Abstract
Endothelial nitric oxide (NO) synthase (eNOS) plays a critical role in the maintenance of blood vessel homeostasis. Recent findings suggest that cytoskeletal dynamics play an essential role in regulating eNOS expression and activation. Here, we sought to test whether modulation of cytoskeletal dynamics through pharmacological regulation of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation affects eNOS expression and endothelial function in vitro and in vivo. We found that tubulin acetylation inducer (tubacin), a compound that appears to selectively inhibit HDAC6 activity, dramatically increased eNOS expression in several different endothelial cell lines, as determined by both immunoblotting and NO production assays. Mechanistically, we found that these effects were not mediated by tubacin's inhibitory effect on HDAC6 activity, but rather were due to its ability to stabilize eNOS mRNA transcripts. Consistent with these findings, tubacin also inhibited proinflammatory cytokine-induced degradation of eNOS transcripts and impairment of endothelium-dependent relaxation in the mouse aorta. Furthermore, we found that tubacin-induced up-regulation in eNOS expression in vivo is associated with improved endothelial function in diabetic db/db mice and with a marked attenuation of ischemic brain injury in a murine stroke model. Our findings indicate that tubacin exhibits potent eNOS-inducing effects and suggest that this compound might be useful for the prevention or management of endothelial dysfunction–associated cardiovascular diseases.
Keywords: endothelial dysfunction; endothelium; nitric oxide synthase; nitric oxide; gene regulation; diabetes; histone acetylase; endothelial cells; endothelial nitric oxide synthase (eNOS); cytoskeleton; histone deacetylase 6 (HDCA6); stroke; cardiovascular disorder, tubulin acetylation inducer (tubacin); inflammation
Introduction
Decreased endothelial nitric oxide synthase (eNOS)3 expression and activity have been implicated in the pathogenesis of a wide range of cardiovascular conditions, including atherosclerosis, essential hypertension, stroke, and coronary restenosis (1, 2). In healthy blood vessels, the expression of eNOS is tightly regulated through complex mechanisms, involving transcriptional, post-transcriptional, and post-translational controls (3). Although eNOS levels fluctuate very little under normal conditions, transcript and protein levels can vary considerably in response to different types of endogenous or exogenous exposures (4). For instance, the pro-inflammatory agonists TNFα, lipopolysaccharide (LPS), and oxidized low-density lipoprotein (LDL) down-regulate eNOS expression in endothelial cells (5, 6), whereas the steroid hormone estrogen and HMG-CoA reductase inhibitors act as potent stimulators of eNOS expression (7–9). Notably, in many of these scenarios, the fluctuation in eNOS levels is due to factors that either shorten or prolong the half-life of eNOS mRNA.
Recently, a growing body of evidence suggests that the composition of cytoskeletal components can influence the stability of eNOS mRNA (10). For example, Laufs and others showed that the half-life of eNOS transcripts can be significantly prolonged by physically disrupting the interactions of actin filaments (11, 12). Moreover, Xue et al. (13) showed that eNOS transcripts physically interact with microtubule proteins and that altering the structure of microtubules can by itself influence eNOS levels and NO production in endothelial cells (11, 14). Taken together, these findings suggest that pharmacological strategies targeting microtubule proteins could be employed for manipulating eNOS levels in cells.
It is now known that post-translational modifications play an important role in regulating the stability of microtubules (15–17). Based on this understanding, we hypothesized that manipulating post-translational modifications of tubulin might have an effect on eNOS levels in endothelial cells. To test this hypothesis, we focused on the enzyme histone deacetylase 6 (HDAC6), which serves as a potent microtubule-associated tubulin deacetylase (18, 19), and pharmacological inhibition of this enzyme is known to regulate acetylation of tubulin and the stability of microtubules in various types of cells (20, 21). Further, HDAC6 knockout mice are not only viable but also have normal blood vessel development (22), suggesting that selective inhibition of HDAC6 might be safe when delivered systemically (20, 23). In this study, we investigated the biological effects of tubacin, a compound with selective HDAC6-inhibitory activity, on the expression of eNOS in endothelial cells as well as examined the effects of this compound on NO production and blood vessel functions in mice.
Results
Tubacin increases eNOS expression in endothelial cells
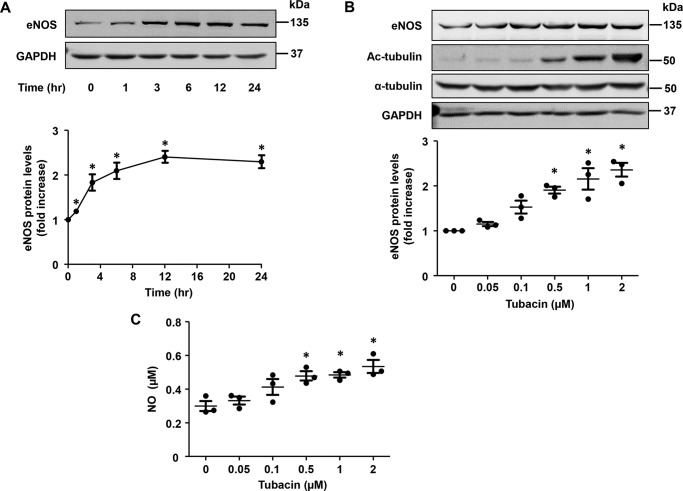
To investigate the role of HDAC6 in eNOS expression, we treated HUVECs with tubacin, a compound discovered from screening over 7000 compounds with an inhibitory effect on tubulin deacetylation (24, 25). As shown in Fig. 1A, we found that tubacin dramatically increased eNOS expression in HUVECs, displaying a maximal effect at 12 h after first exposure. Further, we found that tubacin also increased eNOS expression in a dose-dependent manner, exhibiting an EC50 of ∼0.3 μmol/liter and showing a maximal effect at a concentration of 1–2 μmol/liter. Consistent with the ability to induce eNOS expression, we found that NO levels were also markedly increased in the supernatant of tubacin-exposed cells (Fig. 1, B and C). As expected, tubacin also appeared to inhibit deacetylase activity in our cells, as evidenced by a marked increase in acetylated tubulin levels. Next, to assess whether the above findings were specific to HUVECs, we examined the effects of tubacin on eNOS expression in other EC populations. As shown in Fig. S1, we found that tubacin induced a similar dose-dependent increase in eNOS expression in both bovine aortic endothelial cells (BAECs) and primary bovine brain microvascular endothelial cells (BBMVECs), illustrating the reproducibility of findings across ECs from different species and vascular beds.
Figure 1.
Tubacin increases eNOS expression and NO generation in HUVECs. A, eNOS expression in HUVECs treated with 1 μmol/liter tubacin for different lengths of time. Protein expression was quantified by densitometry analysis. Results are mean ± S.E. (error bars), n = 3; *, p < 0.05 versus 0 h, one-way ANOVA with Tukey's post-test. B, eNOS expression in HUVECs exposed to varying concentrations of tubacin for 12 h. Protein expression was quantified by densitometric analysis; mean ± S.E., n = 3; *, p < 0.05 versus 0 μmol/liter, one-way ANOVA with Tukey's post-test. C, NO levels in supernatant of HUVECs treated with varying concentrations of tubacin for 24 h. Results are mean ± S.E., n = 3; *, p < 0.05 versus 0 μmol/liter, one-way ANOVA with Tukey's post-test.
Tubacin induces eNOS expression through HDAC6-independent mechanisms
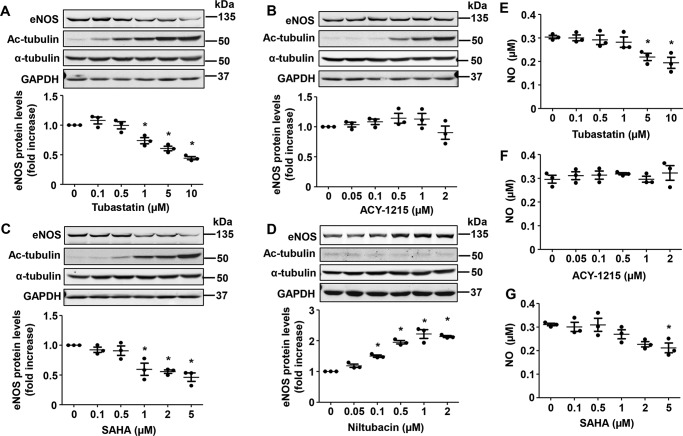
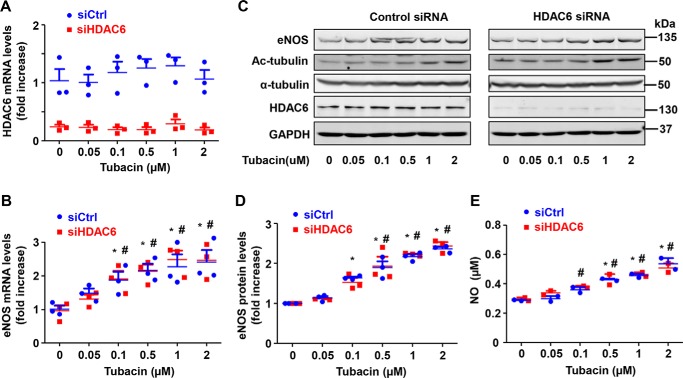
Recently, several different HDAC6 inhibitors have been discovered (20). To assess whether the effects of tubacin on eNOS expression were specific to this compound, we exposed HUVECs to several other HDAC6 inhibitors (Fig. 2), including tubastatin A (selective HDAC6 inhibitor), ACY-1215 (Rocilinostat) (selective HDAC6 inhibitor), and SAHA (vorinostat) (pan-HDAC inhibitor). We also exposed HUVECs to niltubacin, a compound that is structurally similar to tubacin but lacks any known HDAC6-inhibitory activity (24). As shown in Fig. 3, we found that other HDAC6 inhibitors had little or inhibitory effect on eNOS expression in our cells, although each clearly inhibited deacetylase activity, as evidenced by the marked increase in acetylated tubulin levels. In fact, eNOS expression and NO production were actually reduced in response to tubastatin A and SAHA, but not affected by ACY-1215, suggesting that effects of tubacin on eNOS might be independent of actions on HDAC6. Consistent with this line of reasoning, we found that niltubacin, a compound that lacks HDAC6-inhibitory activity, also dramatically increased eNOS expression in our cells. Next, to confirm that tubacin mediates its effect through HDAC6-independent mechanisms, we performed siRNA-mediated knockdown of the HDAC6 gene and examined the effects on eNOS expression. As expected, siRNA knockdown of HDAC6 markedly reduced mRNA and protein levels of HDAC6 in our cells (Fig. 4, A and C). Furthermore, we found that tubacin still readily increased eNOS mRNA and protein levels in HDAC6-deficient cells (Fig. 4, B–E), supporting the notion that tubacin augments eNOS expression largely through HDAC6-independent mechanisms.
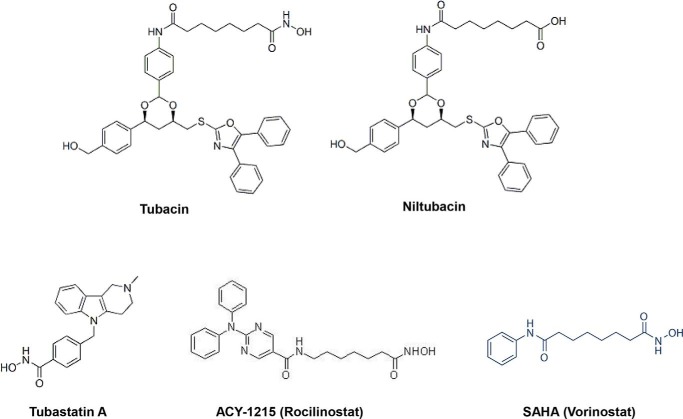
Figure 2.
Chemical structure of different HDAC6 inhibitors. Tubacin is a highly selective and reversible HDAC6 inhibitor that permeates the cell membrane. Tubastatin A and rocilinostat (ACY-1215) are also selective HDAC6 inhibitors. SAHA is a pan-HDAC inhibitor. Niltubacin, which retains the bulk of the chemical structure of tubacin, but lacks HDAC6-inhibitory activity, is normally used as a negative control for studies with tubacin.
Figure 3.
The effects of different HDAC inhibitors on eNOS expression in HUVECs. Protein levels of eNOS, acetylated tubulin (Ac-tubulin), total tubulin, and GAPDH in HUVECs treated with tubastatin (A), ACY-1215 (B), SAHA (C), or niltubacin (D) for 24 h. Representative images of Western blots (top) and densitometry measures (bottom) are shown. Results are mean ± S.E. (error bars), n = 3; *, p < 0.05 versus 0 μmol/liter, one-way ANOVA with Tukey's post-test. E–G, NO levels in the supernatant of HUVECs treated with varying concentrations of tubastatin, ACY-1215, or SAHA for 24 h. Results are mean ± S.E., n = 3; *, p < 0.05 versus 0 μm, one-way ANOVA with Tukey's post-test.
Figure 4.
Tubacin-induced eNOS expression occurs independent of HDAC6 inhibition. A and B, transcript levels for HDAC6 or eNOS in control and HDAC6-deficient cells exposed to varying concentrations of tubacin for 6 h. C, protein levels for eNOS, acetylated α-tubulin, total α-tubulin, HDAC6, and GAPDH in control and HDAC6-deficient cells exposed to different concentrations of tubacin for 24 h. D, densitometry measurements of eNOS and HDAC6 in control and HDAC6-deficient cells exposed to different concentrations of tubacin for 24 h. E, NO levels in supernatants of HUVECs exposed to 0–2 μmol/liter tubacin for 24 h. Results are mean ± S.E. (error bars), n = 3; *, p < 0.05 versus 0-h control siRNA, one-way ANOVA with Tukey's post-test; #, p < 0.05 versus 0-h HDAC6 siRNA, one-way ANOVA with Tukey's post-test.
Tubacin increases eNOS expression by enhancing eNOS mRNA stability
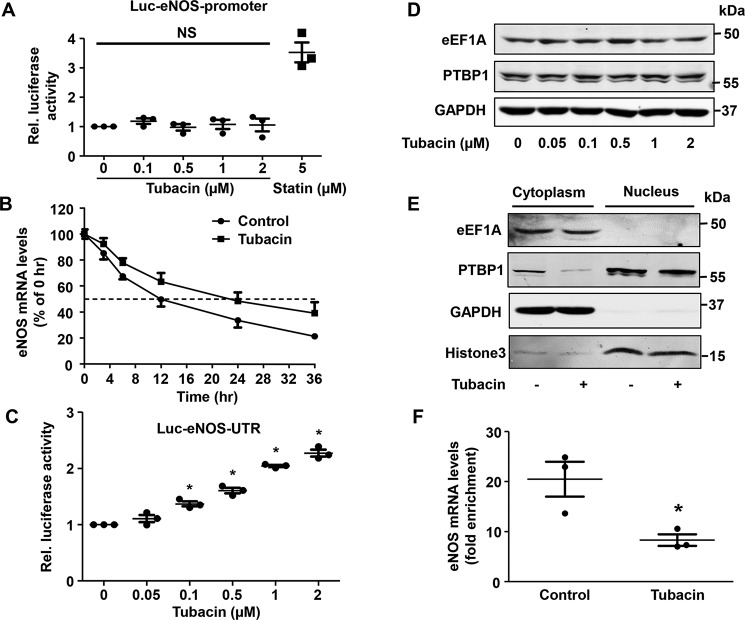
Because eNOS mRNA levels were significantly increased following tubacin exposure, we hypothesized that this might relate to an increase in gene transcription. To test this, we next transfected COS-7 cells with a luciferase reporter plasmid linked to the eNOS promotor (F1: −1600 nucleotides from the transcription start site) (26). As shown in Fig. 5, we found that transcription of eNOS was readily increased in cells exposed to simvastatin, which has been shown to increase eNOS promoter activity (27). In contrast, tubacin had little to no effect on gene transcription of eNOS in our cells (Fig. 5A). Because post-transcriptional mechanisms can also have an influence on eNOS transcript levels, we next examined whether tubacin acts to prolong the half-life of eNOS mRNA in our cells. To test this, we inhibited gene transcription with actinomycin D and measured transcript levels at various time points after tubacin exposure. As shown in Fig. 5B, we found that treatment with tubacin had a dramatic effect on eNOS mRNA stability, as demonstrated by a doubling of its half-life from 12 to 24 h. Further, we confirmed these findings using a unique reporter system in which the luciferase gene was linked to the 3′-UTR of eNOS (Fig. 5C) as we reported previously (28).
Figure 5.
Tubacin increases eNOS mRNA stability in HUVECs. A, relative luciferase activity in COS-7 cells treated with tubacin or 5 μmol/liter simvastatin for 12 h. Cell lysates were assayed for luciferase activities. Results are mean ± S.E. (error bars), n = 3. B, eNOS transcript levels in HUVECs treated with or without 1 μm tubacin for 3 h. eNOS mRNA stability was determined by exposing cells to 5 μm actinomycin D. Results are mean ± S.E., n = 3. C, relative luciferase activity in COS-7 cells treated with different concentrations of tubacin. Results are mean ± S.E., n = 3; *, p < 0.05 versus 0 μmol/liter, one-way ANOVA with Tukey's post-test. D, the protein levels of PTBP1 and eEF1A in HUVECs treated with different concentrations of tubacin for 24 h. E, PTBP1 and eEF1A levels in the cytoplasmic or nuclear fraction of HUVECs treated with either vehicle or 1 μmol/liter tubacin for 24 h. GAPDH was used as a cytoplasmic marker, and histone 3 was used as a nuclear marker. F, eNOS mRNA levels in the anti-PTBP1 immunoprecipitates were quantified by real-time PCR of control and tubacin-treated cells. Data are shown as enrichment over the control IgG antibody. Results are mean ± S.E., n = 3; *, p < 0.05 versus control, unpaired Student's t test.
In recent work, we reported that the cytoplasmic proteins eEF1A and PTBP1 play a crucial role in regulating the stability of eNOS mRNA in ECs (28, 29). To assess whether tubacin alters the expression of these proteins, we compared levels of eEF1A and PTBP1 between control and tubacin-exposed cells. Although tubacin was found to have little to no effect on total protein levels of either protein (Fig. 5D), we detected a substantial reduction in cytoplasmic levels of PTBP1 in our cells (Fig. 5E). Because the binding of cytoplasmic PTBP1 to the eNOS 3′-UTR is known to facilitate mRNA degradation (28), we hypothesized that tubacin might also act to reduce this protein-transcript interaction. To test this, we immunoprecipitated PTBP1 from the cytoplasmic fraction of control and tubacin-exposed cells and quantified eNOS transcript levels in this protein fraction. Consistent with our proposed hypothesis, we found that eNOS transcript levels were markedly reduced in PTBP1 immunocomplexes after tubacin exposure (Fig. 5F).
Tubacin ameliorates TNFα-induced endothelial dysfunction through increasing eNOS expression
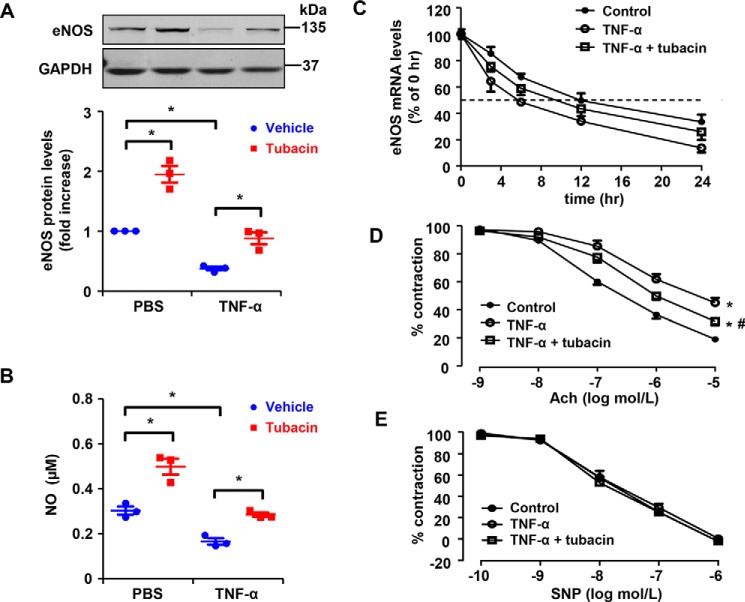
The pro-inflammatory cytokine TNFα is known to cause endothelial cell dysfunction, in part, by targeting eNOS mRNA for degradation (28, 29). With this in mind, we next sought to determine whether tubacin can mitigate eNOS mRNA degradation in response to TNFα exposure. Consistent with previous reports (28, 29), we found that eNOS expression and NO production were dramatically reduced in TNFα-exposed cells (20 ng/ml) and this was associated with a decrease in eNOS mRNA stability. Conversely, we found that eNOS expression, NO production, and the half-life of eNOS transcripts were markedly increased in cells treated with tubacin (Fig. 6, A–C). Because down-regulation of eNOS expression is known to contribute to endothelial dysfunction in various model systems, we next sought to determine whether augmenting eNOS expression with tubacin could restore vascular function in TNFα-exposed blood vessels. After confirming that tubacin (1 μmol/liter) can restore eNOS expression in the isolated mouse aorta after TNFα exposure (20 ng/ml), we examined whether this associated with improved acetylcholine (Ach)-mediated vasorelaxation. As shown in Fig. 6D, we found that treatment with tubacin largely reversed TNFα-induced endothelial dysfunction in isolated aorta segments. As expected, tubacin did not have an effect on vasodilatory responses to sodium nitroprusside (Fig. 6E), supporting the notion that tubacin's effects are mediated specifically through augmenting eNOS expression.
Figure 6.
Tubacin inhibits TNFα-induced down-regulation of eNOS in HUVECs and ameliorates TNFα-induced endothelial dysfunction in the aorta of mice. A, protein levels of eNOS in HUVECs treated with or without TNFα (20 ng/ml) in the presence or absence of tubacin. Results are mean ± S.E. (error bars), n = 3; *, p < 0.05, one-way ANOVA with Tukey's post-test. B, NO levels in the supernatant of HUVECs pretreated with 1 μmol/liter tubacin for 1 h and then exposed to vehicle or 20 ng/ml TNFα for 24 h. Results are mean ± S.E. n = 3; *, p < 0.05, one-way ANOVA with Tukey's post-test. C, eNOS mRNA half-life in control and tubacin-treated cells exposed to PBS versus TNFα. The data are representative of five independent experiments. D, Ach-induced endothelium-dependent relaxation in aortas treated with or without tubacin (1 μmol/liter) for 24 h while also being exposed to control or TNFα. Endothelium-dependent vasorelaxation was determined by measuring Ach-induced relaxation in rings precontracted with phenylephrine. Endothelium-dependent vasorelaxation was determined by measuring Ach-induced relaxation in rings precontracted with phenylephrine; n = 8; *, p < 0.05 versus control group at 10−5 mol/liter acetylcholine; #, p < 0.05 versus TNFα group at 10−5 mol/liter acetylcholine, one-way ANOVA with Tukey's post-test. E, endothelium-independent vasorelaxation to sodium nitroprusside (SNP) was examined.
Tubacin enhances endothelium-dependent vasorelaxation in murine aorta
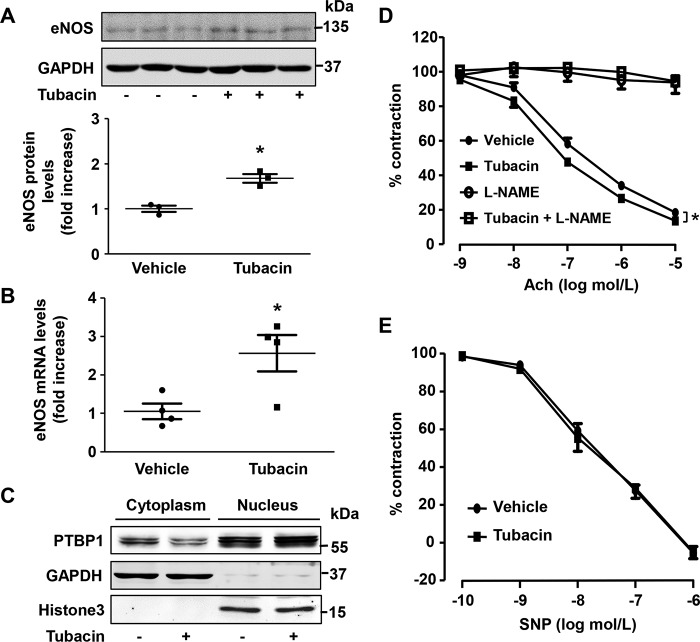
To examine whether tubacin mediates similar effects in vivo, we next treated mice with a one-time intraperitoneal injection of tubacin (5 mg/kg) or vehicle control and examined eNOS expression in the mouse aorta. As shown in Fig. 7 (A and B), we found that both mRNA and protein levels for eNOS were significantly increased in the aorta of tubacin-treated mice. Moreover, this was associated with a substantial reduction in cytoplasmic levels of PTBP1 (Fig. 7C) and a weak, but significant, increase in Ach-induced vasorelaxation (Fig. 7D). Importantly, in the presence of a NOS inhibitor (NG-nitro-l-arginine methyl ester) or NO donor sodium nitroprusside, tubacin's effects on Ach-mediated vasorelaxation were largely abolished (Fig. 7, D and E), indicating that tubacin mediates its effects on blood vessel tone via eNOS-dependent mechanisms.
Figure 7.
The effect of tubacin on eNOS expression and endothelium-dependent relaxation in mice. Mice were intraperitoneally injected with 5 mg/kg tubacin. 24 h after injection, the eNOS protein levels (A) and eNOS mRNA levels (B) in mouse aorta were measured by Western blotting and qRT-PCR, respectively. Results are mean ± S.E. (error bars), n = 3–4; *, p < 0.05 versus control group, unpaired Student's t test. C, the levels of PTBP1 in cytosolic and nuclear subcellular fractions were determined by Western blotting in mouse aorta treated with or without tubacin for 24 h. GAPDH was used as a cytoplasmic marker, and histone 3 was used as a nuclear marker. D, representative traces of Ach-induced endothelium-dependent relaxations in aortas from mice treated with or without tubacin for 24 h. Endothelium-dependent vasorelaxation was determined by measuring Ach-induced relaxation in rings precontracted with phenylephrine; n = 8; *, p < 0.05 versus control group at 10−5 mol/liter acetylcholine, one-way ANOVA with Tukey's post-test. E, endothelium-independent vasorelaxation to sodium nitroprusside (SNP) was examined.
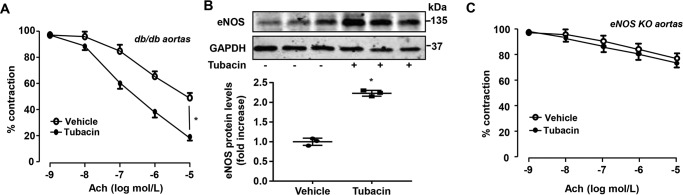
To determine whether tubacin is able to attenuate endothelial dysfunction in disease, we employed db/db mice, which are known to manifest an impaired endothelial function at 4 months of age (30). Using these mice, we found that treatment with tubacin (5 mg/kg/day, intraperitoneally, 1 week) significantly improved endothelium-dependent vasorelaxation to ACh in aortas of db/db mice (Fig. 8A) and the expression of eNOS, as determined by Western blotting (Fig. 8B). Consistent with a previous report (31), Ach-mediated vasorelaxation in the aorta of eNOS knockout mice was significantly impaired, which was not affected by tubacin treatment (Fig. 8C), further illustrating the vasoprotective effects of tubacin in vivo through eNOS-dependent mechanisms.
Figure 8.
Tubacin improves endothelial function in diabetic mice. A, db/db mice received intraperitoneal injection of either vehicle or 5 mg/kg/day of tubacin for a week, and Ach-induced endothelium-dependent relaxation of aortic ring was determined. Results are mean ± S.E. (error bars); n = 8; *, p < 0.05 versus vehicle at 10−5 mol/liter acetylcholine, one-way ANOVA with Tukey's post-test. B, db/db mice received intraperitoneal injection of either vehicle or 5 mg/kg/day of tubacin for a week. Expression of eNOS was determined by Western blotting. n = 3; *, p < 0.05 versus vehicle control group, unpaired Student's t test. C, eNOS knockout (KO) mice received intraperitoneal injection of vehicle or 5 mg/kg/day of tubacin for a week, and Ach-induced endothelium-dependent relaxation of aortic ring was determined. n = 8.
Tubacin attenuates cerebral injury in mice
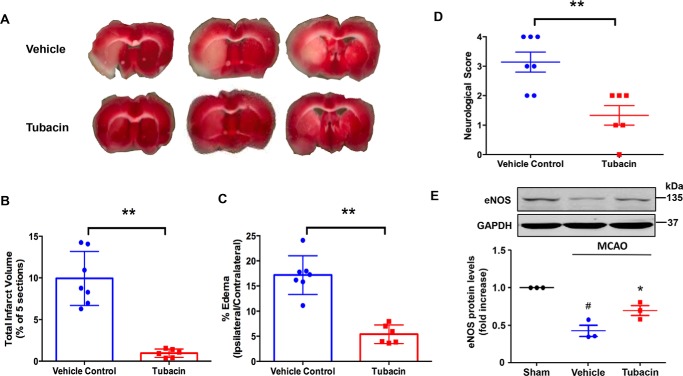
Finally, because NO is known to have potent neuroprotective and anti-apoptotic properties (11, 32), we hypothesized that tubacin might reduce brain injury in a cerebral ischemia model. To this end, we employed the well-established mouse middle cerebral artery occlusion (MCAO) model to assess the effects of tubacin on ischemia-induced brain injury. As shown in Fig. 9, we found that pretreatment with tubacin (5 mg/kg) significantly reduced cerebral infarct size and the severity of cortical edema at 24 h after arterial occlusion (Fig. 9, A and B) when compared with controls. Consistent with a reduction in brain injury, we found that functional neurological scores were also significantly improved in tubacin-treated mice (Fig. 9C). Moreover, these findings were associated with a marked increase in eNOS expression in the ischemic penumbra at 24 h after MCAO (Fig. 9D), supporting the concept that tubacin mediates its effects by augmenting eNOS expression.
Figure 9.
Tubacin reduces cerebral infarct size, improves neurological function, and increases eNOS expression in MCAO mice. A, representative TTC-stained cerebral sections of the MCAO model showing decrease infarct size (white area) in tubacin-treated mice as compared with the vehicle-treated group. B and C, quantitative measurement of infarct size and cerebral edema in control and tubacin-treated mice after MCAO. n = 6–7 for each group; **, p < 0.01, unpaired Student's t test. D, neurological deficit scores in control and tubacin-treated mice at 24 h after MCAO. Data are presented as mean ± S.E. (error bars) n = 6–7 for each group; **, p < 0.01, unpaired Student's t test. E, eNOS protein levels in brain penumbra after reperfusion for 24 h. Densitometry measurements are depicted in a bar graph. Each bar represents the mean ± S.E. n = 3; *, p < 0.05 compared with vehicle-treated group, one-way ANOVA with Tukey's post-test; #, p < 0.05 compared with the sham group, one-way ANOVA with Tukey's post-test.
Discussion
In this study, we show that tubacin acts to increase eNOS expression and NO production in vitro and in vivo. However, in contrast to previous reports, we found that tubacin does not mediate its effects through inhibiting HDAC6 activity but rather through stabilizing eNOS mRNA. Because many pathological conditions are known to decrease eNOS expression in endothelial cells by reducing mRNA stability (5, 6, 28, 29), identification of tubacin as a potent eNOS enhancer suggests that tubacin or its analogues might be effective in ameliorating systemic vascular complications of disease. Along these lines, we found that tubacin ameliorated endothelial dysfunction in a mouse diabetic model and reduced the size of cerebral infarction in mice after transient occlusion of the middle cerebral artery.
The interplay between histone deacetylases (HDACs) and histone acetyltransferases differentially regulates the acetylation status of histone and nonhistone proteins and regulates gene expression in various ways (33, 34). In addition to regulating gene expression, HDACs are also involved in a number of other important biological pathways, including those involved in growth, proliferation, and differentiation of cells (35, 36). Indeed, in the vessel wall, several HDACs have been identified to regulate eNOS through both transcriptional and post-translational mechanisms (37, 38). For example, at post-translational levels, both HDAC1 and HDAC3 have been shown to induce lysine deacetylation of eNOS, thereby inhibiting eNOS activity (38). Furthermore, treatment of endothelial cells with pan-HDAC inhibitors, such as trichostatin A and butyric acid, has been shown to decrease eNOS expression at post-transcriptional levels through yet unidentified mechanisms (37). Consistent with the previous report (37), we found that tubastatin A (selective HDAC6 inhibitor) and SAHA (pan-HDAC inhibitor) reduced eNOS protein expression in HUVECs. Recent studies have demonstrated that modulation of the endothelial actin cytoskeleton represents a major mechanism in regulating eNOS expression and mRNA stability (39, 40). Among 18 HDACs in mammals, HDAC6 is a unique member of the type II HDACs that has been shown to regulate cytoskeleton dynamics by acting as a specific α-tubulin deacetylase (18, 22), which prompted us to investigate whether inhibition of HDAC6 could alter eNOS gene expression through modulating cytoskeleton dynamics in endothelial cells. In the present study, we treated endothelial cells with three structurally unrelated inhibitors of HDAC6 and found that only tubacin potently increased eNOS expression, despite all three inhibitors exhibiting inhibitory effects on α-tubulin deacetylation. Furthermore, we found that niltubacin, an inactive tubacin analog without inhibitory effect on HDAC6, also increased eNOS protein expression in a dose-dependent manner, with no changes on the acetylated levels of α-tubulin. Most importantly, our experiments performed in HDAC6 knockdown cells demonstrated that eNOS expression increased by tubacin was largely, if not wholly, independent of HDAC6, as tubacin increased eNOS expression to a similar extent in control siRNA– and HADC6 siRNA–transfected cells. Together, these results identified tubacin as a unique compound that potently increases eNOS expression and NO production through mechanisms independent of its effects on HDAC6.
Endothelially derived NO is a critical mediator of vascular integrity (41). Although the expression of eNOS is regulated at multiple levels, it has been increasingly appreciated that posttranscriptional regulation plays an important role (40). Our laboratory has recently reported that PTBP1, also known as hnRNP1 (heterogeneous nuclear ribonucleoprotein 1), is an essential trans-acting factor that binds to eNOS 3′-UTR in the cytoplasm, leading to eNOS mRNA destabilization and impairment of endothelium-dependent vasorelaxation (28). In our study, we found that tubacin up-regulated eNOS expression, in part, through suppressing cytoplasmic PTBP1 levels in endothelial cells. At this point, the molecular mechanism underlying tubacin-mediated suppression of PTBP1 remains unknown. PTBP1 is a nucleocytoplasmic shuttling protein that has been shown to regulate RNA metabolism, including mRNA stability, translation, and localization (42, 43). For example, glucose stimulation of pancreatic beta cells can induce redistribution of PTBP1 from the nucleus to the cytoplasm, thus promoting PTBP1 binding to 3′-UTR of insulin to enhance mRNA stability (44). Interestingly, PTBP1 has been shown to bind to the mRNAs of actin and the focal adhesion proteins vinculin and α-actinin 4 to regulate cytoskeletal assembly (45, 46). Thus, it is tempting to speculate that tubacin may increase eNOS expression through at least two mechanisms, which include the disruption of PTBP1 binding to eNOS 3′-UTR and the inhibition of PTBP1-mediated cytoskeleton assembly. Further, it would be interesting to investigate whether tubacin can also regulate nucleocytoplasmic shuttling of PTBP1 by affecting its phosphorylation status, as reported previously in the neuronal PC12 cells (47).
Vascular NO production regulates cerebrovascular perfusion and protects against stroke by increasing collateral flow to the ischemic area (48, 49). In general, preclinical studies demonstrate that NO generated by the neuronal and inducible NOS after stroke is detrimental to neuronal survival (30), whereas eNOS and endothelial NO are neuroprotective (49). Mice lacking eNOS exhibit larger cerebral infarctions, and further inhibition of NOS activity by Nω-nitro-l-arginine methyl ester (l-NAME) increases infarct size (11, 48, 50). In contrast, up-regulation of eNOS by estrogen, statins, and Rho kinase inhibitors has consistently been shown to confer protection from ischemic stroke in mice (8, 51). Therefore, conditions that enhance eNOS activity could have beneficial effects on cerebrovascular disease. In our study, we show that treatment of mice with tubacin for only 2 days prior to ischemic injury markedly reduced infarct size and brain edema after MCAO. Furthermore, our findings suggest that vascular protective effects of tubacin might be mediated through its ability to increase eNOS expression. This was suggested based on finding elevated eNOS levels in the ischemic penumbra and our other findings showing that tubacin's effects on vessel relaxation were dependent on the expression of eNOS. Interestingly, the increased relaxing response to acetylcholine in normal mice is relatively week. This is not surprising because eNOS gene expression and the resulting content of eNOS protein is not a limiting factor under normal conditions (i.e. in the absence of endothelial dysfunction). In fact, this effect is more remarkable in db/db mice, indicating that tubacin is capable of restoring altered eNOS expression under pathological conditions.
Several lines of evidence suggest that HDAC6 is essentially involved in the development of cancer and neurodegenerative disorders, such as Parkinson disease and Huntington disease (20, 23, 52, 53). Thus, HDAC6 has emerged as a promising therapeutic target for treatment of these diseases. Investigation of HDAC6 inhibitors on eNOS expression is not only important to identify eNOS regulators, but also allows us to predict potential side effects of these inhibitors in the cardiovascular system. Tubastatin was previously shown to have selectivity and potency similar to those of tubacin in inhibiting HDAC6 activity (54), but we found that each exerted opposite effects on eNOS expression. Moreover, we found that rocilinostat (ACY-1215), which is structurally similar to tubacin (55), had no effect on eNOS expression. In this regard, it is tempting to speculate that in vivo application of tubastatin may lead to cardiovascular complications through inhibiting eNOS activity, as compared with other inhibitors, such as tubacin and rocilinostat.
In conclusion, we provide compelling evidence that tubacin is a potent eNOS enhancer and can induce the production of NO both in vitro and in vivo as well as exert vascular protective effects in various clinical scenarios. Importantly, we found that these unique activities were independent of its HDAC6 inhibitor effects and related more to its ability to selectively increase eNOS mRNA stability. Despite its high lipophilicity and difficulties in synthesis, successful identification of tubacin as a promising vasoprotective agent suggests that further optimizing its structure for in vivo use might be worthwhile because of its potential as a treatment for a wide range of cardiovascular diseases.
Experimental procedures
Cell culture
HUVECs were purchased from ATCC and cultured in EBM-2 Basal Medium (Lonza) supplemented with EGM-2 BulletKit (Lonza). COS-7 cells and BAECs were also purchased from ATCC but cultured in Dulbecco's modified Eagle's medium. BBMECs were purchased from Lonza and grown in EBM-2 basal medium (Lonza) supplemented with EMVB SingleQuots (Lonza). In some studies, endothelial cells were exposed to tubacin (Tocris Bioscience), tubastatin A (Tocris Bioscience), ACY-1215 (Cayman Chemical), or vorinostat (Cayman Chemical). Niltubacin (Enzo Life Sciences) served as a negative control in HDAC6 inhibitor experiments.
Gene silencing with small interference RNA
Human HDAC6 and scrambled (Mission siRNA Universal Negative Control) siRNA were transfected into HUVECs using the Lipofectamine RNAiMAX transfection Reagent (Invitrogen) according to the manufacturer's recommendations.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted using the TRIzol reagent kit (Invitrogen). qRT-PCR analysis was performed as we described previously (56). Briefly, cDNA was synthesized from total RNA using the High-Capacity cDNA Archive Kit (Applied Biosystems). qRT-PCR was performed using the MyiQTM single-color real-time PCR detection system (Bio-Rad) and HotStart-IT SYBR Green one-step qRT-PCR master mix kit (AB Science).
Luciferase assay
COS-7 cells were transfected with the firefly luciferase F1-eNOS-promoter reporter plasmid or firefly luciferase-eNOS-UTR reporter plasmid, together with control luciferase reporter plasmid (28, 29). 24 h after transfection, cells were incubated with tubacin or simvastatin (eNOS-inducing agent) for an additional 24 h. Cell lysates were then collected and assayed for luciferase activity using the Dual-Luciferase assay system (Promega).
Determination of eNOS mRNA stability
The effects of tubacin on eNOS mRNA stability were determined by comparing mRNA levels in control and actinomycin D (5 μm)-exposed cells. -Fold change in gene expression was calculated using the 2−ΔΔCT method with 18S rRNA as an internal control as described previously (28). The relative amount of eNOS mRNA at 0 h AcD was set at 100%.
Western blot analysis
Western blot analysis was performed as described previously (28). In brief, cell lysates were resolved by SDS-PAGE and transferred to nitrocellulose membrane (Bio-Rad). Blots were incubated with diluted primary antibodies against histone 3 (Santa Cruz Biotechnology, Inc., 10809, 1:1000), GAPDH (Santa Cruz Biotechnology, 32233 and 25778, 1:1000), α-tubulin (Cell Signaling, 2144, 1:2000), acetylated α-tubulin (Cell Signaling, 3971, 1:1000), HDAC6 (Cell Signaling, 7558, 1:500) and eNOS (BD Biosciences, 610297, 1:500), PTBP1 (Abcam, ab5642, 1:1000), eEF1A (Thermo Fisher Scientific, PA5-17213, 1:1000) followed by either IRDye 700– or 800–labeled secondary antibodies (1:10,000, LI-COR, 926-32212, 926-68073, and 925-68074) and then were visualized on an Odyssey Imaging System (LI-COR).
Measurement of NO production
The NO production was assessed by quantifying nitrite and nitrate levels in the supernatant of cells. Nitrite (NO2−) and nitrate (NO3−) levels were determined using a chemiluminescence NO detector (Siever 280i NO Analyzer), as described previously (57).
Preparation of nuclear and cytoplasmic extracts
HUVECs were lysed in hypotonic buffer (20 mm Tris-HCl, pH 7.4, 10 mm NaCl, 3 mm MgCl2, 100 units of RNase OUT (Invitrogen), protease inhibitor mixture (Roche Applied Science)) on ice for 15 min, and then 25 μl of detergent (10% Nonidet P-40) was added. The mixture was vortexed for 10 s, followed by centrifugation at 3000 rpm for 10 min at 4 °C. After centrifugation, the supernatant was removed and represents the cytoplasmic fraction for Western blotting and RNA Immunoprecipitation. Nuclear extract was suspended by Cell Extraction Buffer (10 mm Tris-HCl, pH 7.4, 100 mm NaCl, 10% glycerol, 1% Triton X-100, 1 mm EDTA, 0.1% SDS mixture) with protease inhibitors, followed by centrifugation at 16,000 rpm for 30 min at 4 °C.
RNA immunoprecipitation
Cytoplasmic fraction was incubated with 4 μg of anti-PTBP1 mAb (Abcam) 2 h at 4 °C and 20 μl of Protein A/G (Santa Cruz Biotechnology) to perform immunoprecipitation. Immune complexes were then washed multiple times with hypotonic buffer, total RNA was extracted using the TRIzol reagent (Invitrogen), and eNOS mRNA levels were quantified by qRT-PCR (29).
Arterial ring preparation and vascular tension recording
Studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health following protocols that were reviewed and approved by the Institutional Animal Care and Use Committee at Thomas Jefferson University. All experiments were performed on adult (8–12-week-old) male C57BL/6J mice, male eNOS knockout mice (stock no. 002684, Jackson Laboratory, Bar Harbor, ME), and male BKS-db/db mice (stock no. 000642, Jackson Laboratory). All animals were euthanized by carbon dioxide. Experimental mice were randomized to receive either 5 mg/kg tubacin or DMSO by intraperitoneal injection. 24 h after injection, mice were sacrificed, and segments of the descending aorta were recovered. Aortic tissues were maintained in ice-cold Krebs–Henseleit buffer consisting of 118 mm NaCl, 25 mm NaHCO3, 4.5 mm KCl, 1.2 mm KH2PO4, 1.2 mm MgSO4, 2.5 mm CaCl2, and 11 mm glucose. Loose fat and connective tissue were removed, and 2–3-mm aorta rings were isometrically mounted on a Multi-Wire Myograph System (DMT620M). Resting tension for each aortic ring was set at 4 millinewtons and maintained at this level throughout the experiment. During equilibration, the rings were exposed to Krebs–Henseleit buffer (replaced every 15 min) at 37 °C and continuously gassed with 95% O2, 5% CO2. After 2 h, rings were exposed to phenylephrine (1 × 10−6 m) to elicit contraction, followed by monitoring of endothelium-dependent vasorelaxation to acetylcholine (10−9 to 10−5 m) over time. eNOS-independent vasorelaxation was assessed by measuring the cumulative response to acetylcholine in rings pretreated with the eNOS inhibitor l-NAME (10−4 m). Endothelium-independent vasorelaxation was measured by assessing response to sodium nitroprusside (10−10 to 10−6 m). Data were analyzed using the Powerlab system (AD Instruments). Vasorelaxation was expressed as a percentage based on the percentage change from the preconstricted state.
MCAO model
Both male and female mice (12 weeks old) were injected with either vehicle (DMSO) or tubacin (5 mg/kg, intraperitoneally) twice at 24 and 3 h, respectively, before surgical occlusion. Focal cerebral ischemia was induced by transient MCAO as previously described (58). Animals were randomly divided into three groups: the sham group, the MCAO with vehicle-treated group, and the MCAO with tubacin-treated group. In the tubacin-treated group, 5 mg/kg tubacin was intraperitoneally injected 3 h before MCAO. Mice were subjected to MCAO by transient right MCA occlusion (60 min) under isoflurane (3%) anesthesia, followed by 24 h of reperfusion. Body temperature was controlled at 37 °C. Occlusion and reperfusion were verified in each animal by laser speckle contrast imaging (Pericam PSI). All animals were euthanized by carbon dioxide. For the quantification of infarct size, brain tissue was collected at 24 h for standard 2,3,5-triphenyltetrazolium chloride (TTC) histology and digital image analysis of infarct volume. Neurological function was evaluated using a 0–4-point neurological score: 0 = no neurological dysfunction; 1 = failure to extend left forelimb fully when lifted by tail: 2 = circling to the contralateral side; 3 = falling to the left; 4 = no spontaneous walk or in a comatose state, or barrel rolling. All scores were performed while blinded to study groups.
Statistical analysis
All values are expressed as the mean ± S.E. Comparisons between two groups were analyzed by t test, whereas comparisons between more than two groups were made using one-way ANOVA followed by Tukey's post-test. p < 0.05 was considered statistically significant. All statistical analyses were performed via GraphPad Prism version 5.
Author contributions
J. C., J. Z., N. F. S., B. Y., X.-F. Y., U. P. N., R. S., G. Y., X. X., and J. S. conceptualization; J. C., J. Z., N. F. S., B. Y., X. W., G. Y., X. X., and J. S. data curation; J. C., J. Z., N. F. S., B. Y., X. W., U. P. N., R. S., G. Y., and J. S. formal analysis; J. C., J. Z., N. F. S., B. Y., X. W., G. Y., and J. S. validation; J. C., J. Z., N. F. S., B. Y., X. W., G. Y., and J. S. investigation; J. C., J. Z., N. F. S., B. Y., X. W., U. P. N., R. S., G. Y., X. X., and J. S. methodology; J. C., R. S., and J. S. writing-original draft; J. Z., X.-F. Y., U. P. N., R. S., and J. S. supervision; J. Z., X.-F. Y., U. P. N., R. S., X. X., and J. S. funding acquisition; J. Z., X.-F. Y., U. P. N., X. X., and J. S. project administration; N. F. S. and J. S. writing-review and editing; B. Y., X.-F. Y., R. S., G. Y., X. X., and J. S. resources; X.-F. Y. and J. S. visualization.
Supplementary Material
This work was supported by National Institutes of Health Grants R01HL103869 and R01GM123047 and American Heart Association Established Investigator Award 16EIA27710023 (to J. S.) and Chinese Natural Science Foundation Grant 81672927 (to X. X.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Fig. S1.
- eNOS
- endothelial nitric oxide synthase
- NOS
- nitric oxide synthase
- HDAC
- histone deacetylase
- PTBP1
- polypyrimidine tract–binding protein 1
- qRT-PCR
- quantitative real-time PCR
- HUVECs
- human umbilical vein endothelial cells
- MCAO
- middle cerebral artery occlusion
- TNFα
- tumor necrosis factor α
- Ach
- acetylcholine
- BBMVECs
- bovine brain microvascular endothelial cells
- eEF1A
- translation elongation factor 1-α1
- BAECs
- bovine aortic endothelial cells
- LPS
- lipopolysaccharide
- LDL
- low-density lipoprotein
- HMG
- 3-hydroxy-3-methylglutaryl
- EC
- endothelial cell
- l-NAME
- Nω-nitro-l-arginine methyl ester
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- TTC
- 2,3,5-triphenyltetrazolium chloride
- ANOVA
- analysis of variance.
References
- 1. Oemar B. S., Tschudi M. R., Godoy N., Brovkovich V., Malinski T., and Lüscher T. F. (1998) Reduced endothelial nitric oxide synthase expression and production in human atherosclerosis. Circulation 97, 2494–2498 10.1161/01.CIR.97.25.2494 [DOI] [PubMed] [Google Scholar]
- 2. Minamino T., Miyauchi H., Yoshida T., Ishida Y., Yoshida H., and Komuro I. (2002) Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 105, 1541–1544 10.1161/01.CIR.0000013836.85741.17 [DOI] [PubMed] [Google Scholar]
- 3. Rafikov R., Fonseca F. V., Kumar S., Pardo D., Darragh C., Elms S., Fulton D., and Black S. M. (2011) eNOS activation and NO function: structural motifs responsible for the posttranslational control of endothelial nitric oxide synthase activity. J. Endocrinol. 210, 271–284 10.1530/JOE-11-0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tai S. C., Robb G. B., and Marsden P. A. (2004) Endothelial nitric oxide synthase: a new paradigm for gene regulation in the injured blood vessel. Arterioscler. Thromb. Vasc. Biol. 24, 405–412 10.1161/01.ATV.0000109171.50229.33 [DOI] [PubMed] [Google Scholar]
- 5. Yoshizumi M., Perrella M. A., Burnett J. C. Jr, and Lee M. E. (1993) Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life. Circ. Res. 73, 205–209 10.1161/01.RES.73.1.205 [DOI] [PubMed] [Google Scholar]
- 6. Liao J. K., Shin W. S., Lee W. Y., and Clark S. L. (1995) Oxidized low-density lipoprotein decreases the expression of endothelial nitric oxide synthase. J. Biol. Chem. 270, 319–324 10.1074/jbc.270.1.319 [DOI] [PubMed] [Google Scholar]
- 7. Xiao Z., Zhang Z., Ranjan V., and Diamond S. L. (1997) Shear stress induction of the endothelial nitric oxide synthase gene is calcium-dependent but not calcium-activated. J. Cell. Physiol. 171, 205–211 [DOI] [PubMed] [Google Scholar]
- 8. Endres M., Laufs U., Huang Z., Nakamura T., Huang P., Moskowitz M. A., and Liao J. K. (1998) Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 95, 8880–8885 10.1073/pnas.95.15.8880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weber M., Hagedorn C. H., Harrison D. G., and Searles C. D. (2005) Laminar shear stress and 3′ polyadenylation of eNOS mRNA. Circ. Res. 96, 1161–1168 10.1161/01.RES.0000170651.72198.fa [DOI] [PubMed] [Google Scholar]
- 10. Fletcher D. A., and Mullins R. D. (2010) Cell mechanics and the cytoskeleton. Nature 463, 485–492 10.1038/nature08908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Laufs U., Endres M., Stagliano N., Amin-Hanjani S., Chui D. S., Yang S. X., Simoncini T., Yamada M., Rabkin E., Allen P. G., Huang P. L., Böhm M., Schoen F. J., Moskowitz M. A., and Liao J. K. (2000) Neuroprotection mediated by changes in the endothelial actin cytoskeleton. J. Clin. Invest. 106, 15–24 10.1172/JCI9639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Searles C. D., Ide L., Davis M. E., Cai H., and Weber M. (2004) Actin cytoskeleton organization and posttranscriptional regulation of endothelial nitric oxide synthase during cell growth. Circ. Res. 95, 488–495 10.1161/01.RES.0000138953.21377.80 [DOI] [PubMed] [Google Scholar]
- 13. Xue C., Botkin S. J., and Johns R. A. (1996) Localization of endothelial NOS at the basal microtubule membrane in ciliated epithelium of rat lung. J. Histochem. Cytochem. 44, 463–471 10.1177/44.5.8627003 [DOI] [PubMed] [Google Scholar]
- 14. Su Y., Zharikov S. I., and Block E. R. (2002) Microtubule-active agents modify nitric oxide production in pulmonary artery endothelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 282, L1183–L1189 10.1152/ajplung.00388.2001 [DOI] [PubMed] [Google Scholar]
- 15. Hammond J. W., Cai D., and Verhey K. J. (2008) Tubulin modifications and their cellular functions. Curr. Opin. Cell Biol. 20, 71–76 10.1016/j.ceb.2007.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang F., Su B., Wang C., Siedlak S. L., Mondragon-Rodriguez S., Lee H. G., Wang X., Perry G., and Zhu X. (2015) Posttranslational modifications of α-tubulin in alzheimer disease. Transl. Neurodegener. 4, 9 10.1186/s40035-015-0030-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fernández-Barrera J., and Alonso M. A. (2018) Coordination of microtubule acetylation and the actin cytoskeleton by formins. Cell Mol. Life Sci. 75, 3181–3191 10.1007/s00018-018-2855-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hubbert C., Guardiola A., Shao R., Kawaguchi Y., Ito A., Nixon A., Yoshida M., Wang X. F., and Yao T. P. (2002) HDAC6 is a microtubule-associated deacetylase. Nature 417, 455–458 10.1038/417455a [DOI] [PubMed] [Google Scholar]
- 19. Matthias P., Yoshida M., and Khochbin S. (2008) HDAC6 a new cellular stress surveillance factor. Cell Cycle 7, 7–10 10.4161/cc.7.1.5186 [DOI] [PubMed] [Google Scholar]
- 20. Wang X. X., Wan R. Z., and Liu Z. P. (2018) Recent advances in the discovery of potent and selective HDAC6 inhibitors. Eur. J. Med. Chem. 143, 1406–1418 10.1016/j.ejmech.2017.10.040 [DOI] [PubMed] [Google Scholar]
- 21. Seidel C., Schnekenburger M., Dicato M., and Diederich M. (2015) Histone deacetylase 6 in health and disease. Epigenomics 7, 103–118 10.2217/epi.14.69 [DOI] [PubMed] [Google Scholar]
- 22. Zhang Y., Kwon S., Yamaguchi T., Cubizolles F., Rousseaux S., Kneissel M., Cao C., Li N., Cheng H. L., Chua K., Lombard D., Mizeracki A., Matthias G., Alt F. W., Khochbin S., and Matthias P. (2008) Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol. Cell Biol. 28, 1688–1701 10.1128/MCB.01154-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang P. H., Zhang L., Zhang Y. J., Zhang J., and Xu W. F. (2013) HDAC6: physiological function and its selective inhibitors for cancer treatment. Drug. Discov. Ther. 7, 233–242 10.5582/ddt.2013.v7.6.233 [DOI] [PubMed] [Google Scholar]
- 24. Haggarty S. J., Koeller K. M., Wong J. C., Grozinger C. M., and Schreiber S. L. (2003) Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc. Natl. Acad. Sci. U.S.A. 100, 4389–4394 10.1073/pnas.0430973100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Namdar M., Perez G., Ngo L., and Marks P. A. (2010) Selective inhibition of histone deacetylase 6 (HDAC6) induces DNA damage and sensitizes transformed cells to anticancer agents. Proc. Natl. Acad. Sci. U.S.A. 107, 20003–20008 10.1073/pnas.1013754107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang R., Min W., and Sessa W. C. (1995) Functional analysis of the human endothelial nitric oxide synthase promoter. Sp1 and GATA factors are necessary for basal transcription in endothelial cells. J. Biol. Chem. 270, 15320–15326 10.1074/jbc.270.25.15320 [DOI] [PubMed] [Google Scholar]
- 27. Martínez-González J., Raposo B., Rodriguez C., and Badimon L. (2001) 3-Hydroxy-3-methylglutaryl coenzyme a reductase inhibition prevents endothelial NO synthase downregulation by atherogenic levels of native LDLs: balance between transcriptional and posttranscriptional regulation. Arterioscler. Thromb. Vasc. Biol. 21, 804–809 10.1161/01.ATV.21.5.804 [DOI] [PubMed] [Google Scholar]
- 28. Yi B., Ozerova M., Zhang G. X., Yan G., Huang S., and Sun J. (2015) Post-transcriptional regulation of endothelial nitric oxide synthase expression by polypyrimidine tract-binding protein 1. Arterioscler. Thromb. Vasc. Biol. 35, 2153–2160 10.1161/ATVBAHA.115.305750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yan G., You B., Chen S. P., Liao J. K., and Sun J. (2008) Tumor necrosis factor-α downregulates endothelial nitric oxide synthase mRNA stability via translation elongation factor 1-α 1. Circ. Res. 103, 591–597 10.1161/CIRCRESAHA.108.173963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang Z., Huang P. L., Panahian N., Dalkara T., Fishman M. C., and Moskowitz M. A. (1994) Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science 265, 1883–1885 10.1126/science.7522345 [DOI] [PubMed] [Google Scholar]
- 31. Chataigneau T., Félétou M., Huang P. L., Fishman M. C., Duhault J., and Vanhoutte P. M. (1999) Acetylcholine-induced relaxation in blood vessels from endothelial nitric oxide synthase knockout mice. Br. J. Pharmacol. 126, 219–226 10.1038/sj.bjp.0702300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mayanagi K., Katakam P. V., Gáspár T., Domoki F., and Busija D. W. (2008) Acute treatment with rosuvastatin protects insulin resistant (C57BL/6J ob/ob) mice against transient cerebral ischemia. J. Cereb. Blood Flow Metab. 28, 1927–1935 10.1038/jcbfm.2008.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Olzscha H., Sheikh S., and La Thangue N. B. (2015) Deacetylation of chromatin and gene expression regulation: a new target for epigenetic therapy. Crit. Rev. Oncog. 20, 1–17 10.1615/CritRevOncog.2014012463 [DOI] [PubMed] [Google Scholar]
- 34. Dekker F. J., van den Bosch T., and Martin N. I. (2014) Small molecule inhibitors of histone acetyltransferases and deacetylases are potential drugs for inflammatory diseases. Drug Discov. Today 19, 654–660 10.1016/j.drudis.2013.11.012 [DOI] [PubMed] [Google Scholar]
- 35. Pasyukova E. G., and Vaiserman A. M. (2017) HDAC inhibitors: a new promising drug class in anti-aging research. Mech. Ageing Dev. 166, 6–15 10.1016/j.mad.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 36. Damaskos C., Garmpis N., Valsami S., Kontos M., Spartalis E., Kalampokas T., Kalampokas E., Athanasiou A., Moris D., Daskalopoulou A., Davakis S., Tsourouflis G., Kontzoglou K., Perrea D., Nikiteas N., and Dimitroulis D. (2017) Histone deacetylase inhibitors: an attractive therapeutic strategy against breast cancer. Anticancer Res. 37, 35–46 10.21873/anticanres.11286 [DOI] [PubMed] [Google Scholar]
- 37. Rössig L., Li H., Fisslthaler B., Urbich C., Fleming I., Förstermann U., Zeiher A. M., and Dimmeler S. (2002) Inhibitors of histone deacetylation downregulate the expression of endothelial nitric oxide synthase and compromise endothelial cell function in vasorelaxation and angiogenesis. Circ. Res. 91, 837–844 10.1161/01.RES.0000037983.07158.B1 [DOI] [PubMed] [Google Scholar]
- 38. Jung S. B., Kim C. S., Naqvi A., Yamamori T., Mattagajasingh I., Hoffman T. A., Cole M. P., Kumar A., Dericco J. S., Jeon B. H., and Irani K. (2010) Histone deacetylase 3 antagonizes aspirin-stimulated endothelial nitric oxide production by reversing aspirin-induced lysine acetylation of endothelial nitric oxide synthase. Circ. Res. 107, 877–887 10.1161/CIRCRESAHA.110.222968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Takemoto M., Sun J., Hiroki J., Shimokawa H., and Liao J. K. (2002) Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation 106, 57–62 10.1161/01.CIR.0000020682.73694.AB [DOI] [PubMed] [Google Scholar]
- 40. Searles C. D. (2006) Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression. Am. J. Physiol. Cell Physiol. 291, C803–C816 10.1152/ajpcell.00457.2005 [DOI] [PubMed] [Google Scholar]
- 41. Alderton W. K., Cooper C. E., and Knowles R. G. (2001) Nitric oxide synthases: structure, function and inhibition. Biochem. J. 357, 593–615 10.1042/0264-6021:3570593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sawicka K., Bushell M., Spriggs K. A., and Willis A. E. (2008) Polypyrimidine-tract-binding protein: a multifunctional RNA-binding protein. Biochem. Soc. Trans. 36, 641–647 10.1042/BST0360641 [DOI] [PubMed] [Google Scholar]
- 43. Romanelli M. G., Diani E., and Lievens P. M. (2013) New insights into functional roles of the polypyrimidine tract-binding protein. Int. J. Mol. Sci. 14, 22906–22932 10.3390/ijms141122906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fred R. G., Tillmar L., and Welsh N. (2006) The role of PTB in insulin mRNA stability control. Curr. Diabetes Rev. 2, 363–366 10.2174/157339906777950570 [DOI] [PubMed] [Google Scholar]
- 45. Ma S., Liu G., Sun Y., and Xie J. (2007) Relocalization of the polypyrimidine tract-binding protein during PKA-induced neurite growth. Biochim. Biophys. Acta 1773, 912–923 10.1016/j.bbamcr.2007.02.006 [DOI] [PubMed] [Google Scholar]
- 46. Babic I., Sharma S., and Black D. L. (2009) A role for polypyrimidine tract binding protein in the establishment of focal adhesions. Mol. Cell Biol. 29, 5564–5577 10.1128/MCB.00590-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xie J., Lee J. A., Kress T. L., Mowry K. L., and Black D. L. (2003) Protein kinase A phosphorylation modulates transport of the polypyrimidine tract-binding protein. Proc. Natl. Acad. Sci. U.S.A. 100, 8776–8781 10.1073/pnas.1432696100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morikawa E., Moskowitz M. A., Huang Z., Yoshida T., Irikura K., and Dalkara T. (1994) l-Arginine infusion promotes nitric oxide-dependent vasodilation, increases regional cerebral blood flow, and reduces infarction volume in the rat. Stroke 25, 429–435 10.1161/01.STR.25.2.429 [DOI] [PubMed] [Google Scholar]
- 49. Dalkara T., Morikawa E., Panahian N., and Moskowitz M. A. (1994) Blood flow-dependent functional recovery in a rat model of focal cerebral ischemia. Am. J. Physiol. 267, H678–H683 10.1152/ajpheart.1994.267.2.H678 [DOI] [PubMed] [Google Scholar]
- 50. Huang Z., Huang P. L., Ma J., Meng W., Ayata C., Fishman M. C., and Moskowitz M. A. (1996) Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-l-arginine. J. Cereb. Blood Flow Metab. 16, 981–987 10.1097/00004647-199609000-00023 [DOI] [PubMed] [Google Scholar]
- 51. Shin H. K., Salomone S., Potts E. M., Lee S. W., Millican E., Noma K., Huang P. L., Boas D. A., Liao J. K., Moskowitz M. A., and Ayata C. (2007) Rho-kinase inhibition acutely augments blood flow in focal cerebral ischemia via endothelial mechanisms. J. Cereb. Blood Flow Metab. 27, 998–1009 10.1038/sj.jcbfm.9600406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aldana-Masangkay G. I., and Sakamoto K. M. (2011) The role of HDAC6 in cancer. J. Biomed. Biotechnol. 2011, 875824 10.1155/2011/875824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li G., Jiang H., Chang M., Xie H., and Hu L. (2011) HDAC6 α-tubulin deacetylase: a potential therapeutic target in neurodegenerative diseases. J. Neurol. Sci. 304, 1–8 10.1016/j.jns.2011.02.017 [DOI] [PubMed] [Google Scholar]
- 54. Butler K. V., Kalin J., Brochier C., Vistoli G., Langley B., and Kozikowski A. P. (2010) Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A. J. Am. Chem. Soc. 132, 10842–10846 10.1021/ja102758v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Santo L., Hideshima T., Kung A. L., Tseng J. C., Tamang D., Yang M., Jarpe M., van Duzer J. H., Mazitschek R., Ogier W. C., Cirstea D., Rodig S., Eda H., Scullen T., Canavese M., et al. (2012) Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma. Blood 119, 2579–2589 10.1182/blood-2011-10-387365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. You X., Guo Z. F., Cheng F., Yi B., Yang F., Liu X., Zhu N., Zhao X., Yan G., Ma X. L., and Sun J. (2018) Transcriptional up-regulation of relaxin-3 by Nur77 attenuates β-adrenergic agonist-induced apoptosis in cardiomyocytes. J. Biol. Chem. 293, 14001–14011 10.1074/jbc.RA118.003099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen M., Yi B., Zhu N., Wei X., Zhang G. X., Huang S., and Sun J. (2016) Pim1 kinase promotes angiogenesis through phosphorylation of endothelial nitric oxide synthase at Ser-633. Cardiovasc. Res. 109, 141–150 10.1093/cvr/cvv250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kleinschnitz C., Pozgajova M., Pham M., Bendszus M., Nieswandt B., and Stoll G. (2007) Targeting platelets in acute experimental stroke: impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation 115, 2323–2330 10.1161/CIRCULATIONAHA.107.691279 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.