parameters in singly housed cynomolgus macaques after routine domestic ground transportation were significantly different compared with baseline. In addition, we found a number of statistically significant short-term (that is, day 2) changes in circulating lymphocyte numbers and function. Our finding of increased circulating CD4+ and CD3+ T cells on day 2 is consistent with the expected rebound in peripheral lymphocyte cell numbers during the recovery phase after a stressful stimulus is discontinued.13 In contrast, NK cell numbers initially increase but then quickly decrease, due to a redistribution of cells from lymphoid organs and blood vessels to other body tissues.12,30,56 The acute decline in NK cells that we observed is also consistent with a recent study in which low-ranking (that is, stressed) socially housed female rhesus macaques had fewer NK cells than high-ranking females.60 Expression of NK markers is different in both humans and NHP.46 We defined NK cells as CD3−CD16+ cells, as described previously.35 The majority (more than 85%) of circulating NK cells are single-positive for CD16.46 Other NK subsets including CD16+, CD3−CD16−, CD16+CD56+ double-positive, and double-negative populations represent only a small fraction of the total NK count and therefore were not measured in our current study.46
The effects of stress on circulating NKT cell numbers is not well described in the literature, although one study involving adult men found increased numbers of circulating NKT cells at 12 min after a mild stressor.3 In our study, the numbers of NKT cells decreased approximately 24 h after the stressor (day 2). This decrease in NKT cells mirrored the decrease we found in NK cells, and given that our sampling occurred well over 12 min after the stressor, this finding likely reflects a redistribution of these cells into other body tissues as well.
Circulating monocytes are currently divided into 3 subsets: classic (CD14+CD16−), intermediate (CD14++CD16+), and nonclassic (CD14+CD16+). One hypothesis is that these subsets represent a developmental continuum in which the circulating proportion of each subset changes according to health status.65 On day 2, nonclassic CD14+CD16+ monocytes were significantly below baseline and returned to baseline counts by day 30. Nonclassic monocytes are thought to play an important role in tissue repair and the response to bacterial and viral infections through the production of high amounts of TNFα and IL1β and by patrolling the vascular endothelium42,44,52 The functional and phenotypic changes in monocytes from patients with tuberculosis are reversed with treatment.53 The role of CD14highCD16+ rather than CD14low CD16+ monocytes correlates with disease progression in chronic HIV-infected patients.21 Studies in mice have shown that nonclassic monocytes slowly patrol the vascular endothelium and express different adhesion receptors in different organs and vascular beds during homeostasis and vascular inflammation5 Thus, the significant decrease we observed on day 2 may be the result of the stress response directing these cells to increase patrolling activity of the vascular endothelium where their presence may be advantageous in the case of an injury or infection. By day 30, nonclassic monocytes had returned to baseline levels, suggesting a return to homeostasis. However, the significant increase that we observed in the overall absolute numbers of circulating monocytes compared with baseline indicates that a full return to homeostasis had not yet been achieved and is consistent with the work showing that stress hormones, through a β-adrenoceptor-mediated mechanism, increase the myelopoietic output of monocytes.47,64 Moreover, further evidence for a failure to return to baseline homeostasis on day 30 is reflected in the significant decreased circulating B-cell numbers on that day compared with baseline. Monocytosis and decreased B cell counts are associated with a response to acute stress, suggesting that by 30 d after transportation and relocation, the effects of the initial relatively large stressor (transportation) were abating, and we instead were detecting signs of a different acute stress, such as stress due to phlebotomy, at this later time point.12,60
Based on our previous study of similarly transported and relocated rhesus macaques (M. mulatta), which showed a heightened proliferative response of PBMC to mitogens on day 30 compared with baseline,36 we were surprised to find that the lymphocyte proliferation data from this study supports a return to homeostasis by day 30. However, we did find a significant decrease in proliferative response to LPS on day 30 compared with baseline. In contrast to the enhanced proliferative responses to mitogens, the production of IFNγ by PBMC stimulated with phytohemagglutinin or PWM was significantly elevated on days 2 and 30 compared with baseline. The persistent increase in IFNγ production in our current study is consistent with our previous findings in relocated rhesus macaques.36 IFNγ is a potent proinflammatory cytokine, so its presence may indicate an increased inflammatory response after relocation, even after 30 d of acclimation. The persistence of a proinflammatory response in our study is of note since a proinflammatory response to stress is more likely to be associated with acute stressors compared with the potentially profound immunosuppression associated with chronic stress.11 Therefore, one hypothesis for why we found persistent signs of a proinflammatory response after relocation is that the macaques in our study were reacting to the study manipulations related to obtaining the blood sample as an acute stressor in their new environment, compared with the sample obtained at the pretransportation baseline time point in a familiar environment. This situation poses an interesting interpretation, given that we might expect macaques that had previously been acclimated to ‘routine’ animal handling procedures, such as a blood draw, to acclimate fairly quickly to such routine procedures at a new institution, especially once other purported measures of acclimation have been observed such as normalization of the complete blood count or a return to baseline cortisol levels. However, these findings indicate that it may take longer than 30 d for M. fascicularis to acclimate to routine procedures performed at a new location when immune responses are of interest.
While we observed changes in the phenotype and function of PBMC in this study, we found no transportation- and relocation-related differences in plasma cytokines. In humans, stress has been shown to dysregulate the immune response by altering the response from a type-1 to a type-2 response based on changes detected in intracellular cytokine concentrations.18,19 Therefore, future cytokine studies may focus on changes in intracellular cytokine levels.
Our finding that some immune parameters normalized by day 30 after transportation and some did not is consistent with the complex interaction between the central nervous system, endocrine system, and immune system. The psychologic and immunologic consequences of stress are so complex and interdependent that the field of psychoneuroimmunology is dedicated to understanding the underlying mechanisms of their interaction. One mechanism that may be important in our study is the binding of stress hormones to their cognate receptors on immune cells. While chronic stress is well known to have immunosuppressive effects, primarily through the action of the stress hormone cortisol, stress has also been shown to have a sensitizing effect on the inflammatory response, resulting in increased responsiveness to immune challenges.11,20,26,28 Investigating the effects of stress hormones is complicated further since the immune altering effects may persist even after the stress has resolved and stress hormones have returned to baseline through mechanisms such as upregulation of toll-like receptors or decreased expression of Fox p3.17,22,45,66 Although we did not measure stress hormones in this study, the published data have shown that blood cortisol will be elevated in NHP 2 d after transportation and relocation to a new facility, with a return to baseline after 30 d of acclimation.27,36,41 Thus, while the blood concentrations of cortisol are typically normalized after 30 d of acclimation, our study indicates that the effects of the transportation and relocation event remain evident in the immune response even after 30 d. However, interpretation of these data is limited by the absence of a control group and unavailability of samples after day 30. Additional studies are needed to determine when the long-term effects of transportation and relocation on the immune system resolve and immune response homeostasis is achieved and to examine sex-associated differences in immune system acclimation after transportation and relocation.
The data presented here can help guide researchers in determining an appropriate acclimation period for their study; that can influence how long it takes for acclimation to occur. These factors include the intensity and duration of stress, as well as the species, sex, age, genotype, health status, previous life experience, allometric differences, and even the time of year that acclimation occurs.4,9,10,15,38,39,49 These factors add variability regarding when each subject in a group of animals will achieve homeostasis. For example, in our study, we hypothesize that the different responses to the mitogens that we used resulted from interplay of variables in our study population such as heterogeneous life histories and genetics. Particularly lengthy transportation events likely will increase the magnitude of physiologic changes and the length of time needed for these measures to return to normal. Investigators who have linked the duration of transport with loss of body weight have reported that the longer the animals are transported, the more body weight an animal loses and the longer it takes for animals to regain their initial condition. In another study, physiologic measures were affected by transport duration in a linear fashion, including serum glucose, urea nitrogen, and urea nitrogen: creatinine ratio.8 Based on these data, we can expect that the immune response to transportation and relocation will also vary due to the interplay of multiple factors.
Although our study involved singly housed animals, a critical factor in providing for the health and wellbeing of NHP is social housing. Social housing impacts the immune response, and the presence of social partners has been shown either to modulate the perceived intensity of relocation stress or to downregulate the magnitude of the physiologic response to stress in group-living NHP.14,16,39,63,64 Furthermore, among socially housed animals, social rank can affect the neuroendocrine system resulting in differences in basal plasma cortisol levels.1,26,30,48,54,59 Disruption of social groups is an additional source of stress to which animals must acclimate if they have been singly housed during transportation and relocation. Fortunately, socially housing NHP during, or soon after, transportation likely will mitigate the stress response and is likely to be an effective way to ensure a more rapid return to physiologic homeostasis while also providing for their overall health and wellbeing.
Together, these factors have an interdependent and dynamic effect on the magnitude and duration of physiologic changes related to stress. Consequently, no formula is available for calculating exactly when the immune system of transported animals will regain homeostasis. Furthermore, although our study focused on macaques that underwent a ‘major’ transportation and relocation event, it is important to consider that any transportation and relocation of NHP (for example, within the same building within the same campus) will introduce potential stressors, requiring acclimation of the animal, along with recovering from the acute stress of transportation. Therefore, in addition to reviewing the literature, we advise investigators to evaluate their dependent measures for acclimation when data in the literature are insufficient and to consider the animals’ individual characteristics and circumstances.
In conclusion, our findings show that 30 d of acclimation after transportation and relocation to a new facility is insufficient for all measures of immune function to return to baseline levels in singly housed cynomolgus macaques. Additional time to acclimate beyond 30 d may be necessary before conducting studies involving immune outcome measures. The immune system is exquisitely sensitive to stress, and additional studies are needed to determine when the M. fascicularis immune system fully returns to homeostasis after transportation and relocation to minimize confounding effects on research.
Acknowledgments
This study was partly supported by Cattleman for Cancer Research (to PNN) and the Michale E Keeling Center for Comparative Medicine and Research, University of Texas Anderson Cancer Center. We appreciate Luke Segura's assistance in providing blood samples for this study.
References
- 1.Adams MR, Kaplan JR, Clarkson TB, Koritnik DR. 1985. Ovariectomy, social status, and atherosclerosis in cynomolgus monkeys. Arteriosclerosis 5:192–200. 10.1161/01.ATV.5.2.192. [DOI] [PubMed] [Google Scholar]
- 2.Animal Welfare Act as Amended. 2013. 7 USC §2131–2159.
- 3.Atanackovic D, Nowottne U, Freier E, Weber CS, Meyer S, Bartels K, Hildebrandt Y, Cao Y, Kröger N, Brunner-Weinzierl MC, Bokemeyer C, Deter HC. 2013. Acute psychologic stress increases peripheral blood CD3+CD56+ natural killer T cells in healthy men: possible implications for the development and treatment of allergic and autoimmune disorders. Stress 16:421–428. 10.3109/10253890.2013.777702. [DOI] [PubMed] [Google Scholar]
- 4.Bilbo SD, Dhabhar FS, Viswanathan K, Saul A, Yellon SM, Nelson RJ. 2002. Short day lengths augment stress-induced leukocyte trafficking and stress-induced enhancement of skin immune function. Proc Natl Acad Sci USA 99:4067–4072. 10.1073/pnas.062001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buscher K, Marcovecchio P, Hedrick CC, Ley K. 2017. Patrolling mechanics of nonclassical monocytes in vascular inflammation. Front Cardiovasc Med 4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capitanio JP, Kyes RC, Fairbanks LA. 2006. Considerations in the selection and conditioning of Old World monkeys for laboratory research: animals from domestic sources. ILAR J 47:294–306. 10.1093/ilar.47.4.294. [DOI] [PubMed] [Google Scholar]
- 7.Cinque C, De Marco A, Mairesse J, Giuli C, Sanna A, De Marco L, Zuena AR, Casolini P, Catalani A, Thierry B, Cozzolino R. 2016. Relocation stress induces short-term fecal cortisol increase in Tonkean macaques (Macaca tonkeana). Primates 58:315–321. 10.1007/s10329-016-0590-7. [DOI] [PubMed] [Google Scholar]
- 8.Cole NA, Camp TH, Rowe LD, Jr, Stevens DG, Hutcheson DP. 1988. Effect of transport on feeder calves. Am J Vet Res 49:178–183. [PubMed] [Google Scholar]
- 9.Davenport MD, Lutz CK, Tiefenbacher S, Novak MA, Meyer JS. 2008. A rhesus monkey model of self-injury: effects of relocation stress on behavior and neuroendocrine function. Biol Psychiatry 63:990–996. 10.1016/j.biopsych.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis N, Schaffner CM, Smith TE. 2005. Evidence that zoo visitors influence HPA activity in spider monkeys (Ateles geoffroyii rufiventris). Appl Anim Behav Sci 90:131–141. 10.1016/j.applanim.2004.08.020. [DOI] [Google Scholar]
- 11.Dhabhar FS. 2008. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection versus immunopathology. Allergy Asthma Clin Immunol 4:2–11. 10.1186/1710-1492-4-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhabhar FS, Malarkey WB, Neri E, McEwen BS. 2012. Stress-induced redistribution of immune cells—from barracks to boulevards to battlefields: a tale of 3 hormones. Curt Richter award winner. Psychoneuroendocrinology 37:1345–1368. 10.1016/j.psyneuen.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhabhar FS, Miller AH, McEwen BS, Spencer RL. 1995. Effects of stress on immune cell distribution. Dynamics and hormonal mechanisms. J Immunol 154:5511–5527. [PubMed] [Google Scholar]
- 14.Duarte RB, Maior RS, Barros M. 2018. Behavioral and cortisol responses of adult marmoset monkeys (Callithrix penicillata) to different home cage social-disruption intervals. Appl Anim Behav Sci 201:117–124. 10.1016/j.applanim.2017.12.005. [DOI] [Google Scholar]
- 15.Fairbanks LA, Jorgensen MJ, Bailey JN, Breidenthal SE, Grzywa R, Laudenslager ML. 2011. Heritability and genetic correlation of hair cortisol in vervet monkeys in low and higher stress environments. Psychoneuroendocrinology 36:1201–1208. 10.1016/j.psyneuen.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernström AL, Sutian W, Royo F, Westlund K, Nilsson T, Carlsson HE, Paramastri Y, Pamungkas J, Sajuthi D, Schapiro SJ, Hau J. 2008. Stress in cynomolgus monkeys (Macaca fascicularis) subjected to long-distance transport and simulated transport housing conditions. Stress 11:467–476. 10.1080/10253890801903359. [DOI] [PubMed] [Google Scholar]
- 17.Frank MG, Miguel ZD, Watkins LR, Maier SF. 2010. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun 24:19–30. 10.1016/j.bbi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Glaser R, Kiecolt-Glaser JK. 2005. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol 5:243–251. 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 19.Glaser R, MacCallum RC, Laskowski BF, Malarkey WB, Sheridan JF, Kiecolt-Glaser JK. 2001. Evidence for a shift in the Th1 to Th2 cytokine response associated with chronic stress and aging. J Gerontol A Biol Sci Med Sci 56:M477–M482. 10.1093/gerona/56.8.M477. [DOI] [PubMed] [Google Scholar]
- 20.Glaser R, Robles TF, Sheridan J, Malarkey WB, Kiecolt-Glaser JK. 2003. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry 60:1009–1014. 10.1001/archpsyc.60.10.1009. [DOI] [PubMed] [Google Scholar]
- 21.Han J, Wang B, Han N, Zhao Y, Song C, Feng X, Mao Y, Zhang F, Zhao H, Zeng H. 2009. CD14highCD16+ rather than CD14low CD16+ monocytes correlate with disease progression in chronic HIV-infected patients. J Acquir Immune Defic Syndr 52:553–559. 10.1097/QAI.0b013e3181c1d4fe. [DOI] [PubMed] [Google Scholar]
- 22.Hermoso MA, Matsuguchi T, Smoak K, Cidlowski JA. 2004. Glucocorticoids and tumor necrosis factor alpha cooperatively regulate toll-like receptor 2 gene expression. Mol Cell Biol 24:4743–4756. 10.1128/MCB.24.11.4743-4756.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honess PE, Johnson PJ, Wolfensohn SE. 2004. A study of behavioral responses of nonhuman primates to air transport and rehousing. Lab Anim 38:119–132. 10.1258/002367704322968795. [DOI] [PubMed] [Google Scholar]
- 24.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 25.Johnson JD, O'Connor KA, Deak T, Stark M, Watkins LR, Maier SF. 2002. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun 16:461–476. 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- 26.Keverne EB, Meller RE, Eberhart JA. 1982. Social influences on behavior and neuroendocrine responsiveness of talapoin monkeys. Scand J Psychol 23 Suppl 1:37–47. 10.1111/j.1467-9450.1982.tb00450.x. [DOI] [PubMed] [Google Scholar]
- 27.Kim CY, Han JS, Suzuki T, Han SS. 2005. Indirect indicator of transport stress in hematologic values in newly acquired cynomolgus monkeys. J Med Primatol 34:188–192. 10.1111/j.1600-0684.2005.00116.x. [DOI] [PubMed] [Google Scholar]
- 28.Maes M, Ombelet W, De Jongh R, Kenis G, Bosmans E. 2001. The inflammatory response following delivery is amplified in women who previously suffered from major depression, suggesting that major depression is accompanied by a sensitization of the inflammatory response system. J Affect Disord 63:85–92. 10.1016/S0165-0327(00)00156-7. [DOI] [PubMed] [Google Scholar]
- 29.Michopoulos V, Reding KM, Wilson ME, Toufexis D. 2012. Social subordination impairs hypothalamic-pituitary-adrenal function in female rhesus monkeys. Horm Behav 62:389–399. 10.1016/j.yhbeh.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mills PJ, Meck JV, Waters WW, D'Aunno D, Ziegler MG. 2001. Peripheral leukocyte subpopulations and catecholamine levels in astronauts as a function of mission duration. Psychosom Med 63:886–890. 10.1097/00006842-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 32.National Academy of Sciences. 1975. The National Academies collection: reports funded by National Institutes of Health. Washington (DC): National Academies Press. [Google Scholar]
- 33.Nehete PN, Gambhira R, Nehete BP, Sastry KJ. 2003. Dendritic cells enhance detection of antigen-specific cellular immune responses by lymphocytes from rhesus macaques immunized with an HIV envelope peptide cocktail vaccine. J Med Primatol 32:67–73. 10.1034/j.1600-0684.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- 34.Nehete PN, Hanley PW, Nehete BP, Yang G, Ruiz JC, Williams L, Abee CR, Sastry KJ. 2013. Phenotypic and functional characterization of lymphocytes from different age groups of Bolivian squirrel monkeys (Saimiri boliviensis boliviensis). PLoS One 8:1–11. 10.1371/journal.pone.0079836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nehete PN, Nehete BP, Chitta S, Williams LE, Abee CR. 2017. Phenotypic and functional characterization of peripheral blood lymphocytes from various age- and sex-specific groups of owl monkeys (Aotus nancymaae). Comp Med 67:67–78. [PMC free article] [PubMed] [Google Scholar]
- 36.Nehete PN, Shelton KA, Nehete BP, Chitta S, Williams LE, Schapiro SJ, Abee CR. 2017. Effects of transportation, relocation, and acclimation on phenotypes and functional characteristics of peripheral blood lymphocytes in rhesus monkeys (Macaca mulatta). PLoS One 12:1–15. 10.1371/journal.pone.0188694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norcross JL, Newman JD. 1999. Effects of separation and novelty on distress vocalizations and cortisol in the common marmoset (Callithrix jacchus). Am J Primatol 47:209–222. . [DOI] [PubMed] [Google Scholar]
- 38.Novak MA, Hamel AF, Kelly BJ, Dettmer AM, Meyer JS. 2013. Stress, the HPA axis, and nonhuman primate wellbeing: A review. Appl Anim Behav Sci 143:135–149. 10.1016/j.applanim.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Connor KA, Brindle E, Shofer J, Trumble BC, Aranda JD, Rice K, Tatar M. 2011. The effects of a long-term psychosocial stress on reproductive indicators in the baboon. Am J Phys Anthropol 145:629–638. 10.1002/ajpa.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Obernier JA, Baldwin RL. 2006. Establishing an appropriate period of acclimatization following transportation of laboratory animals. ILAR J 47:364–369. 10.1093/ilar.47.4.364. [DOI] [PubMed] [Google Scholar]
- 41.Ochi T, Yamada A, Naganuma Y, Nishina N, Koyama H. 2016. Effect of road transportation on the serum biochemical parameters of cynomolgus monkeys and beagle dogs. J Vet Med Sci 78:889–893. 10.1292/jvms.15-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogle ME, Segar CE, Sridhar S, Botchwey EA. 2016. Monocytes and macrophages in tissue repair: implications for immunoregenerative biomaterial design. Exp Biol Med (Maywood) 241:1084–1097. 10.1177/1535370216650293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Office of Laboratory Animal Welfare. [Internet]. 2002. Public health service policy on humane care and use of laboratory animals. [Cited 3 October 2019]. Available at: http://grants.nih.gov/grants/olaw/references/phspol.htm.
- 44.Olingy CE, San Emeterio CL, Ogle ME, Krieger JR, Bruce AC, Pfau DD, Jordan BT, Peirce SM, Botchwey EA. 2017. Nonclassical monocytes are biased progenitors of wound-healing macrophages during soft tissue injury. Sci Rep 7:447 10.1038/s41598-017-00477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olsen PC, Kitoko JZ, Ferreira TP, de-Azevedo CT, Arantes AC, Martins Mu A. 2015. Glucocorticoids decrease Treg cell numbers in lungs of allergic mice. Eur J Pharmacol 747:52–58. 10.1016/j.ejphar.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 46.Pomplun N, Weisgrau KL, Evans DT, Rakasz EG. 2015. OMIP-028: activation panel for rhesus macaque NK cell subsets. Cytometry A 87:890–893. 10.1002/cyto.a.22727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Powell ND, Sloan EK, Bailey MT, Arevalo JMG, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, Cole SW. 2013. Social stress upregulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc Natl Acad Sci USA 110:16574–16579. 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qin DD, Dominic Rizak J, Feng XL, Chu XX, Yang SC, Li CL, Lv LB, Ma YY, Hu XT. 2013. Social rank and cortisol among female rhesus macaques (Macaca mulatta). Dongwuxue Yanjiu 34:E42–E49. [DOI] [PubMed] [Google Scholar]
- 49.Reimers M, Schwarzenberger F, Preuschoft S. 2007. Rehabilitation of research chimpanzees: stress and coping after long-term isolation. Horm Behav 51:428–435. 10.1016/j.yhbeh.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 50.Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL. 1991. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J Immunol Methods 142:257–265. 10.1016/0022-1759(91)90114-U. [DOI] [PubMed] [Google Scholar]
- 51.Sackett GP. 1981. Pregnancy outcome following jet transport stress in nonhuman primates. J Med Primatol 10:149–154. 10.1159/000460066. [DOI] [PubMed] [Google Scholar]
- 52.San Emeterio CL, Olingy CE, Chu Y, Botchwey EA. 2017. Selective recruitment of nonclassical monocytes promotes skeletal muscle repair. Biomaterials 117:32–43. 10.1016/j.biomaterials.2016.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sánchez MD, García Y, Montes C, París SC, Rojas M, Barrera LF, Arias MA, García LF. 2006. Functional and phenotypic changes in monocytes from patients with tuberculosis are reversed with treatment. Microbes Infect 8:2492–2500. 10.1016/j.micinf.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 54.Sapolsky RM. 1995. Social subordinance as a marker of hypercortisolism. Some unexpected subtleties. Ann N Y Acad Sci 771 1 Stress:626–639. 10.1111/j.1749-6632.1995.tb44715.x. [DOI] [PubMed] [Google Scholar]
- 55.Schapiro SJ, Lambeth SP, Jacobsen KR, Williams LE, Nehete BN, Nehete PN. 2012. Physiologic and welfare consequences of transport, relocation, and acclimatization of chimpanzees (Pan troglodytes). Appl Anim Behav Sci 137:183–193. 10.1016/j.applanim.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schedlowski M, Jacobs R, Stratmann G, Richter S, Hädicke A, Tewes U, Wagner TO, Schmidt RE. 1993. Changes of natural killer cells during acute psychologic stress. J Clin Immunol 13:119–126. 10.1007/BF00919268. [DOI] [PubMed] [Google Scholar]
- 57.Shively CA, Willard SL. 2012. Behavioral and neurobiologic characteristics of social stress versus depression in nonhuman primates. Exp Neurol 233:87–94. 10.1016/j.expneurol.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Snyder-Mackler N, Sanz J, Kohn JN, Brinkworth JF, Morrow S, Shaver AO, Grenier JC, Pique-Regi R, Johnson ZP, Wilson ME, Barreiro LB, Tung J. 2016. Social status alters immune regulation and response to infection in macaques. Science 354:1041–1045. 10.1126/science.aah3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stammen RL, Cohen JK, Meeker TL, Crane MM, Amara RR, Hicks SL, Meyer JS, Ethun KF. 2018. Effect of chronic social stress on prenatal transfer of antitetanus immunity in captive breeding rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 57:357–367. 10.30802/AALAS-JAALAS-17-000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steppich B, dayyani F, Gruber R, Lorenz R, Mack M, Ziegler-Heitbrock HW. 2000. Selective mobilization of CD14+CD16+ monocytes by exercise. Am J Physiol Cell Physiol 279:C578–C586. 10.1152/ajpcell.2000.279.3.C578. [DOI] [PubMed] [Google Scholar]
- 61.Suomi SJ, Eisele CD, Grady SA, Harlow HF. 1975. Depressive behavior in adult monkeys following separation from family environment. J Abnorm Psychol 84:576–578. 10.1037/h0077066. [DOI] [PubMed] [Google Scholar]
- 62.Suomi SJ, Harlow HF. 1975. Effects of differential removal from group on social development of rhesus monkeys. J Child Psychol Psychiatry 16:149–164. 10.1111/j.1469-7610.1975.tb01264.x. [DOI] [PubMed] [Google Scholar]
- 63.Weaver EA, Nehete PN, Nehete BP, Yang G, Buchl SJ, Hanley PW, Palmer D, Montefiori DC, Ferrari G, Ng P, Sastry KJ, Barry MA. 2013. Comparison of systemic and mucosal immunization with helper-dependent adenoviruses for vaccination against mucosal challenge with SHIV. PLoS One 8:1–12. 10.1371/journal.pone.0067574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wohleb ES, Powell ND, Godbout JP, Sheridan JF. 2013. Stress-induced recruitment of bone marrow–derived monocytes to the brain promotes anxiety-like behavior. J Neurosci 33:13820–13833. 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong KL, Yeap WH, Tai JJY, Ong SM, Dang TM, Wong SC. 2012. The 3 human monocyte subsets: implications for health and disease. Immunol Res 53:41–57. 10.1007/s12026-012-8297-3. [DOI] [PubMed] [Google Scholar]
- 66.Wüst S, van den Brandt J, Tischner D, Kleiman A, Tuckermann JP, Gold R, Luhder F, Reichardt HM. 2008. Peripheral T cells are the therapeutic targets of glucocorticoids in experimental autoimmune encephalomyelitis. J Immunol 180:8434–8443. 10.4049/jimmunol.180.12.8434. [DOI] [PubMed] [Google Scholar]