Abstract
The importance of frailty in heart failure (HF) patients has been increasingly recognized because of its high prevalence and its significantly adverse impact on prognosis and quality of life. Due to the impact of frailty on both prognosis and treatment of HF patients, all patients with HF, regardless of their chronological age, should be evaluated for the presence of, or the risk for developing frailty. However, although several instruments are available, there is still no consensus as to which is the best method to assess frailty in patients with HF. Therefore, a validated and easy to apply instrument to assess frailty in HF patients in daily practice is warranted.
Keywords: Heart failure, Frailty, Aging, Prognosis, Multidimensional
Introduction
Heart failure (HF) is one of the most important and rapidly growing diseases due to its high prevalence worldwide and the significant impact on morbidity and mortality.1–4 The phenotype of patients with HF is often complex because of their improved survival from other chronic conditions and the progressive aging of developed societies,5 leading to an increased prevalence in particular if heart failure with preserved ejection fraction (HFpEF) in the elderly.6,7 The typical patient with HF presents with an involvement and impairment of multiple organs and body systems that require complex and intricate poly-therapeutic schemes.8 Common clinical impairments and comorbidities in HF patients include kidney injury, diabetes, arrhythmias, anaemia, depression, while common non-clinical comorbidities are isolation, living alone, dependency, institutionalization, sub-optimal self-care (Table 1).9–11
Table 1.
Main clinical and non-clinical comorbidities in patients with heart failure
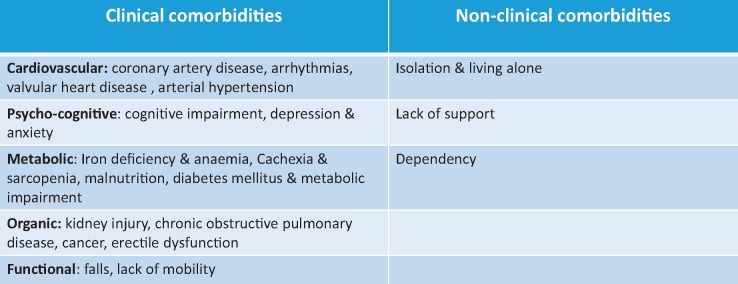
|
Comorbidities present with different patterns in patients with HF and can cause an accumulation of defects that makes patients more vulnerable to stressors with consequential negative outcomes and prognosis. This increased patient’s vulnerability is commonly described with the term frailty, that origins from the French ‘frêle’ and means little resistance.12,13
According to a recent HFA position paper on frailty in HF patients, frailty is defined as a multidimensional dynamic state, independent of age, that makes the individual with HF more vulnerable to the effect of stressors.14 When exposed to clinical and non-clinical stressors, acute or chronic, HF patients are at higher risk for the occurrence of decompensation and negative outcomes, such as adverse clinical events, prolonged recovery times, functional decline, disability, and mortality compared to those patients without frailty. Therefore, all patients with HF, due to their state of vulnerability, are at higher risk to be frail. Several studies have shown that frailty is common in HF patients with an estimated prevalence of around 45%.1,15,16 The overlap between frailty and HF is complex: patients with HF were up to six times more likely to be frail and frail people had a significantly increased risk of developing new-onset HF. Although both frailty and HF are more common in older than younger adults, their occurrence is independent of age, and frailty should be not considered as an inevitable part of aging or as a state exclusive of the elderly.17 All patients with HF, irrespective of their chronological age, should be evaluated for the presence or the risk of frailty.
Although the precise pathogenesis of frailty has yet to be elucidated, the concomitant presence of frailty and HF worsen each another via multiple complex pathogenic mechanisms, such as disorders and dysregulation in neuro-hormonal, muscular,18–20 immune, metabolic,21 and endocrine systems and up-regulation of inflammatory cytokines.22–25 This leads to an imbalance between the anabolic and catabolic state in HF patients that may exacerbate the decline in muscle mass and strength,26,27 thus favouring the occurrence of reduced lean muscle mass (sarcopaenia) and cachexia (defined as a generalized wasting process affecting all body compartments).28–31 Both sarcopaenia and cachexia are common in patients with HF, particularly in those at an advanced stage of the disease. The higher risk of disability and dependency in performing simple daily activities (activities of daily living and instrumental activities of daily living; Table 2) impairs quality of life of HF patients and it is associated with a higher occurrence of depression and isolation, in particular, in those HF patients that do not have a familiar and social support.32–36
Table 2.
Activities of Daily Living (ADLs) and Instrumental Activities of Daily Living (IADLs)
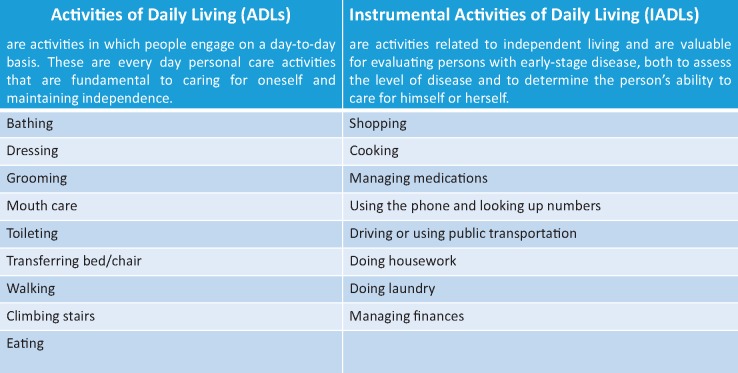
|
Problems in self-care and difficulties in leaving home may reduce also patient’s access to healthcare, which also contributes to insufficient treatment surveillance, delayed responses, and untimely treatment modifications, thus increasing the risk of negative outcomes.37
Frailty has an important prognostic role in patients with HF, as can exacerbate the progression of HF as well as the occurrence of negative outcomes such as mortality, lower probability of surviving more than 10 years, and increased health care use (higher risk of hospitalization, prolonged recovery, institutionalization, etc.).38–43
Therefore, the identification of frailty is of utmost importance in patients with HF. Recognizing those HF patients who are not only frail but also at risk of frailty (‘pre-frail’), may allow an early and immediate multidisciplinary therapeutic intervention with the aim to improve their prognosis, outcomes, and management. To this aim, possible treatments for frailty in HF are, besides those indicated as GDMT by the scientific guidelines,44 exercise (resistance and aerobic), caloric and protein support, vitamin D supplementation, and a reduction in polypharmacy. The therapeutic approach should also take into consideration the non-clinical components of frailty and should include occupational therapy, psychological and social support as well as education of patients and their families.
The disease-centred approach (organ system-based model) is no longer acceptable in the management of frail multi-morbid HF patients and has to be abandoned in favour of a holistic and multidimensional approach. This approach will allow us to recognize all the impairments, clinical and non-clinical, present in HF patients and to identify those patients with or at risk of frailty. Only using an holistic approach, is it possible to identify those impairments that have to be addressed with priority thus building a personalized and tailored healthcare program.
However, although several instruments are available to assess frailty, there is still no agreement on which is the best method to assess frailty in patients with HF. In brief, two main models are used to identify frailty: the physical frail phenotype45 and the cumulative deficit model.46,47
The Physical Frailty Phenotype is defined by the presence of three or more of the following physical components: unintentional weight loss; self-reported exhaustion; weakness (reduced hand grip strength); slow walking speed, low self-reported physical activity. A pre-frail status is described when one or two criteria are present and this identifies an individual at high risk of progressing to frailty. The cumulative deficit model, describes frailty as an accumulation of individual impairments and conditions, such as cognition, activities of daily living, comorbid diseases, deficits of social relations, and social support present or abnormal laboratory results, thus creating a Frailty Index. The greater the number of deficits the higher the degree of frailty.
Both the Fried phenotype and the cumulative index definitions have been widely used and have demonstrated their predictive value; however, their applicability in HF patients is limited by major weaknesses. Although, the Fried phenotype is easier to apply compared to the cumulative model, it is burdened by the fact that it does not take into consideration all the main domains: clinical, physical–functional, cognitive-psychological, and social, which combine to be responsible for frailty, as it is focused mainly on the physical component of frailty.
In addition, the overlap between the presence of symptoms shared between HF and frailty, such as the limited physical activity in performing basic activities of daily living, weakness, fatigue and shortness of breath, or the difficulty in correctly identifying weight loss in HF patients taking diuretics can be responsible for frequently missing the diagnosis of frailty in HF patients.
Conversely, the cumulative model is more reliable to evaluate all the clinical and non-clinical aspects of frailty, but its assessment is time consuming. Therefore, the routine use of the Frailty index in the busy cardiac clinic in which HF patients are followed is difficult.
These major limitations as well as and the availability of a plethora of assessment instruments have limited the routine assessment of frailty in daily practice. This has facilitated the use of the clinical subjective judgments (eyeball test or foot-of-the-bed assessment) to define frailty in HF patients.48
However, due to the prognostic and therapeutic implications of frailty in HF patients, the use of a subjective assessment in routine daily practice is no longer acceptable. To this end, the aim to find validated and prognostic instruments to evaluate frailty in an objective, easy and reliable way is essential.
Conclusion
The presence of a complex overlap between frailty and HF, the emerging and increasing data on the prognostic role of frailty, as well as the interference of frailty with the possible treatments for HF patients are only some of the reasons why frailty should be routinely identified in HF patients. An accurate assessment of frailty in HF patients is the first and mandatory step to build a tailored and individualized healthcare program in order to reduce dependency, increase quality of life, and improve prognosis.
Funding
This paper is part of a supplement funded by the Heart Failure Association of the European Society of Cardiology.
Conflict of interest: none declared.
References
- 1. Savarese G, Lund LH.. Global public health burden of heart failure. Card Fail Rev 2017;03:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 3. Crespo-Leiro MG, Metra M, Lund LH, Milicic D, Costanzo MR, Filippatos G, Gustafsson F, Tsui S, Barge-Caballero E, De Jonge N, Frigerio M, Hamdan R, Hasin T, Hülsmann M, Nalbantgil S, Potena L, Bauersachs J, Gkouziouta A, Ruhparwar A, Ristic AD, Straburzynska-Migaj E, McDonagh T, Seferovic P, Ruschitzka F.. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018;20:1505–1535. [DOI] [PubMed] [Google Scholar]
- 4. Spoletini I, Lainscak M.. Epidemiology and prognosis of heart failure. Int Cardiovasc Forum J 2017;10:8–11. [Google Scholar]
- 5. Martone AM, Bianchi L, Abete P, Bellelli G, Bo M, Cherubini A, Corica F, Di Bari M, Maggio M, Manca GM, Marzetti E, Rizzo MR, Rossi A, Volpato S, Landi F.. The incidence of sarcopenia among hospitalized older patients: results from the Glisten study. J Cachexia Sarcopenia Muscle 2017;8:907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hill E, Taylor J.. Chronic heart failure care planning: considerations in older patients. Card Fail Rev 2017;03:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jankowska EA, Coats AJS, Anker SD.. Approach to the treatment of heart failure with preserved ejection fraction and mid-range ejection fraction. Int Cardiovasc Forum J 2017;10:34–36. [Google Scholar]
- 8. Metra M. September 2018 at a glance: co-morbidities, heart failure with preserved ejection fraction and mineralocorticoid receptor antagonists. Eur J Heart Fail 2018;20:1245–1246. [DOI] [PubMed] [Google Scholar]
- 9. Pan A. The real-world evidence of heart failure co-morbidities. Eur J Heart Fail 2017;19:434.. [DOI] [PubMed] [Google Scholar]
- 10. Iorio A, Senni M, Barbati G, Greene SJ, Poli S, Zambon E, Di Nora C, Cioffi G, Tarantini L, Gavazzi A, Sinagra G, Di Lenarda A.. Prevalence and prognostic impact of non-cardiac co-morbidities in heart failure outpatients with preserved and reduced ejection fraction: a community-based study. Eur J Heart Fail 2018;20:1257–1266. [DOI] [PubMed] [Google Scholar]
- 11. Von Haehling S. Co-morbidities in heart failure beginning to sprout-and no end in sight? Eur J Heart Fail 2017;19:1566–1568. [DOI] [PubMed] [Google Scholar]
- 12. Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, Wolfson C.. Frailty: an emerging research and clinical paradigm issues and controversies. J Gerontol A Biol Sci Med Sci 2007;62:731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shamliyan T, Talley KMC, Ramakrishnan R, Kane RL.. Association of frailty with survival: a systematic literature review. Ageing Res Rev 2013;12:719–736. [DOI] [PubMed] [Google Scholar]
- 14. Vitale C, Jankowska E, Hill L, Piepoli M, Doehner W, Anker SD, Lainscak M, Jaarsma T, Ponikowski P, Rosano GMC, Seferovic P, Coats AJ.. Heart Failure Association/European Society of Cardiology position paper on frailty in patients with heart failure. Eur J Heart Fail 2019;21:1299–1305. [DOI] [PubMed] [Google Scholar]
- 15. Dent E, Kowal P, Hoogendijk EO.. Frailty measurement in research and clinical practice: a review. Eur J Intern Med 2016;31:3–10. [DOI] [PubMed] [Google Scholar]
- 16. McDonagh J, Martin L, Ferguson C, Jha SR, Macdonald PS, Davidson PM, Newton PJ.. Frailty assessment instruments in heart failure: a systematic review. Eur J Cardiovasc Nurs 2018;17:23–35. [DOI] [PubMed] [Google Scholar]
- 17. Khan H, Kalogeropoulos AP, Georgiopoulou VV, Newman AB, Harris TB, Rodondi N, Bauer DC, Kritchevsky SB, Butler J.. Frailty and risk for heart failure in older adults: the health, aging, and body composition study. Am Heart J 2013;166:887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tieland M, Trouwborst I, Clark BC.. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle 2018;9:3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brzeszczyńska J, Meyer A, McGregor R, Schilb A, Degen S, Tadini V, Johns N, Langen R, Schols A, Glass DJ, Roubenoff R, Ross JA, Fearon KCH, Greig CA, Jacobi C.. Alterations in the in vitro and in vivo regulation of muscle regeneration in healthy ageing and the influence of sarcopenia. J Cachexia Sarcopenia Muscle 2018;9:93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nijholt W, Scafoglieri A, Jager-Wittenaar H, Hobbelen JSM, van der Schans CP.. The reliability and validity of ultrasound to quantify muscles in older adults: a systematic review. J Cachexia Sarcopenia Muscle 2017;8:702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tkaczyszyn M, Drozd M, Węgrzynowska-Teodorczyk K, Flinta I, Kobak K, Banasiak W, Ponikowski P, Jankowska EA.. Depleted iron stores are associated with inspiratory muscle weakness independently of skeletal muscle mass in men with systolic chronic heart failure. J Cachexia Sarcopenia Muscle 2018;9:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anker SD, Ponikowski PP, Clark AL, Leyva F, Rauchhaus M, Kemp M, Teixeira MM, Hellewell PG, Hooper J, Poole-Wilson PA, Coats AJ.. Cytokines and neurohormones relating to body composition alterations in the wasting syndrome of chronic heart failure. Eur Heart J 1999;20:683–693. [DOI] [PubMed] [Google Scholar]
- 23. Joseph SM, Rich MW.. Targeting frailty in heart failure. Curr Treat Options Cardiovasc Med 2017;19:31. [DOI] [PubMed] [Google Scholar]
- 24. Junius-Walker U, Onder G, Soleymani D, Wiese B, Albaina O, Bernabei R, Marzetti E; ADVANTAGE JA WP4 group. The essence of frailty: a systematic review and qualitative synthesis on frailty concepts and definitions. Eur J Intern Med 2018;56:3–10. [DOI] [PubMed] [Google Scholar]
- 25. Bellumkonda L, Tyrrell D, Hummel SL, Goldstein DR.. Pathophysiology of heart failure and frailty: a common inflammatory origin? Aging Cell 2017;16:444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peng LN, Lee WJ, Liu LK, Lin MH, Chen LK.. Healthy community-living older men differ from women in associations between myostatin levels and skeletal muscle mass. J Cachexia Sarcopenia Muscle 2018;9:635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gonzalez-Freire M, Adelnia F, Moaddel R, Ferrucci L.. Searching for a mitochondrial root to the decline in muscle function with ageing. J Cachexia Sarcopenia Muscle 2018;9:435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Von Haehling S, Anker SD.. Prevalence, incidence and clinical impact of cachexia: facts and numbers—update 2014. J Cachexia Sarcopenia Muscle 2014;5:261–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vitale C, Spoletini I, Rosano GM.. Frailty in heart failure: implications for management. Card Fail Rev 2018;4:104–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsutsumimoto K, Doi T, Makizako H, Hotta R, Nakakubo S, Makino K, Suzuki T, Shimada H.. Aging-related anorexia and its association with disability and frailty. J Cachexia Sarcopenia Muscle 2018;9:834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Distefano G, Standley RA, Zhang X, Carnero EA, Yi F, Cornnell HH, Coen PM.. Physical activity unveils the relationship between mitochondrial energetics, muscle quality, and physical function in older adults. J Cachexia Sarcopenia Muscle 2018;9:279–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dunlay SM, Manemann SM, Chamberlain AM, Cheville AL, Jiang R, Weston SA, Roger VL.. Activities of daily living and outcomes in heart failure. Circ Heart Fail 2015;8:261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gobbens RJ, van Assen MA.. The prediction of ADL and IADL disability using six physical indicators of frailty: a longitudinal study in the Netherlands. Curr Gerontol Geriatr Res 2014;2014:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Uchmanowicz I, Młynarska A, Lisiak M, Kałuz·na-Oleksy M, Wleklik M, Chudiak A, Dudek M, Migaj J, Hinterbuchner L, Gobbens R.. Heart failure and problems with frailty syndrome: why it is time to care about frailty syndrome in heart failure. Card Fail Rev 2019;5:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Uchmanowicz I, Gobbens RJ.. The relationship between frailty, anxiety and depression, and health-related quality of life in elderly patients with heart failure. Clin Interv Aging 2015;10:1595–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Uchmanowicz I, Chudiak A, Jankowska-Polańska B, Gobbens R.. Hypertension and frailty syndrome in old age: current perspectives. Card Fail Rev 2017;3:102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Flint K. Frailty in TOPCAT: a deep dive into the deficit index approach for defining frailty. Eur J Heart Fail 2018;20:1578–1579. [DOI] [PubMed] [Google Scholar]
- 38. Sanders NA, Supiano MA, Lewis EF, Liu J, Claggett B, Pfeffer MA, Desai AS, Sweitzer NK, Solomon SD, Fang JC.. The frailty syndrome and outcomes in the TOPCAT trial. Eur J Heart Fail 2018;20:1570–1577. [DOI] [PubMed] [Google Scholar]
- 39. Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo-Leiro MG, Harjola VP, Parissis J, Laroche C, Piepoli MF, Fonseca C, Mebazaa A, Lund L, Ambrosio GA, Coats AJ, Ferrari R, Ruschitzka F, Maggioni AP, Filippatos G.. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail 2017;19:1574–1585. [DOI] [PubMed] [Google Scholar]
- 40. Bottle A, Kim D, Hayhoe B, Majeed A, Aylin P, Clegg A, Cowie MR.. Frailty and comorbidity predict first hospitalisation after heart failure diagnosis in primary care: population-based observational study in England. Age Ageing 2019;48:347–354. [DOI] [PubMed] [Google Scholar]
- 41. Goldfarb M, Sheppard R, Afilalo J.. Prognostic and therapeutic implications of frailty in older adults with heart failure. Curr Cardiol Rep 2015;17:92.. [DOI] [PubMed] [Google Scholar]
- 42. Sokoreli I, Pauws SC, Steyerberg EW, de Vries GJ, Riistama JM, Tesanovic A, Kazmi S, Pellicori P, Cleland JG, Clark AL.. Prognostic value of psychosocial factors for first and recurrent hospitalizations and mortality in heart failure patients: insights from the OPERA-HF study. Eur J Heart Fail 2018;20:689–696. [DOI] [PubMed] [Google Scholar]
- 43. Uchmanowicz I, Łoboz-Rudnicka M, Szeląg P, Jankowska-Polańska B, Łoboz-Grudzień K.. Frailty in heart failure. Curr Heart Fail Rep 2014;11:266–273. [DOI] [PubMed] [Google Scholar]
- 44. Vitale C, Ilaria S, Rosano GM.. Pharmacological interventions effective in improving exercise capacity in heart failure. Card Fail Rev 2018;4:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 46. Mitnitski AB, Mogilner AJ, Rockwood K.. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001;1:323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A.. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Afilalo J. The clinical frailty scale: upgrade your eyeball test. Circulation 2017;135:2025–2022. [DOI] [PubMed] [Google Scholar]


