Abstract
Endoplasmic reticulum (ER) stress occurs when the abundance of unfolded proteins in the ER exceeds the capacity of the folding machinery. Despite the expanding cadre of characterized cellular adaptations to ER stress, knowledge of the effects of ER stress on cellular physiology remains incomplete. We investigated the impact of ER stress on ER and inner nuclear membrane protein quality control mechanisms in Saccharomyces cerevisiae. We analyzed the turnover of substrates of four ubiquitin ligases (Doa10, Rkr1/Ltn1, Hrd1, and the Asi complex) and the metalloprotease Ste24 in induced models of ER stress. ER stress did not substantially impact Doa10 or Rkr1 substrates. However, Hrd1-mediated destruction of a protein that aberrantly engages the translocon (Deg1-Sec62) and substrates with luminal degradation signals was markedly impaired by ER stress; by contrast, Hrd1-dependent degradation of proteins with intramembrane degrons was largely unperturbed by ER stress. ER stress impaired the degradation of one of two Asi substrates analyzed and caused a translocon-clogging Ste24 substrate to accumulate in a form consistent with persistent translocon occupation. Degradation of Deg1-Sec62 in the absence of stress and stabilization during ER stress were independent of four ER stress–sensing pathways. Our results indicate ER stress differentially impacts degradation of protein quality control substrates, including those mediated by the same ubiquitin ligase. These observations suggest the existence of additional regulatory mechanisms dictating substrate selection during ER stress.
Keywords: endoplasmic reticulum stress (ER stress), endoplasmic reticulum–associated protein degradation (ERAD), E3 ubiquitin ligase, Saccharomyces cerevisiae, protein translocation, yeast genetics, inner nuclear membrane-associated degradation (INMAD), protein quality control, Ste24, translocon
Introduction
Eukaryotic cells possess sensitive mechanisms to detect and respond to a variety of external and intrinsic stresses. One such stress is the increased abundance of misfolded and unfolded proteins in the endoplasmic reticulum (ER).6 Cells and organisms have evolved a multipronged approach to cope with ER stress.
Much of what has been learned about the cellular response to ER stress was first discovered in Saccharomyces cerevisiae. The prototypical branch of the ER stress response is the unfolded protein response (UPR) (1). In budding yeast, accumulation of misfolded proteins in the ER activates the transmembrane protein Ire1 (in mammals, the UPR has expanded to include two additional transmembrane signal transducers, PERK and ATF6). The cytoplasmic portion of Ire1 carries both kinase and RNase domains. Binding to unfolded proteins by the luminal domain triggers multimerization of Ire1, which undergoes trans-autophosphorylation and RNase activation. This, in turn, stimulates Ire1-dependent noncanonical splicing of the mRNA encoding the Hac1 transcription factor (Xbp1 in mammals), allowing Hac1 protein translation. Hac1 activates an expansive gene expression program to restore ER homeostasis.
Genes induced by Hac1 include those encoding ER-localized chaperones (such as Kar2/BiP) to facilitate protein folding and components of the ER-Associated Degradation (ERAD) machinery to promote proteasomal turnover of aberrant polypeptides that cannot be correctly folded (2). At least four ubiquitin ligases (E3s), Doa10, Hrd1, Rkr1 (also called Ltn1), and Ubr1, promote ERAD of aberrant ER proteins in yeast cells (3–9). Doa10 and Hrd1 are transmembrane proteins with cytosolic catalytic domains (10, 11), whereas Rkr1 and Ubr1 are soluble cytosolic enzymes (12, 13). Doa10 also resides in the inner nuclear membrane (INM), which is physically continuous with the ER, where it promotes the destruction of nucleoplasmic and integral membrane proteins (14, 15). Two additional E3 complexes, the transmembrane Asi complex (Asi1, Asi2, and Asi3) and the anaphase-promoting complex (APC), mediate protein quality control at the INM (16–18).
In general, ERAD E3s target distinct proteins based on the location and nature of the proteins' degradation signals, or degrons. Proteins possessing cytosolic and nucleoplasmic degrons are ubiquitylated by Doa10 in ERAD-C (19–21). Proteins with degrons in the ER lumen or within membrane-spanning segments are generally ubiquitylated by Hrd1 in ERAD-L or ERAD-M, respectively (22–24). Hrd1 also recognizes proteins that aberrantly or persistently engage the translocon via ERAD-T (25). The E3 Rkr1 targets translationally stalled ER-targeted proteins in ERAD-RA (ribosome-associated) (8, 26, 27). Doa10, Asi, and APC promote turnover of nuclear envelope proteins in INM-associated degradation (INMAD) (15–18, 28). These functional distinctions are not absolute. For example, Doa10 promotes degradation of some proteins with intramembrane degrons (29, 30), and Ubr1 redundantly recognizes a subset of Doa10 and Hrd1 substrates (9, 30). An E3-independent degradation mechanism for relieving obstructed translocons was recently identified in which the zinc metalloprotease Ste24 cleaves engineered translocon-clogging proteins (31).
Following the discovery of the UPR, additional ER stress-sensing mechanisms have been identified that reduce the burden of aberrant proteins in the ER. The ER stress surveillance (ERSU) signaling pathway, mediated by the Slt2 mitogen-activated protein kinase, prevents inheritance of cortical ER containing aggregated proteins during ER stress (32, 33). ER stress also promotes lysosomal destruction of ER-resident proteins by at least two mechanisms: the rapid ER stress-induced export (RESET) pathway, in which misfolded glycosylphosphatidylinositol (GPI)-anchored proteins are trafficked to the lysosome via the secretory pathway (34), and the activation of autophagy of ER subdomains (micro-ER-phagy) (35). In macro-ER-phagy, segments of ER are also targeted for lysosomal destruction (36, 37); however, this mechanism has not been shown to be induced by ER stress. In the recently identified stress-induced homeostatically regulated protein degradation (SHRED) pathway, ER stress accelerates Ubr1-dependent degradation of misfolded cytosolic proteins and ER-localized proteins with misfolded cytosolic domains (38). In mammals, a subset of ER-targeted proteins exhibit translocational attenuation during ER stress, presumably as a preemptive measure to reduce the burden of unfolded proteins in the ER (39). This is likely mediated in part by the recently identified function of HRD1 in targeting secretory proteins prior to translocon insertion in a mechanism termed ER preemptive quality control (ERpQC) (40, 41).
Despite the expanding catalogue of characterized ER stress-response mechanisms, not all consequences of ER stress are known or understood. ER stress is a feature of several human diseases, including metabolic and neurodegenerative disorders, some forms of cancer, inflammation and immune syndromes, and mental illness (42–46). Prolonged ER stress leads to cell death, and at least one class of anticancer medications exerts its toxic effects on malignant cells by inducing ER stress (47, 48).
In this work, we systematically analyzed the effect of ER stress on degradation of a panel of ER and INM quality control substrates. We found that ER stress did not substantially alter the degradation profiles of model substrates for Doa10 or Rkr1. However, despite well-documented induction of Hrd1 machinery by the UPR (2), degradation of model luminal and translocon-associated substrates of Hrd1 exhibited strong sensitivity to ER stress. By contrast, degradation of Hrd1 substrates with intramembrane degrons proceeded with normal kinetics in the face of ER stress, indicating Hrd1 is not broadly inhibited during stress. Furthermore, two Asi substrates demonstrated different sensitivities to ER stress. Divergent responses of ERAD and INMAD pathways mediated by the same ubiquitin ligase suggest novel layers of regulation of protein degradation by ER stress. Finally, a translocon-clogging substrate of Ste24 accumulated in a form consistent with prolonged translocon engagement. Impaired degradation of proteins that persistently engage the translocon is expected to curtail ER import of other proteins, thereby mitigating the impact of ER stress.
Results
ER stress impairs degradation of a Hrd1 ERAD-T substrate
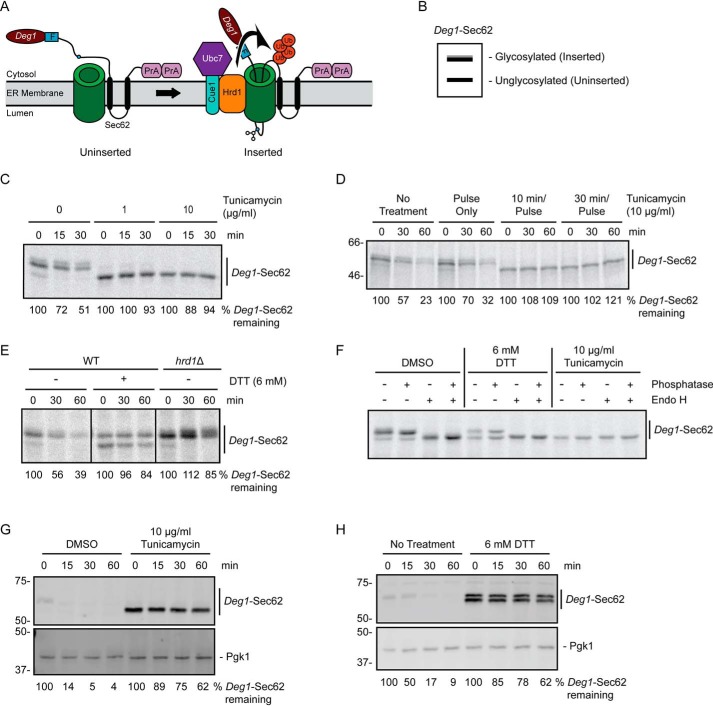
N-terminal fusion of the Deg1 degron from MATα2 to Sec62 converts the protein to a Hrd1 ERAD-T substrate (25). Following co-translational insertion of two transmembrane segments of Sec62, a portion of the N-terminal tail aberrantly translocates into the translocon (Fig. 1A). A disulfide bond forms between a portion of the Deg1-Sec62 N-terminal tail and the interior of the translocon, contributing to persistent channel engagement (25, 49). After aberrant translocon engagement, Deg1-Sec62 becomes progressively modified by glycosylation, causing the protein to migrate as multiple species by SDS-PAGE (Fig. 1B) (25). Deg1-Sec62 is also acetylated on its N terminus and on two internal lysine residues (50). Neither acetylation nor glycosylation are required for Deg1-Sec62 degradation (25, 50). However, glycosylation serves as a visual indicator of aberrant translocon engagement. This aberrant translocon engagement triggers Hrd1-dependent degradation of Deg1-Sec62, although the mechanism by which Hrd1 recognizes the protein remains unclear (25).
Figure 1.
ER stress impairs degradation of a Hrd1 ERAD-T substrate. A, schematic depiction of Deg1-Sec62 prior to (uninserted) and following (inserted) aberrant translocon engagement. Deg1-Sec62 consists of Deg1 (the N-terminal 67 amino acids from the yeast transcriptional repressor MATα2), a FLAG (F) epitope, the two-transmembrane protein Sec62, and two copies of the S. aureus protein A (PrA). Following translocon engagement, Deg1-Sec62 undergoes extensive PTM, including N-linked glycosylation, and is polyubiquitylated by Hrd1. The primary glycosylated asparagine residue is indicated as a blue circle. Ub, ubiquitin. B, virtual SDS-PAGE illustrates differential migration of Deg1-Sec62 based on glycosylation status. C, pulse-chase analysis of WT yeast expressing Deg1-Sec62 cultured in the presence of DMSO or tunicamycin at the indicated concentrations for 30 min. DMSO and tunicamycin were maintained at the same concentrations throughout pulse labeling. D, pulse-chase analysis of WT yeast expressing Deg1-Sec62 cultured in the presence of 10 μg/ml tunicamycin for the indicated times (or DMSO for 30 min). Tunicamycin and DMSO were maintained at the same concentrations throughout pulse labeling. E, pulse-chase analysis of WT or hrd1Δ yeast expressing Deg1-Sec62 cultured in the presence of 6 mm DTT (or no treatment) for 30 min. DTT was maintained at the same concentrations throughout pulse labeling. F, doa10Δ hrd1Δ yeast cells expressing Deg1-Sec62 were cultured in the presence 6 mm DTT, 10 μg/ml tunicamycin, or DMSO control for 30 min. DTT, tunicamycin, and DMSO were maintained at the indicated concentrations throughout pulse labeling. Immunoprecipitated Deg1-Sec62 was incubated in the presence or absence of Endo H and calf intestinal phosphatase as indicated. G and H, cycloheximide chase analysis of WT yeast expressing Deg1-Sec62 cultured in the presence of 10 μg/ml tunicamycin or DMSO (G) or 6 mm DTT or no treatment (H) for 1 h. Tunicamycin, DTT, or DMSO were maintained at the same concentration during incubation with cycloheximide. Deg1-Sec62 was detected with AlexaFluor-680–conjugated rabbit anti-mouse antibodies. Pgk1 served as a loading control. Where indicated, the percentage of Deg1-Sec62 remaining at each time point is presented below the image. For cycloheximide chase experiments, Deg1-Sec62 signal intensity was normalized to Pgk1. The experiment depicted in C was performed three times with tunicamycin at 0 and 10 μg/ml, and one time with tunicamycin at 1 μg/ml. Experiments depicted in D and F were performed one time. Experiments depicted in E, G, and H were performed three times.
To determine the impact of ER stress on ERAD-T, we analyzed the degradation of Deg1-Sec62 by pulse-chase in the presence and absence of the ER-specific stressor tunicamycin, which blocks N-linked glycosylation. In untreated cells, Deg1-Sec62 exhibited characteristic modification and degradation over time (Fig. 1C). Strikingly, treatment of yeast with 1 μg/ml tunicamycin for 30 min prior to radioactive labeling of nascent protein profoundly impaired Deg1-Sec62 degradation. Observable post-translational modification (PTM) of Deg1-Sec62 was also strongly impaired. Increasing tunicamycin concentration to 10 μg/ml completely abrogated observable Deg1-Sec62 PTM. The effects of tunicamycin were rapid. Degradation and PTM were strongly impaired when tunicamycin incubation was reduced to 10 min prior to radiolabeling, but not when tunicamycin was present only during pulse labeling (Fig. 1D). Similarly, treatment with the reducing agent dithiothreitol (DTT) at a concentration of 6 mm strongly impaired Deg1-Sec62 turnover (Fig. 1E); DTT causes ER stress by countering the normally oxidizing environment of the ER lumen. DTT also reduced PTM of Deg1-Sec62, but its impact on PTM was less pronounced than that of tunicamycin.
To characterize the PTM impaired by ER stressors, we incubated pulse-labeled Deg1-Sec62 with endoglycosidase H (Endo H) or calf intestinal phosphatase. The electrophoretic mobility of Deg1-Sec62 from unstressed cells exhibited strong sensitivity to Endo H, consistent with N-linked glycosylation (Fig. 1F). By contrast, Deg1-Sec62 exhibited little if any change in mobility after treatment with phosphatase, which is consistent with failure to detect Deg1-Sec62 phosphorylation in a recent biochemical analysis (50). Loss of Endo H-sensitive Deg1-Sec62 species following tunicamycin treatment confirmed that the compound completely inhibited Deg1-Sec62 N-glycosylation. DTT reduced the extent of or delayed glycosylation.
The pulse-chase experiments described above evaluated the degradation and modification of nascent Deg1-Sec62. Treatment for 1 h with either tunicamycin or DTT also strongly stabilized and impaired PTM of the steady-state Deg1-Sec62 population in cycloheximide-chase experiments (Fig. 1, G and H). Taken together, our results indicate that two different forms of ER stress strongly impair degradation and PTM of nascent and steady-state pools of a Hrd1 substrate that aberrantly engages the ER translocon.
ER stress differentially impacts degradation of Hrd1 substrates
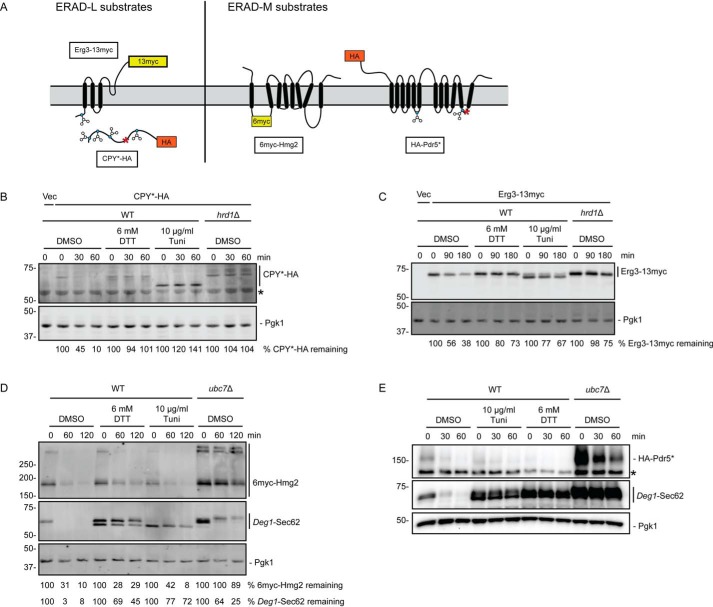
Previous work indicated that degradation of the soluble Hrd1 ERAD-L substrate CPY* (a misfolded variant of carboxypeptidase Y possessing the G255R mutation) is inhibited by ER stress (2). Thus, one possible explanation for impairment of Deg1-Sec62 degradation is that Hrd1 catalytic activity is impaired by such stress. We analyzed the impact of ER stress on the degradation of a panel of Hrd1 substrates (depicted in Fig. 2A). Consistent with earlier reports, HA-tagged CPY* was strongly stabilized by both DTT and tunicamycin (Fig. 2B). Similar to Deg1-Sec62, both treatments impeded PTM of CPY*-HA, which is N-glycosylated. DTT and tunicamycin also stabilized the transmembrane ERAD-L substrate Erg3-13myc (51) to a similar extent as HRD1 deletion (Fig. 2C).
Figure 2.
ER stress impairs degradation of Hrd1 ERAD-L substrates but not Hrd1 ERAD-M substrates. A, schematic of Hrd1 substrates investigated in this figure. Blue circles represent glycosylated amino acids. Red asterisks indicate destabilizing point mutations. B–E, cycloheximide chase analysis of yeast of the indicated genotypes harboring an empty vector (Vec) or expressing CPY*-HA (B), Erg3-13myc (C), 6myc-Hmg2 (D), or HA-Pdr5* (E) cultured in the presence of 6 mm DTT, 10 μg/ml tunicamycin, or DMSO for 1 h. DTT, tunicamycin, and DMSO were maintained at the same concentration during incubation with cycloheximide. Asterisks in B and E denote nonspecific bands. Cells analyzed in D and E also expressed Deg1-Sec62 as a control for ER stress induction. CPY*-HA and Pdr5*-HA were detected with anti-HA antibodies. Erg3-13myc and 6myc-Hmg2 were detected with anti-Myc antibodies. Deg1-Sec62 was detected with AlexaFluor-680–conjugated rabbit anti-mouse antibodies (D) or peroxidase anti-peroxidase antibodies (E). Pgk1 served as a loading control. Where indicated, the percentage of substrate remaining (normalized to Pgk1) at each time point is presented below the image. We note that, relative to other experiments, Deg1-Sec62 exhibited weaker stabilization in the absence of UBC7 in the cycloheximide chase presented in D; this may be related to differences in genetic background in the strains analyzed. Experiments depicted in B–D were performed three times. The experiment depicted in E was performed two times.
We next evaluated the effect of ER stress on degradation of ERAD-M substrates 6myc-Hmg2 (3) and Pdr5*-HA (52). In contrast to ERAD-T and -L substrates, degradation of 6myc-Hmg2 (Fig. 2D) and Pdr5*-HA (Fig. 2E) was largely insensitive to DTT and tunicamycin; degradation of Deg1-Sec62 expressed in the same cells was markedly impaired during ER stress. These substrates were substantially stabilized by loss of Ubc7, the primary ubiquitin-conjugating enzyme that functions with Hrd1 (4). Finally, we evaluated the impact of ER stress on turnover of a self-ubiquitylating substrate (SUS-GFP) in which GFP and the Hrd1 RING domain are fused to a Myc-tagged transmembrane portion of Hmg1 (53, 54). Neither form of ER stress impaired turnover of SUS-GFP (Fig. S1). Thus, stabilization of model ERAD-T and ERAD-L substrates by ER stress is not due to broad impairment of Hrd1 ubiquitin ligase activity.
ER stress does not impair Doa10-dependent degradation
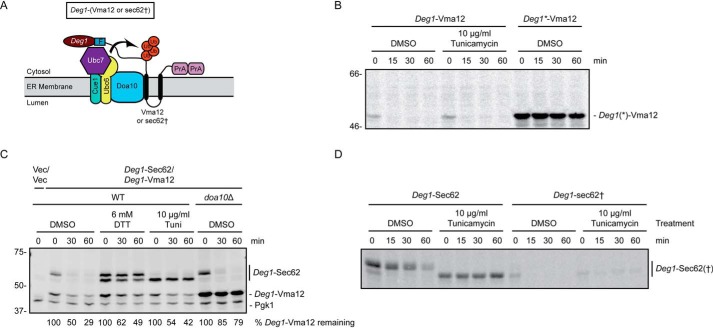
We evaluated the impact of ER stress on ERAD substrates targeted by Doa10. The nascent population of the transmembrane Doa10 ERAD-C substrate Deg1-Vma12 (Fig. 3A) (21) exhibited rapid degradation in the presence of ER stress in pulse-chase experiments (Fig. 3B). By contrast, this model Doa10 substrate was strongly stabilized by point mutations in the Deg1 degron (Deg1*, F18S and I22T (55)) that prevent Doa10-dependent degradation (21). Similarly, ER stress only mildly perturbed degradation of the steady-state population of Deg1-Vma12. This contrasted sharply with the strong stabilization of Deg1-Sec62 in the same cells (Fig. 3C).
Figure 3.
ER stress does not impair degradation of Doa10 ERAD-C substrates. A, schematic of Doa10 substrates investigated in this figure. Deg1-Vma12 consists of Deg1, a FLAG (F) epitope, the two-transmembrane protein Vma12, and two copies of the S. aureus protein A (PrA). Deg1-sec62† is Deg1-Sec62 with a point mutation that prevents aberrant translocon engagement, thus rendering the protein a Doa10 substrate. Ub, ubiquitin. B and D, pulse-chase analysis of WT yeast expressing Deg1(*)-Vma12 or Deg1-Sec62(†) cultured in the presence of 10 μg/ml tunicamycin or DMSO for 30 min. Tunicamycin and DMSO were maintained at the same concentration throughout pulse labeling. C, cycloheximide chase analysis of yeast of the indicated genotypes expressing Deg1-Vma12 and Deg1-Sec62 or harboring empty vectors (Vec/Vec), cultured in the presence of 6 mm DTT, 10 μg/ml tunicamycin, or DMSO for 1 h. DTT, tunicamycin, and DMSO were maintained at the same concentration during incubation with cycloheximide. Deg1-Sec62 and Deg1-Vma12 were detected with AlexaFluor-680–conjugated rabbit anti-mouse antibody. Pgk1 served as a loading control. The percentage of Deg1-Vma12 remaining at each time point (normalized to Pgk1) is indicated below the image. Experiments depicted in B and D were performed one time. The experiment depicted in C was performed three times.
Deg1-sec62† possesses a mutation in Sec62 (G127D of the fusion protein, equivalent to G37D of untagged Sec62) that substantially reduces aberrant translocation of its N-terminal tail (25, 56, 57). This variant is degraded predominantly by the Doa10 ERAD-C pathway. A minor subpopulation of this protein still undergoes aberrant translocation and retains Hrd1-dependent degradation (25). Deg1-sec62† was highly unstable, regardless of the presence of tunicamycin (Fig. 3D). Interestingly, a small fraction of Deg1-sec62† was stabilized by tunicamycin; this likely reflects the fraction of Deg1-sec62† that has become an ERAD-T (Hrd1) substrate by virtue of having undergone aberrant translocation.
ER stress does not impact Rkr1 substrate abundance
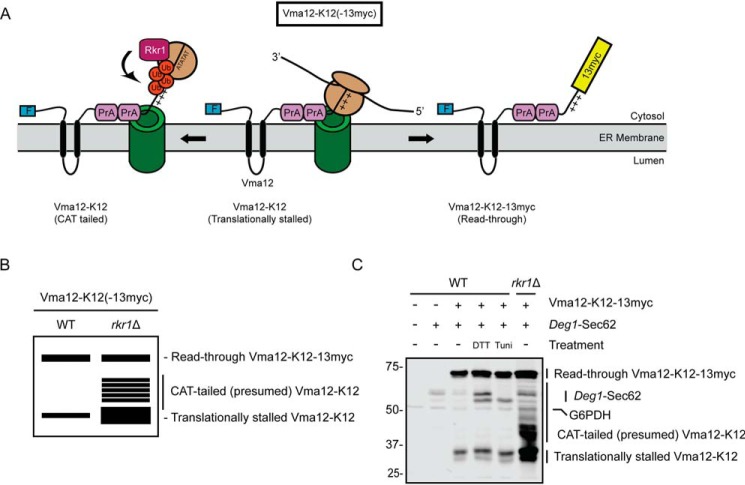
Vma12-K12-13myc is a model Rkr1 ERAD-RA substrate (Fig. 4A) (8). In this construct, N-terminally FLAG-tagged Vma12 is followed, in sequence, by two copies of protein A, 12 lysine residues (K12), and a 13myc epitope. The positively charged K12 sequence triggers ribosome stalling, likely via ionic attraction to the negatively charged ribosome exit tunnel (12, 58, 59). A virtual SDS-PAGE of lysates from WT and rkr1Δ cells expressing Vma12-K12-13myc is depicted in Fig. 4B. When Vma12-K12-13myc expressed in WT yeast cells is analyzed by SDS-PAGE and immunoblotting, two major populations are observed: a translationally stalled product (Vma12-K12) and the full-length translational read-through product (Vma12-K12-13myc). RKR1 deletion increases abundance of the translationally-stalled protein relative to the full-length read-through product (8). Loss of RKR1 also results in the appearance of multiple species migrating more slowly than Vma12-K12. We speculate these species represent Vma12-K12 molecules that have been modified by the C-terminal addition of alanine and threonine (CAT tails) as observed for soluble translationally-stalled substrates of Rkr1 (60, 61).
Figure 4.
ER stress does not alter abundance of an Rkr1 ERAD-RA substrate. A, schematic of Vma12-K12-13myc, which consists of a FLAG epitope tag (F), the two-transmembrane protein Vma12, two copies of the S. aureus protein A (PrA), 12 lysine (K12) residues (depicted as sequential “+” symbols), and a 13myc epitope tag. After insertion of the two transmembrane segments of Vma12, K12 triggers translational stalling (middle). This is resolved by predicted C-terminal addition of alanine and threonine (AT) residues (CAT tailing), Rkr1-mediated ubiquitylation, and degradation (left) or release from stalling and translation of 13myc (right). Ub, ubiquitin. B, virtual SDS-PAGE illustrates differential migration of translationally stalled Vma12-K12, CAT-tailed Vma12-K12, and read-through product Vma12-K12-13myc in WT and rkr1Δ yeast lysates. C, yeast of the indicated genotypes harboring plasmids encoding Vma12-K12-13myc and Deg1-Sec62 (as a control for ER stress induction) or empty vectors were cultured in the presence of 6 mm DTT, 10 μg/ml tunicamycin, or DMSO (−) for 1 h prior to lysis and separation by SDS-PAGE. Vma12-K12-13myc was detected using anti-Myc antibodies, which bind to both 13myc and protein A. Deg1-Sec62 was detected with AlexaFluor-680–conjugated rabbit anti-mouse antibodies. G6PDH served as a loading control. Expression of Vma12-K12(-13myc) in rkr1Δ cells may increase Deg1-Sec62 levels, suggesting stabilized ERAD-RA substrates may cross-inhibit ERAD-T. The experiment depicted was performed four times.
Incubation with ER stressors did not substantially alter Vma12-K12 mobility or abundance (Fig. 4C). We also have not observed an increase in the abundance of translationally stalled species of model soluble ER-targeted Rkr1 substrates in cells exposed to ER stress.7 Our results suggest that ER stress does not inhibit Rkr1-dependent destruction of translationally stalled ERAD-RA substrates.
ER stress differentially impacts degradation of Asi substrates
We investigated the effect of ER stress on turnover of Erg11-FLAG and Vtc1-3HA, which are degraded via Asi-mediated INMAD upon mislocalization to the INM (Fig. 5A) (16, 17). Whereas ER stress perturbed Erg11-FLAG steady-state abundance, its turnover rate was largely unaffected relative to the impact of ASI1 deletion (Fig. 5B). By contrast, ER stress stabilized Vtc1-3HA to a similar degree as loss of ASI1 (Fig. 5C). Thus, Asi substrates are modestly, but differentially, sensitive to ER stress.
Figure 5.
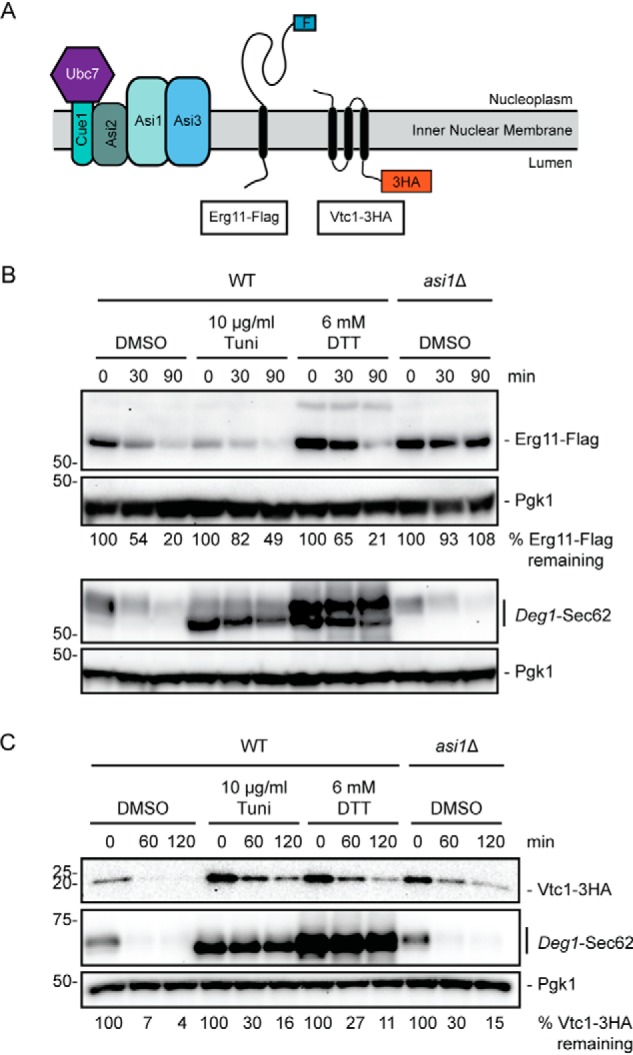
ER stress differentially affects degradation of Asi INMAD substrates. A, schematic of Asi substrates investigated in this figure. B, cycloheximide chase analysis of yeast of the indicated genotypes expressing Erg11-FLAG or Deg1-Sec62 cultured in the presence of 6 mm DTT, 10 μg/ml tunicamycin, or DMSO for 1 h. C, cycloheximide chase analysis of yeast of the indicated genotypes expressing Vtc1-3HA and Deg1-Sec62 cultured in the absence or presence of 6 mm DTT for 1 h. DTT, tunicamycin, and DMSO were maintained at the same concentration during incubation with cycloheximide. Erg11-FLAG was detected with anti-FLAG antibodies. Vtc1-3HA was detected with anti-HA antibodies. Deg1-Sec62 was detected with peroxidase anti-peroxidase antibodies. Pgk1 served as a loading control. The experiment depicted in B was performed two times. The experiment depicted in C was performed three times (with the exception of tunicamycin treatment, which was performed one time).
ER stress impairs degradation of a translocon-clogging substrate of Ste24
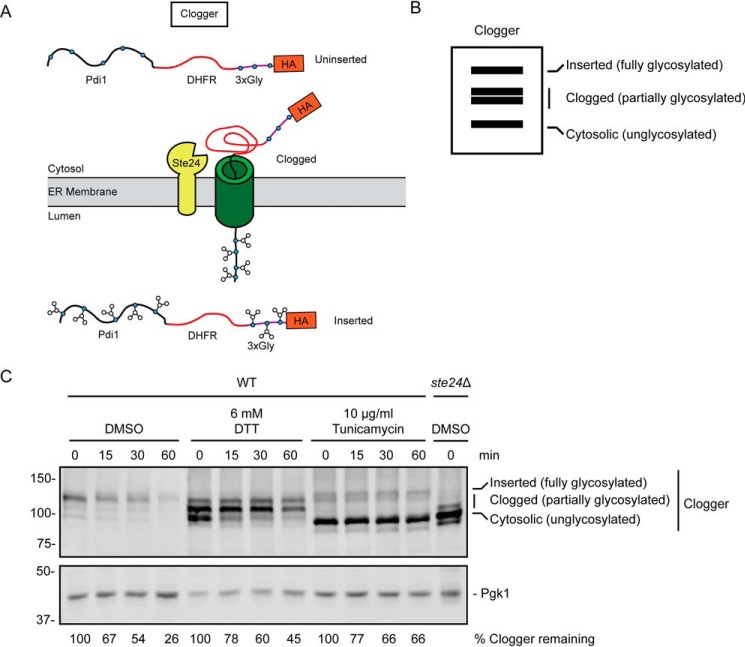
We analyzed the effects of ER stress on a second protein engineered to clog the ER translocon. This protein, dubbed “Clogger,” consists of the soluble ER luminal Pdi1 protein followed, in sequence, by a rapidly folding variant of DHFR, three engineered glycosylation acceptor sequences, and an HA epitope (Fig. 6A) (31). The Pdi1 signal sequence promotes post-translational translocation. However, the rapidly folding DHFR causes a substantial fraction of the protein to clog the translocon. Because sequences upstream and downstream of the clogging moiety possess N-glycosylation acceptor sites, Clogger's ER insertion status can be assessed by comparing the relative abundance of differently migrating species (Fig. 6B). The fastest migrating species are nonglycosylated, cytosolic (uninserted) molecules. The slowest migrating species are fully glycosylated, completely translocated molecules. Species exhibiting intermediate migration are partially glycosylated, translocationally stalled molecules (31). The metalloprotease Ste24 cleaves the clogged form of this protein, thereby relieving translocon obstruction. Loss of Ste24 causes accumulation of faster migrating (i.e. clogged and cytosolic) forms of Clogger.
Figure 6.
ER stress impairs degradation of a translocon-clogging substrate of Ste24. A, schematic of Clogger protein prior to (uninserted), during (clogged), and following (inserted) translocon engagement. Clogger consists of Pdi1 (which possesses glycosylation sites), DHFR, three additional glycosylation sites, and an HA epitope. Glycosylated amino acids are depicted as blue circles. B, virtual SDS-PAGE illustrates differential migration of uninserted, clogged, and inserted Clogger. C, cycloheximide chase analysis of yeast of the indicated genotypes expressing Clogger cultured in the presence of 6 mm DTT, 10 μg/ml tunicamycin, or DMSO for 1 h. DTT, tunicamycin, and DMSO were maintained at the same concentration during incubation with cycloheximide. Clogger was detected with anti-HA antibodies. Pgk1 served as a loading control. The percentage of Clogger remaining at each time point (normalized to Pgk1) is indicated below the image. The experiment depicted was performed three times.
In the presence of DTT, faster migrating (presumably clogged and preinserted) Clogger species accumulated (Fig. 6C). Tunicamycin profoundly stabilized and impaired modification of Clogger. Thus, ER stress impairs degradation of Clogger and causes it to accumulate in a form consistent with persistent translocon engagement.
Deg1-Sec62 induces the UPR
ER stress impairs degradation of Deg1-Sec62 (Fig. 1) and Clogger (Fig. 6), two proteins that aberrantly engage the translocon. High-level expression of Clogger induces the UPR (31). To determine whether Deg1-Sec62 also induces the UPR, we transformed WT and ubc7Δ yeast with an empty vector or a plasmid encoding Deg1-Sec62 driven by the strong glyceraldehyde-3-phosphate dehydrogenase (GPD) promoter. These yeast expressed GFP under the control of the unfolded protein response element (UPRE). Fluorescence intensity, measured by flow cytometry, is therefore a readout of ER UPR induction. Deg1-Sec62 expression caused an ∼2-fold induction of the UPR in ubc7Δ cells, consistent with exacerbation of ER stress by persistent translocon engagement (Fig. 7).
Figure 7.
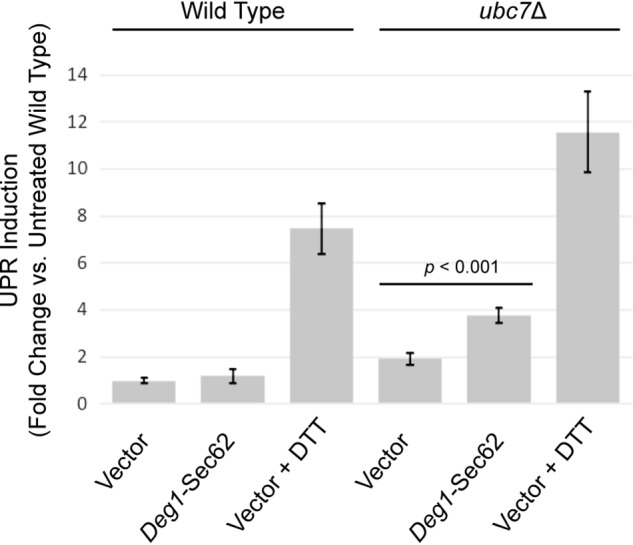
Deg1-Sec62 induces the unfolded protein response. Abundance of the UPR reporter GFP (driven by the UPRE) was analyzed by flow cytometry in WT and ubc7Δ yeast. Mid-exponential–phase cells harboring an empty vector or a plasmid encoding Deg1-Sec62 under the control of the GPD promoter were incubated in the absence (vector and Deg1-Sec62) or presence (vector) of 6 mm DTT for 1 h. The mean fluorescence intensity of 10,000 cells from each of nine cultures for each condition was normalized to the average mean fluorescence intensity of nine cultures of untreated WT cells harboring a vector. Mean fluorescence intensity ± standard error of the mean is presented. A two-tailed unpaired t test was performed to determine the significance of the difference between ubc7Δ cells harboring an empty vector and those expressing Deg1-Sec62.
ERAD-T and its inhibition during ER stress are independent of characterized ER stress-responsive pathways
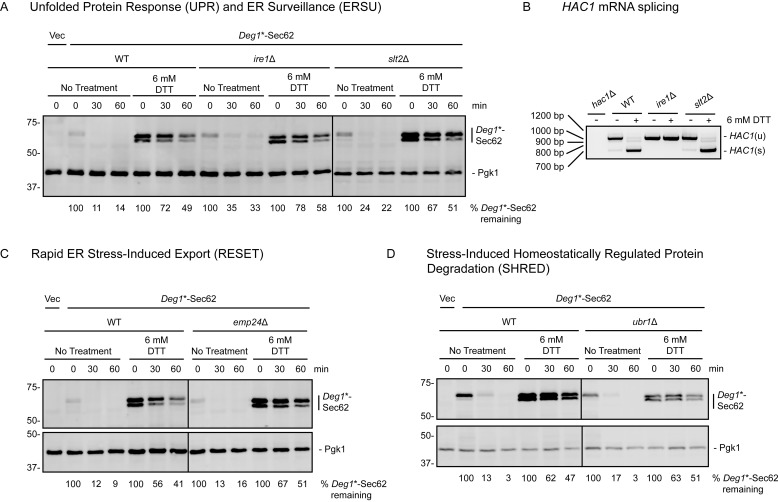
ERAD-T impairment during ER stress was surprising, given that the UPR increases expression of ubiquitin ligases (including Hrd1) and chaperone proteins involved in ER quality control (2). We tested the hypothesis that the UPR selectively inhibits ERAD-T during stress. In this case, restoration of ERAD-T during stress in cells lacking the UPR transducer Ire1 would be predicted. However, impairment of Deg1*-Sec62 degradation by ER stress was not mitigated by loss of Ire1. Furthermore, ERAD-T proceeded largely unimpeded in ire1Δ cells (Fig. 8A). Failure to splice HAC1 mRNA confirmed UPR deficiency in ire1Δ cells (Fig. 8B).
Figure 8.
Characterized ER stress-sensing pathways do not regulate ERAD-T in the presence or absence of ER stress. A, C, and D, cycloheximide chase analysis of yeast of the indicated genotypes expressing Deg1*-Sec62 or harboring an empty vector (Vec) cultured in the presence or absence of 6 mm DTT for 1 h. DTT was maintained at the same concentration during incubation with cycloheximide. Deg1*-Sec62 was detected with peroxidase anti-peroxidase antibodies. Pgk1 served as a loading control. The percentage of Deg1*-Sec62 remaining at each time point (normalized to Pgk1) is indicated below the images. IRE1 (A), SLT2 (A), EMP24 (C), and UBR1 (D) gene deletions were verified by PCR genotyping.8 B, yeast of the indicated genotypes were incubated in the presence or absence of 6 mm DTT for 1 h prior to RNA extraction and RT-PCR to analyze HAC1 mRNA splicing. Expected product sizes are 969 bp for unspliced HAC1 (HAC1(u)) and 717 bp for spliced HAC1 (HAC1(s)). Experiments depicted in A, C, and D were performed three times (with the exception of the right panel of A, which was performed two times). The experiment depicted in B was performed one time.
We investigated whether mediators of other ER stress-responsive pathways are required for Deg1*-Sec62 degradation or stabilization by ER stress. Deletion of the gene encoding the Slt2 kinase, which prevents transmission of stressed ER to daughter cells via the ERSU mechanism (32), did not stabilize Deg1*-Sec62 under nonstress conditions or prevent its stabilization by tunicamycin (Fig. 8A). In mammalian cells, where the RESET pathway was first characterized, the p24 family member Tmp21 mediates ER export of GPI-anchored proteins during ER stress (34). Loss of the Tmp21 homologue Emp24, which results in a p24-null phenotype in yeast (62), did not impair Deg1*-Sec62 degradation or ER stress-dependent stabilization (Fig. 8C). Finally, mutation of the SHRED mediator Ubr1, which ubiquitylates misfolded proteins during cellular stress (38), did not detectably alter Deg1*-Sec62 degradation kinetics in the presence or absence of ER stress (Fig. 8D).
We note that the variant of Deg1-Sec62 (Deg1*-Sec62) employed in experiments presented in Fig. 8 (and in Fig. 11, A and C) possesses point mutations in Deg1 (F18S and I22T). These alterations do not affect aberrant translocon engagement or Hrd1-dependent degradation (25). This construct has been used interchangeably with Deg1-Sec62 to investigate Hrd1-dependent ERAD-T (25, 50).
Figure 11.
ERAD-T is not broadly stress-sensitive. A, cycloheximide chase analysis of WT yeast harboring an empty vector (Vec) or expressing Deg1*-Sec62 cultured in inositol-rich medium in the presence or absence of 6 mm DTT for 1 h or shifted to inositol-free medium for 5 h. Cells also possessed a plasmid encoding GFP (driven by the UPRE). DTT concentration and inositol abundance were maintained during incubation with cycloheximide. B, cycloheximide chase analysis of WT yeast expressing Deg1-Sec62 cultured at 30 °C in the presence or absence of 6 mm DTT or shifted to 42 °C in the absence of 6 mm DTT for 1 h. Temperatures were maintained during incubation with cycloheximide. C, cycloheximide chase analysis of WT yeast harboring an empty vector or expressing Deg1*-Sec62 in the presence or absence of 0.4 mm hydrogen peroxide (H2O2) for 1h. H2O2 concentrations were maintained during incubation with cycloheximide. D, in parallel to experiment depicted in C, mid-exponential phase yeast expressing oxidant-responsive Rtc3-GFP were analyzed by flow cytometry following incubation in the presence of 0.4 mm H2O2 for 1 h. The mean fluorescence intensity for each culture was normalized to the average mean fluorescence intensity of three repeats of untreated cells. Mean fluorescence intensity ± standard error of the mean is presented for three repeats of 10,000 cells for each condition. A–C, Pgk1 served as a loading control. A and C, percentage of Deg1*-Sec62 remaining at each time point (normalized to Pgk1) is indicated below the images. Experiments depicted were performed three times.
Kar2 overexpression does not rescue Deg1-Sec62 degradation during ER stress
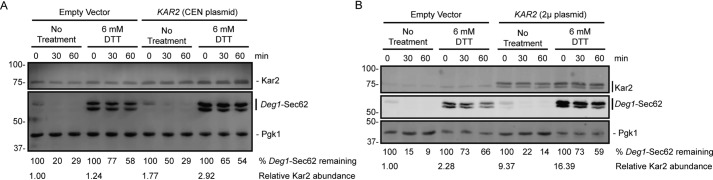
We tested whether overexpression of the multifunctional ER chaperone Kar2 suppresses inhibition of Deg1-Sec62 degradation during ER stress. We mildly overexpressed Kar2 by transforming WT yeast expressing Deg1-Sec62 with a centromeric plasmid encoding Kar2 (Fig. 9A). Western blot analysis confirmed a mild plasmid-dependent increase in Kar2 abundance. This mildly increased KAR2 gene dosage did not accelerate Deg1-Sec62 degradation in the presence of DTT.
Figure 9.
Kar2 overexpression does not rescue impaired ERAD-T during ER stress. Cycloheximide chase analysis of WT yeast expressing Deg1-Sec62 and harboring low-copy (centromeric) (A) or high-copy (2μ) (B) plasmids encoding Kar2 (or matching empty vector controls) cultured in the presence of 6 mm DTT or no treatment for 1 h. DTT was maintained at the same concentration during incubation with cycloheximide. Deg1-Sec62 was detected with AlexaFluor-680–conjugated rabbit anti-mouse antibodies. Kar2 was detected with anti-Kar2 antibodies. Pgk1 served as a loading control. The percentage of Deg1-Sec62 remaining at each time point (normalized to Pgk1) and Kar2 steady-state abundance relative to Empty Vector/No Treatment controls (normalized to Pgk1) are indicated below the images. Experiments depicted were performed three times.
To more dramatically increase KAR2 gene dosage, we transformed yeast expressing Deg1-Sec62 with a 2μ plasmid expressing KAR2 from a yeast genomic library clone (63). Western blot analysis confirmed overexpression of Kar2 (Fig. 9B). In cells harboring the KAR2 plasmid, we observed the appearance of a second, more slowly migrating species of Kar2, which likely corresponds to cytosolic Kar2 precursor that has retained its signal peptide prior to translocation (64). The accumulation of immature Kar2 in cells expressing Kar2 from a 2μ plasmid suggests an effective upper limit for ER-specific Kar2 overexpression. This moderately increased KAR2 expression also did not accelerate Deg1-Sec62 degradation in the presence of DTT.
ER stress does not alter membrane association of Deg1-Sec62
ER stress limits Deg1-Sec62 PTM. Because these modifications largely occur in the ER lumen, we considered the possibility that ER stress alters translocation and membrane association of Deg1-Sec62, thereby impairing its ability to be recognized or degraded by Hrd1. We subjected ER-derived microsomes prepared from nonstressed and DTT-stressed cells to a variety of conditions to analyze the membrane association of Deg1-Sec62 (Fig. 10). Deg1-Sec62 was solubilized by detergent (Triton X-100). Consistent with membrane association, Deg1-Sec62 was not substantially solubilized following incubation with sodium chloride (which solubilizes cytosolic peripheral proteins) or sodium carbonate (which solubilizes both luminal and cytosolic peripheral proteins). Incubation of cells with DTT did not markedly alter the membrane association properties of Deg1-Sec62. Therefore, impairment of Deg1-Sec62 degradation by ER stress is not due to a stress-dependent change in Deg1-Sec62 membrane association.
Figure 10.
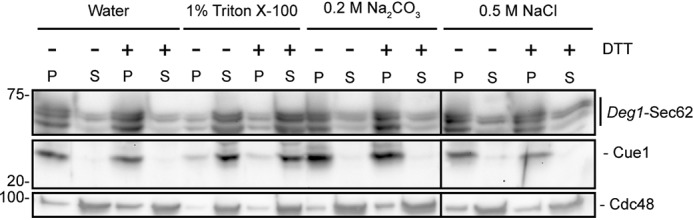
ER stress does not alter Deg1-Sec62 membrane association. ER-derived microsomes were prepared from hrd1Δ yeast expressing Deg1-Sec62 that had been cultured in the presence or absence of 6 mm DTT for 1 h. Microsomal fractions were incubated in the presence of water, 1% Triton X-100, 0.5 m sodium carbonate, or 0.5 m sodium chloride before being separated into pellet (P) and supernatant (S) fractions and solubilized. Deg1-Sec62 was detected with peroxidase anti-peroxidase antibodies. Cue1 and Cdc48 were detected by anti-Cue1 and anti-Cdc48 antibodies, respectively. The experiment depicted was performed two times (with the exception of sodium chloride treatment, which was performed one time).
ERAD-T is not broadly stress-sensitive
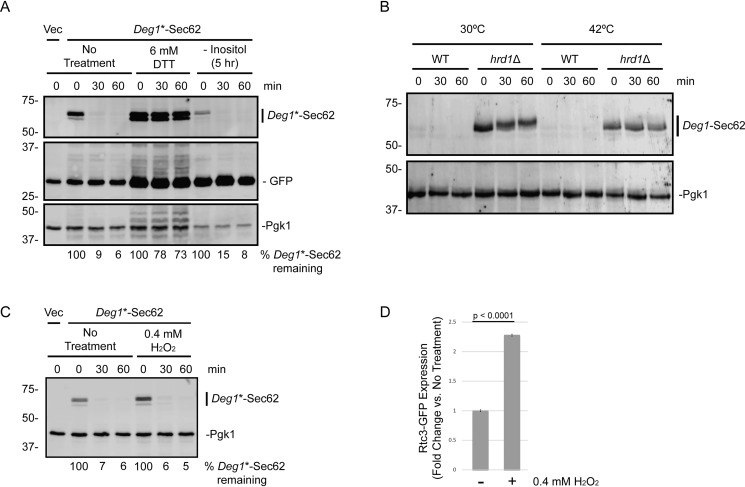
ER stress can also be induced by membrane aberrancy caused by inositol depletion (65). We cultured exponential-phase cells expressing Deg1*-Sec62 in the presence or absence of inositol for 5 h. Inositol depletion induced the UPR, as evidenced by increased UPRE-driven GFP expression (Fig. 11A). However, the rate of Deg1*-Sec62 degradation was not impacted by inositol limitation.
DTT and tunicamycin induce ER stress by specifically promoting misfolding of ER-localized proteins. We asked whether heat shock, which causes protein misfolding throughout the cell, impairs Deg1-Sec62 destruction. We performed cycloheximide chase experiments to analyze Deg1-Sec62 degradation in cells cultured at 30 °C and shifted to 42 °C for 1 h and for the duration of the chase. Exposure to elevated temperatures did not stabilize Deg1-Sec62 in WT cells (Fig. 11B). Therefore, conditions associated with global protein misfolding are not sufficient to impair Hrd1-dependent destruction of an aberrant translocon-associated protein.
Finally, we determined whether oxidative stress affects Deg1*-Sec62 turnover. The oxidant hydrogen peroxide induces the UPR in cultured myoblasts (66). Incubation of yeast in the presence of 0.4 mm hydrogen peroxide for 1 h did not affect Deg1*-Sec62 degradation (Fig. 11C). Induction of oxidative stress-responsive GFP-tagged Rtc3 (67) in a parallel culture confirmed hydrogen peroxide activity (Fig. 11D).
Discussion
Our results reveal that ER stress differentially impacts ER and INM protein quality control proteolytic pathways. Model Doa10 ERAD-C and Rkr1 ERAD-RA substrates were largely unaffected by ER stress. Similarly, Hrd1-dependent degradation of proteins with intramembrane degrons proceeded with similar kinetics regardless of ER stress induction. However, destruction of three Hrd1 substrates (a soluble ERAD-L substrate, a transmembrane ERAD-L substrate, and an ERAD-T substrate) was specifically impaired by ER stress. Modification and turnover of a translocon-clogging substrate of Ste24 was also perturbed by ER stress. Finally, degradation of one of two tested Asi INMAD substrates was sensitive to ER stress.
During ER stress, translocation of a subset of proteins into the ER is slowed (39). Reduced ER chaperone availability correlates with and likely contributes to translocational attenuation, which is thought to be protective, reducing the load for the already burdened proteostasis machinery. Dampened translocon quality control during ER stress may be an additional adaptive mechanism in which undegraded channel-engaged proteins temporarily impede translocation of other polypeptides into the stressed ER.
Yeast and mammalian homologues of Hrd1 have been reported to physically interact with the translocon (41, 68). Interaction of mammalian HRD1 with the translocon was reported to increase ∼1.5-fold during ER stress (41), an observation that would not be predicted by our results. Increased association of HRD1 with the translocon correlates with HRD1-dependent turnover of ER-targeted proteins prior to their translocation via ERpQC. By preemptively promoting degradation of secretory proteins prior to translocation, ERpQC may protect the stressed ER. We therefore speculate that regulation of Hrd1 by ER stress reduces protein load in the ER by two mechanisms: 1) stimulation of preemptive Hrd1-mediated targeting of secretory proteins prior to ER insertion, and 2) inhibition of Hrd1-dependent targeting of proteins that are already translocon-engaged.
Sensitivity of Deg1-Sec62 degradation to ER stress is consistent with an earlier observation that high-level expression of polyQ-expanded huntingtin protein both induces ER stress and impairs Deg1-Sec62 degradation (69). ERAD-T impairment is specific to particular subtypes of ER stress, as several different forms of stress expected to disrupt global proteostasis or ER homeostasis (elevated temperature, inositol limitation, and oxidative stress) did not impair Deg1-Sec62 degradation. Thus, misfolded proteins per se are likely not the direct signal impairing Hrd1-mediated destruction of translocon-associated proteins.
One potential trivial explanation for impairment of Deg1-Sec62 degradation is that ER stress prevents aberrant translocon engagement by Deg1-Sec62, which converts the protein into a Hrd1 substrate in the first place (25). We do not believe this is the cause for Deg1-Sec62 stabilization by ER stress for two reasons. First, Deg1-Sec62 becomes N-glycosylated in the presence of DTT (albeit in a delayed fashion), strongly suggesting that aberrant translocation of the fusion protein does occur. Second, mutations that prevent aberrant translocon engagement of Deg1-Sec62 cause a reversion of dependence of Deg1-Sec62 degradation from Hrd1 to Doa10 (25). Therefore, if ER stress blocks Hrd1-dependent degradation of Deg1-Sec62 by preventing translocon engagement, we would expect Deg1-Sec62 to become a Doa10 substrate and still be rapidly degraded; this is not observed. Consistently, we did not observe a difference in association of Deg1-Sec62 with ER membrane fractions in the presence or absence of ER stress.
Another possible explanation for impaired Deg1-Sec62 degradation is that ER stress prevents one or more PTMs required for recognition by Hrd1. However, neither glycosylation nor acetylation are required for Deg1-Sec62 degradation (25, 50). Although it remains possible that uncharacterized PTMs contribute to Deg1-Sec62 degradation, no available evidence suggests this is the case. The mechanism by which DTT delays modification of Deg1-Sec62 remains unclear. Redox perturbation may alter the structure or intermolecular interactions of enzymes required for glycosylation or substrate molecules in a manner that precludes efficient modification.
Unperturbed degradation of a subset of Hrd1 substrates argues against complete Hrd1 inhibition during ER stress. We speculate that one or more proteins sense ER stress and specifically inhibit Hrd1-dependent degradation of translocon-associated proteins. Our experiments argue against roles for the UPR, ERSU, RESET, and SHRED stress-sensing pathways in impeding the destruction of translocon-associated proteins during stress, unless they function redundantly.
It is possible that an as yet unidentified ERAD-T co-factor functions in a novel ER stress-sensing mechanism and becomes limiting for translocon-associated protein degradation. Stress-dependent changes in expression and complex association of Hrd1 cofactors have been observed (70). Another possibility is that one or more protein(s) sensitive to redox and glycosylation state mediate the effect of ER stress on degradation. Efforts to identify and characterize factors that regulate Hrd1-dependent degradation of translocon-engaged proteins are underway.
ER stress may similarly impair targeting of the translocon-associated clientele of Ste24. Accumulation of higher-mobility species suggests that the translocon-engaged form of Clogger is enriched in the presence of DTT. DTT-dependent changes in Clogger mobility were not observed in a previous investigation (31). This difference may be related to differences in treatment protocol between the two studies. In our experiments, cultures were incubated in the presence of 6 mm DTT for 1 h; in the experiments described in Ref. 31, yeast were incubated in the presence of 5 mm DTT for 4 h. It is possible that short- and long-term responses to ER stress differ.
Both Clogger and Deg1-Sec62 overexpression mildly induce the UPR (Fig. 7) (31). On its face, this runs counter to our suggestion that persistent translocon engagement protects against ER stress by reducing inward flux of nascent proteins. However, these two ideas can be reconciled with the following model. At basal levels of unfolded proteins in the ER, translocon clogging may increase stress by preventing inward movement of proteostasis machinery. By contrast, at higher levels of stress, nonspecifically stemming ER import may minimize the luminal burden of unfolded proteins.
Why does ER stress impair ERAD-L and not ERAD-M? Following Hrd1-mediated ubiquitylation, both ERAD-L and ERAD-M substrates must undergo protein extraction from the ER into the cytosol (retrotranslocation) prior to proteasome-mediated degradation (71). ERAD-L and ERAD-M substrates have different requirements for retrograde transport, and ER stress may only impair ERAD-L retrotranslocation (54, 71).
Finally, ER stress impacts Asi complex substrates in subtly different ways. Induction of ER stress alters the steady-state abundance of Erg11-FLAG without dramatically changing its rate of degradation. By contrast, ER stress impedes turnover of Vtc1-3HA to a similar degree as deletion of ASI1. These results imply that Asi substrates may be degraded and regulated via distinct modalities reminiscent of the multitude of Hrd1-mediated degradation mechanisms.
Unperturbed degradation of several proteins in the presence of tunicamycin and DTT appears to contradict a previous report of global proteasome inhibition by ER stress (72). However, although the earlier study reported acute stabilization of a subset of unstable proteins, degradation of a metabolically-labeled cytosolic protein was observed to proceed with similar kinetics in the absence and presence of stress. Mild accumulation of this protein during stress was observed over a longer time course following transient proteasome inhibition. These results argue for minor, indirect effects of ER stress on global protein degradation. In a more recent proteomics investigation, ER stress accelerated the proteasomal degradation of a subset of physiological proteins, while stabilizing others (73), consistent with divergent effects of ER stress on different protein populations.
Experimental procedures
Yeast and plasmid methods
Yeast were cultured at 30 °C in standard growth medium as described previously (49). Plasmids were introduced to yeast by the lithium acetate transformation procedure (74). Yeast strains and plasmids used in this study are presented in Tables 1 and 2, respectively. Yeast strains were generated by standard gene replacement methods and yeast mating, sporulation, and haploid selection (74, 75). Plasmids pVJ343, pVJ411, and pVJ463 were constructed by subcloning BamHI/HindIII fragments containing Deg1-Vma12, Deg1*-Sec62, or Deg1-Sec62, respectively, from plasmids constructed in a previous study (25) into expression vectors with the desired promoter and auxotrophic marker gene (76, 77).
Table 1.
Yeast strains used in this study
| Name | Alias | Genotype | Source | Figs. |
|---|---|---|---|---|
| VJY6 | MHY500 | MATa his3-200 leu2-3,112 ura3-52 lys2-801 trp1-1 gal2 | 78 | 1, C, E, G, and H, 2, B, C, and E, 3, B–D, 5B, 7, 9, A and B, and 11, A and B |
| VJY7 | MHY1685 | MATa his3-200 leu2-3,112 ura3-52 lys2-801 trp1-1 gal2 doa10Δ::HIS3 | 19 | 3C |
| VJY8 | MHY1702 | MATa his3-200 leu2-3,112 ura3-52 lys2-801 trp1-1 gal2 doa10Δ::HIS3 hrd1Δ::LEU2 | 19 | 1F and 10 |
| VJY9 | MHY2822 | MATa his3-200 leu2-3,112 ura3-52 lys2-801 trp1-1 gal2 hrd1Δ::LEU2 | 19 | 1E |
| VJY10 | MHY6198 | MATa his3-200 leu2-3,112 ura3-52 lys2-801 trp1-1 gal2 hrd1Δ::KanMX4 | This study | 2, B and C |
| VJY29 | MHY7719 | MATα ade2–101 met2 lys2-801 his3Δ200 trp1Δ leu2Δ ura3-52::6MYC-HMG2 hmg1Δ::LYS2 hmg2Δ::HIS3 | This study | 2D |
| VJY33 | MHY2972 | MATa his3Δ1 leu2Δ0 ura3Δ0 | 88 | 1D and 4C |
| VJY35 | MHY1661/RHY665 | MATα ade2–101 met2 lys2-801 his3Δ200 trp1::hisG ura3-52::6MYC-HMG2 hmg1Δ::LYS2 hmg2Δ::HIS3 ubc7Δ::HIS3 | 89 | 2D |
| VJY38 | MHY7723 | MATa his3Δ1 leu2Δ0 ura3Δ0 met15Δ0 slt2Δ::kanMX4 | 90 | 8, A and B |
| VJY50 | MHY551 | MATa his3-200 leu2-3,112 ura3-52 lys2-801 trp1-1 gal2 ubc7Δ::LEU2 | 78 | 2E and 7 |
| VJY166 | MHY5978 | MATa his3Δ1 leu2Δ0 ura3Δ0 met15Δ0 hac1Δ::kanMX4 | 90 | 8B |
| VJY173 | MHY2177 | MATa his3Δ1 leu2Δ0 ura3Δ0 met15Δ0 ire1Δ::kanMX4 | 90 | 8, A and B |
| VJY306 | SKY342 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 rkr1Δ::hphMX4 | 8 | 4C |
| VJY404 | 2378/29 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 HO::NatR-Galp-PDIClogger | 31 | 6C |
| VJY405 | 2379/29 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 HO::NatR-Galp-PDIClogger ste24Δ::pcgURA | 31 | 6C |
| VJY476 | BY4741 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | 90 | 8, A–8C and 11, B and C |
| VJY536 | SSY122 | MATa leu2-3,112 trp1-1 ura3-1 his3-11,15 | 38 | 8D |
| VJY540 | SSY1782 | MATa leu2-3,112 trp1-1 ura3-1 his3-11,15 ubr1Δ::HIS3 | 38 | 8D |
| VJY572 | yMaM791 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 VTC1-3HA:hphNT1 | 17 | 5C |
| VJY574 | yMaM801 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 VTC1-3HA:hphNT1 asi1Δ::kanMX6 | 17 | 5C |
| VJY616 | MATa his3Δ1 leu2Δ0 ura3Δ0 met15Δ0 RTC3-GFP:his5Sp | 91 | 11D | |
| VJY620 | MATa his3Δ1 leu2Δ0 ura3Δ0 met15Δ0 emp24Δ::kanMX4 | 90 | 8C | |
| MHY8941 | MATa his3-200 leu2-3,112 ura3-52 lys2-801 trp1-1 gal2 asi1Δ::KanMX6 | Gift of C. Hickey | 5B | |
| MHY10483 | ABM124 | MATα his3-200 leu2-3,112 ura3-52 lys2-801 trp1-1 gal2 PTDH3-SUS-GFP::TRP1 | This study | S1 |
| MHY10487 | ABM128 | MATα his3-200 leu2-3,112 ura3-52 lys2-801 trp1-1 gal2 PTDH3-SUS-GFP::TRP1 ubc7Δ::LEU2 | This study | S1 |
Table 2.
Plasmids used in this study
| Name | Alias | Yeast selection marker | Yeast plasmid type | Description | Source | Figs. |
|---|---|---|---|---|---|---|
| pHA-Pdr5* | pVJ1/pRH2312 | HIS3 | CEN | HA-tagged Pdr5*; Pdr5* = C1427Y | 24 | 2E |
| YCp50-PPRC1-CPY*-HA | pVJ2/pDN431 | URA3 | CEN | HA-tagged CPY* driven by native promoter; CPY* = G255R | 92 | 2B |
| pRS313 | pVJ26 | HIS3 | CEN | Empty vector | 93 | 4C and 7 |
| pRS316 | pVJ27 | URA3 | CEN | Empty vector | 93 | 2, B and C, 3C, 4C, 8, A and C, and 11C |
| p416-PMET25-Deg1-FLAG-Sec62-2×ProtA | pVJ29 | URA3 | CEN | Deg1-Sec62 driven by MET25 promoter | 25 | 1, D, E, G, and H, 4C, 5, B and C, 10, and 11B, and S1 |
| p414-PMET25-Deg1-FLAG-Sec62-2×ProtA | pVJ30 | TRP1 | CEN | Deg1-Sec62 driven by MET25 promoter | 94 | 1, C and F, 2, D and E, 3, C and D, and 9, A and B |
| pRS314 | pVJ39 | TRP1 | CEN | Empty vector | 93 | 3C |
| pRS425 | pVJ43 | LEU2 | 2μ | Empty vector | 95 | 9B |
| p415-PMET25 | pVJ122 | LEU2 | CEN | Empty vector with MET25 promoter | 76 | 8D and 11A |
| p414- PMET25-Deg1-FLAG-Vma12-2×ProtA | pVJ172 | TRP1 | CEN | Deg1-Vma12 driven by MET25 promoter | 21 | 3B |
| p414-PMET25-Deg1*-FLAG-Vma12-2×ProtA | pVJ177 | TRP1 | CEN | Deg1*-Vma12 driven by MET25 promoter; Deg1* = F18S, I22T | 21 | 3B |
| p414-PMET25-Deg1-FLAG-sec62†-2×ProtA | pVJ204 | TRP1 | CEN | Deg1-sec62† driven by MET25 promoter; sec62† = sec62-1 = G127D | 25 | 3D |
| p416-PMET25-Deg1*-Sec62-2×ProtA | pVJ317 | URA3 | CEN | Deg1*-Sec62 driven by MET25 promoter; Deg1* = F18S, I22T | 25 | 8, A and C, and 11C |
| p416-PGPD-Deg1-FLAG-Vma12-2×ProtA | pVJ343 | TRP1 | CEN | Deg1-Vma12 driven by TDH3 (GPD) promoter | This study | 3C |
| p415-P PMET25-Deg1*-Sec62-2×ProtA | pVJ411 | LEU2 | CEN | Deg1*-Sec62 driven by MET25 promoter; Deg1* = F18S, I22T | This study | 8D and 11A |
| p413-PGPD-FLAG-Vma12-ProtA-K12-13myc | pVJ457 (STK 07.4.3) | HIS3 | CEN | Vma12-K12-13myc driven by TDH3 (GPD) promoter | 8 | 4C |
| p413-PGPD-Deg1-Sec62-2×ProtA | pVJ463 | HIS3 | CEN | Deg1-Sec62 driven by TDH3 (GPD) promoter | Gift of S. Kreft | 7 |
| pMRS366 | pVJ512 | URA3 | CEN | Empty vector | 96 | 9A |
| pMR397 (CEN) | pVJ513 | URA3 | CEN | Kar2 expression plasmid | 96 | 9A |
| YGPM17a24 | pVJ532 | LEU2 | 2μ | Plasmid from Yeast Genomic Tiling Collection with genomic segment, including KAR2 gene and promoter | 63 | 9B |
| p416-PERG3-ERG3-13Myc | pVJ533/pLJ001 | URA3 | CEN | 13myc-tagged Erg3 driven by native promoter | 51 | 2C |
| pRS314-UPRE-GFP | pVJ552 | TRP1 | CEN | GFP driven by unfolded Protein Response Element (UPRE) | 97 | 7 and 11A |
| pTDH3-SUS-GFP | pRH2900 | TRP1 | YIp | SUS-GFP driven by TDH3 (GPD) promoter | 54 | Used to generate MHY10483 |
| pRS316-Erg11-FLAG | URA3 | CEN | FLAG-tagged Erg11 driven by native promoter | This study | 5B |
For galactose induction of Clogger, which is driven by the GAL1/10 promoter, yeast were cultured overnight in selective medium containing 2% raffinose as the carbon source. Overnight cultures were diluted in fresh medium containing 4% galactose and cultured until cells reached mid-exponential growth.
For inositol limitation experiments, cells were cultured until they reached mid-exponential growth in medium containing inositol (prepared using yeast nitrogen base without amino acids). Cells were washed six times in medium lacking inositol (prepared using yeast nitrogen base without amino acids and inositol) and incubated in medium lacking inositol for 5 h.
Pulse-chase analysis
Pulse-chase analysis was performed as described previously (25, 78). Briefly, yeast cells were labeled with 20 μCi of Tran35S-label (MP Biomedicals) per 1 OD600 unit of cells at 30 °C for 10 min in medium lacking methionine and cysteine. Chases were performed in the presence of excess unlabeled methionine and cysteine. Deg1 fusion proteins were immunoprecipitated with anti-FLAG M2 affinity resin (Sigma) (Fig. 1, D–F) or sequential incubation with anti-Deg1 antibody (79) and recombinant protein A–cross-linked agarose A (Repligen; Figs. 1C and 3, B and D). Immunoprecipitated proteins were separated by SDS-PAGE. Gels were analyzed by autoradiography, using a Storm 860 Phosphorimager system and ImageQuant 5.2 software (Molecular Dynamics). Figures for which molecular weight markers are not available (Figs. 1, C, E and F, and 3D) portray experiments performed on variants of proteins analyzed multiple times elsewhere in this study in experiments that include molecular weight markers.
Endoglycosidase H and calf intestinal phosphatase treatment
Cells were radiolabeled as described for pulse-chase experiments, and FLAG-tagged Deg1-Sec62 was immunoprecipitated with anti-FLAG M2 affinity resin. After five washes of resin with wash buffer (150 mm NaCl, 50 mm Hepes, pH 7.5, 5 mm EDTA, 1% Triton X-100, and 0.1% SDS), two additional equilibrating washes were performed with NEBuffer 4 (New England Biolabs). The resin was resuspended in 50 μl of NEBuffer 4 that had been supplemented with potassium acetate, pH 5.6, to a final concentration of 80 mm. 0.005 units of Endo H (Roche Applied Science), 10 units of calf intestinal phosphatase (New England Biolabs), both, or neither were added to the resin suspension. Samples were incubated at 37 °C for 3 h with gentle mixing approximately every 10 min. 1× Laemmli sample buffer was added, and samples were heated to 100 °C for 5 min.
Cell lysis
For experiments presented in Figs. 1, G and H, 2, B–D, 3C, 4C, 6C, 8, A, C, and D, 9, A and B, and 11, A–C, yeast were lysed as described previously (80, 81). Briefly, 2.5 OD600 units of yeast were harvested, suspended in 200 μl of 0.1 m NaOH, and incubated for 5 min at room temperature. Cells were pelleted, resuspended in 1× Laemmli sample buffer, and heated to 100 °C for 5 min. Lysates were subject to centrifugation to clear the preparations of insoluble material. The soluble fraction was separated by SDS-PAGE.
For experiments presented in Figs. 2E, and 5, B and C, and Fig. S1, yeast were lysed as described previously (82). Briefly, 2.5 OD600 units of yeast were harvested. NaOH was added to a final concentration of 0.26 m, and β-mercaptoethanol was added to a final concentration of 0.13 m. Cells were incubated on ice for 15 min. To precipitate proteins, TCA was added to a final concentration of 5%. Proteins were collected by centrifugation at 4 °C. Protein pellets were resuspended in 50 μl of TCA sample buffer (3.5% SDS, 0.5 m DTT, 80 mm Tris, 8 mm EDTA, 15% glycerol, and 0.1 mg/ml bromphenol blue) and incubated at 37 °C for 30 min. Insoluble material was pelleted by centrifugation prior to electrophoretic separation.
Cycloheximide-chase analysis
Cycloheximide-chase experiments were performed as described previously (83). Briefly, yeast were grown to mid-exponential phase at 30 °C, unless otherwise specified. Cells were concentrated to 2.5 OD600 units/ml in fresh media. Cycloheximide was added to a final concentration of 250 μg/ml. Aliquots of 2.4 OD600 units of cells (950 μl) were harvested at the indicated times following cycloheximide addition, added to stop mix (final concentration 10 mm sodium azide, 0.25 mg/ml BSA), and placed on ice until the end of the chase, when all cells were lysed.
Microsome preparation and protein-membrane association analyses
Yeast microsomal membranes were prepared essentially as described previously (10, 25, 49). 10 OD600 units of cells were harvested, suspended in 1 ml of resuspension buffer (10 mm Tris, pH 9.4, and 10 mm DTT), and incubated at room temperature for 10 min. Cells were harvested by centrifugation, washed with spheroplast buffer (1 m sorbitol, 20 mm sodium phosphate, pH 7.5, 150 mm NaCl, and 2 mm DTT), and digested with 140 μg of zymolyase 100T (MP Biomedicals)/10 OD600 units of cells in spheroplast buffer for 20 min at 30 °C. Spheroplasts were harvested (5 min at 600 × g at 4 °C) and washed in spheroplast buffer containing 20 μg/ml pepstatin A, 1 mm EDTA, and 1× EDTA-free Complete Protease Inhibitor Mixture (Roche Applied Science). Spheroplasts were centrifuged again (5 min at 600 × g at 4 °C), resuspended in fractionation buffer (200 mm d-mannitol, 20 mm sodium phosphate, pH 7.5, and 150 mm NaCl) with protease inhibitors, and lysed by vortexing in the presence of glass beads for three 30-s pulses (1 min on ice between pulses). Unbroken cells and cellular debris were pelleted by centrifugation (5 min at 600 × g at 4 °C), and the supernatant was used as the microsomal preparation. Microsomes were incubated with sodium carbonate (final concentration 0.2 m, pH 11), Triton X-100 (final concentration 1% v/v), sodium chloride (final concentration 0.5 m), or no additive. Samples were maintained on ice for 15 min with occasional vortexing and then centrifuged (15 min at 13,000 × g at 4 °C). Supernatants were transferred to fresh tubes and preserved as the soluble fraction. To ensure samples had equal detergent concentrations, Triton X-100 was added to all supernatants that had not been initially solubilized with Triton X-100 (final concentration 1%); an equal volume of water was added to samples that had been previously solubilized by Triton X-100. Pellets were washed with fractionation buffer containing protease inhibitors and resuspended in fractionation buffer with 1% Triton X-100. 1× Laemmli sample buffer was added to all samples, prior to heating at 100 °C for 8 min and separation by SDS-PAGE.
Western blotting
Proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride membrane via wet transfer at 20 V for 1 h, 70 V for 2.5 h, or 30 V for 8 h at 4 °C. Membranes were blocked in a solution containing 5% skim milk in Tris-buffered saline (TBS: 50 mm Tris-base, 150 mm NaCl) at room temperature for 1 h or at 4 °C overnight. Membranes were probed in a solution containing 1% skim milk in TBS with 1% Tween 20 (TBS/T) and the appropriate antibody. The membranes were incubated in the presence of antibodies for 1 h at room temperature followed by three 5-min washes in TBS/T.
The following antibody dilutions were used: mouse anti-HA.11 (Clone 16B12; Covance and BioLegend) at 1:1,000–1:2,000; mouse anti-GFP (Clone JL-8; Clontech) at 1:1,000–1:2,000; mouse anti-phosphoglycerate kinase 1 (Pgk1; clone 22C5D8; Life Technologies, Inc.) at 1:5,000–1:40,000; mouse anti-FLAG (M2; Sigma) at 1:5,000; rabbit anti-glucose-6-phosphate dehydrogenase (G6PDH; Sigma) at 1:10,000; rabbit anti-KAR2 (clone y-115; Santa Cruz Biotechnology) at 1:2,000; rabbit anti-Cue1 (Hochstrasser laboratory) at 1:1,000; and rabbit anti-Cdc48 (gift of Thomas Sommer) at 1:1,000. Mouse primary antibodies were followed by incubation with either AlexaFluor-680–conjugated rabbit anti-mouse secondary antibody (Life Technologies, Inc.) at 1:20,000–1:40,000 or peroxidase-coupled sheep anti-mouse secondary antibody (GE Healthcare) at 1:5,000. Rabbit primary antibodies were followed by incubation with either peroxidase-coupled goat anti-rabbit (GE Healthcare) at 1:4,000 or IRDye-680RD–conjugated goat anti-rabbit secondary antibody (Li-Cor) at 1:40,000. AlexaFluor-680–conjugated rabbit anti-mouse antibody (Figs. 1, G and H, 2D, 3C, 4C, 8, A, C, and D, 9, A and B, and 11, A–C) or peroxidase anti-peroxidase (Sigma; 1:5,000; Figs. 2E, 5, B and C, and 10 and Fig. S1) were also used to directly detect the Staphylococcus aureus protein A epitope (found in variants of Deg1-Sec62, Deg1-Vma12, and Vma12-K12-13myc), which binds to mammalian Igs (84).
Membranes probed with fluorescently-labeled antibodies (Figs. 1, G and H, 2, B–D, 3C, 4C, 6C, 8, A, C, and D, 9, A and B, and 11, A–C) were imaged using an Odyssey CLx IR Imaging System and Image Studio Software (Li-Cor). Membranes probed with peroxidase-conjugated antibodies (Figs. 2E, 5, B and C, and 10, and Fig. S1) were visualized using enhanced chemiluminescence (GE Healthcare) and the G:BOX gel-imaging system and software (Syngene).
Flow cytometry
Flow cytometry was performed using cells expressing the indicated GFP-tagged proteins. Cells were cultured until they reached mid-exponential growth and were subjected to experimental treatments, as indicated. Mean GFP fluorescence of 10,000 cells in synthetic-defined medium was measured using the MACSquant Analyzer X.
Analysis of HAC1 mRNA splicing
HAC1 mRNA splicing was assessed using previously described methods with modifications (85–87). RNA was extracted from 1 OD600 unit of cells cultured to mid-exponential growth using RNeasy Mini (Qiagen) and DNA-free (Ambion) kits. cDNA was synthesized from 1 μg of total RNA using the iScript cDNA synthesis kit (Bio-Rad). The resulting cDNA was used as a template for PCRs as described (85). PCR products were analyzed on a 1.5% agarose gel and confirmed by DNA sequencing.
Author contributions
B. W. B., A. B. M., C. L. B., B. J. S., L. N. S., and E. M. R. formal analysis; B. W. B., C. L. B., A. M. R., B. J. S., M. H., and E. M. R. funding acquisition; B. W. B., A. B. M., C. L. B., A. M. R., B. J. S., L. N. S., and E. M. R. investigation; B. W. B., A. B. M., C. L. B., A. M. R., B. J. S., L. N. S., M. H., and E. M. R. writing-review and editing; M. H. and E. M. R. conceptualization; M. H. and E. M. R. supervision; E. M. R. project administration; E. M. R. writing-original draft.
Supplementary Material
Acknowledgments
We thank Andrew Kusmierczyk for insightful conversations at the genesis of this project. We thank Andrew Kusmierczyk, Douglas Bernstein, and members of the Hochstrasser, Kusmierczyk, Bernstein, and Rubenstein labs for helpful discussions throughout the project. We thank Christopher Hickey, Stefan Kreft, Randy Hampton, Christian Hirsch, Ernst Jarosch, Michael Knop, Matthias Meurer, Davis Ng, James Olzmann, Mark Rose, Randy Schekman, Sebastian Schuck, Maya Schuldiner, and Thomas Sommer for sharing plasmids, yeast strains, and antibodies. We thank Heather Bruns for assistance with flow cytometry. We thank Seth Horowitz for laboratory assistance during the project.
This work was supported by Ball State University ASPiRE Research Awards (to B. W. B., C. L. B., and A. M. R.), a Ball State University Department of Biology Sigma Zeta research award (to B. J. S.), National Institutes of Health Grant GM046904 (to M. H.), a Ball State University Faculty Advance research award (to E. M. R.), an Indiana Academy of Science senior research grant (to E. M. R.), and National Institutes of Health Grant GM111713 (to E. M. R.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Fig. S1.
B. W. Buchanan, L. N. Scanameo, and E. M. Rubenstein, unpublished observations.
C. L. Broshar and E. M. Rubenstein, unpublished observations.
- ER
- endoplasmic reticulum
- APC
- anaphase-promoting complex
- CAT tail
- C-terminal addition of alanine and threonine
- CPY
- carboxypeptidase Y
- DHFR
- dihydrofolate reductase
- Endo H
- endoglycosidase H
- ERAD
- ER-associated degradation
- ERpQC
- ER preemptive quality control
- ERSU
- ER surveillance
- GPD
- glyceraldehyde-3-phosphate dehydrogenase
- GPI
- glycosylphosphatidylinositol
- INM
- inner nuclear membrane
- INMAD
- inner nuclear membrane-associated degradation
- K12
- tract of twelve lysine residues
- PTM
- post-translational modification
- RESET
- rapid ER stress-induced export
- SHRED
- stress-induced homeostatically regulated protein degradation
- SUS–GFP
- self-ubiquitylating substrate tagged with green fluorescent protein
- TBS
- Tris-buffered saline
- TBS/T
- TBS supplemented with Tween 20
- UPR
- unfolded protein response
- UPRE
- unfolded protein response element
- G6PDH
- glucose-6-phosphate dehydrogenase.
References
- 1. Wu H., Ng B. S., and Thibault G. (2014) Endoplasmic reticulum stress response in yeast and humans. Biosci. Rep. 34, e00118 10.1042/BSR20140058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Travers K. J., Patil C. K., Wodicka L., Lockhart D. J., Weissman J. S., and Walter P. (2000) Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101, 249–258 10.1016/S0092-8674(00)80835-1 [DOI] [PubMed] [Google Scholar]
- 3. Hampton R. Y., Gardner R. G., and Rine J. (1996) Role of 26S proteasome and HRD genes in the degradation of 3-hydroxy-3-methylglutaryl–CoA reductase, an integral endoplasmic reticulum membrane protein. Mol. Biol. Cell 7, 2029–2044 10.1091/mbc.7.12.2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Plemper R. K., Bordallo J., Deak P. M., Taxis C., Hitt R., and Wolf D. H. (1999) Genetic interactions of Hrd3p and Der3p/Hrd1p with Sec61p suggest a retro-translocation complex mediating protein transport for ER degradation. J. Cell Sci. 112, 4123–4134 [DOI] [PubMed] [Google Scholar]
- 5. Wilhovsky S., Gardner R., and Hampton R. (2000) HRD gene dependence of endoplasmic reticulum-associated degradation. Mol. Biol. Cell 11, 1697–1708 10.1091/mbc.11.5.1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bays N. W., Gardner R. G., Seelig L. P., Joazeiro C. A., and Hampton R. Y. (2001) Hrd1p/Der3p is a membrane-anchored ubiquitin ligase required for ER-associated degradation. Nat. Cell Biol. 3, 24–29 10.1038/35050524 [DOI] [PubMed] [Google Scholar]
- 7. Swanson R., Locher M., and Hochstrasser M. (2001) A conserved ubiquitin ligase of the nuclear envelope/endoplasmic reticulum that functions in both ER-associated and Matα2 repressor degradation. Genes Dev. 15, 2660–2674 10.1101/gad.933301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crowder J. J., Geigges M., Gibson R. T., Fults E. S., Buchanan B. W., Sachs N., Schink A., Kreft S. G., and Rubenstein E. M. (2015) Rkr1/Ltn1 ubiquitin ligase-mediated degradation of translationally stalled endoplasmic reticulum proteins. J. Biol. Chem. 290, 18454–18466 10.1074/jbc.M115.663559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stolz A., Besser S., Hottmann H., and Wolf D. H. (2013) Previously unknown role for the ubiquitin ligase Ubr1 in endoplasmic reticulum-associated protein degradation. Proc. Natl. Acad. Sci. U.S.A. 110, 15271–15276 10.1073/pnas.1304928110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kreft S. G., Wang L., and Hochstrasser M. (2006) Membrane topology of the yeast endoplasmic reticulum-localized ubiquitin ligase Doa10 and comparison with its human ortholog TEB4 (MARCH-VI). J. Biol. Chem. 281, 4646–4653 10.1074/jbc.M512215200 [DOI] [PubMed] [Google Scholar]
- 11. Deak P. M., and Wolf D. H. (2001) Membrane topology and function of Der3/Hrd1p as a ubiquitin-protein ligase (E3) involved in endoplasmic reticulum degradation. J. Biol. Chem. 276, 10663–10669 10.1074/jbc.M008608200 [DOI] [PubMed] [Google Scholar]
- 12. Bengtson M. H., and Joazeiro C. A. (2010) Role of a ribosome-associated E3 ubiquitin ligase in protein quality control. Nature 467, 470–473 10.1038/nature09371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heck J. W., Cheung S. K., and Hampton R. Y. (2010) Cytoplasmic protein quality control degradation mediated by parallel actions of the E3 ubiquitin ligases Ubr1 and San1. Proc. Natl. Acad. Sci. U.S.A. 107, 1106–1111 10.1073/pnas.0910591107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deng M., and Hochstrasser M. (2006) Spatially regulated ubiquitin ligation by an ER/nuclear membrane ligase. Nature 443, 827–831 10.1038/nature05170 [DOI] [PubMed] [Google Scholar]
- 15. Boban M., Pantazopoulou M., Schick A., Ljungdahl P. O., and Foisner R. (2014) A nuclear ubiquitin-proteasome pathway targets the inner nuclear membrane protein Asi2 for degradation. J. Cell Sci. 127, 3603–3613 10.1242/jcs.153163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Foresti O., Rodriguez-Vaello V., Funaya C., and Carvalho P. (2014) Quality control of inner nuclear membrane proteins by the Asi complex. Science 346, 751–755 10.1126/science.1255638 [DOI] [PubMed] [Google Scholar]
- 17. Khmelinskii A., Blaszczak E., Pantazopoulou M., Fischer B., Omnus D. J., Le Dez G., Brossard A., Gunnarsson A., Barry J. D., Meurer M., Kirrmaier D., Boone C., Huber W., Rabut G., Ljungdahl P. O., and Knop M. (2014) Protein quality control at the inner nuclear membrane. Nature 516, 410–413 10.1038/nature14096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koch B. A., Jin H., Tomko R. J. Jr., and Yu H. G. (2019) The anaphase-promoting complex regulates the degradation of the inner nuclear membrane protein Mps3. J. Cell Biol. 218, 839–854 10.1083/jcb.201808024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huyer G., Piluek W. F., Fansler Z., Kreft S. G., Hochstrasser M., Brodsky J. L., and Michaelis S. (2004) Distinct machinery is required in Saccharomyces cerevisiae for the endoplasmic reticulum-associated degradation of a multispanning membrane protein and a soluble luminal protein. J. Biol. Chem. 279, 38369–38378 10.1074/jbc.M402468200 [DOI] [PubMed] [Google Scholar]
- 20. Metzger M. B., Maurer M. J., Dancy B. M., and Michaelis S. (2008) Degradation of a cytosolic protein requires endoplasmic reticulum-associated degradation machinery. J. Biol. Chem. 283, 32302–32316 10.1074/jbc.M806424200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ravid T., Kreft S. G., and Hochstrasser M. (2006) Membrane and soluble substrates of the Doa10 ubiquitin ligase are degraded by distinct pathways. EMBO J. 25, 533–543 10.1038/sj.emboj.7600946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carvalho P., Goder V., and Rapoport T. A. (2006) Distinct ubiquitin-ligase complexes define convergent pathways for the degradation of ER proteins. Cell 126, 361–373 10.1016/j.cell.2006.05.043 [DOI] [PubMed] [Google Scholar]
- 23. Gauss R., Sommer T., and Jarosch E. (2006) The Hrd1p ligase complex forms a linchpin between ER-lumenal substrate selection and Cdc48p recruitment. EMBO J. 25, 1827–1835 10.1038/sj.emboj.7601088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sato B. K., Schulz D., Do P. H., and Hampton R. Y. (2009) Misfolded membrane proteins are specifically recognized by the transmembrane domain of the Hrd1p ubiquitin ligase. Mol. Cell 34, 212–222 10.1016/j.molcel.2009.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rubenstein E. M., Kreft S. G., Greenblatt W., Swanson R., and Hochstrasser M. (2012) Aberrant substrate engagement of the ER translocon triggers degradation by the Hrd1 ubiquitin ligase. J. Cell Biol. 197, 761–773 10.1083/jcb.201203061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arakawa S., Yunoki K., Izawa T., Tamura Y., Nishikawa S., and Endo T. (2016) Quality control of nonstop membrane proteins at the ER membrane and in the cytosol. Sci. Rep. 6, 30795 10.1038/srep30795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. von der Malsburg K., Shao S., and Hegde R. S. (2015) The ribosome quality control pathway can access nascent polypeptides stalled at the Sec61 translocon. Mol. Biol. Cell 26, 2168–2180 10.1091/mbc.E15-01-0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smoyer C. J., and Jaspersen S. L. (2019) Patrolling the nucleus: inner nuclear membrane-associated degradation. Curr. Genet. 65, 1099–1106 10.1007/s00294-019-00971-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Habeck G., Ebner F. A., Shimada-Kreft H., and Kreft S. G. (2015) The yeast ERAD-C ubiquitin ligase Doa10 recognizes an intramembrane degron. J. Cell Biol. 209, 261–273 10.1083/jcb.201408088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ruggiano A., Mora G., Buxó L., and Carvalho P. (2016) Spatial control of lipid droplet proteins by the ERAD ubiquitin ligase Doa10. EMBO J. 35, 1644–1655 10.15252/embj.201593106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ast T., Michaelis S., and Schuldiner M. (2016) The protease Ste24 clears clogged translocons. Cell 164, 103–114 10.1016/j.cell.2015.11.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Babour A., Bicknell A. A., Tourtellotte J., and Niwa M. (2010) A surveillance pathway monitors the fitness of the endoplasmic reticulum to control its inheritance. Cell 142, 256–269 10.1016/j.cell.2010.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Piña F. J., and Niwa M. (2015) The ER Stress Surveillance (ERSU) pathway regulates daughter cell ER protein aggregate inheritance. Elife 2015 4, 10.7554/eLife.06970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Satpute-Krishnan P., Ajinkya M., Bhat S., Itakura E., Hegde R. S., and Lippincott-Schwartz J. (2014) ER stress-induced clearance of misfolded GPI-anchored proteins via the secretory pathway. Cell 158, 522–533 10.1016/j.cell.2014.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schuck S., Gallagher C. M., and Walter P. (2014) ER-phagy mediates selective degradation of endoplasmic reticulum independently of the core autophagy machinery. J. Cell Sci. 127, 4078–4088 10.1242/jcs.154716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lipatova Z., and Segev N. (2015) A role for Macro-ER-Phagy in ER quality control. PLoS Genet. 11, e1005390 10.1371/journal.pgen.1005390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mochida K., Oikawa Y., Kimura Y., Kirisako H., Hirano H., Ohsumi Y., and Nakatogawa H. (2015) Receptor-mediated selective autophagy degrades the endoplasmic reticulum and the nucleus. Nature 522, 359–362 10.1038/nature14506 [DOI] [PubMed] [Google Scholar]
- 38. Szoradi T., Schaeff K., Garcia-Rivera E. M., Itzhak D. N., Schmidt R. M., Bircham P. W., Leiss K., Diaz-Miyar J., Chen V. K., Muzzey D., Borner G. H. H., and Schuck S. (2018) SHRED is a regulatory cascade that reprograms Ubr1 substrate specificity for enhanced protein quality control during stress. Mol. Cell 70, 1025–1037.e5 10.1016/j.molcel.2018.04.027 [DOI] [PubMed] [Google Scholar]
- 39. Kang S. W., Rane N. S., Kim S. J., Garrison J. L., Taunton J., and Hegde R. S. (2006) Substrate-specific translocational attenuation during ER stress defines a pre-emptive quality control pathway. Cell 127, 999–1013 10.1016/j.cell.2006.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kadowaki H., Nagai A., Maruyama T., Takami Y., Satrimafitrah P., Kato H., Honda A., Hatta T., Natsume T., Sato T., Kai H., Ichijo H., and Nishitoh H. (2015) Pre-emptive quality control protects the ER from protein overload via the proximity of ERAD components and SRP. Cell Rep. 13, 944–956 10.1016/j.celrep.2015.09.047 [DOI] [PubMed] [Google Scholar]
- 41. Kadowaki H., Satrimafitrah P., Takami Y., and Nishitoh H. (2018) Molecular mechanism of ER stress-induced pre-emptive quality control involving association of the translocon, Derlin-1, and HRD1. Sci. Rep. 8, 7317 10.1038/s41598-018-25724-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Corazzari M., Gagliardi M., Fimia G. M., and Piacentini M. (2017) Endoplasmic reticulum stress, unfolded protein response, and cancer cell fate. Front. Oncol. 7, 78 10.3389/fonc.2017.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grootjans J., Kaser A., Kaufman R. J., and Blumberg R. S. (2016) The unfolded protein response in immunity and inflammation. Nat. Rev. Immunol. 16, 469–484 10.1038/nri.2016.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim Y., Santos R., Gage F. H., and Marchetto M. C. (2017) Molecular mechanisms of bipolar disorder: progress made and future challenges. Front. Cell. Neurosci. 11, 30 10.3389/fncel.2017.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sozen E., and Ozer N. K. (2017) Impact of high cholesterol and endoplasmic reticulum stress on metabolic diseases: an updated minireview. Redox Biol. 12, 456–461 10.1016/j.redox.2017.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xiang C., Wang Y., Zhang H., and Han F. (2017) The role of endoplasmic reticulum stress in neurodegenerative disease. Apoptosis 22, 1–26 10.1007/s10495-016-1296-4 [DOI] [PubMed] [Google Scholar]
- 47. Takenokuchi M., Miyamoto K., Saigo K., and Taniguchi T. (2015) Bortezomib causes ER stress-related death of acute promyelocytic leukemia cells through excessive accumulation of PML-RARA. Anticancer Res. 35, 3307–3316 [PubMed] [Google Scholar]
- 48. Utecht K. N., and Kolesar J. (2008) Bortezomib: a novel chemotherapeutic agent for hematologic malignancies. Am. J. Health Syst. Pharm. 65, 1221–1231 10.2146/ajhp070272 [DOI] [PubMed] [Google Scholar]
- 49. Scott D. C., and Schekman R. (2008) Role of Sec61p in the ER-associated degradation of short-lived transmembrane proteins. J. Cell Biol. 181, 1095–1105 10.1083/jcb.200804053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Engle S. M., Crowder J. J., Watts S. G., Indovina C. J., Coffey S. Z., and Rubenstein E. M. (2017) Acetylation of N terminus and two internal amino acids is dispensable for degradation of a protein that aberrantly engages the endoplasmic reticulum translocon. PeerJ 5, e3728 10.7717/peerj.3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jaenicke L. A., Brendebach H., Selbach M., and Hirsch C. (2011) Yos9p assists in the degradation of certain nonglycosylated proteins from the endoplasmic reticulum. Mol. Biol. Cell 22, 2937–2945 10.1091/mbc.e10-10-0832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Plemper R. K., Egner R., Kuchler K., and Wolf D. H. (1998) Endoplasmic reticulum degradation of a mutated ATP-binding cassette transporter Pdr5 proceeds in a concerted action of Sec61 and the proteasome. J. Biol. Chem. 273, 32848–32856 10.1074/jbc.273.49.32848 [DOI] [PubMed] [Google Scholar]
- 53. Garza R. M., Sato B. K., and Hampton R. Y. (2009) In vitro analysis of Hrd1p-mediated retrotranslocation of its multispanning membrane substrate 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase. J. Biol. Chem. 284, 14710–14722 10.1074/jbc.M809607200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Neal S., Jaeger P. A., Duttke S. H., Benner C., K Glass C., Ideker T., and Hampton R. Y. (2018) The Dfm1 Derlin is required for ERAD retrotranslocation of integral membrane proteins. Mol. Cell 69, 306–320.e4 10.1016/j.molcel.2017.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Johnson P. R., Swanson R., Rakhilina L., and Hochstrasser M. (1998) Degradation signal masking by heterodimerization of MATα2 and MATα1 blocks their mutual destruction by the ubiquitin-proteasome pathway. Cell 94, 217–227 10.1016/S0092-8674(00)81421-X [DOI] [PubMed] [Google Scholar]
- 56. Wittke S., Dünnwald M., and Johnsson N. (2000) Sec62p, a component of the endoplasmic reticulum protein translocation machinery, contains multiple binding sites for the Sec-complex. Mol. Biol. Cell 11, 3859–3871 10.1091/mbc.11.11.3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Deshaies R. J., and Schekman R. (1990) Structural and functional dissection of Sec62p, a membrane-bound component of the yeast endoplasmic reticulum protein import machinery. Mol. Cell. Biol. 10, 6024–6035 10.1128/MCB.10.11.6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dimitrova L. N., Kuroha K., Tatematsu T., and Inada T. (2009) Nascent peptide-dependent translation arrest leads to Not4p-mediated protein degradation by the proteasome. J. Biol. Chem. 284, 10343–10352 10.1074/jbc.M808840200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lu J., and Deutsch C. (2008) Electrostatics in the ribosomal tunnel modulate chain elongation rates. J. Mol. Biol. 384, 73–86 10.1016/j.jmb.2008.08.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shen P. S., Park J., Qin Y., Li X., Parsawar K., Larson M. H., Cox J., Cheng Y., Lambowitz A. M., Weissman J. S., Brandman O., and Frost A. (2015) Protein synthesis. Rqc2p and 60S ribosomal subunits mediate mRNA-independent elongation of nascent chains. Science 347, 75–78 10.1126/science.1259724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kostova K. K., Hickey K. L., Osuna B. A., Hussmann J. A., Frost A., Weinberg D. E., and Weissman J. S. (2017) CAT-tailing as a fail-safe mechanism for efficient degradation of stalled nascent polypeptides. Science 357, 414–417 10.1126/science.aam7787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hirata R., Nihei C., and Nakano A. (2013) Isoform-selective oligomer formation of Saccharomyces cerevisiae p24 family proteins. J. Biol. Chem. 288, 37057–37070 10.1074/jbc.M113.518340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jones G. M., Stalker J., Humphray S., West A., Cox T., Rogers J., Dunham I., and Prelich G. (2008) A systematic library for comprehensive overexpression screens in Saccharomyces cerevisiae. Nat. Methods 5, 239–241 10.1038/nmeth.1181 [DOI] [PubMed] [Google Scholar]
- 64. Ng D. T., Brown J. D., and Walter P. (1996) Signal sequences specify the targeting route to the endoplasmic reticulum membrane. J. Cell Biol. 134, 269–278 10.1083/jcb.134.2.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Promlek T., Ishiwata-Kimata Y., Shido M., Sakuramoto M., Kohno K., and Kimata Y. (2011) Membrane aberrancy and unfolded proteins activate the endoplasmic reticulum stress sensor Ire1 in different ways. Mol. Biol. Cell 22, 3520–3532 10.1091/mbc.e11-04-0295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pierre N., Barbé C., Gilson H., Deldicque L., Raymackers J. M., and Francaux M. (2014) Activation of ER stress by hydrogen peroxide in C2C12 myotubes. Biochem. Biophys. Res. Commun. 450, 459–463 10.1016/j.bbrc.2014.05.143 [DOI] [PubMed] [Google Scholar]
- 67. Tsang C. K., Liu Y., Thomas J., Zhang Y., and Zheng X. F. (2014) Superoxide dismutase 1 acts as a nuclear transcription factor to regulate oxidative stress resistance. Nat. Commun. 5, 3446 10.1038/ncomms4446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schäfer A., and Wolf D. H. (2009) Sec61p is part of the endoplasmic reticulum-associated degradation machinery. EMBO J. 28, 2874–2884 10.1038/emboj.2009.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Duennwald M. L., and Lindquist S. (2008) Impaired ERAD and ER stress are early and specific events in polyglutamine toxicity. Genes Dev. 22, 3308–3319 10.1101/gad.1673408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nakatsukasa K., Brodsky J. L., and Kamura T. (2013) A stalled retrotranslocation complex reveals physical linkage between substrate recognition and proteasomal degradation during ER-associated degradation. Mol. Biol. Cell 24, 1765–1775, S1–S8 10.1091/mbc.e12-12-0907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mehrtash A. B., and Hochstrasser M. (2019) Ubiquitin-dependent protein degradation at the endoplasmic reticulum and nuclear envelope. Semin. Cell Dev. Biol. 93, 111–124 10.1016/j.semcdb.2018.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Menéndez-Benito V., Verhoef L. G., Masucci M. G., and Dantuma N. P. (2005) Endoplasmic reticulum stress compromises the ubiquitin-proteasome system. Hum. Mol. Genet. 14, 2787–2799 10.1093/hmg/ddi312 [DOI] [PubMed] [Google Scholar]
- 73. Fabre B., Livneh I., Ziv T., and Ciechanover A. (2019) Identification of proteins regulated by the proteasome following induction of endoplasmic reticulum stress. Biochem. Biophys. Res. Commun. 517, 188–192 10.1016/j.bbrc.2019.07.040 [DOI] [PubMed] [Google Scholar]
- 74. Guthrie C., and Fink G. R. (eds) (2004) Guide to Yeast Genetics and Molecular and Cell Biology, pp. 1–933, Elsevier, San Diego, CA [Google Scholar]
- 75. Goldstein A. L., and McCusker J. H. (1999) Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15, 1541–1553 [DOI] [PubMed] [Google Scholar]
- 76. Mumberg D., Müller R., and Funk M. (1994) Regulatable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use for heterologous expression. Nucleic Acids Res. 22, 5767–5768 10.1093/nar/22.25.5767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mumberg D., Müller R., and Funk M. (1995) Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156, 119–122 10.1016/0378-1119(95)00037-7 [DOI] [PubMed] [Google Scholar]
- 78. Chen P., Johnson P., Sommer T., Jentsch S., and Hochstrasser M. (1993) Multiple ubiquitin-conjugating enzymes participate in the in vivo degradation of the yeast MATα2 repressor. Cell 74, 357–369 10.1016/0092-8674(93)90426-Q [DOI] [PubMed] [Google Scholar]
- 79. Hochstrasser M., and Varshavsky A. (1990) In vivo degradation of a transcriptional regulator: the yeast α2 repressor. Cell 61, 697–708 10.1016/0092-8674(90)90481-S [DOI] [PubMed] [Google Scholar]
- 80. Kushnirov V. V. (2000) Rapid and reliable protein extraction from yeast. Yeast 16, 857–860 [DOI] [PubMed] [Google Scholar]
- 81. Watts S. G., Crowder J. J., Coffey S. Z., and Rubenstein E. M. (2015) Growth-based determination and biochemical confirmation of genetic requirements for protein degradation in Saccharomyces cerevisiae. J. Vis. Exp. 2015, e52428 10.3791/52428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Loayza D., and Michaelis S. (1998) Role for the ubiquitin-proteasome system in the vacuolar degradation of Ste6p, the a-factor transporter in Saccharomyces cerevisiae. Mol. Cell. Biol. 18, 779–789 10.1128/MCB.18.2.779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Buchanan B. W., Lloyd M. E., Engle S. M., and Rubenstein E. M. (2016) Cycloheximide chase analysis of protein degradation in Saccharomyces cerevisiae. J. Vis. Exp. 2016, e53975 10.3791/53975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hjelm H., Hjelm K., and Sjöquist J. (1972) Protein A from Staphylococcus aureus. Its isolation by affinity chromatography and its use as an immunosorbent for isolation of immunoglobulins. FEBS Lett. 28, 73–76 10.1016/0014-5793(72)80680-X [DOI] [PubMed] [Google Scholar]
- 85. Miyazaki T., Nakayama H., Nagayoshi Y., Kakeya H., and Kohno S. (2013) Dissection of Ire1 functions reveals stress response mechanisms uniquely evolved in Candida glabrata. PLoS Pathog. 9, e1003160 10.1371/journal.ppat.1003160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Scrimale T., Didone L., de Mesy Bentley K. L., and Krysan D. J. (2009) The unfolded protein response is induced by the cell wall integrity mitogen-activated protein kinase signaling cascade and is required for cell wall integrity in Saccharomyces cerevisiae. Mol. Biol. Cell 20, 164–175 10.1091/mbc.e08-08-0809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ryu H. Y., Wilson N. R., Mehta S., Hwang S. S., and Hochstrasser M. (2016) Loss of the SUMO protease Ulp2 triggers a specific multichromosome aneuploidy. Genes Dev. 30, 1881–1894 10.1101/gad.282194.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lewis A., Felberbaum R., and Hochstrasser M. (2007) A nuclear envelope protein linking nuclear pore basket assembly, SUMO protease regulation, and mRNA surveillance. J. Cell Biol. 178, 813–827 10.1083/jcb.200702154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hampton R. Y., and Bhakta H. (1997) Ubiquitin-mediated regulation of 3-hydroxy-3-methylglutaryl–CoA reductase. Proc. Natl. Acad. Sci. U.S.A. 94, 12944–12948 10.1073/pnas.94.24.12944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tong A. H., Evangelista M., Parsons A. B., Xu H., Bader G. D., Pagé N., Robinson M., Raghibizadeh S., Hogue C. W., Bussey H., Andrews B., Tyers M., and Boone C. (2001) Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science 294, 2364–2368 10.1126/science.1065810 [DOI] [PubMed] [Google Scholar]
- 91. Huh W. K., Falvo J. V., Gerke L. C., Carroll A. S., Howson R. W., Weissman J. S., and O'Shea E. K. (2003) Global analysis of protein localization in budding yeast. Nature 425, 686–691 10.1038/nature02026 [DOI] [PubMed] [Google Scholar]
- 92. Ng D. T., Spear E. D., and Walter P. (2000) The unfolded protein response regulates multiple aspects of secretory and membrane protein biogenesis and endoplasmic reticulum quality control. J. Cell Biol. 150, 77–88 10.1083/jcb.150.1.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sikorski R. S., and Hieter P. (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mayer T. U., Braun T., and Jentsch S. (1998) Role of the proteasome in membrane extraction of a short-lived ER-transmembrane protein. EMBO J. 17, 3251–3257 10.1093/emboj/17.12.3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Christianson T. W., Sikorski R. S., Dante M., Shero J. H., and Hieter P. (1992) Multifunctional yeast high-copy-number shuttle vectors. Gene 110, 119–122 10.1016/0378-1119(92)90454-W [DOI] [PubMed] [Google Scholar]
- 96. Rose M. D., Misra L. M., and Vogel J. P. (1989) KAR2, a karyogamy gene, is the yeast homologue of the mammalian BiP/GRP78 gene. Cell 57, 1211–1221 10.1016/0092-8674(89)90058-5 [DOI] [PubMed] [Google Scholar]
- 97. Olzmann J. A., and Kopito R. R. (2011) Lipid droplet formation is dispensable for endoplasmic reticulum-associated degradation. J. Biol. Chem. 286, 27872–27874 10.1074/jbc.C111.266452 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.