Abstract
Excessive collagen deposition by myofibroblasts during adverse cardiac remodeling leads to myocardial fibrosis that can compromise cardiac function. Unraveling the mechanisms underlying collagen gene expression in cardiac myofibroblasts is therefore an important clinical goal. The collagen receptors, discoidin domain receptor 2 (DDR2), a collagen-specific receptor tyrosine kinase, and integrin-β1, are reported to mediate tissue fibrosis. Here, we probed the role of DDR2–integrin-β1 cross-talk in the regulation of collagen α1(I) gene expression in angiotensin II (Ang II)-stimulated cardiac fibroblasts. Results from gene silencing/overexpression approaches, electrophoretic mobility shift assays, and ChIP revealed that DDR2 acts via extracellular signal–regulated kinase 1/2 mitogen-activated protein kinase (ERK1/2 MAPK)-dependent transforming growth factor-β1 (TGF-β1) signaling to activate activator protein-1 (AP-1) that in turn transcriptionally enhances the expression of collagen-binding integrin-β1 in Ang II–stimulated cardiac fibroblasts. The DDR2–integrin-β1 link was also evident in spontaneously hypertensive rats and DDR2-knockout mice. Further, DDR2 acted via integrin-β1 to regulate α-smooth muscle actin (α-SMA) and collagen type I expression in Ang II–exposed cardiac fibroblasts. Downstream of the DDR2–integrin-β1 axis, α-SMA was found to regulate collagen α1(I) gene expression via the Ca2+ channel, transient receptor potential cation channel subfamily C member 6 (TRPC6), and the profibrotic transcription factor, Yes-associated protein (YAP). This finding indicated that fibroblast-to-myofibroblast conversion is mechanistically coupled to collagen expression. The observation that collagen receptor cross-talk underlies α-SMA–dependent collagen type I expression in cardiac fibroblasts expands our understanding of the complex mechanisms involved in collagen gene expression in the heart and may be relevant to cardiac fibrogenesis.
Keywords: fibroblast, fibrosis, integrin, collagen, tyrosine-protein kinase (tyrosine kinase), cardiovascular disease, connective tissue, cardiac fibroblast, cardiac fibrosis, collagen type I, discoidin domain receptor 2, integrin-beta1, alpha-smooth muscle actin, tissue damage, gene regulation
Introduction
Cardiac fibroblasts, the only intracardiac source of collagen types I and III, are a major determinant of wound healing and its unintended consequences in the injured myocardium. In a setting of cardiac muscle damage, normally quiescent cardiac fibroblasts are phenotypically transformed into α-smooth muscle actin (α-SMA)3-expressing myofibroblasts that infiltrate the site of injury, proliferate, and produce matrix components, facilitating the formation of a collagen-rich scar tissue that prevents ventricular rupture and preserves myocardial integrity (1). However, active cardiac fibroblasts persist in the infarct scar long after termination of the healing response, which leads to excessive collagen deposition and fibrosis that contribute significantly to compromised cardiac function post-injury (2). Expression of α-SMA and enhanced collagen deposition, which characterize active myofibroblasts, are critical steps in cardiac tissue repair that profoundly impact cardiac remodeling associated with conditions such as hypertension and myocardial infarction (3). In this regard, angiotensin II (Ang II), whose intracardiac generation is reported to be enhanced following myocardial injury, is a potent profibrotic factor in the myocardium with marked stimulatory effects on collagen expression in cardiac fibroblasts (4). Identification of mechanisms involved in cardiac fibroblast activation and collagen gene expression, especially in response to Ang II, can therefore provide novel insights into the molecular basis of cardiac fibrogenesis and uncover strategies to optimize cardiac fibroblast function following myocardial injury.
In this context, collagen receptors are increasingly implicated in physiological processes and in the pathophysiology of diseases that primarily affect collagen homeostasis (5). Discoidin domain receptor 2 (DDR2) is a mesenchymal cell–specific fibrillar collagen-receptor tyrosine kinase expressed predominantly in fibroblasts and is a fibroblast-selective marker (6, 7), whereas collagen-binding integrin-β1 is expressed in a variety of cell types. DDR2 and integrin-β1 are individually reported to regulate collagen expression and extracellular matrix remodeling (8, 9) and cellular processes such as cell survival (10, 11), proliferation (12), migration (13, 14), and differentiation (15, 16). However, despite the reported synergy between receptor tyrosine kinases and integrins in promoting angiogenesis, tumor metastasis, and atherosclerosis (17), the possibility that a cross-talk between these two collagen receptors may underlie critical cellular functions in the heart has not been explored. Importantly, the regulatory role of DDR2 in integrin-β1 gene expression and its implications in the regulation of cardiac fibroblast function have not hitherto been reported.
This study explored the involvement of collagen receptor cross-talk in the regulation of collagen α1(I) gene expression in Ang II–stimulated cardiac fibroblasts. Specifically, we probed the regulatory role of DDR2 in integrin-β1 gene expression and, downstream of the DDR2–integrin-β1 axis, investigated a hitherto unknown role for α-SMA, a cytoskeletal protein traditionally implicated in cell motility and contraction (18), in the regulation of Ang II–stimulated collagen α1(I) expression. To the best of our knowledge, this report is the first to present robust evidence that DDR2–integrin-β1 cross-talk underlies α-SMA–dependent collagen type I expression in cardiac fibroblasts. The obligate role of α-SMA, downstream of the DDR2–integrin-β1 axis, in collagen expression implies that phenotypic transformation of cardiac fibroblasts and collagen type I expression are mechanistically coupled, representing an exquisite continuum of cellular events, culminating in enhanced collagen expression in Ang II–stimulated cardiac fibroblasts.
Results
DDR2 mediates integrin-β1 expression in cardiac fibroblasts exposed to Ang II
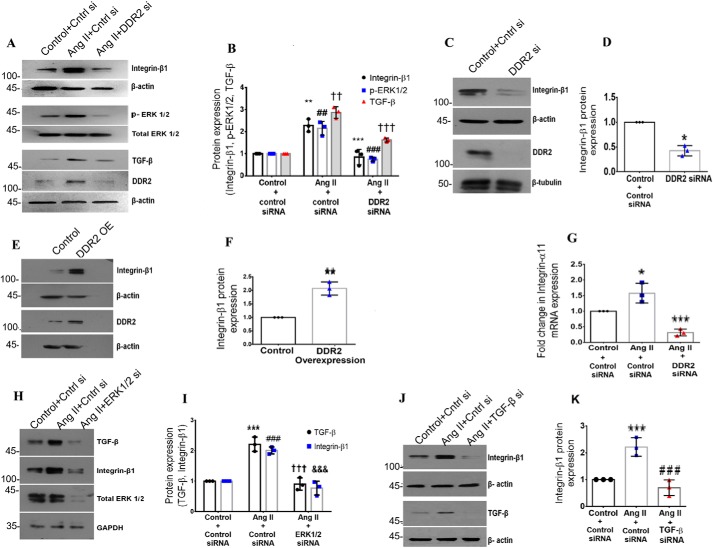
Ang II was found to significantly enhance integrin-β1 protein expression at 12 h post-treatment (Fig. 1, A and B). To test the possibility that DDR2 mediates Ang II–stimulated integrin-β1 expression in cardiac fibroblasts, cells transiently transfected with DDR2 siRNA were exposed to Ang II for 12 h, followed by analysis of integrin-β1 expression. Cell viability post-transfection was affected only minimally (data not shown). DDR2 knockdown significantly attenuated integrin-β1 expression, indicating a role for DDR2 in the regulation of Ang II–dependent integrin-β1 expression in cardiac fibroblasts (Fig. 1, A and B). Interestingly, whereas DDR2 knockdown reduced basal levels of integrin-β1 expression (Fig. 1, C and D), overexpression of DDR2 in unstimulated cells enhanced integrin-β1 expression (Fig. 1, E and F), which indicates the regulatory role of DDR2 in integrin-β1 expression even in unstimulated cardiac fibroblasts maintained under serum-free conditions.
Figure 1.
DDR2 mediates integrin-β1 expression in cardiac fibroblasts exposed to Ang II. A and B, cardiac fibroblasts were transiently transfected with DDR2 siRNA or scrambled siRNA (control). Following exposure of the transfected cells to Ang II for 12 h, integrin-β1 protein expression was examined by Western blot analysis, with β-actin as loading control. **, p < 0.01 versus control; ***, p < 0.001 versus Ang II. Phospho-ERK1/2 activation was examined by Western blot analysis and normalized to total ERK1/2 levels. ##, p < 0.01 versus control; ###, p < 0.001 versus Ang II. TGF-β1 protein expression was examined by Western blot analysis, with β-actin as loading control. ††, p < 0.01 versus control; †††, p < 0.001 versus Ang II. C and D, cardiac fibroblasts were transiently transfected with DDR2 siRNA or scrambled siRNA (control). Following revival and serum deprivation of the transfected cells for 24 h, integrin-β1 protein expression at baseline was examined by Western blot analysis and normalized to β-actin. *, p < 0.05 versus control. E and F, subconfluent cultures of cardiac fibroblasts in M199 were transiently transfected with DDR2 overexpression vector or empty plasmid vector (control). Following revival and serum deprivation of the transfected cells for 24 h, integrin-β1 protein expression was examined by Western blot analysis and normalized to β-actin. **, p < 0.01 versus control. G, cardiac fibroblasts were transiently transfected with DDR2 siRNA or scrambled siRNA (control). Following exposure of the transfected cells to Ang II for 6 h, integrin-α11 mRNA expression was examined by RT-qPCR analysis. 18S rRNA served as the endogenous control. *, p < 0.05 vs. control and ***, p < 0.001 vs. Ang II. H and I, cardiac fibroblasts were transiently transfected with ERK1/2 siRNA or scrambled siRNA (control). Following exposure of the transfected cells to Ang II for 12 h, TGF-β1 and integrin-β1 protein expression was examined by Western blot analysis with GAPDH as loading control. For TGF-β, ***, p < 0.001 versus control; †††, p < 0.001 versus Ang II. For integrin-β1, ###, p < 0.001 versus control; &&&, p < 0.001 versus Ang II. J and K, cardiac fibroblasts were transiently transfected with TGF-β1 siRNA or scrambled siRNA (control). Following exposure of the transfected cells to Ang II for 12 h, integrin-β1 protein expression was examined by Western blot analysis, with β-actin as loading control. ***, p < 0.001 versus control; ###, p < 0.001 versus Ang II. Data are representative of three independent experiments (n = 3). Error bars, S.D.
Our previous studies had demonstrated that inhibitors of NADPH oxidase–dependent reactive oxygen species (ROS), phospholipase C, protein kinase C, and p38 mitogen-activated protein kinase (MAPK) inhibited Ang II–stimulated DDR2 expression in cardiac fibroblasts, implicating the ROS-GPCR-p38-MAPK signaling pathway in Ang II–induced increase in DDR2 (8). In the present study, these inhibitors were found to significantly reduce Ang II-induced integrin-β1 expression as well (Fig. S1, A–H). Because DDR2 regulates integrin-β1, as shown in the present study, the findings collectively show that Ang II activates the ROS-GPCR signaling cascade to enhance DDR2, which in turn enhances the expression of integrin-β1 in cardiac fibroblasts.
Integrin α11β1 is reported to be the dominant collagen-binding integrin in cardiac fibroblasts (19). In the present study, DDR2 knockdown attenuated Ang II–induced expression of integrin-α11 as well, demonstrating a role for DDR2 in the coordinated regulation of the α11 and β1 integrin subunits (Fig. 1G).
Subsequent investigations focused on the regulation of integrin-β1 because the β1 subunit is the major signaling transducer with reported roles in myofibroblast formation and collagen production (20) whereas the α subunit confers ligand specificity (21). Moreover, the role of integrin-β1 in mediating profibrotic signaling events in cardiac fibroblasts remains largely unclear.
DDR2 acts via extracellular signal-regulated kinase 1/2 (ERK1/2)-dependent transforming growth factor-β1 (TGF-β1) to increase integrin-β1 expression in Ang II–stimulated cardiac fibroblasts
Next, the signaling effectors downstream of DDR2 that promote integrin-β1 expression were explored. Based on our earlier observation that DDR2 induces ERK1/2 MAPK (8) and the reported synergy between ERK1/2 MAPK and TGF-β1 signaling, a role for DDR2-dependent ERK1/2 MAPK activation and TGF-β1 in the regulation of integrin-β1 expression was probed. siRNA-mediated knockdown of DDR2 reduced Ang II–induced phosphorylation of ERK1/2 MAPK and TGF-β1 expression (Fig. 1, A and B). ERK1/2 MAPK inhibition attenuated the Ang II–induced increase in TGF-β1 and integrin-β1 expression (see Fig. 1 (H and I) for protein and Fig. S1 (I and J) for mRNA) whereas TGF-β1 inhibition reduced the Ang II–induced increase in integrin-β1 expression (see Fig. 1 (J and K) for protein and Fig. S1K for mRNA). Together, the data show the involvement of DDR2-dependent ERK1/2 MAPK activation and TGF-β1 in the regulation of integrin-β1 expression in response to Ang II in cardiac fibroblasts.
Transcriptional regulation of integrin-β1 by activating protein-1 (AP-1) via DDR2-dependent ERK1/2–TGF-β1 signaling in Ang II–stimulated cardiac fibroblasts
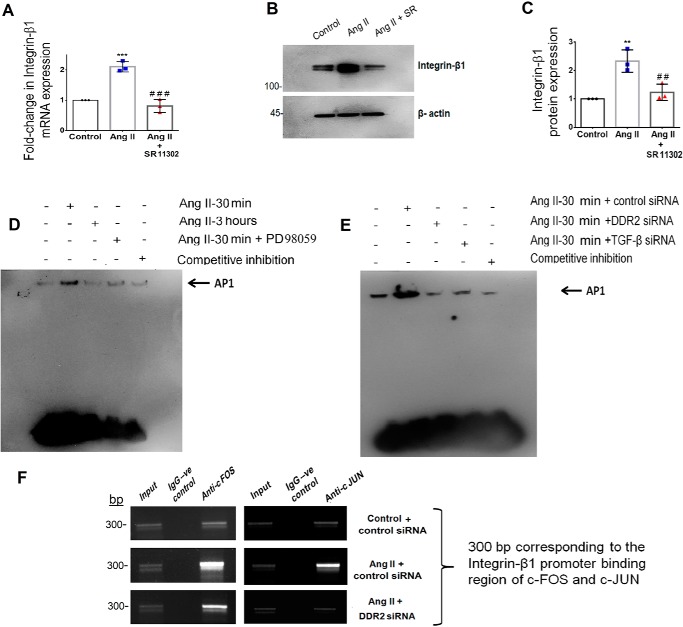
Because AP-1 is a redox-sensitive transcription factor (22) that is reported to be regulated by ERK1/2 MAPK (23) and TGF-β1 (24), the transcriptional regulation of integrin-β1 expression by AP-1 was probed. Inhibition of AP-1 with SR11302 significantly reduced Ang II–induced integrin-β1 mRNA and protein expression (Fig. 2, A–C). EMSA showed Ang II–induced nuclear translocation of AP-1, which occurred maximally at 30 min and was reduced by 3 h (Fig. 2D). Further, inhibition of DDR2, TGF-β1 (using siRNA), and ERK1/2 MAPK (using PD98059) reduced Ang II–induced activation of AP-1 (Fig. 2, D and E), demonstrating their involvement in AP-1 activation in response to Ang II.
Figure 2.
Transcriptional regulation of integrin-β1 by AP-1 via DDR2-dependent ERK1/2 MAPK/TGF-β1 signaling in Ang II–stimulated cardiac fibroblasts. A, subconfluent quiescent cultures of cardiac fibroblasts were pretreated with AP-1 inhibitor (SR11302) for 1 h and, subsequently, with Ang II. integrin-β1 mRNA levels were determined by RT-qPCR analysis at 6 h of Ang II treatment. β-Actin served as the endogenous control. ***, p < 0.001 versus control; ###, p < 0.001 versus Ang II. B and C, protein was isolated at 12 h post-Ang II treatment and subjected to Western blot analysis for detection of integrin-β1, with β-actin as loading control. **, p < 0.01 versus control; ##, p < 0.01 versus Ang II. D, EMSA was performed as described under “Experimental procedures.” Ang II enhanced AP-1 nuclear translocation at 30 min post-treatment, which was attenuated at 3 h. ERK1/2 MAPK inhibition using PD98059 reduced Ang II–induced AP-1 activation at 30 min. E, Ang II enhanced AP-1 nuclear translocation at 30 min post-treatment, which was attenuated upon siRNA-mediated silencing of DDR2 and TGF-β1, respectively. F, DNA binding of AP-1 subunits, c-Fos and c-Jun, to the integrin-β1 (ITGB1) gene promoter was confirmed by ChIP using anti-c-Fos and anti-c-Jun antibody, respectively. A nonspecific anti-rabbit IgG was used as negative control. A representative image showing the PCR amplification product is given. Data are representative of three independent experiments (n = 3). Error bars, S.D.
A ChIP assay was performed to confirm the role of DDR2 in the transcriptional up-regulation of integrin-β1 by AP-1. Subconfluent cultures of cardiac fibroblasts were transiently transfected with DDR2 siRNA. Following Ang II treatment for 30 min, the cells were harvested and chromatin was sheared as mentioned under “Experimental procedures.” Cross-linked chromatin preparations from Ang II–treated cells and DDR2 siRNA–transfected cells exposed to Ang II were immunoprecipitated with anti-c-Fos and anti-c-Jun antibodies. The amplification of input chromatin prior to immunoprecipitation served as positive control, and ChIP using a nonspecific antibody (normal rabbit IgG) served as negative control. Our results, after normalization to input DNA, confirmed enhanced binding of c-Fos and c-Jun to the integrin-β1 (ITGB1) gene promoter. Further, AP-1–binding activity was attenuated upon DDR2 knockdown, confirming the role of DDR2-dependent AP-1 in the transcriptional regulation of integrin-β1 (Fig. 2F).
Corroboration of the DDR2–integrin-β1 link in vivo
Integrin-β1 expression is positively correlated with elevated DDR2 in spontaneously hypertensive rats
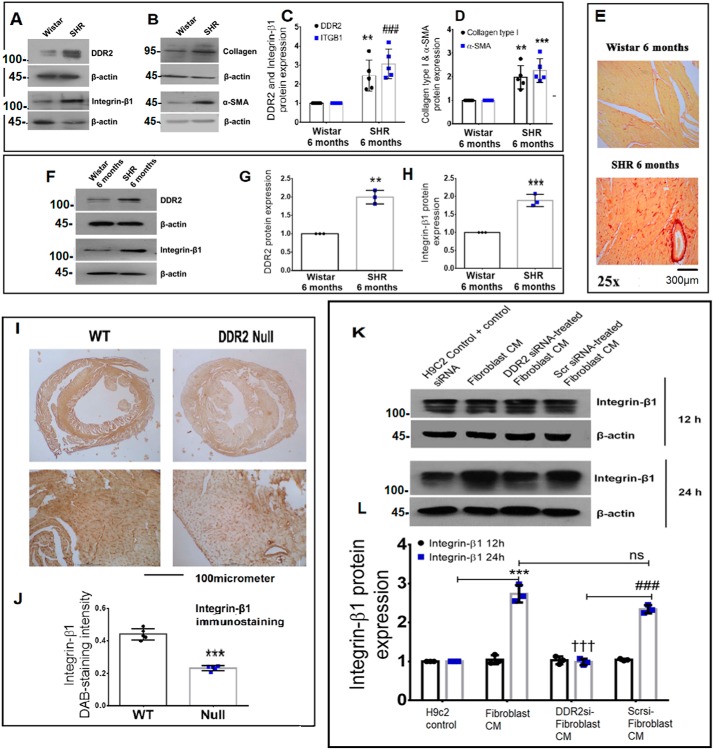
Western blot analysis of whole heart tissue from 6-month-old spontaneously hypertensive rats with myocardial fibrosis, as evidenced by picrosirius red staining, demonstrated an association between DDR2 and integrin-β1, correlating with markers of fibrosis, α-SMA and collagen type I (Fig. 3, A–E). In cardiac fibroblasts isolated from 6-month-old SHRs, a significant elevation in integrin-β1 expression positively correlated with elevated DDR2 (Fig. 3, F–H). These findings point to a possible link between the two collagen receptors in this model of hypertensive heart disease.
Figure 3.
Corroboration of the DDR2–integrin-β1 link in vivo. A–D, cardiac tissues of 6-month-old Wistar rats and SHRs were probed for DDR2, integrin-β1, collagen α1(I), and α-SMA expression by Western blot analysis. **, p < 0.01 versus Wistar for DDR2. ###, p < 0.001 versus Wistar for integrin-β1 (ITGB1). **, p < 0.01 versus Wistar for collagen α1(I). ***, p < 0.001 versus Wistar for α-SMA (n = 5). E, cardiac tissue sections of 6-month-old Wistar rats and SHRs were stained for collagen using picrosirius red (×25 magnification) (n = 4). F–H, cardiac fibroblasts were isolated from 6-month-old Wistar rats and SHRs. The cells were preplated for 2.5 h followed by protein isolation and subjected to Western blot analysis for detection of DDR2 and integrin-β1, with β-actin as loading control. **, p < 0.01 versus Wistar for DDR2; ***, p < 0.001 versus Wistar for integrin-β1 (n = 3). I and J, 3,3′-diaminobenzidine (DAB) staining showing integrin-β1 protein in myocardial tissue sections of 10-week-old WT and DDR2-null mice. ***, p < 0.001 versus WT (n = 5). K and L, subconfluent quiescent cultures of rat ventricular H9c2 cells were treated with conditioned medium (CM) derived from control rat cardiac fibroblasts or DDR2-silenced rat cardiac fibroblasts or control siRNA-treated rat cardiac fibroblasts for 12 or 24 h. Quiescent cultures of H9c2 in M199 without serum were used as control for basal integrin-β1 protein expression in H9c2 cells. Integrin-β1 protein expression in H9c2 cells was examined by Western blot analysis and normalized to β-actin. ***, p < 0.001 versus H9c2 control at 24 h; †††, p < 0.001 versus fibroblast CM at 24 h; ###, p < 0.001 versus DDR2 siRNA–treated fibroblast CM at 24 h; ns, not significant. Three rats (Sprague–Dawley) were used for cardiac fibroblast isolation for the conditioned medium experiments (n = 3). Error bars, S.D.
DDR2 knockout significantly reduces myocardial integrin-β1 in mice
The regulatory role of DDR2 in integrin-β1 expression was evident in vivo in knockout mice carrying a germ-line deletion of DDR2. Previously, knockin of a MerCreMer gene targeting exon 3 of the DDR2 allele was used for germ-line deletion of DDR2 in mice. We had previously described the generation, validation, and initial observations of the DDR2-null mice (25). Immunohistochemical analysis of cardiac tissue sections using an anti-integrin-β1 antibody demonstrated a significant reduction in myocardial integrin-β1 expression in the DDR2-null mice (Fig. 3, I and J). This striking global reduction in integrin-β1 staining intensity pointed to a decrease in integrin-β1 in myocytes in addition to fibroblasts that are reportedly fewer in number in mouse hearts (26). Preliminary experiments were therefore performed to explore the influence of fibroblast-specific DDR2 on myocyte integrin-β1 expression. To this end, the effect of rat cardiac fibroblast-conditioned medium on integrin-β1 expression in rat ventricular H9c2 (cardiomyoblast) cell line was analyzed. Exposure of H9c2 cells to fibroblast-conditioned medium for 24 h enhanced integrin-β1 expression, but, notably, conditioned medium from DDR2-silenced cardiac fibroblasts did not enhance integrin-β1 expression in H9c2 cells (Fig. 3, K and L), suggesting a role for fibroblast-specific DDR2 in integrin-β1 expression in these cells.
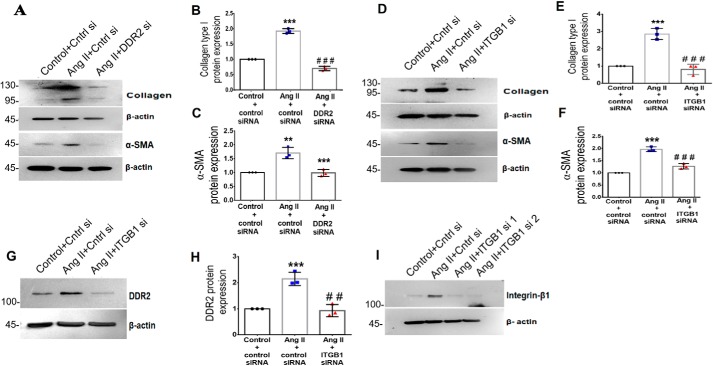
DDR2–integrin-β1 cross-talk regulates α-SMA and collagen α1(I) expression in Ang II–treated cardiac fibroblasts
DDR2 or integrin-β1 knockdown attenuated Ang II–induced α-SMA and collagen α1(I) expression (Fig. 4, A–F). Interestingly, integrin-β1 knockdown also significantly reduced the expression of Ang II–induced DDR2 (Fig. 4, G and H), demonstrating the existence of a reciprocal regulatory relationship between the collagen receptors because DDR2 knockdown abrogated integrin-β1 expression as well (Fig. 1A). The validation of integrin-β1 knockdown by RNAi is shown in Fig. 4I.
Figure 4.
Ang II–induced integrin-β1 regulates DDR2, α-SMA, and collagen type I expression in cardiac fibroblasts. A–C, cardiac fibroblasts were transiently transfected with DDR2 siRNA or scrambled siRNA (control). Following exposure of the transfected cells to Ang II for 12 h, collagen α1(I) and α-SMA protein levels were analyzed, with β-actin as loading control. ***, p < 0.001 versus control; ###, p < 0.001 versus Ang II for collagen α1(I). **, p < 0.01 versus control; ***, p < 0.001 versus Ang II for α-SMA. D–F, cardiac fibroblasts were transiently transfected with integrin-β1 (ITGB1) siRNA or scrambled siRNA (control). Following exposure of the transfected cells to Ang II for 12 h, collagen α1(I) and α-SMA protein levels were analyzed, with β-actin as loading control. ***, p < 0.001 versus control; ###, p < 0.001 versus Ang II. G and H, cardiac fibroblasts were transiently transfected with integrin-β1 (ITGB1) siRNA or scrambled siRNA (control). Following exposure of the transfected cells to Ang II for 12 h, DDR2 protein expression was examined by Western blot analysis and normalized to β-actin. ***, p < 0.001 versus control; ##, p < 0.01 versus Ang II. I, representative image of integrin-β1 siRNA validation. Knockdown efficiency of integrin-β1 siRNA1 or siRNA2 on integrin-β1 protein expression was checked. siRNA2 was used for silencing integrin-β1. Data are representative of three independent experiments (n = 3). Error bars, S.D.
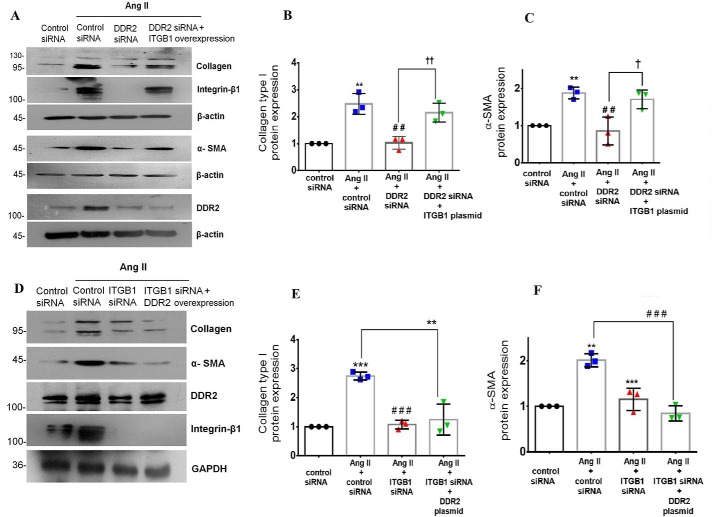
Whereas DDR2 knockdown attenuated the Ang II–induced increase in α-SMA and collagen α1(I), plasmid-based overexpression of integrin-β1 (ITGB1) in DDR2-silenced cells restored the expression of α-SMA and collagen α1(I) upon Ang II treatment (Fig. 5, A–C). However, DDR2 overexpression in integrin-β1–silenced cells failed to restore α-SMA and collagen α1(I) expression in Ang II–treated cells (Fig. 5, D–F), showing that DDR2 acts via integrin-β1 to mediate α-SMA and collagen type I expression.
Figure 5.
DDR2-dependent integrin-β1 expression is a determinant of α-SMA and collagen type I expression in Ang II–treated cardiac fibroblasts. A–C, cardiac fibroblasts were transiently co-transfected with DDR2 siRNA and integrin-β1 (ITGB1) plasmid overexpression vector. Following revival and serum deprivation, the transfected cells were exposed to Ang II for 12 h. Collagen α1(I) protein expression was examined by Western blot analysis and normalized to β-actin. **, p < 0.01 versus control; ##, p < 0.01 versus Ang II; ††, p < 0.01 versus Ang II + DDR2 siRNA. α-SMA protein expression was examined by Western blot analysis and normalized to β-actin. **, p < 0.01 versus control; ##, p < 0.01 versus Ang II; †, p < 0.05 versus Ang II + DDR2 siRNA. D–F, cardiac fibroblasts were transiently co-transfected with integrin-β1 (ITGB1) siRNA and DDR2 overexpression vector. Following revival and serum deprivation, the transfected cells were exposed to Ang II for 12 h. Collagen α1(I) protein expression was examined by Western blot analysis and normalized to GAPDH. ***, p < 0.001 versus control; ###, p < 0.001 versus Ang II; **, p < 0.01 versus Ang II. α-SMA protein expression was examined by Western blot analysis and normalized to GAPDH. **, p < 0.01 versus control; ***, p < 0.001 versus Ang II; ###, p < 0.001 versus Ang II. Data are representative of three independent experiments (n = 3). Error bars, S.D.
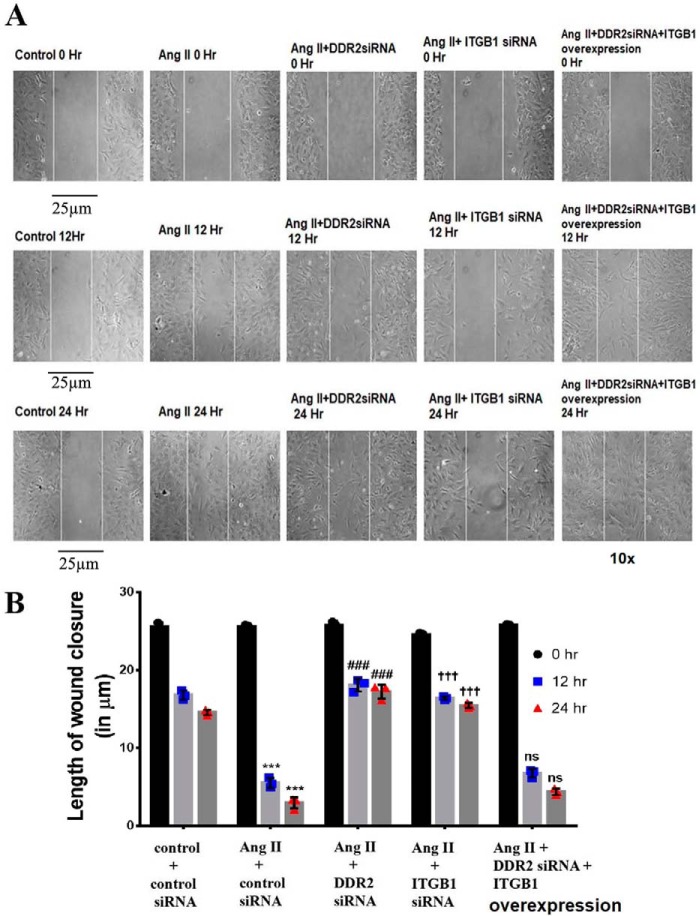
A scratch wound assay showed that, whereas knockdown of either DDR2 or integrin-β1 impaired Ang II–induced wound closure, overexpression of integrin-β1 in DDR2-silenced cells restored the wound-healing response (Fig. 6, A and B). Together, the data show the involvement of the DDR2–integrin-β1 axis in the regulation of α-SMA, collagen type I, and wound healing.
Figure 6.
DDR2-dependent integrin-β1 expression is a determinant of wound healing in Ang II–treated cardiac fibroblasts. A and B, wound-healing ability of cardiac fibroblasts is impaired upon DDR2 or integrin-β1 knockdown and is restored upon integrin-β1 overexpression in DDR2-silenced cells. A scratch wound assay was performed as described under “Experimental procedures.” Cardiac fibroblasts were transiently transfected with DDR2 siRNA or integrin-β1 siRNA or a mixture of DDR2 siRNA and integrin-β1 overexpression vector. Following revival and serum deprivation, the transfected cells were exposed to Ang II, and the wound-healing ability of these cells was examined at 0, 12, and 24 h (A). The length of wound closure was determined in micrometers and quantified as described under “Experimental procedures” (B). Three or four fields were examined per dish. ***, p < 0.001 versus control; ###, p < 0.001 versus Ang II; †††, p < 0.001 versus Ang II; ns, not significant versus Ang II. Scale bar, 25 μm. Magnification is ×10. Data are representative of three independent experiments (n = 3). Error bars, S.D.
α-SMA acts downstream of the DDR2 integrin-β1 axis to regulate collagen α(I) expression via the TRPC6-YAP pathway in Ang II–treated cardiac fibroblasts
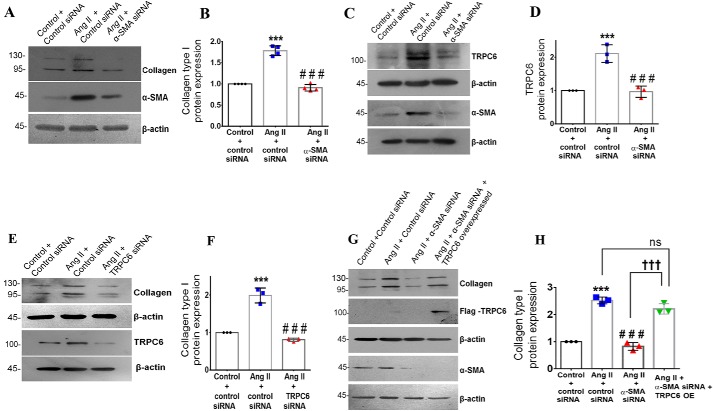
The role of α-SMA as a determinant of collagen type I expression in cardiac fibroblasts exposed to Ang II was examined. α-SMA knockdown significantly reduced Ang II–dependent collagen α1(I) expression in cardiac fibroblasts (Fig. 7, A and B). We probed the role of TRPC6, a Ca2+ channel in fibroblasts with a demonstrated role in myofibroblast transformation (27), as a mediator of α-SMA–dependent collagen type I expression in Ang II–treated cardiac fibroblasts. α-SMA knockdown significantly reduced Ang II–induced TRPC6 expression (Fig. 7, C and D). TRPC6 knockdown in turn significantly attenuated Ang II–stimulated collagen α1(I) expression (Fig. 7, E and F). Notably, plasmid vector–based overexpression of TRPC6 in α-SMA–silenced cardiac fibroblasts rescued collagen α1(I) expression upon Ang II treatment (Fig. 7, G and H), confirming the regulatory link between α-SMA, TRPC6, and collagen type I.
Figure 7.
α-SMA–dependent TRPC6 regulates collagen type I expression in Ang II–treated cardiac fibroblasts. Cardiac fibroblasts were transiently transfected with α-SMA siRNA or scrambled siRNA (control). A and B, following exposure of the transfected cells to Ang II for 12 h, collagen α1(I) protein expression was examined by Western blot analysis and normalized to β-actin. ***, p < 0.001 versus control; ###, p < 0.001 versus Ang II. C and D, following exposure of the transfected cells to Ang II for 12 h, TRPC6 protein expression was examined by Western blot analysis and normalized to β-actin. ***, p < 0.001 versus control; ###, p < 0.001 versus Ang II. E and F, cardiac fibroblasts were transiently transfected with TRPC6 siRNA or scrambled siRNA (control). Following exposure of the transfected cells to Ang II for 12 h, collagen type I protein expression was examined by Western blot analysis and normalized to β-actin. ***, p < 0.001 versus control; ###, p < 0.001 versus Ang II. G and H, cardiac fibroblasts were transiently co-transfected with α-SMA siRNA and TRPC6 plasmid overexpression vector. Following revival of the transfected cells and serum deprivation, cardiac fibroblasts were exposed to Ang II for 12 h. Collagen α1(I) protein expression was examined by Western blot analysis and normalized to β-actin. ***, p < 0.001 versus control; ###, p < 0.001 versus Ang II; †††, p < 0.001 versus Ang II + α-SMA siRNA; ns, not significant versus Ang II. Data are representative of three independent experiments (n = 3). Error bars, S.D.
α-SMA–dependent transcriptional regulation of collagen type I expression
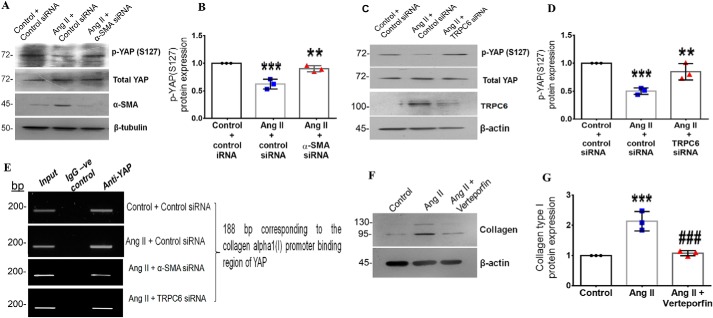
The transcriptional regulation of collagen type I downstream of the α-SMA-TRPC6 signaling axis was checked. Yes-associated protein (YAP) is a mechanosensitive transcription factor that responds to changes in actin filament organization and mediates profibrotic signaling (28, 29). The activation of YAP depends on inhibitory phosphorylation at multiple residues (30). Phosphorylation at serine 127 is reported to inhibit YAP activation by tethering it to the 14-3-3 protein in the cytoplasm and, hence, is used to ascertain YAP activation. In the present study, Ang II enhanced YAP activation at 6 h post-treatment, as evidenced by a decrease in YAP phosphorylation at Ser-127 (Fig. S4, G and H). Importantly, knockdown of α-SMA and TRPC6 attenuated Ang II–stimulated YAP activation, showing that YAP activation depends on α-SMA and TRPC6 (Fig. 8, A–D). A direct role for α-SMA and TRPC6 in YAP activation was confirmed by ChIP that showed reduced binding of YAP to the collagen type I gene promoter upon α-SMA and TRPC6 knockdown in Ang II–stimulated cells (Fig. 8E). Together with the finding that inhibition of YAP by verteporfin attenuated Ang II–stimulated expression of collagen α1(I) (Fig. 8, F and G), the observations indicated a role for YAP activation downstream of α-SMA and TRPC6 in the regulation of collagen type I expression in Ang II–treated cardiac fibroblasts.
Figure 8.
α-SMA–dependent TRPC6 regulates collagen type I expression via YAP activation in Ang II–treated cardiac fibroblasts. Cardiac fibroblasts were transiently transfected with α-SMA siRNA, TRPC6 siRNA, or scrambled siRNA (control). A and B, following exposure of the α-SMA siRNA-transfected cells to Ang II for 6 h, phosphorylation of YAP at Ser-127 was examined by Western blot analysis and normalized to total YAP. ***, p < 0.001 versus control; **, p < 0.01 versus Ang II. C and D, following exposure of the TRPC6 siRNA–transfected cells to Ang II for 6 h, phosphorylation of YAP at Ser-127 was examined by Western blot analysis and normalized to total YAP. ***, p < 0.001 versus control; **, p < 0.01 versus Ang II. E, cardiac fibroblasts were transiently transfected with α-SMA siRNA, TRPC6 siRNA, or scrambled siRNA (control). Following Ang II treatment for 6 h, the ChIP assay was performed as described under “Experimental procedures.” DNA binding of YAP to the collagen α1(I) gene promoter was confirmed using anti-YAP antibody. A nonspecific anti-rabbit IgG was used as negative control. A representative image showing the PCR amplification product is shown. F and G, subconfluent quiescent cultures of cardiac fibroblasts were pretreated with the YAP inhibitor (verteporfin) for 1 h and, subsequently, with Ang II. Protein was isolated at 12 h post-Ang II treatment and subjected to Western blot analysis for detection of collagen α1(I), with β-actin as loading control. ***, p < 0.001 versus control; ###, p < 0.001 versus Ang II. Data are representative of three independent experiments (n = 3). Error bars, S.D.
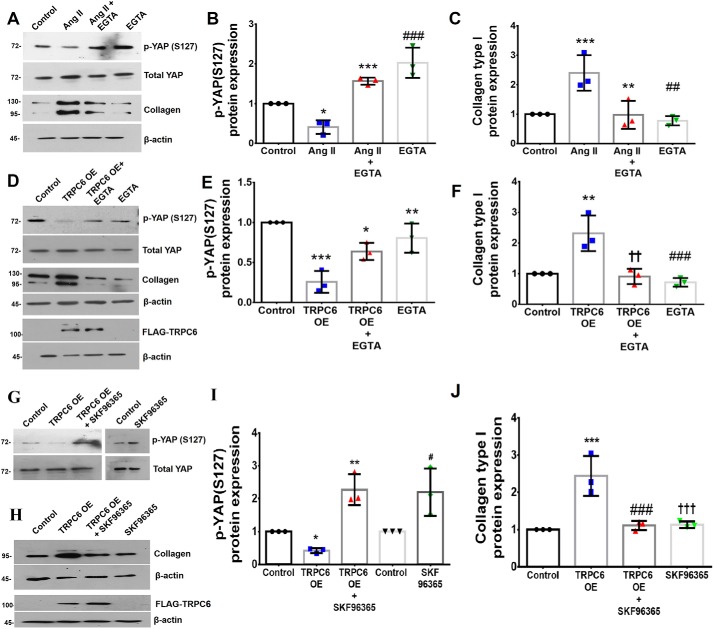
A previous study by Davis et al. (27) had demonstrated a role for TRPC6-mediated Ca2+ influx in cardiac myofibroblast differentiation. Because the present study focused on TRPC6 and its role in collagen expression via activation of YAP, preliminary experiments were performed to confirm the involvement of Ca2+ in the activation of YAP and regulation of collagen type I expression. Ang II–stimulated collagen type I expression and YAP activation were significantly attenuated upon chelation of extracellular Ca2+ using EGTA (1 mm), a specific Ca2+ chelator (31), demonstrating the role of Ca2+ in regulating collagen type I expression and activation of YAP in fibroblasts (Fig. 9, A–C). Furthermore, whereas overexpression of TRPC6 in cardiac fibroblasts enhanced collagen expression and YAP activation, these effects were attenuated in EGTA-treated fibroblasts overexpressing TRPC6 (Fig. 9, D–F), further supporting a role for TRPC6-mediated Ca2+ in the regulation of YAP activation and collagen type I expression. These findings were corroborated using SKF96365, which blocks TRPC-dependent Ca2+ entry (32) and is reported to prevent Ca2+ influx and fibronectin expression in Ang II–treated cardiac fibroblasts (33). Here, whereas overexpression of TRPC6 in cardiac fibroblasts enhanced YAP activation and collagen expression, these effects were attenuated in SKF96365-treated cardiac fibroblasts overexpressing TRPC6 (Fig. 9, G–J). This observation further supports the role of TRPC6-dependent Ca2+ in mediating YAP activation and collagen expression in Ang II–treated cardiac fibroblasts. A schematic representation of the plausible sequence of events that underlie enhanced collagen type 1 expression in cardiac fibroblasts exposed to Ang II is depicted in Fig. 10.
Figure 9.
TRPC6-dependent Ca2+ in mediating YAP activation and collagen expression in Ang II–treated cardiac fibroblasts. Subconfluent quiescent cultures of cardiac fibroblasts were pretreated with EGTA (1 mm) for 1 h and, subsequently, with Ang II. A–C, phosphorylation status of YAP at Ser-127 was examined by Western blot analysis at 6 h post-Ang II treatment and normalized to total YAP. *, p < 0.05 versus control; ***, p < 0.001 versus Ang II; ###, p < 0.001 versus Ang II. Expression of collagen α1(I) was analyzed at 12 h post-Ang II treatment with β-actin as loading control. ***, p < 0.001 versus control; **, p < 0.01 versus Ang II; ##, p < 0.01 versus Ang II. D–F, cardiac fibroblasts were transiently transfected with TRPC6 overexpression plasmid or control plasmid. Following exposure of the transfected cells to EGTA (1 mm), phosphorylation status of YAP at Ser-127 was examined by Western blot analysis at 6 h post-EGTA treatment and normalized to total YAP. ***, p < 0.001 versus control; *, p < 0.05 versus TRPC6 OE; **, p < 0.01 versus TRPC6 OE. Collagen α1(I) protein expression was examined by Western blot analysis at 12 h post-EGTA treatment and normalized to β-actin. **, p < 0.01 versus control; ††, p < 0.01 versus TRPC6 OE; ###, p < 0.001 versus TRPC6 OE. G–J, cardiac fibroblasts were transiently transfected with TRPC6 overexpression plasmid or control plasmid. Following exposure of the transfected cells to SKF96365 (10 μm), phosphorylation status of YAP at Ser-127 was examined by Western blot analysis at 6 h post-treatment with SKF96365 and normalized to total YAP. *, p < 0.05 versus control; **, p < 0.01 versus TRPC6 OE; #, p < 0.05 versus TRPC6 OE. The control and SKF96365 blot from a separate run have been juxtaposed. Collagen α1(I) protein expression was examined by Western blot analysis at 12 h post-treatment with SKF96365 and normalized to β-actin. ***, p < 0.001 versus control; ###, p < 0.001 versus TRPC6 OE; †††, p < 0.001 versus TRPC6 OE. Data are representative of three independent experiments (n = 3). Error bars, S.D.
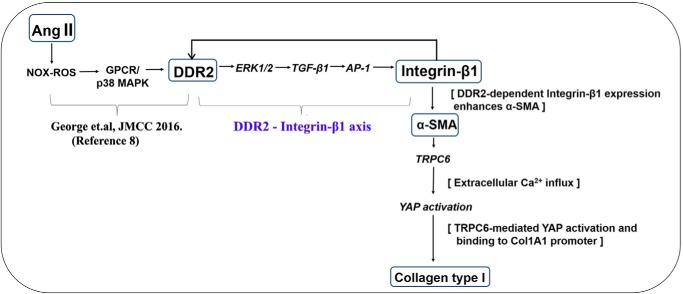
Figure 10.
Schematic representation of the plausible sequence of events that underlie enhanced collagen type I expression in cardiac fibroblasts exposed to Ang II.
Effect of siRNA on unstimulated cells
In all of the experiments, the effect of siRNA on cells maintained under unstimulated (basal) conditions was also analyzed (Figs. S1–S4).
Discussion
Heart failure remains a major cause of morbidity and mortality worldwide despite significant advances in therapy and prevention (34). Apart from progressive myocyte hypertrophy and apoptosis that compromise cardiac function, sustained activation of cardiac interstitial fibroblasts in response to injury leads inadvertently to interstitial fibrosis that contributes to the onset and progression of heart failure (35). There is increasing appreciation that therapy directed at cardiac fibrosis may retard heart failure and other cardiovascular diseases (36). In the current clinical setting, blocking the actions of Ang II, a potent inducer of cardiac fibrosis, is a preferred treatment modality to reduce adverse myocardial remodeling following myocardial infarction. However, Ang II blockade remains only partially effective and can potentially entail unfavorable effects (37, 38), which necessitates identification of other appropriate therapeutic targets through exploration of the molecular basis of cardiac fibrogenesis.
It has been reported that a synergy between receptor tyrosine kinases (RTKs) and integrins underlies the regulation of many pathophysiological events (17). For example, cross-talk between IGF-1 (insulin-like growth factor-1) and integrin-αvβ3 is reported to impact atherosclerotic lesion formation (39). In cancer cells, epidermal growth factor receptor–mediated signaling cooperates with integrin-αvβ5 to promote cell adhesion, migration, and metastasis (40). Additionally, antagonism of integrin-αvβ3 prevents basic fibroblast growth factor–induced angiogenesis in vivo (41). Together, these reports highlight the importance of RTK-integrin cross-talk in the modulation of cell type–specific biological responses.
In the present study, we show that DDR2 knockdown abrogates the effect of Ang II on integrin-β1 expression, which demonstrates the obligate role of DDR2 in integrin-β1 expression in Ang II–stimulated cardiac fibroblasts. A combination of DDR2 knockdown and overexpression approaches established the regulatory role of DDR2 in integrin-β1 gene expression even in unstimulated cells. It is pertinent to note that previous studies exploring links between DDRs and integrin-β1 had focused mainly on the role of the DDR1 isoform in mediating integrin-β1 activation and its effects on cell migration, protein trafficking, and epithelial-mesenchymal transition (42). The focus of the present study was on DDR2 rather than DDR1 because the DDR2 isoform is found in cells of mesenchymal origin, like cardiac fibroblasts (43), whereas DDR1 is expressed mainly in epithelial tissues (44). It was reported that DDR1 and DDR2 induce α1β1 and α2β1 integrin activation to promote adhesion of HEK293 cells to collagen (45). However, no difference in expression levels of the integrins was observed in their study, possibly due to cell type–specific differences. To the best of our knowledge, ours is the first demonstration of the involvement of DDR2 in integrin-β1 gene expression in cardiac fibroblasts. Interestingly, integrin-β1 knockdown attenuated Ang II–induced DDR2 expression, suggesting that DDR2 and integrin-β1 are locked in a cycle of mutual regulation. This feedback loop between integrin-β1 and DDR2 could function to maintain the expression levels of DDR2 in cardiac fibroblasts, enabling RTK signal amplification post-Ang II stimulation.
The SHR represents a genetic model of hypertensive heart disease with a marked degree of myocardial fibrosis (46). A role for the renin-angiotensin system in mediating hypertension-induced fibrosis in SHR is well appreciated (47). The present study provides evidence of a positive correlation between DDR2 and integrin-β1 in the myocardium and in cardiac fibroblasts isolated from 6-month-old SHR with myocardial fibrosis, which was consistent with the demonstration of the DDR2–integrin-β1 link in Ang II–treated cells. Further, in a knockout mouse model carrying a germ-line deletion of DDR2, we observed a significant reduction in integrin-β1 staining intensity in cardiac tissue from DDR2-null mice. Because fibroblasts constitute only about 10% of the total myocardial cell population in mice (26) and cardiac myocytes also express integrin-β1 (48), the global reduction in integrin-β1 staining intensity seems to suggest a decrease in integrin-β1 in cardiomyocytes as well as fibroblasts. This raises the possibility that fibroblast-specific DDR2 contributes to integrin-β1 expression in myocytes via DDR2-dependent paracrine signaling. Cardiac fibroblasts are a major source of humoral factors that regulate transcriptional activity within myocytes (35). Therefore, a loss of DDR2 in fibroblasts could repress paracrine factors regulating myocyte integrin-β1 expression. In support of this, conditioned medium derived from cultured rat cardiac fibroblasts up-regulated integrin-β1 expression in rat ventricular H9c2 cells. However, conditioned medium from DDR2-silenced cardiac fibroblasts failed to enhance integrin-β1 in H9c2 cells, suggesting that DDR2-mediated paracrine signaling may regulate integrin-β1 expression in myocytes. This observation is important because it is consistent with the role of integrin-β1 as an important determinant of cardiac size and organ growth (49). Our previous study on this mouse model had demonstrated, by echocardiography, reduced left ventricular chamber dimensions (25). Cardiomyocyte length was atypically shorter in the DDR2-null mice, resulting in decreased heart size and weight. It is tempting to speculate that the reduction in heart size in DDR2-null mice indicates the importance of DDR2 not only in the regulation of cardiac fibroblast function but also in regulating myocardial size. The possibility that cardiac fibroblasts are involved in myocardial growth via DDR2-dependent paracrine mechanisms warrants investigation.
Having established the regulatory role of DDR2 in integrin-β1 expression, we probed the mechanisms by which DDR2 regulates integrin-β1. We found that DDR2-dependent activation of ERK1/2, as previously shown by us (8), enhanced TGF-β1 expression in Ang II–treated cells. Further, inhibition of ERK1/2 MAPK or TGF-β1 attenuated Ang II–induced integrin-β1 expression, suggesting that DDR2-dependent ERK1/2–TGF-β1 signaling regulates integrin-β1. The transcriptional regulation of integrin-β1 in Ang II–treated cardiac fibroblasts was probed. We focused mainly on AP-1, a redox-sensitive transcription factor known to be a target of Ang II (50). c-Jun, a component of the AP-1 complex, is reported to enhance the proliferation of fibroblasts in idiopathic pulmonary fibrosis (51). Moreover, AP-1 is a target of MAPK signaling (22) and TGF-β1 (52). We observed that DDR2 mediates AP-1 activation via ERK1/2 and TGF-β1 signaling, resulting in AP-1 binding to the integrin-β1 gene promoter and transcriptional up-regulation of integrin-β1. This study provides evidence that Ang II stimulates the expression of DDR2, which in turn enhances integrin-β1 expression in cardiac fibroblasts via ERK1/2– and TGF-β1–mediated AP-1 activation.
An important outcome of the study was the elucidation of the obligate role of DDR2–integrin-β1 cross-talk in α-SMA and collagen expression. Because we found DDR2 to influence integrin-β1 expression, we examined the role of DDR2-dependent integrin-β1 expression in α-SMA and collagen type I expression in Ang II–treated cardiac fibroblasts. Consistent with previous studies (29), we found that integrin-β1 knockdown attenuated α-SMA and collagen type I expression. However, we also found that, whereas DDR2 knockdown inhibited α-SMA and collagen type I expression in Ang II–treated cardiac fibroblasts, overexpression of integrin-β1 in DDR2-silenced cells restored α-SMA and collagen type I expression. Interestingly, DDR2 overexpression in integrin-β1–silenced cells did not restore α-SMA and collagen type I, clearly indicating the centrality of integrin-β1 as an indispensable factor that mediates DDR2-dependent phenotypic transformation of cardiac fibroblasts and collagen expression.
Integrin-β1 is a common subunit of collagen-binding integrins, which generally acts in association with integrin α subunits (α1β1, α2β1, α10β1, and α11β1) to activate downstream signaling events (53). However, different studies have stressed the regulatory role of the α and β subunits in tissue fibrosis individually. For example, the genetic ablation of integrin-β1 per se has been shown to attenuate liver fibrosis and impede dermal wound healing (29, 53). Further, myocardial integrin-β1 expression is reported to be up-regulated after myocardial infarction (54). On the other hand, although germ-line deletion of integrin α11 has been reported to abrogate diabetes-related cardiac fibrosis (55), the role of integrin-β1 in mediating profibrotic signaling events in cardiac fibroblasts remains largely unclear, a lacuna that this study addressed.
To further evaluate the functional significance of DDR2–integrin-β1 cross-talk in cardiac fibroblast function in a context of injury, its involvement in fibroblast response to injury was probed by a scratch wound assay. We found that knockdown of DDR2 or integrin-β1 reduced the wound-healing ability of fibroblasts exposed to Ang II. Importantly, overexpression of integrin-β1 in DDR2-silenced cells restored their wound-healing function, demonstrating the role of collagen receptor cross-talk in cardiac fibroblast function. It is pertinent to note in this context that, in our previous study, collagen synthesis and the rate of collagen deposition were found to be markedly reduced in DDR2-null mice (25). Together, the findings show that reduced expression of integrin-β1 in DDR2-null mice reduces collagen synthesis and deposition and wound healing.
This study uncovers a hitherto unknown role for α-SMA, downstream of integrin-β1, in the regulation of collagen type I expression in Ang II–treated cardiac fibroblasts. Increased collagen synthesis is typically correlated with the transition of cardiac fibroblasts to an α-SMA–positive myofibroblast phenotype (1). α-SMA expression marks myofibroblast differentiation and plays a critical role in wound healing post-injury. Traditionally, the functional roles ascribed to α-SMA involve cell motility and contraction of the fibrotic scar tissue in a setting of injury. However, recent studies exploring a role for α-SMA in cell signaling report α-SMA–dependent activation of ERK1/2 MAPK (18). In lung adenocarcinoma cells, the expression of focal adhesion kinase) and hepatocyte growth factor receptor (or c-MET) has also been shown to be regulated by α-SMA, which leads to augmented metastasis (56). In general, the cellular F/G actin ratio is reported to influence the transcription of smooth muscle differentiation–related genes in cells (57). However, a specific role for α-SMA in the regulation of gene transcription remains largely unexplored. We found that knockdown of α-SMA attenuates Ang II–induced collagen type I expression, showing that α-SMA, beyond its structural importance in myofibroblasts, could be a critical mediator of signaling processes and gene expression following injury. This finding, for the first time, sheds light on a molecular regulatory event that causally links the phenotypic transition of cardiac fibroblasts to enhanced collagen type I production.
Further, we dissected the molecular mechanisms underlying α-SMA–dependent collagen expression. α-SMA constitutes the mechanosensitive stress fibers in myofibroblasts and couples mechanical stretch to Ca2+ influx via cation channels (58). TRPC6 is a cation channel in fibroblasts that mediates Ca2+ influx and is reported to promote fibroblast differentiation into myofibroblasts (27). Because α-SMA mediates collagen expression, as shown here, we proposed a causal role for TRPC6 in mediating the α-SMA–dependent increase in collagen type I in Ang II–treated cardiac fibroblasts. In support of this, we observed that, whereas TRPC6 knockdown abolished collagen type I expression in Ang II–treated cardiac fibroblasts, overexpression of TRPC6 in α-SMA–silenced cells restored collagen type I expression in Ang II–treated cells, demonstrating the importance of α-SMA-TRPC6 signaling in the regulation of collagen type I expression. Further, we probed the transcriptional events downstream of α-SMA and TRPC6 that regulate collagen type I. Considering the role of DDR2, integrin-β1, α-SMA, and TRPC6 as mediators of mechanotransduction, a role for the mechanosensitive transcription factor YAP in the transcriptional regulation of collagen type I seemed plausible. Here, we show that YAP is a positive regulator of collagen expression because inhibition of YAP with verteporfin led to a reduction in collagen type I expression in Ang II–stimulated cardiac fibroblasts. Further, α-SMA and TRPC6, functioning downstream of the DDR2–integrin-β1 axis, were found to mediate Ang II–induced activation and binding of YAP to the collagen type I promoter, facilitating the transcriptional up-regulation of collagen type I. Consistent with its role as a Ca2+ channel mediating Ca2+ influx in cardiac fibroblasts (27), TRPC6 was found to regulate YAP and collagen via Ca2+. This is the first demonstration of a role for mechanosensitive α-SMA and TRPC6 in promoting YAP activation to enhance collagen expression. Together, the findings suggest a role for YAP in cardiac fibrogenesis, consistent with the reported role of YAP in mediating integrin-β1–dependent liver fibrosis (29). Further, our observations imply that YAP may transcriptionally link α-SMA to changes in gene expression.
To summarize the findings, in Ang II–stimulated cardiac fibroblasts, DDR2 acts via ERK1/2 MAPK-dependent TGF-β1 signaling and AP-1 activation to enhance integrin-β1 that in turn increases α-SMA expression. Notably, α-SMA regulates collagen α1(I) gene expression via the mechanosensitive effectors, TRPC6 and YAP. The data also indicate the existence of a reciprocal regulatory relationship between DDR2 and integrin-β1 in which integrin-β1 acts downstream of DDR2, linking DDR2 to α-SMA, collagen type 1, and wound healing. The link between DDR2 and integrin-β1 is also evident in a genetic model of hypertensive heart disease and in DDR2-null mice. Future studies should address the involvement of these regulatory mechanisms in the pathogenesis of myocardial fibrosis in animal models of human disease using knockout strategies.
In conclusion, this study provides credible evidence of an obligate role for collagen receptor cross-talk in Ang II–stimulated cardiac fibroblast function, which is a major determinant of tissue remodeling following myocardial injury. The findings also uncover a mechanistic coupling of the two distinct cellular processes of collagen production and phenotypic transformation of interstitial fibroblasts into an active state. The regulatory role of α-SMA in collagen gene expression is a novel and notable finding of considerable significance insofar as α-SMA has not hitherto been linked to regulation of gene expression. Lastly, whereas it is reasonable to believe that other signaling pathways may also contribute to the regulation of collagen expression or converge upon the regulatory cascade described here, the demonstration that DDR2–integrin-β1 cross-talk underlies α-SMA-TRPC6-YAP–dependent collagen type I expression in cardiac fibroblasts significantly advances our knowledge of the complex mechanisms underlying collagen gene expression in the heart and provides a new paradigm to understand the molecular basis of myocardial fibrosis. Future strategies to therapeutically target cardiac fibrogenesis may lie within this inherent complexity of regulatory mechanisms.
Experimental procedures
Materials
Angiotensin II, PD 98059, chelerythrine, U73122, VAS2870, SB431542 hydrate, EGTA, and M199 were obtained from Sigma-Aldrich. Random primers, reverse transcriptase, RNase inhibitor, dNTPs, and SB203580 were obtained from Promega (Madison, WI). The PureLink RNA isolation kit and Lipofectamine 2000 were from Invitrogen, Inc. (Carlsbad, CA). The Low Cell Number ChIP kit protein A × 48 was from Diagenode (Denville, NJ). NE-PER nuclear and cytoplasmic extraction reagents, the chemiluminescent nucleic acid detection module, the Pierce Biotin 3′-end DNA labeling kit, SYBR Green Master Mix, and TaqMan probes for mRNA expression were from Thermo Fisher Scientific (Waltham, MA). DDR2, TGF-β1, transient receptor potential cation channel subfamily C member 6 (TRPC6), and control siRNAs were from Ambion (Foster City, CA). Integrin-β1 and α-SMA siRNAs were custom-designed from Eurogentec (Liege, Belgium). The rat DDR2/CD167b gene ORF cDNA clone expression plasmid was obtained from Sino Biologicals (Beijing, China). The TRPC6 (NM_053559) rat tagged ORF clone was from Origene (Rockville, MD). Pcax Itgb1-FLAG was a gift from Dennis Selkoe and Tracy Young-Pearse (Addgene plasmid 30153) (59). Opti-MEM and fetal bovine serum (FBS) were from Gibco (Waltham, MA). All cell culture ware was purchased from BD Falcon (Corning, NY). Primary antibodies against DDR2, TGF-β1, ERK1/2, MAPK, and c-Jun were obtained from Cell Signaling Technology (Danvers, MA). The primary antibodies for collagen α1 type I and TRPC6 were from Santa Cruz Biotechnology, Inc. (Dallas, TX). The primary antibodies for integrin-β1, α-SMA, and c-Fos were from Abcam (Cambridge, UK). β-Tubulin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies were purchased from Elabscience (Houston, TX). All antibodies were used after dilution (1:1000), except c-Fos and c-Jun (1:50) for ChIP and α-SMA (1:7000) for Western blotting. The H9c2 cell line was obtained from the American Type Culture Collection. SKF96365 was a kind gift from Prof. Gaiti Hasan (NCBS, Bangalore). XBT X-ray film was from Carestream (Rochester, NY). The study on rats was approved by the Institutional Animal Ethics Committees of Sree Chitra Tirunal Institute for Medical Sciences and Technology, and the study on mice was approved by the Institutional Animal Care and Use Committee of the University of California, San Diego.
Isolation of cardiac fibroblasts
Cardiac fibroblasts were isolated from young adult male Sprague–Dawley rats (2–3 months old) as described earlier (31). Subconfluent cultures of cardiac fibroblasts from passage 2 or 3 were used for the experiments. Cells were serum-deprived for 24 h prior to treatment with 1 μm Ang II.
Quantitative RT-PCR (RT-qPCR) analysis
Subconfluent cultures of cardiac fibroblasts were subjected to the indicated treatments, and total RNA was isolated using the PureLink RNA isolation kit (Invitrogen) according to the manufacturer's instructions. Following DNase I treatment, 2 μg of total RNA was reverse-transcribed to cDNA with random primers and Moloney murine leukemia virus reverse transcriptase. TaqMan RT-qPCR analysis was carried out using the ABI Prism 7500 sequence detection system (Applied Biosystems, Waltham, MA) with specific FAM-labeled probes for Itgb1 (Assay ID: Rn00566727_m1) and TGF-β1 (Assay ID: Rn00572010_m1), and VIC-labeled probes for β-actin (Rn00667869_m1). RT-qPCR analysis using a SYBR Green gene expression assay was used for analyzing -fold change in integrin-α11 expression with ACCGCACGGCATTTGGCAT as the forward primer and TCGTGGGATTCCCCGTCCGT as the reverse primer. 18S rRNA was used as the endogenous control with CCCGCGAGTACAACCTTCT as the forward primer and CGTCATCCATGGCGAACT as the reverse primer. PCRs were performed under the following thermal cycling conditions: 95 °C for 10 min followed by 40 cycles of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 1 min. Gene expression was quantified using Ct values. mRNA expression was normalized to that of β-actin. The relative -fold change in target mRNA levels of treated versus control was quantified using the 2−ΔΔCt method.
Western blot analysis
Subconfluent cultures of cardiac fibroblasts in serum-free M199 were treated with Ang II (1 μm), and relative protein abundance was determined by Western blot analysis following standard protocols (60) and using β-actin, β-tubulin, or GAPDH as loading control. Enhanced chemiluminescence reagent was used to detect the proteins with X-ray film.
RNA interference and overexpression
Cardiac fibroblasts at passage 3 were seeded on 60-mm dishes at equal density. After 24 h, the cells were incubated in Opti-MEM for 5–6 h with Ambion predesigned Silencer-Select siRNA, custom-designed siRNA from Eurogentech, or scrambled siRNA at the given concentrations (10 nm for DDR2, ITGB1, TRPC6, and TGF-β1 and 50 nm for α-SMA) and Lipofectamine 2000 (8 μl).
Constitutive expression of DDR2 and TRPC6 was achieved under the control of a cytomegalovirus promoter. Constitutive expression of integrin-β1 was achieved under the control of an SV-40 promoter. The DDR2 and integrin-β1 plasmids were verified by restriction mapping, whereas the TRPC6 plasmid was verified by PCR amplification using the primers provided in the kit. For overexpression, the plasmid vector for DDR2 (1 μg/μl) was transfected using Lipofectamine 2000. For cotransfection experiments, a mixture of siRNA (5 nm) and plasmid DNA (1 μg/μl) was used and transfected using Lipofectamine 2000. Following a post-transfection recovery phase in M199 with 10% FBS for 12 h, the cells were serum-deprived for 24 h and then treated with Ang II (1 μm) for the indicated durations. Cell lysates were prepared in Laemmli sample buffer, denatured, and used for Western blot analysis.
Electrophoretic mobility shift assay (EMSA)
The DNA-binding activity of AP-1 was assessed by EMSA using the LightShift chemiluminescent EMSA kit. Subconfluent cardiac fibroblast cultures were serum-starved for 24 h followed by incubation in M199 with or without Ang II (1 μm), and nuclear extracts were prepared using the NE-PER nuclear extraction kit. The protein concentration of the nuclear extracts was determined using the bicinchoninic acid (BCA) protein assay method. Single-stranded oligonucleotides containing the consensus sequence for the AP-1 binding site 5′-CGCTTGATGACTCAGCCGGAA-3′ were biotinylated using the Thermo Scientific 3′-end biotin labeling kit and annealed with their complementary strand at 90 °C. The nuclear extracts were incubated with the biotinylated probes and components of the LightShift chemiluminescent kit at 37 °C for 60 min and electrophoresed on a 6% nondenaturing gel. After transfer to a nylon membrane, the DNA was UV-cross-linked at a wavelength of 254 nm for 10 min. After blocking, streptavidin-conjugated horseradish peroxidase was applied, and the bands were visualized by enhanced chemiluminescence.
ChIP assay
The ChIP assay was performed with the Low Cell Number ChIP kit, according to the manufacturer's protocol. Briefly, after treatment of cardiac fibroblasts with 1 μm Ang II for 30 min, the cells were cross-linked with 1% formaldehyde, lysed, and sonicated in a Diagenode Bioruptor to generate ∼600-bp DNA fragments. The respective lysates were incubated with anti-c-Fos/anti-c-Jun/YAP antibody overnight at 4 °C with rotation. Immune complexes were precipitated with protein A–coated magnetic beads. After digestion with proteinase K to remove the DNA-protein cross-links from the immune complexes, the DNA was isolated and subjected to PCR using primers for Itgb1 and collagen α1(I). 5′-TCAGGACCTCTAGAAGAGCAG-3′ and 5′-CTTCCTTCCTTCCTTCCTTCC-3′ were used as the forward and reverse primers, respectively, corresponding to a 300-bp region of the Itgb1 promoter that includes the predicted AP-1–binding sites on the integrin-β1 gene. 5′-CTCAGCACTTTCCTCTTTCT-3′ and 5′-GCCACCTCATCTTTAGGAAA-3′ were used as the forward and reverse primers, respectively, corresponding to a 188-bp region of the collagen α1(I) promoter that includes the predicted YAP-binding sites on the collagen α1(I) gene. DNA isolated from an aliquot of the total sheared chromatin was used as loading control for PCR (input control). ChIP with a nonspecific antibody (normal rabbit IgG) served as negative control. The PCR products were subjected to electrophoresis on a 1% agarose gel.
In vivo study and histology
The generation of DDR2 null mice and genotyping were described in our previous study (25). Mouse tissue sections were prepared as described previously. Briefly, hearts were collected from age-matched male WT and DDR2-null mice, fixed in 4% buffered paraformaldehyde for 2 days, embedded in paraffin, cross-sectioned, and mounted onto slides. Integrin-β1 levels in these sections were analyzed by 3,3′-diaminobenzidine staining and quantified using Fiji-ImageJ software.
Scratch wound assay
Cells were seeded on 35-mm culture dishes and grown to 70–80% confluence. Gene knockdown using siRNA and overexpression using plasmid vectors were performed as described above. Following serum deprivation, a single scratch gap was created using a 10-μl pipette tip. After washing with PBS, the cells were treated with Ang II (1 μm) in serum-free M199 for the indicated durations. Approximately 3–4 fields were examined per dish, and images of the wound closure pattern in the treated and control groups were acquired using a Nikon inverted phase-contrast microscope. Quantification of wound healing was calculated based on the following formula: length of wound closure = (length of wound gap at th − length of wound gap at t0)/length of wound gap at t0, where th is the time of wound gap measurement at 12 or 24 h and t0 is the time of wound gap measurement at the initial time point of 0 h.
Conditioned medium experiments
Cardiac fibroblasts isolated from male Sprague–Dawley rats were used. Rat ventricular H9c2 cells cultured in M199 + 10% FBS were used as an in vitro model for myocytes. DDR2 knockdown in cardiac fibroblasts was achieved using siRNA. Fibroblasts transfected with scrambled siRNA and fibroblasts maintained in M199 without serum served as controls. Six hours post-transfection, the transfection mix was replaced with M199 without serum, and the fibroblast culture was maintained for 24 h. Subsequently, H9c2 cells were exposed to the fibroblast-derived conditioned medium from each of the groups. Lysates were collected at 12 and 24 h, and integrin-β1 protein expression was analyzed and quantified after normalization to β-actin expression.
Statistical analysis
Data are expressed as mean ± S.D. Statistical analysis was performed using Student's t tests for comparisons involving two groups. For comparisons involving more than two groups, the data were analyzed by one-way analysis of variance. p ≤ 0.05 was considered significant. The in vitro data presented are representative of three independent experiments (n = 3). The data presented on experiments involving SHRs are representative of myocardial tissue sections obtained from three age-matched male SHRs. The in vivo data on DDR2-null mice are representative of myocardial tissue sections obtained from five age-matched male mice.
Author contributions
H. V. and S. K. conceptualization; H. V., A. S. T., and S. K. data curation; H. V. and A. S. T. software; H. V., R. T. C., and S. K. formal analysis; H. V., A. S. T., R. T. C., and S. K. validation; H. V., A. S. T., R. T. C., and S. K. investigation; H. V., A. S. T., R. T. C., and S. K. visualization; H. V., A. S. T., R. T. C., and S. K. methodology; H. V. and S. K. writing-original draft; H. V., A. S. T., R. T. C., and S. K. writing-review and editing; R. T. C. and S. K. resources; S. K. supervision; S. K. funding acquisition; S. K. project administration.
Supplementary Material
Acknowledgments
S. K. gratefully acknowledges Dr. Barry H. Greenberg (Division of Cardiovascular Medicine, Department of Medicine, University of California, San Diego) for providing the DDR2 knockout mouse tissue samples. We thank Dr. Deepthi AN and Dr. Neethu Mohan for assistance in the analysis of IHC sections. We thank Prof. Gaiti Hasan (National Centre for Biological Sciences, Bangalore, India) for providing SKF96365. S. K., H. V., and A. S. T. acknowledge the facilities provided by the Sree Chitra Tirunal Institute for Medical Sciences and Technology.
This work was supported by Department of Biotechnology, Government of India, Research Grant BT/PR23486/BRB/10/1589/2017 (to S. K.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S4.
- α-SMA
- α-smooth muscle actin
- Ang II
- angiotensin II
- DDR2
- discoidin domain receptor 2
- ROS
- reactive oxygen species
- MAPK
- mitogen-activated protein kinase
- GPCR
- G protein–coupled receptor
- TGF-β1
- transforming growth factor-β1
- ERK
- extracellular signal–regulated kinase
- SHR
- spontaneously hypertensive rat
- YAP
- Yes-associated protein
- RTK
- receptor tyrosine kinase
- TRPC6
- transient receptor potential cation channel subfamily C member 6
- FBS
- fetal bovine serum
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- RT-qPCR
- quantitative RT-PCR
- EMSA
- electrophoretic mobility shift assay
- AP-1
- activator protein-1
- OE
- overexpression.
References
- 1. Tallquist M. D., and Molkentin J. D. (2017) Redefining the identity of cardiac fibroblasts. Nat. Rev. Cardiol. 14, 484–491 10.1038/nrcardio.2017.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Philip L., and Shivakumar K. (2013) cIAP-2 protects cardiac fibroblasts from oxidative damage: an obligate regulatory role for ERK1/2 MAPK and NF-κB. J. Mol. Cell. Cardiol. 62, 217–226 10.1016/j.yjmcc.2013.06.009 [DOI] [PubMed] [Google Scholar]
- 3. Rog-Zielinska E. A., Norris R. A., Kohl P., and Markwald R. (2016) The living scar–cardiac fibroblasts and the injured heart. Trends Mol. Med. 22, 99–114 10.1016/j.molmed.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sopel M. J., Rosin N. L., Lee T. D., and Légaré J.-F. (2011) Myocardial fibrosis in response to Angiotensin II is preceded by the recruitment of mesenchymal progenitor cells. Lab. Invest. 91, 565–578 10.1038/labinvest.2010.190 [DOI] [PubMed] [Google Scholar]
- 5. Leitinger B. (2011) Transmembrane collagen receptors. Annu. Rev. Cell Dev. Biol. 27, 265–290 10.1146/annurev-cellbio-092910-154013 [DOI] [PubMed] [Google Scholar]
- 6. Vogel W., Gish G. D., Alves F., and Pawson T. (1997) The discoidin domain receptor tyrosine kinases are activated by collagen. Mol. Cell. 1, 13–23 10.1016/S1097-2765(00)80003-9 [DOI] [PubMed] [Google Scholar]
- 7. Souders C. A., Bowers S. L. K., and Baudino T. A. (2009) Cardiac fibroblast: the renaissance cell. Circ. Res. 105, 1164–1176 10.1161/CIRCRESAHA.109.209809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. George M., Vijayakumar A., Dhanesh S. B., James J., and Shivakumar K. (2016) Molecular basis and functional significance of angiotensin II-induced increase in discoidin domain receptor 2 gene expression in cardiac fibroblasts. J. Mol. Cell. Cardiol. 90, 59–69 10.1016/j.yjmcc.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 9. Henderson N. C., and Sheppard D. (2013) Integrin-mediated regulation of TGFβ in fibrosis. Biochim. Biophys. Acta 1832, 891–896 10.1016/j.bbadis.2012.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jia S., Agarwal M., Yang J., Horowitz J. C., White E. S., and Kim K. K. (2018) Discoidin domain receptor 2 signaling regulates fibroblast apoptosis through PDK1/Akt. Am. J. Respir. Cell Mol. Biol. 59, 295–305 10.1165/rcmb.2017-0419OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Popov C., Radic T., Haasters F., Prall W. C., Aszodi A., Gullberg D., Schieker M., and Docheva D. (2011) Integrins α2β1 and α11β1 regulate the survival of mesenchymal stem cells on collagen I. Cell Death Dis. 2, e186 10.1038/cddis.2011.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hou S., Isaji T., Hang Q., Im S., Fukuda T., and Gu J. (2016) Distinct effects of β1 integrin on cell proliferation and cellular signaling in MDA-MB-231 breast cancer cells. Sci. Rep. 6, 18430 10.1038/srep18430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Olaso E., Labrador J.-P., Wang L., Ikeda K., Eng F. J., Klein R., Lovett D. H., Lin H. C., and Friedman S. L. (2002) Discoidin domain receptor 2 regulates fibroblast proliferation and migration through the extracellular matrix in association with transcriptional activation of matrix metalloproteinase-2. J. Biol. Chem. 277, 3606–3613 10.1074/jbc.M107571200 [DOI] [PubMed] [Google Scholar]
- 14. Zhao X.-K., Cheng Y., Liang Cheng M., Yu L., Mu M., Li H., Liu Y., Zhang B., Yao Y., Guo H., Wang R., and Zhang Q. (2016) Focal adhesion kinase regulates fibroblast migration via integrin β-1 and plays a central role in fibrosis. Sci. Rep. 6, 19276 10.1038/srep19276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang K., Corsa C. A., Ponik S. M., Prior J. L., Piwnica-Worms D., Eliceiri K. W., Keely P. J., and Longmore G. D. (2013) The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat. Cell Biol. 15, 677–687 10.1038/ncb2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Streuli C. H. (2009) Integrins and cell-fate determination. J. Cell Sci. 122, 171–177 10.1242/jcs.018945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eliceiri B. P. (2001) Integrin and growth factor receptor crosstalk. Circ. Res. 89, 1104–1110 10.1161/hh2401.101084 [DOI] [PubMed] [Google Scholar]
- 18. Rockey D. C., Weymouth N., and Shi Z. (2013) Smooth muscle α actin (Acta2) and myofibroblast function during hepatic wound healing. PLoS One 8, e77166 10.1371/journal.pone.0077166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Talior-Volodarsky I., Connelly K. A., Arora P. D., Gullberg D., and McCulloch C. A. (2012) α11 integrin stimulates myofibroblast differentiation in diabetic cardiomyopathy. Cardiovasc. Res. 96, 265–275 10.1093/cvr/cvs259 [DOI] [PubMed] [Google Scholar]
- 20. Liu S., Xu S. W., Blumbach K., Eastwood M., Denton C. P., Eckes B., Krieg T., Abraham D. J., and Leask A. (2010) Expression of integrin β1 by fibroblasts is required for tissue repair in vivo. J. Cell Sci. 123, 3674–3682 10.1242/jcs.070672 [DOI] [PubMed] [Google Scholar]
- 21. Soto-Ribeiro M., Kastberger B., Bachmann M., Azizi L., Fouad K., Jacquier M.-C., Boettiger D., Bouvard D., Bastmeyer M., Hytönen V. P., and Wehrle-Haller B. (2019) β1D integrin splice variant stabilizes integrin dynamics and reduces integrin signaling by limiting paxillin recruitment. J. Cell Sci. 132, jcs224493 10.1242/jcs.224493 [DOI] [PubMed] [Google Scholar]
- 22. Anupama V., George M., Dhanesh S. B., Chandran A., James J., and Shivakumar K. (2016) Molecular mechanisms in H2O2-induced increase in AT1 receptor gene expression in cardiac fibroblasts: a role for endogenously generated angiotensin II. J. Mol. Cell. Cardiol. 97, 295–305 10.1016/j.yjmcc.2016.05.010 [DOI] [PubMed] [Google Scholar]
- 23. Karin M. (1995) The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 270, 16483–16486 10.1074/jbc.270.28.16483 [DOI] [PubMed] [Google Scholar]
- 24. Jiang T., Qu J. J., Nishinaka T., and Zhang N. (2008) Transcription factor AP-1 regulates TGF-β(1)-induced expression of aldose reductase in cultured human mesangial cells. Nephrology 13, 212–217 10.1111/j.1440-1797.2007.00913.x [DOI] [PubMed] [Google Scholar]
- 25. Cowling R. T., Yeo S. J., Kim I. J., Park J. I., Gu Y., Dalton N. D., Peterson K. L., and Greenberg B. H. (2014) Discoidin domain receptor 2 germline gene deletion leads to altered heart structure and function in the mouse. Am. J. Physiol. Heart Circ. Physiol. 307, H773–H781 10.1152/ajpheart.00142.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pinto A. R., Ilinykh A., Ivey M. J., Kuwabara J. T., D'Antoni M. L., Debuque R., Chandran A., Wang L., Arora K., Rosenthal N. A., and Tallquist M. D. (2016) Revisiting cardiac cellular composition. Circ. Res. 118, 400–409 10.1161/CIRCRESAHA.115.307778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Davis J., Burr A. R., Davis G. F., Birnbaumer L., and Molkentin J. D. (2012) A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev. Cell. 23, 705–715 10.1016/j.devcel.2012.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reddy P., Deguchi M., Cheng Y., and Hsueh A. J. W. (2013) Actin cytoskeleton regulates Hippo signaling. PLoS One 8, e73763 10.1371/journal.pone.0073763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martin K., Pritchett J., Llewellyn J., Mullan A. F., Athwal V. S., Dobie R., Harvey E., Zeef L., Farrow S., Streuli C., Henderson N. C., Friedman S. L., Hanley N. A., and Piper Hanley K. (2016) PAK proteins and YAP-1 signalling downstream of integrin β-1 in myofibroblasts promote liver fibrosis. Nat. Commun. 7, 12502 10.1038/ncomms12502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhao B., Li L., Tumaneng K., Wang C.-Y., and Guan K.-L. (2010) A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCFβ-TRCP. Genes Dev. 24, 72–85 10.1101/gad.1843810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kumaran C., and Shivakumar K. (2002) Calcium- and superoxide anion-mediated mitogenic action of substance P on cardiac fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 282, H1855–H1862 10.1152/ajpheart.00747.2001 [DOI] [PubMed] [Google Scholar]
- 32. He X., Li S., Liu B., Susperreguy S., Formoso K., Yao J., Kang J., Shi A., Birnbaumer L., and Liao Y. (2017) Major contribution of the 3/6/7 class of TRPC channels to myocardial ischemia/reperfusion and cellular hypoxia/reoxygenation injuries. Proc. Natl. Acad. Sci. U.S.A. 114, E4582–E4591 10.1073/pnas.1621384114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang B., Jiang J., Yue Z., Liu S., Ma Y., Yu N., Gao Y., Sun S., Chen S., and Liu P. (2016) Store-operated Ca2+ entry (SOCE) contributes to angiotensin II-induced cardiac fibrosis in cardiac fibroblasts. J. Pharmacol. Sci. 132, 171–180 10.1016/j.jphs.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 34. Dokainish H., Teo K., Zhu J., Roy A., AlHabib K. F., ElSayed A., Palileo-Villaneuva L., Lopez-Jaramillo P., Karaye K., Yusoff K., Orlandini A., Sliwa K., Mondo C., Lanas F., Prabhakaran D., et al. (2017) Global mortality variations in patients with heart failure: results from the International Congestive Heart Failure (INTER-CHF) prospective cohort study. Lancet Global Health 5, e665–e672 10.1016/S2214-109X(17)30196-1 [DOI] [PubMed] [Google Scholar]
- 35. Moore-Morris T., Guimarães-Camboa N., Yutzey K. E., Pucéat M., and Evans S. M. (2015) Cardiac fibroblasts: from development to heart failure. J. Mol. Med. 93, 823–830 10.1007/s00109-015-1314-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Valiente-Alandi I., Potter S. J., Salvador A. M., Schafer A. E., Schips T., Carrillo-Salinas F., Gibson A. M., Nieman M. L., Perkins C., Sargent M. A., Huo J., Lorenz J. N., DeFalco T., Molkentin J. D., Alcaide P., and Blaxall B. C. (2018) Inhibiting fibronectin attenuates fibrosis and improves cardiac function in a model of heart failure. Circulation 138, 1236–1252 10.1161/CIRCULATIONAHA.118.034609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zablocki D., and Sadoshima J. (2013) Angiotensin II and oxidative stress in the failing heart. Antioxid. Redox Signal. 19, 1095–1109 10.1089/ars.2012.4588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mann J. F. E., Schmieder R. E., McQueen M., Dyal L., Schumacher H., Pogue J., Wang X., Maggioni A., Budaj A., Chaithiraphan S., Dickstein K., Keltai M., Metsärinne K., Oto A., Parkhomenko A., et al. (2008) Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 372, 547–553 10.1016/S0140-6736(08)61236-2 [DOI] [PubMed] [Google Scholar]
- 39. Nichols T. C., du Laney T., Zheng B., Bellinger D. A., Nickols G. A., Engleman W., and Clemmons D. R. (1999) Reduction in atherosclerotic lesion size in pigs by αVβ3 inhibitors is associated with inhibition of insulin-like growth factor-I–mediated signaling. Circ. Res. 85, 1040–1045 10.1161/01.RES.85.11.1040 [DOI] [PubMed] [Google Scholar]
- 40. Klemke R. L., Yebra M., Bayna E. M., and Cheresh D. A. (1994) Receptor tyrosine kinase signaling required for integrin αvβ5-directed cell motility but not adhesion on vitronectin. J. Cell Biol. 127, 859–866 10.1083/jcb.127.3.859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Friedlander M., Brooks P. C., Shaffer R. W., Kincaid C. M., Varner J. A., and Cheresh D. A. (1995) Definition of two angiogenic pathways by distinct αv integrins. Science 270, 1500–1502 10.1126/science.270.5241.1500 [DOI] [PubMed] [Google Scholar]
- 42. Shintani Y., Fukumoto Y., Chaika N., Svoboda R., Wheelock M. J., and Johnson K. R. (2008) Collagen I–mediated up-regulation of N-cadherin requires cooperative signals from integrins and discoidin domain receptor 1. J. Cell Biol. 180, 1277–1289 10.1083/jcb.200708137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kaur H., Takefuji M., Ngai C. Y., Carvalho J., Bayer J., Wietelmann A., Poetsch A., Hoelper S., Conway S. J., Möllmann H., Looso M., Troidl C., Offermanns S., and Wettschureck N. (2016) Targeted ablation of periostin-expressing activated fibroblasts prevents adverse cardiac remodeling in mice. Circ. Res. 118, 1906–1917 10.1161/CIRCRESAHA.116.308643 [DOI] [PubMed] [Google Scholar]
- 44. Yeh Y.-C., Wu C.-C., Wang Y.-K., and Tang M.-J. (2011) DDR1 triggers epithelial cell differentiation by promoting cell adhesion through stabilization of E-cadherin. Mol. Biol. Cell. 22, 940–953 10.1091/mbc.e10-08-0678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xu H., Bihan D., Chang F., Huang P. H., Farndale R. W., and Leitinger B. (2012) Discoidin domain receptors promote α1β1- and α2β1-integrin mediated cell adhesion to collagen by enhancing integrin activation. PLoS One 7, e52209 10.1371/journal.pone.0052209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ren X.-S., Ling L., Zhou B., Han Y., Zhou Y.-B., Chen Q., Li Y.-H., Kang Y.-M., and Zhu G.-Q. (2017) Silencing salusin-β attenuates cardiovascular remodeling and hypertension in spontaneously hypertensive rats. Sci. Rep. 7, 43259 10.1038/srep43259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brilla C. G., Reams G. P., Maisch B., and Weber K. T. (1993) Renin-angiotensin system and myocardial fibrosis in hypertension: regulation of the myocardial collagen matrix. Eur. Heart J. 14, 57–61 10.1093/eurheartj/14.1.57 [DOI] [PubMed] [Google Scholar]
- 48. Israeli-Rosenberg S., Manso A. M., Okada H., and Ross R. S. (2014) Integrins and integrin-associated proteins in the cardiac myocyte. Circ. Res. 114, 572–586 10.1161/CIRCRESAHA.114.301275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ieda M., Tsuchihashi T., Ivey K. N., Ross R. S., Hong T.-T., Shaw R. M., and Srivastava D. (2009) Cardiac fibroblasts regulate myocardial proliferation through β1 integrin signaling. Dev. Cell. 16, 233–244 10.1016/j.devcel.2008.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu S., Gao J., Ohlemeyer C., Roos D., Niessen H., Köttgen E., and Gessner R. (2005) Activation of AP-1 through reactive oxygen species by angiotensin II in rat cardiomyocytes. Free Radic. Biol. Med. 39, 1601–1610 10.1016/j.freeradbiomed.2005.08.006 [DOI] [PubMed] [Google Scholar]
- 51. Wernig G., Chen S.-Y., Cui L., Van Neste C., Tsai J. M., Kambham N., Vogel H., Natkunam Y., Gilliland D. G., Nolan G., and Weissman I. L. (2017) Unifying mechanism for different fibrotic diseases. Proc. Natl. Acad. Sci. U.S.A. 114, 4757–4762 10.1073/pnas.1621375114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yamamura Y., Hua X., Bergelson S., and Lodish H. F. (2000) Critical role of Smads and AP-1 complex in transforming growth factor-β-dependent apoptosis. J. Biol. Chem. 275, 36295–36302 10.1074/jbc.M006023200 [DOI] [PubMed] [Google Scholar]
- 53. Liu S., and Leask A. (2013) Integrin β1 is required for dermal homeostasis. J. Invest. Dermatol. 133, 899–906 10.1038/jid.2012.438 [DOI] [PubMed] [Google Scholar]
- 54. Krishnamurthy P., Subramanian V., Singh M., and Singh K. (2006) Deficiency of β1 integrins results in increased myocardial dysfunction after myocardial infarction. Heart 92, 1309–1315 10.1136/hrt.2005.071001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Civitarese R. A., Talior-Volodarsky I., Desjardins J.-F., Kabir G., Switzer J., Mitchell M., Kapus A., McCulloch C. A., Gullberg D., and Connelly K. A. (2016) The α11 integrin mediates fibroblast-extracellular matrix-cardiomyocyte interactions in health and disease. Am. J. Physiol. Heart Circ. Physiol. 311, H96–H106 10.1152/ajpheart.00918.2015 [DOI] [PubMed] [Google Scholar]
- 56. Lee H. W., Park Y. M., Lee S. J., Cho H. J., Kim D.-H., Lee J.-I., Kang M.-S., Seol H. J., Shim Y. M., Nam D.-H., Kim H. H., and Joo K. M. (2013) α-Smooth muscle actin (ACTA2) is required for metastatic potential of human lung adenocarcinoma. Clin. Cancer Res. 19, 5879–5889 10.1158/1078-0432.CCR-13-1181 [DOI] [PubMed] [Google Scholar]
- 57. Olson E. N., and Nordheim A. (2010) Linking actin dynamics and gene transcription to drive cellular motile functions. Nat. Rev. Mol. Cell Biol. 11, 353–365 10.1038/nrm2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Follonier L., Schaub S., Meister J.-J., and Hinz B. (2008) Myofibroblast communication is controlled by intercellular mechanical coupling. J. Cell Sci. 121, 3305–3316 10.1242/jcs.024521 [DOI] [PubMed] [Google Scholar]
- 59. Young-Pearse T. L., Bai J., Chang R., Zheng J. B., LoTurco J. J., and Selkoe D. J. (2007) A critical function for β-amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J. Neurosci. 27, 14459–14469 10.1523/JNEUROSCI.4701-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wood E. J. (1983) Molecular Cloning: A Laboratory Manual by T. Maniatis, E. F. Fritsch and J. Sambrook. pp. 545. Cold Spring Harbor Laboratory, New York. 1982. $48. Biochem. Educ. 11, 82 10.1016/0307-4412(83)90068-7 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.