Abstract
The population of NIH-owned or NIH-supported captive research chimpanzees is quickly becoming aged, and the 1998 NIH breeding moratorium has resulted in a skewed age distribution. As such, behavioral management programs aimed at refining the care of an aging captive chimpanzee population have become increasingly important. However, little research exists that addresses the ways in which captive chimpanzee behavior differs as a function of the interaction of age and aspects of the captive environment. We examined overall differences in behavior between elderly (35 y and older) and nonelderly (younger than 35 y) captive chimpanzees. Elderly chimpanzees exhibited significantly more rough scratching (a behavioral indicator of anxiety) and inactivity, less behavioral diversity, and less affiliation than their nonelderly counterparts. We also assessed whether elderly chimpanzee behavior and wounding rates differed as a function of housing in geriatric (group average age, 35 y or older) or nongeriatric (group average age, younger than 35 y) groups. In our program, geriatric social groups were characterized by smaller group size, more females within the group, and higher levels of individual mobility impairment compared with nongeriatric groups. Furthermore, elderly chimpanzees housed in geriatric groups displayed significantly increased rough scratching, decreased locomotion and submission than nongeriatric animals but no difference in wounding. These findings suggest that housing elderly chimpanzees in nongeriatric groups may be beneficial, given that doing so may stimulate locomotion. However, the establishment and maintenance of geriatric groups may be unavoidable as the demographics of the population of captive former research chimpanzees continues to age. Therefore, refinements to captive geriatric care strategies for chimpanzees should focus on methods of evaluating and enhancing functionally appropriate captive environments within geriatric groups.
In 1998, the NIH announced a breeding moratorium on all NIH-owned or -supported research chimpanzees in the United States, creating an increasingly skewed age distribution in the research population.19 Advances in health care allow captive chimpanzees to live until well after 50 y of age.12,20,32 As such, this current chimpanzee population is quickly becoming an aged one.
Like humans, elderly chimpanzees face increased risks of experiencing a variety of age-related ailments, including hypertension, heart disease, mobility impairment, arthritis, Alzheimer pathology, and diabetes.13,14,18,24,26,27,32 Although a plethora of research that assesses physiologic and cognitive changes related to advanced age in chimpanzees is available, studies examining the behavioral effects of old age are sparse. In the wild, the social behavior of elderly chimpanzees is characterized by withdrawal from the group and social interactions, and, in particular, female chimpanzees increasingly spend time exclusively with family members.5,15,22 In addition, male dominance rank can drop with old age, but female rank often remains the same.15 In fact, old female chimpanzees may enjoy certain advantages within the group, given that male chimpanzees seem to prefer to mate with older—even geriatric—females.28
Studies that have examined behavioral changes in elderly captive chimpanzees involve fairly small sample sizes, and findings are conflicting. In one study,35 old age (that is, greater than 30 y) in 6 chimpanzees was characterized by lower levels of activity, reduced mobility, and increased self-grooming and other solitary activity. In addition, aged chimpanzees (30 to 44 y; n = 14) housed in pairs or trios exhibited less aggression, locomotion, and object manipulation than younger chimpanzees (11 to 22 y; n = 20) but affiliation did not differ.6 Conversely, a study that examined differences between 12 younger chimpanzees (12 to 26 y) and 12 older chimpanzees (36 to 44 y) reported no significant difference across any of the 70 behaviors evaluated; the authors suggested that there was no evidence of consistent age-associated behavioral differences.38
Behavioral management techniques are designed to enhance the welfare of NHP in captivity. Specific attempts to enhance the welfare of captive elderly chimpanzees are becoming more common, including the use of acupuncture and laser therapy for treatment of arthritis, self-medication paradigms, and systematic programs that use personalized care approaches for geriatric animals.10,23,25-27,30,34,37 In addition, behavioral management techniques have focused on methods to enhance the captive environment of elderly chimpanzees in ways that are functionally appropriate, promote species-typical behaviors, and encourage locomotor activities that are necessary for healthy aging.4 Elderly chimpanzees often experience mobility impairments that may limit their ability to escape aggression during agonistic encounters, potentially increasing rates of wounding and other health-related problems in those that live in large, age- and sex-diverse social groups.1,8,26,30,32 As a way to prevent detrimental effects to elderly chimpanzee welfare resulting from these types of situations, some behavioral management programs establish and maintain ‘geriatric groups.’ Such groups house elderly chimpanzees with health or mobility impairments together, to 1) facilitate access to animals that are in need of specific treatment regimens, 2) provide a physical environment that supports access to all portions of the enclosure, and 3) provide a social environment that minimizes the deleterious effects of intragroup aggression.4,10 Although geriatric groups are becoming more common in captive settings, little research has examined the ways in which housing in different types of groups affects the behavior of chimpanzees of advanced age. As the proportion of captive formerly research chimpanzees that is elderly (35 y of age and older) continues to grow, empirical examination of the environment of these animals becomes increasingly important for facilities that house this currently expanding population.
At our facility, the National Center for Chimpanzee Care, more than 30% of the chimpanzees are considered elderly (or geriatric). These chimpanzees are housed in a variety of group sizes and compositions. As part of a larger project examining the behavioral and welfare effects of refinements to behavioral management techniques and the captive environment at the NCCC, we aimed to examine captive chimpanzee behavior as a function of advanced age and the social environment.29-31 We were specifically interested in the following questions. First, using the definition of elderly as 35 y and older proposed by past literature, in what ways does overall behavior differ between elderly and nonelderly chimpanzees?32,38 Second, at what age does a chimpanzee begin to behave like an elderly chimpanzee? In other words, when does a chimpanzee's behavior begin to deviate from younger chimpanzee behavior? Third, what are the size and compositional characteristics of a geriatric group compared with a nongeriatric group in our program? And fourth, among elderly chimpanzees, does behavior differ as a function of living in a geriatric compared with a nongeriatric group?
Materials and Methods
Subjects and housing.
Subjects comprised a total of 118 chimpanzees (72 females and 46 males) housed at the the National Center for Chimpanzee Care (Michale E Keeling Center for Comparative Medicine and Research, The University of Texas MD Anderson Cancer Center, Bastrop, TX). Chimpanzees were housed in 17 separate social groups of 4 to 10 animals each in corrals or ‘primadomes’.29,30 Of these 118 chimpanzees, 27 were nursery-reared, 76 were mother-reared, and 15 were wild-born. For the purposes of this study, 35 of the 118 chimpanzees were considered elderly (35 y and older), with an average age of 41.62 y (SE, 1.12 y; range, 35 through 56 y), and the remaining 83 were considered nonelderly (mean age, 27.04 y, SE, 0.49 y; range, 15 through 34 y). Of the 35 elderly chimpanzees, 20 were housed in geriatric groups (that is, groups with a mean age of 35 y or older), and 15 were housed in nongeriatric groups (that is, groups with a mean age of younger than 35 y).
As part of an existing behavioral management program for elderly chimpanzees, several adaptations of the standard housing environment, daily healthcare procedures, diet, and training routines were aimed at creating personalized, cageside care for each geriatric animal. These adaptations include ramps within enclosures to facilitate movement and the use of various heights in the enclosure, positive reinforcement training to allow chimpanzees to voluntarily participate in health-related procedures (for example, acupuncture), and additional enrichment and feeding devices that are adapted to address physiologic changes or that may serve as physical therapy.4,10,17,34
The research conducted in this study complied with protocols approved by our facility's IACUC and complied with the legal requirements of the United States and the ethical guidelines put forth by AALAS, the Animal Welfare Act,3 and the Guide for the Care and Use of Laboratory Animals.21
Procedure.
Behavioral observations were conducted by using 15-min focal animal sampling (Altmann, 1974).2 Data were collected (Observer XT, Noldus, Leesburg, VA) between 1200 and 1600, counterbalanced across 1200 to 2400 and 0700 to 1200 from August 2016 through May 2018. Each chimpanzee served as a focal animal in a minimum of 22 observations, for a total of more than 705 h of data, including 200 h of observation of the 35 geriatric chimpanzees. Behavioral categories included inactive, aggressive, object manipulation, locomotive, submissive, affiliative, abnormal, self-directed, and proximity (near, distant, or touching). See Supplementary Figure S1 for the ethogram.
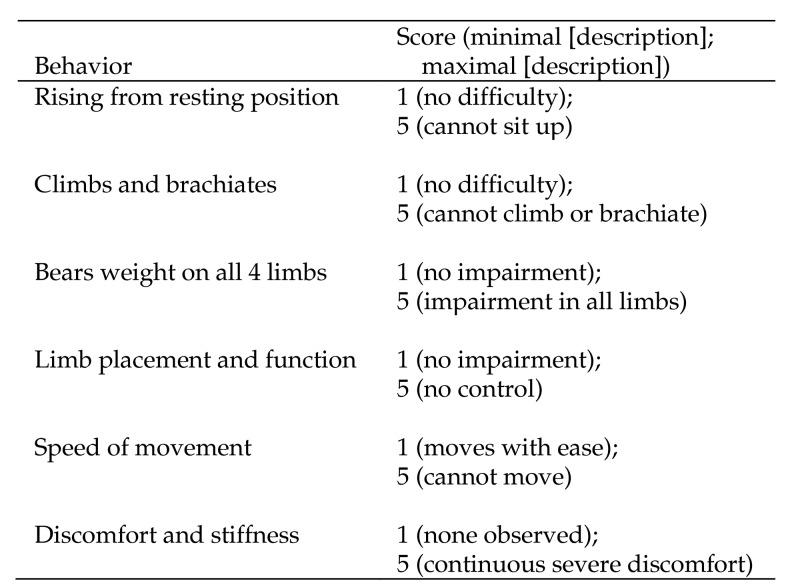
As part of the behavioral management and veterinary programs at our facility, chimpanzees that exhibit mobility issues are assigned to ‘mobility watch,’ whereby veterinary technicians, trainers, and caregivers rate each chimpanzees’ overall mobility by using an established mobility impairment scoring system.30 The scoring system is composed of 6 categories that are rated on a scale of 1 to 5, with 1 indicating no impairment and 5 indicating the most impairment (Figure 1), such that higher scores indicate more severe mobility impairments. Raters reliably score the mobility of these chimpanzees once each month (intraclass correlation, 0.96). During the course of this study, 10 of the 35 elderly chimpanzees had mobility issues and therefore were rated by using this system. Chimpanzee's mobility scores throughout the course of the present study were averaged to create an overall mobility impairment score. Although scores theoretically could range from 6 to 30, observed scores ranged from 6 to 16 only (mean, 8.79; 1 SD, 2.38). Chimpanzees without mobility issues were not scored in regard to mobility (because they had no mobility issues) and were assumed to have a score of 6 (that is, 1 for each of the 6 categories), indicating no impairment.
Figure 1.
Mobility scoring system used in the current study. Higher scores are indicative of greater impairment in mobility.
Data analysis.
Data were analyzed by using the techniques that we have published previously.29-31 Briefly, durations of each behavior were averaged across all observations for each chimpanzee, with ‘in-view’ duration as the denominator. Average duration was then converted into a percentage of total time (that is, percentage of time of behavior = [duration of behavior / in-view duration] × 100%). A behavioral diversity score was calculated by counting the total number of different behaviors that the chimpanzee exhibited, excluding any negative welfare-related behaviors. In addition, we calculated proximity scores for each chimpanzee ([time spent near + time spent touching] / time spent distant), in which a higher score is indicative of closer average proximity to group mates. Lastly, the total number of wounds that required veterinary treatment across the approximately 2-y data collection period was calculated for each chimpanzee. Wounding primarily resulted from intragroup aggression and included punctures, lacerations, abrasions, bites, lameness, swelling, and abscesses. The number of wounds was derived from an existing health database used by veterinarians and veterinary technicians to track and treat health issues. Therefore, the dependent variables for this study included the number of wounds over the 2-y data collection period, behavioral diversity scores, proximity scores, and behavioral percentages (rough scratching, aggressive, locomotive, submissive, affiliative, abnormal, and inactive).
Prior to analyses, data were examined for normality by using Q-Q plots and histograms. Except for behavioral diversity and inactivity, all data were positively skewed, and we therefore used bootstrapping for all analyses. First, bootstrapped independent samples t tests were used to assess behavioral differences between geriatric (35 y and older) and nongeriatric (younger than 35 y) chimpanzees. Second, we divided chimpanzees into 5 age groups to achieve approximately equal age intervals and similar numbers of chimpanzees in each age group: 24 y and younger, n = 19; 25 through 29 y, n = 41; 30 through 34 y, n = 23; 35 through 39 y, n = 19; and 40 y and older, n = 16. Bootstrapped multivariate analysis of covariance with Bonferroni posthoc comparisons was used to assess behavioral differences among age groups. Mobility impairment scores, group size, within-group age range, and percentage of male chimpanzees in the group were used as covariates.31 The remaining analyses focused only on elderly chimpanzees and the effects of social housing conditions on behavior and wounding. Because group size and the average age of the group were significantly negatively correlated (r = –0.78, P = 0.0001), we were unable to assess the effects of these factors independently. Instead, we defined the characteristics of geriatric groups, and then examined the behavioral effects of housing in these types of groups. A series of independent sample t tests were used to characterize geriatric and nongeriatric groups, including average age of the group, average mobility score, group size, percentage of males, and within-group age range. We then used multivariate analysis of covariance with mobility impairment scores as a covariate to examine behavioral differences between elderly chimpanzees housed in geriatric groups compared with those in nongeriatric groups. Correlations were used as posthoc analyses to explore any significant effects of mobility impairment scores as a covariate on behavior. Using the Levene test for equality of variances, we report t values and, where appropriate, the degrees of freedom for unequal variances.
Lastly, independent samples t tests were used to assess differences in number of wounds between elderly and nonelderly chimpanzees and between elderly chimpanzees housed in geriatric and nongeriatric groups. A correlation was then used to examine the relationship between the number of wounds during the 2-y data collection period and mobility scores. All α levels were set to a P value of 0.05 or less.16,33 We also report trending differences, for which the α level was a P value of 0.05 to 0.09. All analyses were conducted by using SPSS 24 (IBM, Armonk, NY).
Results
Behavioral differences between elderly and nonelderly chimpanzees.
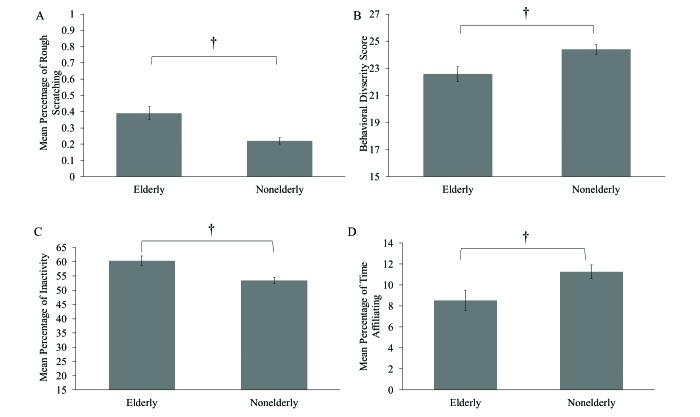
Independent samples t tests were used to examine overall differences in behavior between elderly and nonelderly chimpanzees. Elderly chimpanzees exhibited significantly (P = 0.003) more rough scratching (mean ± SE, 0.39% ± 0.04%) compared with nonelderly chimpanzees (0.22% ± 0.02%; t49.66 = –3.54; Figure 2 A). In addition, elderly chimpanzees had lower (P = 0.008) behavioral diversity scores (22.57 ± 0.54) than nonelderly chimpanzees (24.39 ± 0.37; t116 = 2.72; Figure 2 B) and greater (P = 0.001) inactivity (60.37% ± 1.65%) than nonelderly chimpanzees (53.42% ± 1.10%; t116 = –3.46; Figure 2 C). Lastly, elderly chimpanzees exhibited less (P = 0.018) affiliative behavior (8.53% ± 0.97%) compared with nonelderly chimpanzees (11.27% ± 0.64%; t116 = 2.35; Figure 2 D).
Figure 2.
Behavioral differences between elderly chimpanzees (35 y and older) and nonelderly chimpanzees (younger than 35 y) in (A) rough scratching, (B) behavioral diversity scores, (C) inactivity, and (D) affiliation. Error bars, SEM; †, P ≤ 0.01.
Behavioral differences among age categories.
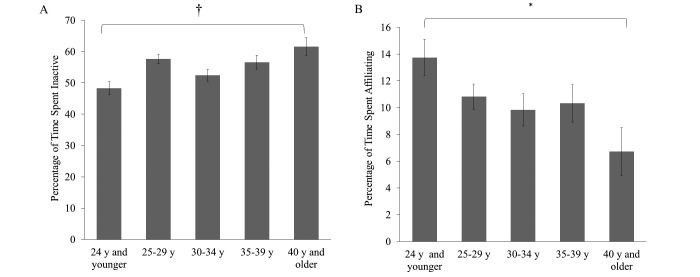
Multivariate analysis of covariance among the 5 age categories revealed significant differences only in inactive behavior (F4,109 = 5.22, P = 0.001; Figure 3 A) and affiliative behavior (F4,109 = 2.43, P = 0.047; Figure 3 B). Specifically, chimpanzees that were 40 y or older exhibited significantly higher levels of inactivity (61.60% ± 2.83%) than chimpanzees that were 24 y or younger (48.30% ± 2.12%, P = 0.005), and chimpanzees that were 35 to 39 y of age exhibited a trend toward greater (P = 0.09) inactivity (56.58% ± 2.20%) compared with chimpanzees that were 24 y or younger. In addition, inactivity levels were higher (P = 0.004) in chimpanzees that were 25 to 29 y of age (57.62% ± 1.48%) compared with chimpanzees that were 24 y or younger. Regarding affiliative behavior, chimpanzees that were 40 y or older exhibited significantly (P = 0.034) lower affiliative behavior (6.71% ± 1.03%) than chimpanzees that were 24 y old or younger (13.74% ± 1.35%). There were no other significant behavioral differences across age categories, and all other posthoc comparisons were nonsignificant (P > 0.05).
Figure 3.
Behavioral differences among chimpanzee age categories. *, P ≤ 0.05; †, P ≤ 0.01.
Characteristics of geriatric groups at our facility.
Among elderly chimpanzees, those housed in geriatric groups were significantly (P = 0.05) older (n = 20; mean ± SEM, 43.40 ± 1.66 y) than geriatric chimpanzees housed in nongeriatric groups (n = 15; 39.26 ± 1.24 y; t32.44 = –2.0). Average group age was significantly (P = 0.0001) older in geriatric groups (40.75 ± 1.21 y) compared with nongeriatric groups (31.14 ± 0.64 y; t28.21 = –7.00). Elderly chimpanzees in geriatric groups had significantly (P = 0.016) higher mobility scores (7.43 ± 0.51), indicative of increased impairment in mobility, compared with those in nongeriatric groups (6.07 ± 0.067; t19.64 = –2.64,). In addition, geriatric groups had 8 animals on mobility watch compared with 3 animals in nongeriatric groups. Geriatric groups were significantly (P = 0.0001) smaller (5.23 ± 0.27 chimpanzees per group) than nongeriatric groups (7.37 ± 0.37 chimpanzees per group; t33 = 4.82). Within-group age range was similar across geriatric (16.75 ± 2.42 y) and nongeriatric (18.40 ± 1.98 y; t33 = 0.50; P = 0.62) groups. Lastly, male chimpanzees comprised a significantly (P = 0.0001) smaller proportion of geriatric groups (20% ± 3%) compared with nongeriatric groups (48% ± 4%; t33 = 5.66).
Elderly chimpanzees: behavioral effects of housing in geriatric groups.
Multivariate analysis of covariance, with mobility impairment scores as a covariate, was used to examine behavioral differences between elderly chimpanzees housed in geriatric groups and those housed in nongeriatric groups. Mobility impairment scores had a significant effect on behavioral diversity scores (P = 0.038) and inactivity (P = 0.012). To further explore these effects, correlations showed that mobility impairment scores were significantly positively correlated with inactivity scores (r35 = 0.49, P = 0.003) and significantly negatively correlated with behavioral diversity (r35 = –0.40, P = 0.02).
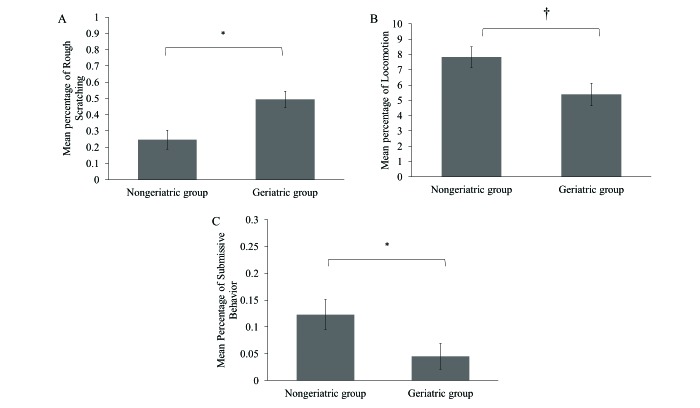
Elderly chimpanzees housed in geriatric groups exhibited significantly (P = 0.004) more rough scratching (0.50% ± 0.05%) than those housed in nongeriatric groups (0.24% ± 0.06%; F1,32 = 9.59; Figure 4 A). In addition, chimpanzees housed in geriatric groups showed lower (P = 0.027) percentages of locomotion (5.38% ± 0.66%) compared with chimpanzees in nongeriatric groups (7.82% ± 0.77%; F1,32 = 5.38; Figure 4 B). Lastly, chimpanzees in geriatric groups showed lower (P = 0.046) levels of submissive behavior (0.05% ± 0.02%) compared with chimpanzees in nongeriatric groups (0.12% ± 0.03%; F1,32 = 4.33; Figure 4 C).
Figure 4.
Behavioral differences among elderly chimpanzees as a function of housing in geriatric and nongeriatric groups in (A) rough scratching, (B) locomotion, and (C) submissive behavior. *, P ≤ 0.05; †, P ≤ 0.01.
Elderly chimpanzees: no. of wounds as a function of geriatric status, mobility impairment, and housing in geriatric groups.
Wounding did not differ regardless of whether chimpanzees were elderly (average no. of wounds per animal, 3.57 ± 0.56) or not (4.52 ± 0.37; P = 0.16). There were no significant differences in wounding between elderly chimpanzees housed in geriatric groups (3.05 ± 0.79) and those housed in nongeriatric groups (4.27 ± 0.71; P = 0.30). Lastly, the number of wounds was not significantly correlated with elderly chimpanzees’ mobility impairment scores (r = –0.28, P = 0.10).
Discussion
The current study aimed to 1) examine behavioral differences between elderly and non elderly chimpanzees, 2) identify a behavioral age category definition for elderly chimpanzees, 3) characterize the composition of geriatric and nongeriatric groups in our program, and 4) assess differences in the behavior of elderly chimpanzees as a function of housing in geriatric and nongeriatric groups. Results showed that—compared with nonelderly chimpanzees—elderly chimpanzees show a number of important, welfare-related differences in behavior, including greater rough scratching and inactivity and lower behavioral diversity and affiliation. In addition, chimpanzees that were 40 y or older exhibited higher levels of inactivity and lower levels of affiliative behavior compared with chimpanzees younger than 24 y. Compared with nonelderly groups, geriatric groups were characterized by higher average group age, higher mobility impairment scores, smaller group size, and a lower percentage of male chimpanzees. Compared with aged animals in nongeriatric groups, elderly chimpanzees housed in geriatric groups exhibited behavioral differences, including lower levels of locomotion, higher levels of rough scratching, and lower levels of submissive behavior. However, although the differences in rough scratching and submissive behavior were statistically significant, they may not be biologically meaningful, as discussed later. Lastly, wounding frequency was unaffected by elderly status, mobility impairment, and housing in geriatric groups.
These findings are consistent with previous reports of decreased aggressive and manipulative behavior and increased inactivity and solitary behavior in older chimpanzees.4,15,22,35 Aged chimpanzees, like their human counterparts, are likely to experience mobility issues and other age-related conditions (that is, hypertension, heart disease, arthritis, diabetes).26,32 As a result, these chimpanzees may begin to display decreasing activity and species-typical behaviors, including affiliative behaviors, thereby perhaps contributing to an overall decreased diversity in behavior, as was seen in the current study. These results have implications on the assessment of welfare. Given that some changes in behavior related to advanced age are likely unavoidable, behavioral managers should account for elderly status when assessing welfare. For example, using measures of levels of behavior that are indicative of positive welfare in younger animals may result in misleading conclusions about diminished welfare when applied to elderly animals. Therefore, it is of utmost importance that the welfare of each chimpanzee be considered in the context of individual variation and normal, age-related changes. Indeed, the behavioral management program at our facility uses a Quality of Life program, whereby the welfare of individual chimpanzees is assessed by using personalized behavioral guidelines to determine any potential decreases in welfare relative to each animal's own baseline level of behavior.23 Systems such as these likely will be valuable when implemented at facilities that house or will house captive elderly chimpanzees.
Data from the current study revealed 2 behavioral differences as a function of the age category: chimpanzees that were 40 y or older had significantly higher inactivity levels and lower affiliative behavior compared with younger chimpanzees. Previous studies have defined the age at which chimpanzees become elderly as 30 or 35 y or older.4,32,35,38 However, we found that chimpanzees that were 30 to 34 y or 35 to 39 y exhibited levels of behavior that were similar to that of chimpanzees in younger age categories. Perhaps aged chimpanzees in the current population do not begin to behave as “elderly” until later in life, likely because of refinements in chimpanzee medical care and behavioral management over the past decades. Indeed, our data suggest that 40 y is the age at which chimpanzees begin to exhibit behavioral signs of being elderly. However, more empirical data are needed to further examine the concept of a behavioral age definition for elderly.
Geriatric and nongeriatric group composition differed in several regards, and elderly chimpanzees exhibited behavioral differences as a function of housing in these groups. Geriatric groups tended to contain fewer chimpanzees and be composed of older animals (mostly females) with more prominent mobility issues. This situation is unsurprising: as the population continues to age, chimpanzees are likely to experience mobility impairment, female chimpanzees are more likely to live longer than male animals, and groups are likely to become smaller as a result of morbidity and mortality.12,26,32 Regarding behavior, elderly chimpanzees housed in nongeriatric groups showed higher levels of locomotion and submissive behavior and lower levels of rough scratching compared with geriatric chimpanzees housed in geriatric groups. These behavioral differences may reflect the smaller percentage of males, the group size, increased mobility impairment, or a combination of these characteristics. These data suggest that housing elderly chimpanzees in nongeriatric groups may have various behavioral advantages, including increased locomotion and decreased anxiety (rough scratching). However, these advantages do not imply that placing elderly animals into newly formed or unfamiliar nongeriatric groups will enhance their welfare. Indeed, the costs of forming new nongeriatric groups likely outweigh the benefits of the familiarity and social stability of existing geriatric groups. Although the levels of submissive behavior and rough scratching differed significantly between geriatric and nongeriatric groups, these differences were small in magnitude (0.07% and 0.2%, respectively) and may not represent biologically meaningful differences in behavior and welfare. Furthermore, the levels of rough scratching, submissive behavior, and locomotion were within ranges reported by other studies of captive chimpanzees, and wounding did not differ as a function of housing in our geriatric and nongeriatric groups.7,11,36 As such, data from the current study suggest comparable welfare between elderly chimpanzees housed in geriatric and nongeriatric groups. The welfare implications of housing aged chimpanzees in geriatric groups will depend on individual chimpanzee needs, and decisions about the compatibility of elderly chimpanzees in certain social groups must be made on a case-by-case basis.6,34
The current study is the first to investigate behavioral differences between chimpanzees housed in geriatric and nongeriatric groups. Unfortunately, as we mentioned in the data analysis section, we were unable to tease apart the effects of group size, average age of the group, and percentage of males within the group on geriatric chimpanzee behavior. As a result, our analyses focused on the behavioral effects of housing in geriatric groups as a whole. Future research should attempt to examine these variables independently. Nevertheless, our current data have practical implications for behavioral management programs across captive settings. The ability of behavioral managers to create compositionally diverse groups will become progressively limited as the population continues to age and all chimpanzees eventually become elderly. In addition, easier access to animals for observation and treatment of age-related ailments will become an increasingly important aspect of captive care as the population continues to age.6,10,19,26,27,34 Future investigations should focus on the implementation and assessment of preventative measures aimed at maintaining appropriate levels of welfare-related behaviors, such as locomotion and species-typical behaviors, within geriatric groups. For example, structural enhancements to the physical environment and increased voluntary participation and choice within the environment have been used to increase locomotion, activity, and behavioral diversity of aged chimpanzees.9,10,17,26,30
Supplemental Materials
Ethogram of behaviors.
Acknowledgments
We thank Dr Michele Mulholland, Lisa Reamer, Mary Catherine Mareno, and Dr Gill Vale for helpful comments during preparation of this paper. We also thank the care staff at the National Center for Chimpanzee Care for their unwavering care of the chimpanzees. This work was supported by NIH grant U42-OD 011197 and the University of Copenhagen.
References
- 1.Alford PL, Bloomsmith MA, Keeling ME, Beck TF. 1995. Wounding aggression during the formation and maintenance of captive, multimale chimpanzee groups. Zoo Biol 14:347–359. 10.1002/zoo.1430140406. [DOI] [Google Scholar]
- 2.Altmann J. 1974. Observational study of behavior: sampling methods. Behaviour 49:227–267. 10.1163/156853974X00534. [DOI] [PubMed] [Google Scholar]
- 3.Animal Welfare Act as Amended. 2013. 7 USC §2131–2159.
- 4.Association of Zoos and Aquariums Ape Taxon Advisory Group. 2010. Chimpanzee (Pan troglodytes) care manual. Silver Spring (MD): Association of Zoos and Aquariums. [Google Scholar]
- 5.Autrey MM, Reamer LA, Mareno MC, Sherwood CC, Herndon JG, Preuss T, Schapiro SJ, Hopkins WD. 2014. Age-related effects in the neocortical organization of chimpanzees: Gray and white matter volume, cortical thickness, and gyrification. Neuroimage 101:59–67. 10.1016/j.neuroimage.2014.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker KC. 2000. Advanced age influences chimpanzee behavior in small social groups. Zoo Biol 19:111–119. . [DOI] [Google Scholar]
- 7.Baker KC. 2000. Benefits of positive human interaction for socially-housed chimpanzees. Anim Welf 13:239–245. [PMC free article] [PubMed] [Google Scholar]
- 8.Baker KC, Seres M, Aureli F, de Waal FB. 2000. Injury risks among chimpanzees in three housing conditions. Am J Primatol 51:161–175. . [DOI] [PubMed] [Google Scholar]
- 9.Bridges JB, Mocarski EC, Reamer LA, Lambeth SP, Schapiro SJ. 2013. Weight management in captive chimpanzees (Pan troglodytes) using a modified feeding device. Am J Primatol 75:51–51. [Google Scholar]
- 10.Bridges JP, Haller RL, Buchl SJ, Magden ER, Lambeth SP, Schapiro SJ. 2015. Establishing a behavioral management program for geriatric chimpanzees. Am J Primatol 77:111–111. [Google Scholar]
- 11.Duncan LM, Jones MA, van Lierop M, Pillay N. 2013. Chimpanzees use multiple strategies to limit aggression and stress during spatial density changes. Appl Anim Behav Sci 147:159–171. 10.1016/j.applanim.2013.06.001. [DOI] [Google Scholar]
- 12.Dyke B, Gage TB, Alford PL, Swenson B, Williams-Blangero S. 1995. Model life table for captive chimpanzees. Am J Primatol 37:25–37. 10.1002/ajp.1350370104. [DOI] [PubMed] [Google Scholar]
- 13.Edler MK, Sherwood CC, Meindl RS, Hopkins WD, Ely JJ, Erwin JM, Mufson EJ, Hof PR, Raghanti MA. 2017. Aged chimpanzees exhibit pathologic hallmarks of Alzheimer's disease. Neurobiol Aging 59:107–120. 10.1016/j.neurobiolaging.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ely JJ, Zavaskis T, Lammey ML. 2012. Hypertension increases with aging and obesity in chimpanzees (Pan troglodytes): Hypertension in chimpanzees. Zoo Biol 32:79–87. 10.1002/zoo.21044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodall J. 1986. The chimpanzees of Gombe: patterns of behavior. Cambridge (MA): Belknap Press of Harvard University Press. [Google Scholar]
- 16.Hair JF, Jr, Black WC, Babin BJ, Anderson RE, Tatham RL. 2010. Multivariate data analysis: A global perspective. Upper Saddle River (NJ): Pearson Education. [Google Scholar]
- 17.Haller RL, Magden ER, Lambeth SP, Schapiro SJ. 2012. Using positive reinforcement techniques to train for acupuncture treatment in chimpanzees (Pan troglodytes): An innovative approach in managing an aging colony. Am J Primatol 74:43–43. [Google Scholar]
- 18.Herndon JG, Moss MB, Rosene DL, Killiany RJ. 1997. Patterns of cognitive decline in aged rhesus monkeys. Behav Brain Res 87:25–34. 10.1016/S0166-4328(96)02256-5. [DOI] [PubMed] [Google Scholar]
- 19.Hopkins WD, Latzman RD. 2017. Future research with captive chimpanzees in the United States: Integrating scientific programs with behavioral management. p 139–155. In: Schapiro SJ, editor. Handbook of Primate Behavioral Management. Boca Raton (FL): CRC Press. [Google Scholar]
- 20.Humle T. 2003. Behavior and ecology of chimpanzees in West Africa, p 13–19. In: Kormos R, Boesch C, Bakarr M, Butynski TM, editors. West African Chimpanzees. Gland (Switzerland) and Cambridge (United Kingdom): International Union for Conservation of Nature and Natural Resources. [Google Scholar]
- 21.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 22.Kawanaka K. 1993. Age differences in spatial positioning of males in a chimpanzee unit-group at the Mahale mountains National Park, Tanzania. Primates 34:255–270. 10.1007/BF02382620. [DOI] [Google Scholar]
- 23.Lambeth S, Schapiro S, Bernacky B, Wilkerson G. 2013. Establishing “quality of life” parameters using behavioural guidelines for humane euthanasia of captive non-human primates. Anim Welf 22:429–435. 10.7120/09627286.22.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowenstine LJ, McManamon R, Terio KA. 2015. Comparative pathology of aging great apes: bonobos, chimpanzees, gorillas, and orangutans. Vet Pathol 53:250–276. 10.1177/0300985815612154. [DOI] [PubMed] [Google Scholar]
- 25.Magden ER. 2017. Positive reinforcement training and health care, p 201–215. In: Schapiro SJ, editor. Handbook of primate behavioral management. Boca Raton (FL): CRC Press. [Google Scholar]
- 26.Magden ER, Haller RL, Thiele EJ, Buchl SJ, Lambeth SP, Schapiro SJ. 2013. Acupuncture as an adjunct therapy for osteoarthritis in chimpanzees (Pan troglodytes). J Am Assoc Lab Anim Sci 52:475–480. [PMC free article] [PubMed] [Google Scholar]
- 27.Magden ER, Sleeper MM, Buchl SJ, Jones RA, Thiele EJ, Wilkerson GK. 2016. Use of an implantable loop recorder in a chimpanzee (Pan troglodytes) to monitor cardiac arrhythmias and assess the effects of acupuncture and laser therapy. Comp Med 66:52–58. [PMC free article] [PubMed] [Google Scholar]
- 28.Muller MN, Thompson ME, Wrangham RW. 2006. Male chimpanzees prefer mating with old females. Curr Biol 16:2234–2238. 10.1016/j.cub.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 29.Neal Webb SJ, Hau J, Schapiro SJ. 2018a. Captive chimpanzee (Pan troglodytes) behavior as a function of space per animal and enclosure type. Am J Primatol 80:1–20. 10.1002/ajp.22749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neal Webb SJ, Hau J, Schapiro SJ. 2018b. Refinements to captive chimpanzee (Pan troglodytes) care: A self-medication paradigm. Anim Welf 27:327–341. 10.7120/09627286.27.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neal Webb SJ, Hau J, Schapiro SJ. 2019. Does group size matter? Captive chimpanzee (Pan troglodytes) behavior as a function of group size and composition. Am J Primatol 81:e22947 10.1002/ajp.22947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nunamaker EA, Lee DR, Lammey ML. 2012. Chronic diseases in captive geriatric female chimpanzees (Pan troglodytes). Comp Med 62:131–136. [PMC free article] [PubMed] [Google Scholar]
- 33.Perneger TV. 1998. What's wrong with Bonferroni adjustments. BMJ 316:1236–1238. 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reamer L, Haller R, Lambeth SP, Schapiro SJ. 2017. Behavioral management of Pan spp, p 395–407. In: Schapiro SJ, editor. Handbook of primate behavioral management. Boca Raton (FL): CRC Press. [Google Scholar]
- 35.Riopelle AJ, Rogers CM. 1965. Age changes in chimpanzees, p 449–462. In: Schrier AM, Harlow HF, Stollnitz F, editors. Behavior of nonhuman primates: Modern research trends. New York (NY): Academic Press. [Google Scholar]
- 36.Ross SR, Wagner KE, Schapiro SJ, Hau J. 2010. Ape behavior in two alternating environments: comparing exhibit and short-term holding areas. Am J Primatol 72:951–959. 10.1002/ajp.20857. [DOI] [PubMed] [Google Scholar]
- 37.Schapiro SJ, Reamer LA, Mareno MC, Lambeth SP. 2014. Providing chimpanzees with opportunities to voluntarily participate in their own care: Choice of medications. Am J Primatol 76:73–73. [Google Scholar]
- 38.Tarou LR, Bloomsmith MA, Hoff MP, Erwin JM, Maple TL. 2002. The behavior of aged great apes, p 209–231. In: Erwin JM, Hoff PR, editors. Aging in nonhuman primates. New York (NY): Karger; 10.1159/000061467 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ethogram of behaviors.