Abstract
Current methods for detecting mites in mouse colonies have limitations in terms of cost, accuracy, and throughput. To address these limitations, we developed PCR assays to detect Myocoptes musculinus in fecal samples. Using a newly generated ribosomal RNA sequence of M. musculinus (MC28S), we developed PCR and qPCR assays capable of detecting M. musculinus mites or eggs ingested during grooming. To determine our ability to detect mites, we tested fur swabs and feces from mouse colonies experimentally infested with M. musculinus and Demodex musculi, 2) Myobia musculi and Radfordia affinis, 3) M. musculinus and M. musculi, and 4) no mites (negative control). The MC28S PCR and qPCR assays positively identified M. musculinus in groups 1 and 3. The MC28S PCR assay detected M. musculinus in 9 of 10 fecal samples from known-positive animals, whereas the qPCR assay correctly identified M. musculinus in all 10 fecal samples. To our knowledge, this report is the first description of PCR-based detection of murine mites in feces. By eliminating the need for pelt examinations, mite detection from fecal samples can facilitate mite detection in sentinel or quarantine programs.
Successful diagnosis of the common mites Myocoptes musculinus, Myobia musculi, Radfordia affinis, and Demodex musculi is important for the management of research rodent colonies due to the organisms’ ability to persistently infect mice, leading to potential clinical disease and research complications. These adverse clinical signs and research-affecting effects include—but are not limited to—ulcerative dermatitis, self-excoriation, erythema, alopecia, reduced weight gain, pruritus, decreased life span, altered IgE levels, and changes in inflammatory cytokines.5,12,14,17,23 M. musculinus is a parasitic fur mite that populates the inguinal region, abdomen, and dorsum, as well as the head and neck.4,20 Other common fur mites, such as M. musculi and R. affinis, infest the skin and hair shafts of the intrascapular and dorsal cervical regions, whereas D. musculi primarily resides in the pilosebaceous unit of mice.19,26
Various methods of detecting murine mites are well established in the literature; however, each methodology presents particular advantages and disadvantages in terms of accuracy, efficiency, ease of use, cost, time, and labor. Some of the most common diagnostic methods for detecting murine mites include fur plucks, deep skin scrapings, superficial skin scrapings, tape impressions, pelt exams, IgE detection, and PCR-based assays on samples from fur, cages, and air exhaust plenums.11,13,18,22,23 Observation-based methods, such as fur plucks, skin scrapings, and pelt exams, involve simple collection techniques that are easily performed by a trained technician. In a recent study, deep skin scrapings and a 18S rRNA PCR assay performed equally well in detecting Demodex musculi on laboratory mice.18 However, disadvantages of these techniques include 1) the necessity to sample multiple sites per mouse,18 2) the need for specialized training for microscopic detection, and 3) low throughput. Variability between users in terms of collection or detection can affect the accuracy of diagnosis. In recent years, murine mite detection has shifted toward PCR-based assays.2,8,11,13,18,22 PCR testing can increase throughput and may reduce concerns regarding sampling error, because swabbing techniques cover a greater surface area than a single pluck or scraping. In addition, the sensitivity of PCR assays have enabled scientists to detect mites at the cage and ventilated-rack levels, allowing more flexibility in sampling.6,11,22 However, inhouse PCR testing requires specialized equipment, whereas sending samples to outside commercial diagnostic labs may be cost-prohibitive for some laboratories. To simplify mite detection, our goal was to develop PCR-based assays for M. musculinus that could detect mite DNA in fecal samples, given the assumption that mites or mite eggs (or both) are ingested during grooming. To accomplish this goal, we generated ribosomal RNA PCR products from M. musculinus for next-generation sequencing using generic mite primers. Using the newly generated DNA sequences,we developed M. musculinus–specific PCR assays. After validating these primers on fur swabs, we demonstrated the detection of M. musculinus in fecal samples from mice with known mite infestations.
Materials and Methods
Collection of mite samples from mouse colonies.
Samples used in this study originated from mice infested during naturally occurring outbreaks at Memorial Sloan Kettering. Sentinel Tac:SW and NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice were cohoused with known-infested NSG mice to expand each group. Mice in each group were confirmed as mite-positive by using microscopic methods (that is, superficial skin scrapes) and pelt swabs sent to a commercial laboratory for confirmation by PCR analysis. For negative controls, culled aged breeders free of known murine viruses, Helicobacter spp., mites, and other parasites were sampled postmortem at the Massachusetts Institute of Technology. Mice at Memorial Sloan Kettering were housed on autoclaved aspen chip bedding (PWI Industries Canada, Quebec, Canada) in solid-bottom polysulfone ‘shoebox’ containment cages maintained in an IVC system (Thoren Caging Systems, Hazelton, PA). γ-irradiated feed (LabDiet 5053, PMI, St Louis, MO) and acidified water (pH 2.5 to 2.8 in a polysulfone bottle [Techniplast, West Chester, PA]) were provided without restriction. Mice at the Massachusetts Institute of Technology were maintained in static microisolation cages and were fed a commercial chow (Prolab RMH3000, PMI Nutrition International, Richmond, IN) without restriction. At both institutions, 12:12-h light:dark photocycles were maintained. Room temperature was maintained at 72 ± 2 °F (22.2 ± 1.1 °C) and relative humidity at 30% to 70%. The animal care and use programs at Memorial Sloan Kettering and the Massachusetts Institute of Technology are accredited by AAALAC, and all animals were maintained in accordance with the recommendations provided in the Guide for the Use and Care of Laboratory Animals.10 The use of these animals was approved by the respective IACUC.
The experiments described were completed in 2 phases requiring separate sample collections. First, fur plucks were collected and analyzed through microscopy to identify samples containing M. musculinus mites and eggs. DNA from M. musculinus–positive samples was used to generate PCR templates for sequencing M. musculinus rRNA genes to develop Myocoptes-specific PCR primers. The second phase of experiments consisted of validating the M. musculinus–specific primers using fecal and fur swab samples. For the validation studies, mouse samples were collected from negative control mice and those infested with different species of mites. Group 1 mice were infested with M. musculinus and D. musculi; group 2 mice were infested with M. musculi and R. affinis; and group 3 mice were infested with M. musculinus and M. musculi. For groups 1 through 3, a Sticky Head Cotton Bud swab (Daiso, Hiroshima, Japan) was used to sample the entire mouse body by rubbing against the direction of fur growth. The swab was rotated during collection to maximize sampling of mites, mite eggs, and mite debris on the fur. Given the preferred niches of murine mites, we heavily sampled the regions around the ears, back of the neck, periscapular area, head (especially under the chin), inguinal area, and base of the tail. In addition, paired fecal samples were collected from swabbed mice to determine whether mite DNA was present in their feces. A total of 5 mice were sampled for each group.
DNA extraction.
For the DNA extraction from fur plucks in the sequencing experiments, M. musculinus samples were processed by using the Isolation of Genomic DNA from Tissues protocol provided with the QIAmp DNA Micro Kit (Qiagen, Hilden, Germany). Samples were incubated for 5 h to complete the lysis process, and carrier RNA was added to buffer AL to maximize yield. For the validation studies using fur swabs, DNA was extracted by using the High Pure PCR Template Preparation Kit (version 20, Roche Molecular Systems, Mannheim, Germany) according to the manufacturer's instructions. Fecal DNA was extracted with the DNeasy PowerSoil Kit (Qiagen) according to the manufacturer's instructions. DNA from all samples was stored at –20 °C until tested.
Sequencing the 18S and 28S rRNA genes of M. musculinus.
To generate PCR products from M. musculinus, ribosomal (18S and 28S rRNA) genes from the suborder Psoroptidia were aligned by using Geneious software (Biomatters, Auckland, New Zealand). 18S rRNA sequences from this suborder included Myocoptes japonensis (NCBI accession nos. EU152597.1 and JQ000333.1), Sturnophagoides bakeri (JQ000246.1), Dermatophagoides farinae (JQ000247.1), and Dermatophagoides pteronyssinus (JQ000249.1). 28S rRNA sequences included M. japonensis (EU152723.1 and JQ000641.1), Sturnophagoides bakeri (JQ000554.1), D. farinae (JQ000555.1), and D. pteronyssinus (JQ000557.1). Based on consensus regions, forward and reverse primers spanning the 18S and 28S rRNA region were designed using Primer3.28 These generic Psoroptidia primers (Table 1) were used to generate 18S and 28S rRNA PCR products from M. musculinus DNA by using Phusion High-Fidelity DNA Polymerase (New England Bio Labs, Ipswich, MA). The MCrRNA thermocycling program used to generate PCR products for MiSeq sequencing consisted of: 30 s at 98 °C for initial denaturation; 36 cycles of denaturation at 98 °C for 10 s, annealing at 59 °C for 30 s, and extension at 72 °C for 1 min; final extension at 72 °C for 7 min; and a hold at 6 °C thereafter.
Table 1.
Primers used in PCR amplifications for M. musculinus
| Target | Forward primer (5ʹ to 3ʹ) | Reverse primer (5ʹ to 3ʹ) | Probe | Program |
| rRNA (sequencing) | MC_18S_seq F1 5ʹ AGG TGA AAC CGC GAA TGG CTC A 3ʹ | MC_28S_seq R1 5ʹ AAC GCC GCA TCT CTT GAA CGC 3ʹ | MCrRNA | |
| rRNA (sequencing) | MC_18S_seq F1 5ʹ AGG TGA AAC CGC GAA TGG CTC A 3ʹ | MC_28s_seq_R2 5ʹ AGT ACA TCA CCC CGG CAG GT 3ʹ | MCrRNA | |
| rRNA (sequencing) | MC_18S_seq F2 5ʹ CGC ACG CGC GCT ACA CTG AAA A 3ʹ | MC_28s_seq_R2 5ʹ AGT ACA TCA CCC CGG CAG GT 3ʹ | MCrRNA | |
| 28S rRNA (PCR or qPCR) | MC28s F1 5ʹ GAG ACC GAT AGC AAA CAA GTA C 3ʹ | MC28s R1 5ʹ CAT AGC CTC CGA AAA GAC CCA CTG 3ʹ | 5ʹ Cy5-GCG CTT CGG TCG ATT CAA TC–IBRQ 3ʹ | MITE58 or default qPCR |
PCR products were pooled for sequencing at the BioMicro Center at the Massachusetts Institute of Technology. Briefly, the sequencing library was prepared by using NexteraXT and sequenced on an MiSeq sequencer (Illumina, San Diego, CA) to generate two 150-bp paired reads by using the 300-cycle MiSeq Reagent Kit (version 2; MS-102-2002, Illumina, San Diego). FASTQC was used to check read quality (http://www.bioinformatics.babraham.ac.uk/projects/fastqc). Paired reads (n = 3,118,749) were generated but were down-sampled to 3000 reads for assembly by using the Trinity Assembler7 for rRNA gene assembly.
Primer design and PCR reactions.
Multiple primer pairs were designed to target the newly sequenced 28S rRNA gene of M. musculinus (Table 1). PCR reactions were run in a thermocycler (MJ Research PTC-200, Gradient Thermal Cycler, Marshall Scientific, Hampton, NH) using the Phusion Hot-Start Flex 2× master mix (New England Bio Labs, Ipswich, MA), water, 250 nM each of forward and reverse primers, and 3 µL DNA. The following conditions were used for the PCR reactions (MITE58): initial denaturation for 3 min at 94 °C; 35 cycles of denaturation at 94 °C for 45 s, annealing at 58 °C for 45 s, and extension at 72 °C for 1 min; with a final extension at 72 °C for 5 min and a hold at 6 °C thereafter. Select PCR products from these reactions were confirmed by Sanger sequencing at a commercial laboratory (Quintara Biosciences, Boston, MA).
Cloning PCR products to generate standards.
A positive control was developed by cloning M. musculinus PCR products generated by using MC28S into the pCR 2.1 TOPO plasmid vector by using the TOPO TA Cloning Kit (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Briefly, plasmids were transformed into Transform One TOP10 cells (Invitrogen) by using the manufacturer's chemical transformation protocol. Eight M. musculinus–transformed colonies were selected and cultured in medium containing ampicillin and X-gal. The PureLink Plasmid Miniprep Kit (Invitrogen) was then used to isolate the plasmid from the bacteria. All colonies underwent PCR verification to confirm the presence of M. musculinus-specific inserts in the plasmids. Three colonies yielding M. musculinus–specific bands at 178 bases were selected. To generate standard curves, we used the known product sizes and the vector size of 3900 bases and estimated the number of plasmid copies of M. musculinus as (mass of plasmids [in ng] × 6.0221 × 1023 molecules/mole) / (4078 bases × 650 g/mole × 109 ng/g). DNA concentration was quantified (ThermoFisher Scientific, Waltham, MA), and standards for qPCR reactions were generated by diluting M. musculinus plasmid stocks.
qPCR reaction.
qPCR reactions included 2× PrimeTime Gene Master Mix (Integrated DNA Technologies, Coralville, IA), Primetime qPCR Primer/Probe Mix for MC_28S (M. musculinus), water, and 5 µL DNA. Technical duplicates or triplicates were placed in the wells of a MicroAmp Fast Optical 96-Well Reaction Plate with Barcode (Applied Biosystems, Foster City, CA) and sealed (MicroAmp Optical Adhesive Film, Applied Biosystems) prior to running on a Fast Real-Time thermocycler (model 7500, Applied Biosystems); the default qPCR program (20 s at 95 °C; 40 cycles at 95 °C for 20 s and 30 s at 60 °C). To determine the limit of detection for the MC28S qPCR assay, 10-fold dilutions ranging from 106 to 0 copies of M. musculinus plasmid were replicated 8 times over 4 separate runs. To establish the limit of detection, the lowest dilution level at which all 8 replicates were positive was determined.9,15,16 Linear regression analysis was performed by using Excel 2016 (Microsoft, Redmond, WA); the correlation coefficient was calculated by using the CORREL function, and the amplification efficiency was computed as 10(–1/slope) – 1.
Results
M. musculinus ribosomal RNA sequencing.
Prior to this study, the only published sequence of M. musculinus was a 935-bp partial sequence from 18S rRNA. Here we obtained sequences for the 18S and 28S rRNA genes within the suborder Psoroptidia to which Myocoptes belong, for the closely related Myocoptes japonensis and several dust mite species (Dermatophagoides spp. and Sturnophagoides bakeri). Alignment of both 18S and 28S rRNA genes revealed conserved regions. The compared regions among these species were 93.8% and 86.1% identical for the 18S and 28S rRNA genes, respectively. Using the alignment of related species, we designed forward primers within the 18S rRNA gene and reverse primers within the 28S rRNA gene that would target the Psoroptidia mite species. Using these primers, we generated 3 PCR products from the M. musculinus DNA, which we sent for MiSeq sequencing. A 6351-base contig was generated that spanned both the 18S and 28S rRNA gene of M. musculinus. Homology analysis of assembled contigs with published mite sequences confirmed the correct assembly of the short reads (Table 2). Sequences for the 18S (NCBI accession no., KT384411.1) and 28S (KT384412.1) rRNA genes of M. musculinus were deposited at NCBI. These sequences were used to generate species-specific PCR primers to identify M. musculinus.
Table 2.
Pairwise identity of mite sequences available in NCBI compared with sequenced M. musculinus (MC) contig
| 18S rRNA sequence used | 18S rRNA % pairwise identity with MC contig | 28S rRNA sequence used | 28S rRNA % pairwise identity with MC contig | |
| M. musculinus | JF834893 (partial, 935 bp) | 98.4%a | not applicable | not applicable |
| M. japonensis | JQ000333 | 97.2% | JQ000641 | 95.7% |
| D. pteronyssus | JQ000249 | 93.7% | JQ000557 | 89.9% |
| D. farinae | JQ000247 | 92.9% | JQ000555 | 89.4% |
| M. musculi | JF834895 | 83.0% | JF934703 (partial, 444 bp) | 68.7% |
14 mismatches in first 28 bases of JF834893
Development of PCR assay for the detection of M. musculinus.
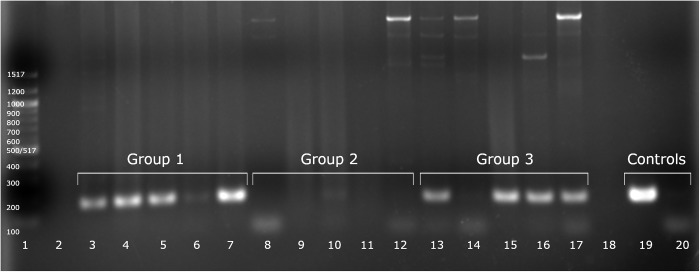
After multiple rounds of testing, we selected the MC28S primer pair (Table 1) because it specifically targeted M. musculinus DNA and did not yield similarly sized bands when tested against mouse DNA (Figure 1). Furthermore, BLAST and Primer-BLAST analysis of the MC28S primer pair demonstrates specificity for the Myocoptes genus, and the only predicted PCR products with one or fewer mismatches are from M. musculinus and M. japonensis.1 To validate the MC28S primers, swab samples were collected from experimentally maintained mouse colonies with known mite infestations. Specifically, we used samples from mice infested with M. musculinus and D. musculi (n = 5), M. musculi and R. affinis (n = 5), or M. musculinus and M. musculi (n = 5). MC28S primers positively identified all 10 samples containing M. musculinus but none of the 5 samples devoid of M. musculinus (Figure 2, Table 3). To confirm the accuracy of our results, we sequenced the PCR product generated from sample 1E by using MC28S primers, which verified the positive identification of M. musculinus (99.4% sequence identity over 154 bases).
Figure 1.
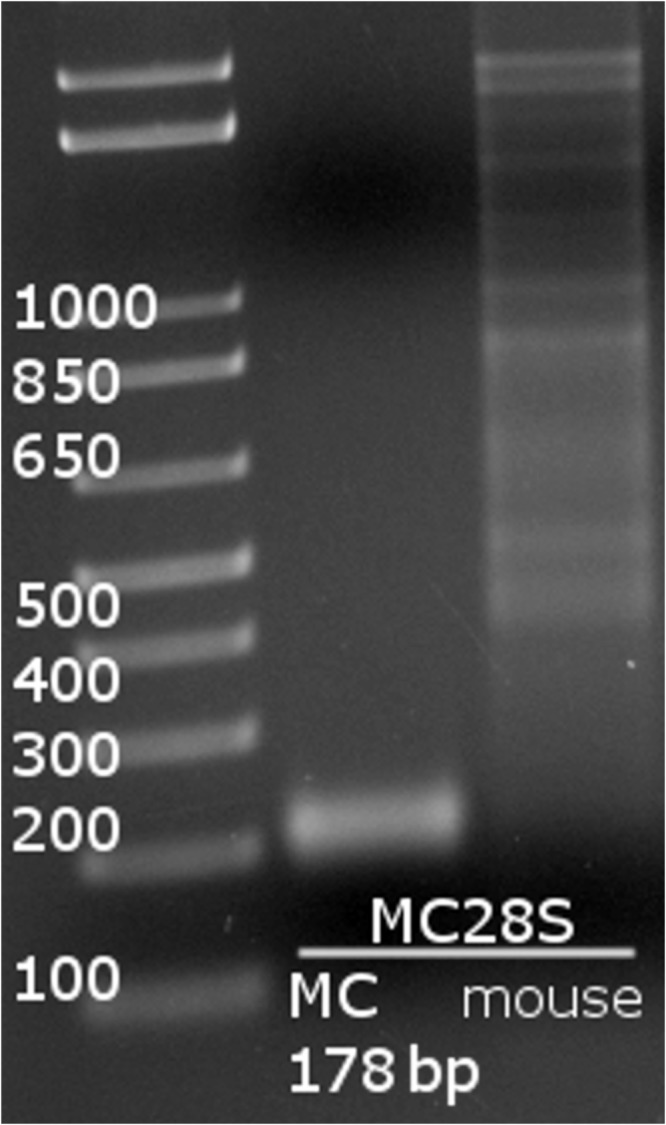
PCR detection using MC28S primers. MC28S primers generate a 178-bp product in the presence of M. musculinus DNA. Lane 1, 1-kb ladder; lane 2, MC28S primers with M. musculinus DNA; lane 3, MC28S primers with mouse DNA.
Figure 2.
PCR detection of M. musculinus by using MC28S primers against fur swab samples. Lane 1, 100-bp ladder; lanes 3 through 7, samples containing M. musculinus and D. musculi (group 1); lanes 8 through 12, samples containing M. musculi and R. affinis (group 2); lanes 13 through 17, samples containing M. musculinus and M. musculi (group 3); lane 19, positive control; and lane 20, negative control.
Table 3.
Results of M. musculinus PCR and qPCR assays
| PCR | qPCR | |||||
| Group | Infested mites | Swab | Feces | Swab | Feces | |
| 1 | M. musculinus and D. musculi | 5 | 5 | 5 | 5 | |
| 2 | M. musculi and R. affinis | 0 | 0 | 0 | 0 | |
| 3 | M. musculinus and M. musculi | 5 | 4 | 5 | 5 | |
Data in each column are given as the number of mite-positive samples among a total of 5 samples.
Quantification of detection by using a qPCR assay for M. musculinus.
Because the MC28S PCR assay showed specificity for M. musculinus, we developed a probe-based qPCR assay was developed as qPCR analysis is faster than conventional PCR amplification and may show increased assay sensitivity and specificity. Relying on the specificity of the PCR primers, we designed a probe within the target region of the MC28S primers. Using 8 replicates of the M. musculinus standard curve, we determined that the limit of detection for the MC28S qPCR assay was 100 copies of M. musculinus, because this dilution was the lowest at which 100% of the replicates were positive. At 10 copies, M. musculinus was detected in 75% of the replicates, whereas at 100 copies M. musculinus was detected in 100% of the samples. (Figure 3 A).9,15,16 Using samples from fur swabs, the MC28S qPCR assay correctly identified all 10 mice in groups 1 and 3 as positive for M. musculinus and all 5 group 2 samples as negative (Figure 3 B, Table 3).
Figure 3.
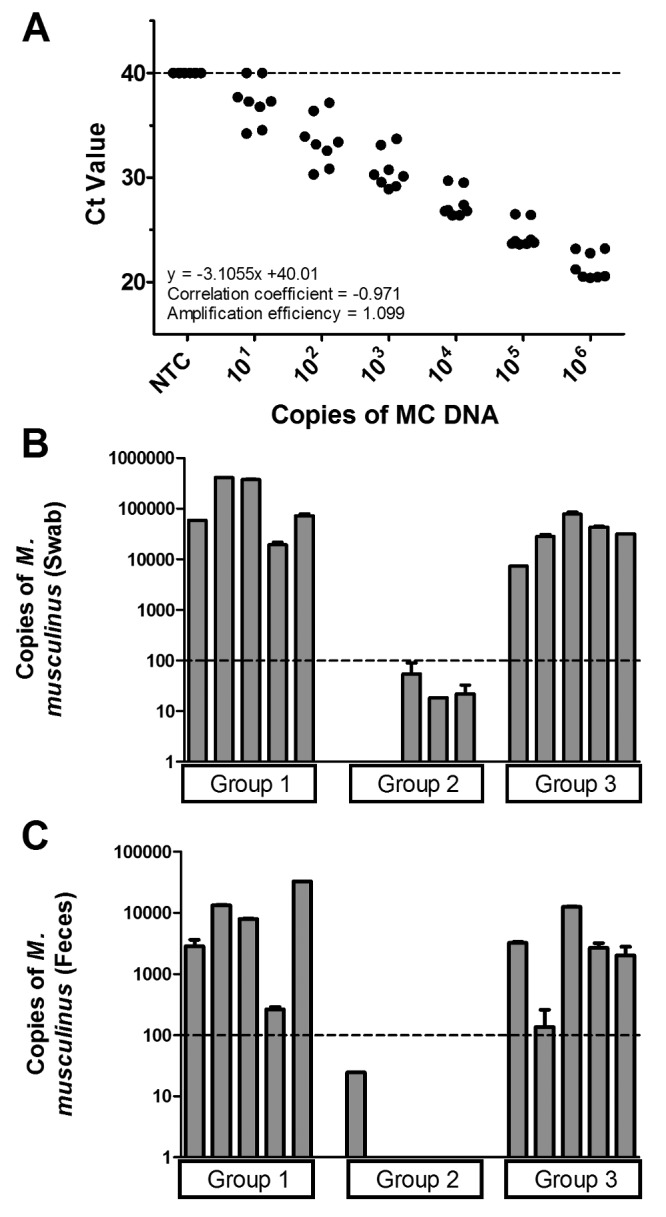
(A) Replicates of the standard curve analysis show that the limit of detection of the M. musculinus PCR assay is 100 copies. (B) Detection of M. musculinus in swab samples by using MC28S qPCR assay for groups 1 (infested with M. musculinus and D. musculi), 2 (M. musculi and R. affinis), and 3 (M. musculinus and M. musculi). (C). Detection of M. musculinus in fecal samples by using the MC28S qPCR assay for groups 1, 2, and 3.
Detection of murine mites in fecal DNA by using PCR-based assays.
Because swabbing for mites may increase the workload within surveillance programs, MC28S assays were tested against DNA extracted from feces, which are commonly collected for colony health surveillance. The MC28S assay correctly detected M. musculinus in all 5 fecal samples from group 1 mice and 4 of the 5 samples from group 3; in addition, sample 3B yielded a very faint band (Figure 4). Furthermore, we obtained a weak band from sample 2C, which lacked M. musculinus (Figure 4). Leveraging the increased sensitivity of qPCR analysis, we tested the 15 fecal samples with the MC28S qPCR assay and confirmed that all group 1 and 3 samples were indeed positive, but all group 2 samples were considered negative (Figure 3 C, Table 3).
Figure 4.
PCR detection of M. musculinus by using the MC28S primer set with fecal samples. Lane 1, 100-bp ladder; lanes 3 through 7, samples containing M. musculinus and D. musculi (group 1); lanes 8 through 12, samples containing M. musculi and R. affinis (group 2); lanes 13 through 17, samples containing M. musculinus and M. musculi (group 3); lane 19, positive control; and lane 20, negative control.
Discussion
PCR-based assays provide advantages in terms of the ability to 1) detect few copies of DNA in complex samples, 2) comprehensively sample whole animals, cages, racks, and so forth by using swabs, and 3) test a large number of samples without linearly increasing time and labor.6,11,22 PCR testing has been used to detect mites in a variety of animal species, including humans, dogs, bats, and marmots.21,25,27 In addition, many publications have shown the successful detection of murine mites in laboratory settings using external diagnostic labs.6,11,22 However, the costs associated with external testing may limit clinicians’ ability to test mice with suspected acariasis. Inhouse PCR testing can provide laboratories the ability to cost-efficiently and rapidly screen for mites. To date, we have found only 2 publications that describe primer sequences to detect murine mites in laboratory mice; these studies focused on pelt and skin samples.8,24
To further simplify the process of inhouse testing for mites in laboratory mice, we developed PCR assays that could detect M. musculinus DNA in fecal samples. Because constant grooming suggests that mites and mite eggs might be ingested, veterinarians have speculated about the ability of PCR tests to detect mites in feces. Anecdotally, we understand that some technicians have reported observing mites in fecal exams. To our knowledge, this report is the first to describe M. musculinus detection in feces using PCR. We here present robust M. musculinus-specific PCR and qPCR assays capable of detecting M. musculinus DNA in complex mixtures including fecal and fur swab samples. In silico analysis of both the PCR and qPCR binding sites predicted efficient binding to Myocoptes species only. While the qPCR probe shares sequence homology with other mite species within the suborder Psoroptidia to which Myocoptes belongs, none of these mites naturally infest mice. In addition, no homology was observed to M. musculi,3 R. affinis or D. musculi, which are taxonomically distinct from Myocoptes. Because specific binding of both primers and probes is necessary for efficient 5′ nuclease qPCR assays, our in silico analysis only predicts the efficient binding of all 3 components to Myocoptes DNA sequences. Validation by using both types of samples with known combinations of mites and Sanger sequencing of select PCR products demonstrated that the PCR assay was indeed specific and detected M. musculinus. The developed probe-based qPCR assay can be used by laboratories with real-time PCR capabilities that are interested in reducing the duration of the assay and hands-on time. In summary, our results demonstrate that fecal samples can be used in the PCR assays presented to screen for M. musculinus infestations in laboratory mice, thus giving laboratory animal veterinarians additional options when designing health surveillance programs.
Acknowledgments
This work was supported in part by the National Institute of Environmental Health Sciences of the NIH under award P30-ES002109 (JGF), by NIH T32-OD010978 (JGF), and the ACLAM Foundation grant #021954-00001 (AS).
References
- 1.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelone-Alasaad S, Molinar Min A, Pasquetti M, Alagaili AN, Berrilli F, Obanda V, Gebely MA, Soriguer RC, Rossi L. 2015. Universal conventional and real-time PCR diagnosis tools for Sarcoptes scabiei. Parasit Vectors 8:587 10.1186/s13071-015-1204-8. Erratum: Universal conventional and real-time PCR diagnosis tools for Sarcoptes scabiei [Parasit Vectors 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldman SH, Ntenda AM. 2011. Phylogenetic analysis of Myobia musculi (Schranck, 1781) by using the 18s small ribosomal subunit sequence. Comp Med 61:484–491. [PMC free article] [PubMed] [Google Scholar]
- 4.Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL. 2006. The mouse in biomedical research, vol 2, 2nd ed: Diseases. San Diego (CA): Academic Press. [Google Scholar]
- 5.Franklin ML, Bennett JC. 2002. Laboratory animal medicine: historical perspectives. p 1–16. In: Fox JG, Anderson LC, Loew FM, Quimby FW, editors. Laboratory animal medicine, 2nd ed San Diego (CA): Academic Press. [Google Scholar]
- 6.Gerwin PM, Ricart Arbona RJ, Riedel ER, Henderson KS, Lipman NS. 2017. PCR Testing of IVC filter tops as a method for detecting murine pinworms and fur mites. J Am Assoc Lab Anim Sci 56:752–761. [PMC free article] [PubMed] [Google Scholar]
- 7.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, Chen Z, Mauceli E, Hacohen N, Gnirke A, Rhind N, di Palma F, Birren BW, Nusbaum C, Lindblad-Toh K, Friedman N, Regev A. 2011. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29:644–652. 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grove KA, Smith PC, Booth CJ, Compton SR. 2012. Age-associated variability in susceptibility of Swiss Webster mice to MPV and other excluded murine pathogens. J Am Assoc Lab Anim Sci 51:789–796. [PMC free article] [PubMed] [Google Scholar]
- 9.Hue ES, Fortier CI, Laurent AM, Quesnelle YF, Fortier GD, Legrand LJ, Pronost SL. 2016. Development and validation of a quantitative PCR method for equid herpesvirus-2 diagnostics in respiratory Fluids. J Vis Exp 109:1–13. 10.3791/53672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 11.Jensen ES, Allen KP, Henderson KS, Szabo A, Thulin JD. 2013. PCR testing of a ventilated caging system to detect murine fur mites. J Am Assoc Lab Anim Sci 52:28–33. [PMC free article] [PubMed] [Google Scholar]
- 12.Jungmann P, Freitas A, Bandeira A, Nobrega A, Coutinho A, Marcos MA, Minoprio P. 1996. Murine acariasis. II. Immunological dysfunction and evidence for chronic activation of Th2 lymphocytes. Scand J Immunol 43:604–612. 10.1046/j.1365-3083.1996.d01-259.x. [DOI] [PubMed] [Google Scholar]
- 13.Karlsson EM, Pearson LM, Kuzma KM, Burkholder TH. 2014. Combined evaluation of commonly used techniques, including PCR, for diagnosis of mouse fur mites. J Am Assoc Lab Anim Sci 53:69–73. [PMC free article] [PubMed] [Google Scholar]
- 14.Lindstrom KE, Carbonne LG, Kellar DE, Mayorga MS, Wilkerson JD. 2011. Soiled bedding sentinels for the detection of fur mites in mice. J Am Assoc Lab Anim Sci 50:54–60. [PMC free article] [PubMed] [Google Scholar]
- 15.Liu C, Guo YM, Cao JZ, Zhang DF, Chang OQ, Li K, Wang F, Shi CB, Jiang L, Wang Q, Lin L. 2018. Detection and quantification of Aeromonas schubertii in Channna maculate by TaqMan MGB probe fluorescence real-time quantitative PCR. J Fish Dis 42:109–117. 10.1111/jfd.12911. [DOI] [PubMed] [Google Scholar]
- 16.Ma L, Zeng F, Huang B, Cong F, Huang R, Ma J, Guo P. 2018. Development of a conventional RT-PCR assay for rapid detection of porcine deltacoronavirus with the same detection limit as a SYBR green-based real-time RT-PCR assay. BioMed Res Int 2018:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morita E, Kaneko S, Hiragun T, Shindo H, Tanaka T, Furukawa T, Nobukiyo A, Yamamoto S. 1999. Fur mites induce dermatitis associated with IgE hyperproduction in an inbred strain of mice, NC/Kuj. J Dermatol Sci 19:37–43. 10.1016/S0923-1811(98)00047-4. [DOI] [PubMed] [Google Scholar]
- 18.Nashat MA, Arbona RJR, Riedel ER, Francino O, Ferrer L, Luchins KR, Lipman NS. 2018. Comparison of diagnostic methods and sampling sites for the detection of Demodex musculi. J Am Assoc Lab Anim Sci 57:173–185. [PMC free article] [PubMed] [Google Scholar]
- 19.Nashat MA, Luchins KR, Lepherd ML, Riedel ER, Izdebska JN, Lipman NS. 2017. Characterization of Demodex musculi infestation, associated comorbidities, and topographic distribution in a mouse strain with defective adaptive immunity. Comp Med 67:315–329. [PMC free article] [PubMed] [Google Scholar]
- 20.National Research Council, Institute for Laboratory Animal Research, Commission on Life Sciences, Committee on Infectious Diseases of Mice and Rats. 1991. Skin and joints, Chapter 8. p 179–182. In: Infectious diseases of mice and rats. Washington (DC): National Academies Press; 10.17226/1429. [DOI] [Google Scholar]
- 21.Ravera I, Altet L, Francino O, Bardagi M, Sanchez A, Ferrer L. 2011. Development of a real-time PCR to detect Demodex canis DNA in different tissue samples. Parasitol Res 108:305–308. 10.1007/s00436-010-2062-0. [DOI] [PubMed] [Google Scholar]
- 22.Rice KA, Albacarys LK, Metcalf Pate KA, Perkins C, Henderson KS, Watson J. 2013. Evaluation of diagnostic methods for Myocoptes musculinus according to age and treatment status of mice (Mus musculus). J Am Assoc Lab Anim Sci 52:773–781. [PMC free article] [PubMed] [Google Scholar]
- 23.Roble GS, Boteler W, Riedel E, Lipman NS. 2012. Total IgE as a serodiagnostic marker to aid murine fur mite detection. J Am Assoc Lab Anim Sci 51:199–208. [PMC free article] [PubMed] [Google Scholar]
- 24.Sastre N, Calvete O, Martinex-Vargas J, Medarde N, Casellas J, Altet L, Sanchez A, Francino O, Ventura J. 2018. Skin mites in mice (Mus musculus): high prevalence of Myobia sp. (Acari, Arachnida) in Robertsonian mice. Parasitol Res 117:2139–2148. 10.1007/s00436-018-5901-z. [DOI] [PubMed] [Google Scholar]
- 25.Sastre N, Francino O, Curti JN, Armenta TC, Fraser DL, Kelly RM, Hunt E, Silbermayr K, Zewe C, Sanchez A, Ferrer L. 2016. Detection, Prevalence and Phylogenetic Relationships of Demodex spp and further Skin Prostigmata mites (Acari, Arachnida) in wild and domestic mammals. PLoS One 11:1–20. 10.1371/journal.pone.0165765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith PC, Zeiss CJ, Beck AP, Scholz JA. 2016. Demodex musculi infestation in genetically immunomodulated mice. Comp Med 66:278–285. [PMC free article] [PubMed] [Google Scholar]
- 27.Thoemmes MS, Fergus DJ, Urban J, Trautwein M, Dunn RR. 2014. Ubiquity and diversity of human-associated Demodex mites. PLoS One 9:1–8. 10.1371/journal.pone.0106265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. 2012. Primer3—new capabilities and interfaces. Nucleic Acids Res 40:1–12. 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]