Abstract
A combination of cytoreductive surgery, either primary (PCS) or interval (ICS), and chemotherapy with a platinum-paclitaxel regimen is the well-accepted treatment for advanced-stage epithelial ovarian cancer (EOC), fallopian tube cancer (FTC), and primary peritoneal serous carcinoma (PPSC), but it is still uncertain whether a combination of dose-dense weekly paclitaxel and low-dose triweekly cisplatin is useful in the management of these patients. Therefore, we retrospectively evaluated the outcomes of women with advanced-stage EOC, FTC, and PPSC treated with PCS and subsequent dose-dense weekly paclitaxel (80 mg/m2) and low-dose triweekly cisplatin (20 mg/m2). Between January 2011 and December 2017, 32 women with International Federation of Gynecology and Obstetrics (FIGO) stage IIIC–IV EOC, FTC, or PPSC were enrolled. Optimal PCS was achieved in 63.5% of patients. The mean and median progression-free survival was 36.5 and 27.0 months, respectively (95% confidence interval (CI): 26.8–46.2 and 11.3–42.7 months, respectively). The mean overall survival was 56.0 months (95% CI: 43.9–68.1 months), and the median overall survival could not be obtained. The most common all-grade adverse events (AEs) were anemia (96.9%), neutropenia (50%), peripheral neuropathy (28.1%), nausea and vomiting (34.4%), and thrombocytopenia (15.6%). These AEs were predominantly grade 1/2, and only a few patients were complicated by grade 3/4 neutropenia (21.9%) and anemia (6.3%). A multivariate analysis indicated that only suboptimal PCS was significantly correlated with a worse prognosis, resulting in an 11.6-fold increase in the odds of disease progression. In conclusion, our data suggest that dose-dense weekly paclitaxel (80 mg/m2) combined with low-dose triweekly cisplatin (20 mg/m2) is a potentially effective and highly tolerable front-line treatment in advanced EOC, FTC, and PPSC. Randomized trials comparing the outcome of this regimen to other standard therapies for FIGO stage IIIC–IV EOC, FTC, and PPSC are warranted.
Keywords: dose-dense weekly paclitaxel, epithelial ovarian cancer, fallopian tube cancer, FIGO stage IIIC–IV, low-dose triweekly cisplatin, primary peritoneal serous carcinoma
1. Introduction
Over the past decade, the predominant treatment for advanced epithelial ovarian cancer, fallopian tube cancer, and primary peritoneal serous cancer (EOC, FTC, and PPSC, respectively) has been primary cytoreductive surgery (PCS) plus adjuvant triweekly paclitaxel and carboplatin. This treatment strategy was based on a study by McGuire et al. [1] that demonstrated the superiority of incorporating paclitaxel into cisplatin-based regimens compared to cyclophosphamide plus cisplatin in patient survival. Further studies have confirmed significant survival benefits in women with EOC, FTC, and PPSC treated with a combination of triweekly cisplatin and paclitaxel in place of the original cisplatin–cyclophosphamide regimen [2,3]. Due to potential neural and renal toxicity as well as the high emetic effects of cisplatin, carboplatin has replaced cisplatin in this combination and has become a standard postoperative adjuvant therapy in the management of women with EOC, FTC, and PPSC after PCS [4,5,6,7,8,9,10,11,12]. In patients not suitable for PCS that require interval cytoreductive surgery (ICS), this triweekly carboplatin and paclitaxel regimen is also used as a neoadjuvant chemotherapy (NACT) for advanced-stage EOC, FTC, and PPSC patients [13,14,15,16,17,18,19]. Under this standard therapy, the median progression-free survival (PFS) is considered to be only between 16 and 21 months, and the median overall survival (OS) is between 32 and 57 months [1,3,4,5,6,7,8,9,12]. Therefore, many efforts have been made to enhance therapeutic effects and subsequently increase PFS and OS. These new modalities of treatment include altered delivery methods of antineoplastic drugs (intravenous or intraperitoneal routes), hyperthermia therapy, and the application of new agents, such as antiangiogenic drugs, immune checkpoint inhibitors, immune system modulators, and targeted therapy, including poly(adenosine diphosphate (ADP)-ribose) polymerase (PARP) inhibitors [11,12,15,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. However, the high cost of all these treatment types is a cause for concern, which is further compounded by the need for long-term maintenance therapy in some agents.
The dose-dense regimen of paclitaxel stems from the Norton–Simon hypothesis, with the rationale that smaller tumors are more prone to eradication and that the chance of tumor regrowth could be decreased by administering an agent more frequently (metronomic therapy) [37,38]. An initial study of the dose-dense administration of weekly paclitaxel with triweekly carboplatin in Japanese Gynecologic Oncology Group (JGOG) 3016 showed significant improvements in median PFS and median OS for stage II to IV EOC [39], resulting in subsequent phase III trials of dose-dense paclitaxel versus triweekly paclitaxel combined with carboplatin in EOC treatment in predominantly western populations [40,41,42]. In addition to the Japanese trial, a study by Chan et al. revealed evidence of superior PFS with weekly paclitaxel and triweekly carboplatin compared to the standard regimen in patients who did not receive concurrent bevacizumab treatment [41]. Moreover, many studies using weekly paclitaxel in the management of patients with EOC have not limited its use to adjuvant therapy after PCS but have also found it acceptable in NACT [43,44,45,46,47,48]. However, the survival benefits found in dose-dense treatments have not always been reproducible in these studies [40,42].
Carboplatin is more myelosuppressive than cisplatin but has less gastrointestinal, renal, and neurologic toxicity, which is the main reason that it has replaced cisplatin in the platinum–paclitaxel regimen [6,7,8]. In this study, we would like to reconsider the role of cisplatin in the management of women with advanced-stage EOC, FTC, and PPSC, because cisplatin toxicity can be reduced through a reduction of the dosage. This regimen of dose-dense weekly paclitaxel plus low-dose triweekly cisplatin can be considered a modified form of previous dose-dense regimens in JGOG 3016 [39]. Our regimen uses low-dose cisplatin rather than carboplatin, with the expectation that this reduces myelosuppression and improves treatment tolerability while not compromising the therapeutic effects. This study aimed to explore the efficacy and safety of this new combination regimen of dose-dense weekly paclitaxel plus low-dose triweekly cisplatin for advanced EOC, FTC, and PPSC in an Asian population (a Chinese population).
2. Materials and Methods
2.1. Patient Population
This was a single-arm, single-institution retrospective cohort study. The eligible inclusion criteria were patients with International Federation of Gynecology and Obstetrics (FIGO) stage IIIC–IV histologically confirmed ovarian, primary peritoneal, or fallopian tube cancer who underwent PCS following a total of six cycles of dose-dense chemotherapy (weekly paclitaxel and triweekly cisplatin). Patients were excluded if they had NACT; had other newly diagnosed cancer, previous chemotherapy, or radiotherapy in the past two years; had a total relative dose intensity (RDI) less than 70% of standard doses for either paclitaxel or cisplatin after six cycles of treatment; or had simultaneous use of other antineoplastic agents, antiangiogenic agents, or targeted therapy. Informed consent was obtained from all eligible participants. This study was approved by the institutional review board.
2.2. Treatment
All patients received a dose-dense regimen of weekly paclitaxel and triweekly cisplatin. Paclitaxel 80 mg/m2 was administered over 2 h intravenously on days 1, 8, and 15, followed by intravenous infusion of cisplatin 20 mg/m2 for 1 h on day 1. Standard premedication with dexamethasone (20 mg), 2000 ml of normal saline, and palonosetron (250 ug) was prescribed intravenously to all patients on treatment day. Granulocyte colony-stimulating factor (GCSF) was administered to patients with grade 3/4 neutropenia for three days before chemotherapy. Treatment was delayed for 7 days in patients with febrile neutropenia to allow for antibiotics administration. Paclitaxel was reduced by 20% and cisplatin was withheld if febrile neutropenia or grade 3/4 neutropenia was noted. The estimated glomerular filtration rate (eGFR) was calculated according to the Cockcroft–Gault formula [49,50,51], and cisplatin was reduced by 50% if the eGFR decreased to 45–60 ml/min or was held for one cycle if the eGFR was less than 45 ml/min.
2.3. Assessments
The first cycle of chemotherapy was administered after PCS within one week. Adverse effects (AEs) were evaluated before every cycle of treatment according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 5.0 [52,53]. Objective follow-up of disease status was assessed with computed tomography (CT) or magnetic resonance imaging (MRI), combined with clinical and CA-125 (cancer antigen 125, carcinoma antigen 125, or carbohydrate antigen 125) examinations [54,55,56]. An imaging evaluation for the response to treatment was performed according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 [54,55,56]. Raised CA-125 levels alone did not indicate disease progression if measurable disease was not available in radiological or clinical examinations. For patients who had ever had normalized CA-125 levels after treatment, the CA-125 criteria for defining disease progression were raised values greater than two times the upper normal limit. For patients who did not have normalized CA-125 during treatment, disease progression was defined as a CA-125 value greater than 2 times the nadir value. Response to treatment was evaluated upon the completion of six cycles of chemotherapy and was reassessed every six months during the first two years and then every year thereafter. Additional imaging and CA-125 evaluation could be performed if there were clinical signs of suspected progressive disease (PD).
2.4. Statistical Analysis
The primary endpoint was PFS, which was defined as the time from the date of primary operation to the earliest date of disease progression, death from any cause, or the date of the last known follow-up. The secondary endpoints were OS, the overall response rate (ORR), the clinical benefit rate (CBR), and safety. OS was defined as the time from the date of primary operation to the date of death from any cause or the date of the last known follow-up. Patients receiving optimal PCS without clinically or instrumentally measurable disease before the first cycle of treatment were not evaluated for response. PFS and OS were calculated using the Kaplan–Meier method. Risk factors for disease progression were evaluated with a logistic multivariate regression model. All statistical analyses were conducted using SPSS v. 24.0 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Clinical Characteristics and Pathological Status
Between January 2011 and December 2017, 32 eligible patients were identified through our electronic prescribing system. Table 1 summarizes the characteristics of all patients. The median age was 57 years (range: 33–79 years). All patients were primarily diagnosed with EOC, FTC, or PPSC that was stage IIIC (81.3%) or stage IV (18.8%) and that originated from bilateral ovaries (81.3%), fallopian tubes (6.3%), or the peritoneum (12.5%). The most prevalent histological type was high-grade serous carcinoma (56.3%). All patients received PCS, and the optimal debulking rate was 62.5%. Eighteen patients (56.3%) completed the six cycles of weekly paclitaxel and triweekly cisplatin without any delay or reduction of dosage for both antineoplastic agents (cisplatin and paclitaxel). Eleven patients (34.4%) had treatment delays of no more than one week, and only three patients (9.4%) had a delay time of more than two weeks.
Table 1.
Demographic and clinicopathological characteristics.
| Characteristics | Total (n = 32) |
|---|---|
| Age at diagnosis (years) | 57 (33–79) |
| Age > 60 years | 11 (34.4%) |
| FIGO stage | |
| IIIC | 26 (81.3%) |
| IV | 6 (18.8%) |
| Cancer type | |
| Ovarian | 26 (81.3%) |
| Peritoneum | 4 (12.5%) |
| Fallopian tube | 2 (6.3%) |
| Histology | |
| High-grade serous | 18 (56.3%) |
| Mucinous | 1 (3.1%) |
| Clear cell | 3 (9.4%) |
| Endometrioid | 8 (25%) |
| Others (mixed high-grade serous) | 2 (6.3%) |
| Size of residual tumor | |
| ≤1 cm | 20 (62.5%) |
| >1 cm | 12 (37.5%) |
| Site of residual tumor | |
| Lower abdomen | 12 (37.5%) |
| Upper abdomen | 11 (34.4%) |
| Whole abdomen | 9 (28.1%) |
| Time of treatment | |
| 18 weeks | 18 (56.3%) |
| 18–21 weeks | 11 (34.4%) |
| 21–24 weeks | 0 (0) |
| >24 weeks | 3 (9.4%) |
| ECOG | |
| 0–1 | 30 (93.8%) |
| 2–3 | 2 (6.3%) |
FIGO: International Federation of Gynecology and Obstetrics; ECOG: Eastern Cooperative Oncology Group Performance Status; Data are presented as a number (%) or the median (range).
3.2. Outcomes
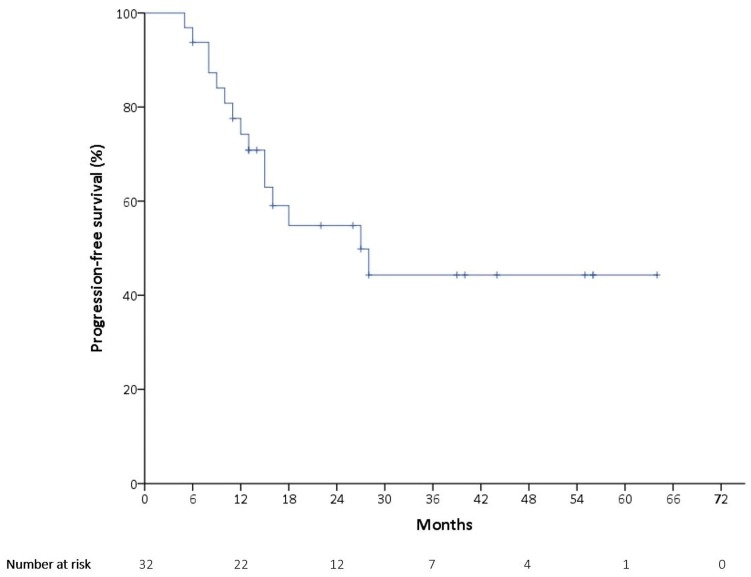
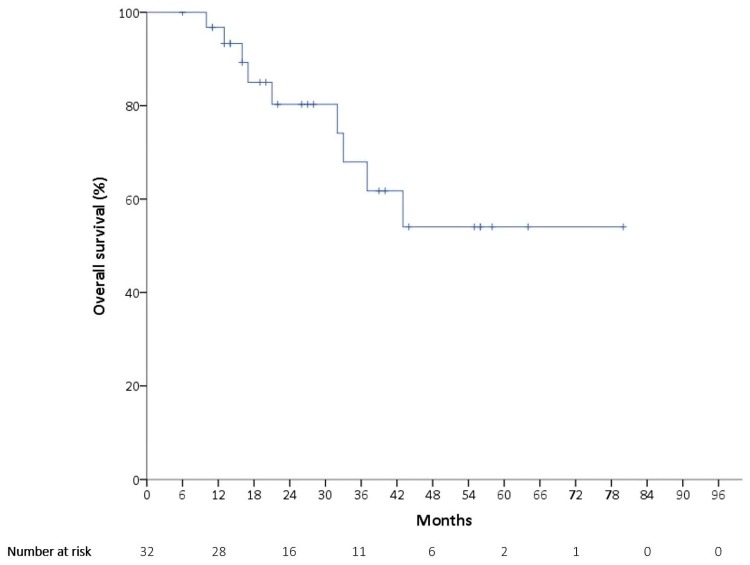
At the time of the data cutoff on 31 January 2019, the median follow-up time was 24 months, with disease progression occurring in 15 patients (46.9%). As is presented in Figure 1, the median PFS was 27 months (95% confidence interval (CI): 11.3–42.7 months). The mean PFS was 36.5 months, with a 95% CI of 26.8–46.2 months. There were a total of nine deaths (28.1%). The mean OS was 56.0 months (95% CI: 43.9–68.1 months), as is shown in Figure 2, and the median OS could not be obtained.
Figure 1.
Kaplan–Meier estimates of progression-free survival (PFS).
Figure 2.
Kaplan–Meier estimates of overall survival (OS).
Objective response was evaluated by RECIST in the 12 patients who received suboptimal cytoreductive surgery with measurable disease at baseline. Two complete responses (16.7%) were observed, and both responding patients had a 4.5-month duration of response. Two patients had stable disease (16.7%), and the CBR was 33.3%.
3.3. Prognostic Factors
To clarify the prognostic factors for disease progression, a univariate analysis of clinicopathologic factors showed that endometrioid histology tended to be a relatively poor prognosis predictor (Table 2). However, it was not statistically significant in the multivariate analysis. After adjusting for histology, residual tumor size, and time of treatment, a multivariate analysis indicated a significantly worse prognosis in residual tumor sizes greater than 1 cm, with an 11.6-fold increase in the odds of disease progression. This in turn also implied a better prognosis in patients receiving optimal cytoreductive surgery. To investigate the possible interactions between variables, a variance inflation factor (VIF) was used for assessing multicollinearity. All variables displayed VIF values less than 5, which ensured the absence of collinearity in the multivariate regression analysis.
Table 2.
Association between baseline characteristics and the progression of disease.
| Characteristic | Number (%) | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| Odds Ratio (95% Confidence Interval (Cl)) | p-Value | Odds Ratio (95% Cl) | p-Value | ||
| Age | |||||
| ≤60 years | 21 (65.6) | Reference | |||
| >60 years | 11 (34.4) | 1.6 (0.37–6.95) | 0.53 | ||
| FIGO stage | |||||
| IIIC | 26 (81.3) | Reference | |||
| IV | 6 (18.8) | 0.5 (0.08–3.22) | 0.466 | ||
| Histology | |||||
| Serous/others | 20 (62.5) | Reference | Reference | ||
| Mucinous/clear cell | 4 (12.5) | 0.67 (0.08–5.75) | 0.712 | 0.41 (0.03–5.25) | 0.494 |
| Endometrioid | 8 (25) | 0.1 (0.01–0.93) | 0.043 | 0.11 (0.01–1.35) | 0.085 |
| Size of residual tumor | |||||
| ≤1 cm | 20 (62.5) | Reference | Reference | ||
| >1 cm | 12 (37.5) | 3.71 (0.82–16.84) | 0.089 | 11.6 (1.07–125.92) | 0.044 |
| Site of residual tumor | |||||
| Lower abdomen | 12 (37.5) | Reference | |||
| Upper abdomen | 11 (34.4) | 1.67 (0.31–9.01) | 0.553 | ||
| Whole abdomen | 9 (28.1) | 4 (0.64–25.02) | 0.138 | ||
| Time of treatment | |||||
| 18 weeks | 18 (56.3) | Reference | Reference | ||
| 18–21 weeks | 11 (34.4) | 2.75 (0.58–12.98) | 0.201 | 5.17 (0.63–42.45) | 0.126 |
| >24 weeks | 3 (9.4) | 0.79 (0.06–10.38) | 0.855 | 0.10 (0.004–2.26) | 0.146 |
| ECOG | |||||
| 0–1 | 30 (93.8) | Reference | |||
| 2–3 | 2 (6.3) | 1.14 (0.07–20.02) | 0.927 | ||
Data are presented as numbers (%).
3.4. Adverse Events
Table 3 lists the adverse events, and no treatment-related death was observed. The most common all-grade AEs were anemia (96.9%), neutropenia (50%), and nausea and vomiting (34.4%). However, only 21.9% and 6.3% of patients had grade 3/4 neutropenia and anemia, respectively. There was no grade 3/4 thrombocytopenia, and only five patients (15.6%) had grade 1/2 thrombocytopenia. Of note, there was no grade 3/4 kidney injury, proteinuria, sensory neuropathy, nausea, or vomiting given the propensity for these cisplatin toxicities. There were no patients withheld from cisplatin administration due to impaired renal function.
Table 3.
Adverse events (n = 32).
| Events | Any Grade, n (%) | Grade 1/2, n (%) | Grade 3/4, n (%) |
|---|---|---|---|
| Neutropenia | 16 (50) | 9 (28.1) | 7 (21.9) |
| Anemia | 31 (96.9) | 29 (90.6) | 2 (6.3) |
| Thrombocytopenia | 5 (15.6) | 5 (15.6) | 0 |
| Renal toxicity | 3 (9.4%) | 3 (9.4) | 0 |
| Proteinuria | 6 (18.8) | 6 (18.8) | 0 |
| Peripheral neuropathy | 9 (28.1) | 9 (28.1) | 0 |
| Nausea | 11 (34.4) | 11 (34.4) | 0 |
n: Number of patients; data are presented as numbers and percentages.
4. Discussion
The modification of dose scheduling and intensity is one of the targeted strategies for improving the prognosis of advanced EOC, FTC, and PPSC [39,40,43,50]. Our study tried to evaluate the outcome of patients with FIGO stage IIIC–IV EOC, FTC, and PPSC treated with dose-dense weekly paclitaxel and low-dose triweekly cisplatin regimen. The primary outcome of the current study was PFS, and the results seem to be promising because the median PFS (27 months) was longer than in previous western trials regarding dose-dense chemotherapy for advanced EOC, FTC, and PPSC (median PFS: 14.2–24.9 months) [41,42]. Moreover, it was even longer than in results from the experimental and control arms of many studies [57] that have attempted to add another agent to standard chemotherapy, regardless of whether the agents were given simultaneously during front-line chemotherapy or during maintenance therapy after standard chemotherapy [10,11,12,57,58,59,60,61,62,63,64,65]. These adding agents have included antiangiogenic drugs, PARP inhibitors, immune system modulators, and many multitarget compounds that were used as upfront therapy [10,11,12,23,27,28,29,30,31,32,57,58,59,60,61,62,63,64,65].
The first two positive advanced-stage frontline ovarian cancer randomized phase III trials that added bevacizumab to chemotherapy were Gynecologic Oncology Group study 0218 (GOG-0218) and Gynecologic Cancer InterGroup (GCIG) International Collaboration on Ovarian Neoplasms (ICON7) [57,58,59,60,61]. These two trials used different treatment durations and dosages of bevacizumab (a dose of 15 mg/kg for 22 cycles in GOG-0218 and a dose of 7.5 mg/kg for 18 cycles in ICON7), but they both showed an increase in PFS [57]. In GOG-0218, the median PFS was 14.1 months in the bevacizumab-concurrent plus maintenance arm compared to 10.3 months in the standard chemotherapy arm, with a statistically significant increase of 4 months [10,57,59]. A similar positive finding of prolonged PFS in patients treated with bevacizumab-concurrent plus maintenance therapy was noted in ICON7, with an increase of 1.5 months (from 20.3 months to 21.8 months) compared to standard chemotherapy alone [57,60,61]. The ICON7 study further identified the apparent benefits of adding bevacizumab in selective highly risky patients, such as patients with FIGO IIIC and FIGO V, who could not reach initially optimal PCS, where the estimated median PFS was 10.5 months in the standard chemotherapy arm compared to 15.9 months in the bevacizumab-concurrent plus maintenance arm [57]. Our results seemed to be not inferior to the results from patients treated with standard therapy plus bevacizumab treatment, as shown above [10,57,58,59,60,61], and also not inferior to the data from the Japanese trial (median PFS: 28.2 months) [39].
One multitargeted compound, nintedanib (an oral triple angiokinase inhibitor of the vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), and fibroblast growth factor receptor (FGFR)), has been used for maintenance therapy in patients with advanced-stage EOC who were given standard-of-care PCS and carboplatin plus paclitaxel chemotherapy, and the results showed that the median PFS was significantly longer in the nintedanib group than in the placebo group (17.2 months vs 16.6 months) [62]. The maintenance of pazopanib, another oral multikinase inhibitor of VEGFR -1/-2/-3, PDGFR -α/-β, and c-Kit, also prolonged PFS compared to a placebo (a median of 17.9 months vs 12.3 months) in patients with advanced EOC who had not progressed after first-line standard chemotherapy [63]. The median PFS in our current study seemed to be not inferior to the patients treated with maintenance therapy, as is shown above.
The input of immune system modulators in ovarian cancer is based on the observation that immunosuppressive microenvironments can affect tumor growth, metastasis, and even treatment resistance [64]. Therefore, additional therapies might be needed. There are many new cancer-targeted strategies available in the management of patients with advanced-stage EOC, and some of them are new combinations [57,64,65]. For example, several phase III trials, including NRG-GY009 (NCT02839707), ATLANTE (NCT02891824), IMagyn050 (NCT03038100), NRG-GY005 (NCT02502266) phase II/III, and NRG-GY004 (NCT02446600), are ongoing, and many are combinations of multiagents during or after standard-of-care PCS/ICS and carboplatin plus paclitaxel chemotherapy (adjuvant chemotherapy or NACT) [57,64].
The biggest change in EOC treatment might have been explored by the SOLO-1 study, which demonstrated that the risk of disease progression was 70% lower with olaparib (estimated median PFS ≥49 months) than with a placebo (median PFS of 13 months) in patients with newly diagnosed advanced ovarian cancer who had a complete or partial response to platinum-based chemotherapy [25]. However, the benefits might be limited to certain populations, such as patients with a mutation of breast cancer gene 1 (BRCA1), breast cancer gene 2 (BRCA2), or both (BRCA1/2).
Studies by Katsumata et al. [39], Chan et al. [41], and Walker et al. [42] have shown an optimal debulking rate ranging from 37% to 92%. The distinctly lower PFS in the study by Chan et al. could be explained by the lower optimal debulking rate and the more advanced-stage disease [41]. In our study, the optimal debulking rate was 63.5%, which was comparable to previous trials [39,42].
In the subgroup of patients who received suboptimal PCS, the change in tumor size could be used to evaluate the treatment response, since residual tumors over 1 cm can be detected by CT or MRI. As expected, the ORR of the suboptimal debulking group was low, which could be explained by the highly resistant nature of grossly larger tumors under the Norton–Simon hypothesis [37]: through the multivariate analysis in our study, we reaffirmed the necessity of achieving optimal PCS to significantly reduce the disease progression rate. Despite the poor prognosis for advanced disease with grossly residual tumors, the CBR could reach as high as 33.3% following dose-dense paclitaxel with low-dose cisplatin chemotherapy. Patients receiving NACT were not eligible in the current study to avoid possible interference with the follow-up evaluation of treatment effects. In contrast, NACT therapy has been included in previous studies of dose-dense chemotherapy regimens [39,41,42,48].
The current low-dose cisplatin plus dose-dense paclitaxel regimen was associated with lower rates of hematologic toxicities compared to the conventional dose-dense paclitaxel plus carboplatin regimen. The most prevalent AE in the current study was anemia of any grade (96.9%), and the second most prevalent was neutropenia of any grade (50%). However, in terms of grade 3 and grade 4 neutropenia, anemia, and thrombocytopenia, the low-dose cisplatin regimen had much fewer AEs than the standard-dose carboplatin regimen did, with the former only causing 21.9% of patients to have grade 3/4 neutropenia and 6.3% to have grade 3/4 anemia: there was an absence of grade 3/4 thrombocytopenia. Conventional dose-dense paclitaxel plus carboplatin studies have displayed grade 3/4 neutropenia, grade 3/4 anemia, and grade 3/4 thrombocytopenia in the range of 72–92%, 27–69%, and 18–44% (Table 4), respectively. The variation could have resulted from different carboplatin doses equivalent to the area under the curve (AUC), with both 5 and 6 used [39,41,42]. Concerning specific AEs associated with cisplatin, our study showed a low frequency of gastrointestinal, renal, and peripheral neuropathies of any grade, as well as an absence of grade 3 and grade 4 events, which were relatively lower than the reported grade 3 and grade 4 data in studies with carboplatin regimens [38,41,42]. These findings are noteworthy, since compared to conventional dose-dense paclitaxel plus carboplatin regimens, this current low-dose cisplatin plus dose-dense paclitaxel regimen had significantly lower rates of severe hematologic toxicities, and cisplatin-specific AEs were not evident with this relatively lower dose of cisplatin.
Table 4.
Summary of treatment efficacy and safety of weekly paclitaxel and triweekly carboplatin in phase III RCTs.
| Authors | Population | n | Regimen (Intravenous) |
Median PFS | Median OS | Wbc | Plt | Rbc | SN | V |
|---|---|---|---|---|---|---|---|---|---|---|
| Katsumata et al. [39] | EOC FIGO II–IV |
312 | P 80mg/m2 (D1,8,15), C AUC 6 (D1) |
28.2 months | 100.5 months | 92% | 44% | 69% | 7% | 3% |
| Chan et al. [41] | EOC FIGO II–IV |
340 | P 80mg/m2 (D1,8,15), C AUC 6 (D1), optional bevacizumab 15mg/kg (D1) |
14.7 months | - | 72% | 20% | 36% | 3% | 6% |
| 55 | P 80mg/m2 (D1,8,15), C AUC 6 (D1), without bevacizumab |
14.2 months | - | - | - | - | - | - | ||
| Walker et al. [42] | EOC FIGO II–IV |
521 | P 80mg/m2 (D1,8,15), C AUC 6 (D1), bevacizumab 15mg/kg (D1) |
24.9 months | 75.5 months | 72% | 18% | 27% | 6% | 5% |
All studies permitted the inclusion of patients receiving neoadjuvant chemotherapy; RCT: randomized control trial; n: number of patients; PFS: progression-free survival; OS: overall survival; Wbc: neutropenia; Plt: thrombocytopenia; Rbc: anemia; SN: sensory neuropathy; V: vomiting; EOC: epithelial ovarian cancer; FIGO: International Federation of Gynecology and Obstetrics; P: paclitaxel; D: day; C: carboplatin; AUC: area under the curve; kg: kilograms. The current adverse events (neutropenia, thrombocytopenia, anemia, sensory neuropathy, and vomiting) are limited to grade 3 and grade 4.
The limitations of this study included its retrospective design and small sample size, since we were practicing a relatively new regimen of chemotherapy in the primary treatment of advanced EOC, FTC, and PPSC. In addition, we did not evaluate new therapeutic strategies, such as maintenance therapy, in the current report. The nature of a single-arm study design did not allow us to compare treatment effects between our newly proposed therapy and current standard therapies.
5. Conclusions
In conclusion, our data suggest that weekly dose-dense paclitaxel combined with triweekly low-dose cisplatin is a potentially effective and highly tolerable front-line treatment in advanced EOC, FTC, and PPSC. Randomized trials comparing the therapeutic outcomes of this regimen to other standard therapies for FIGO stage IIIC–IV EOC, FTC, and PPSC patients are warranted.
Abbreviations
| AEs | Adverse events |
| AUC | Area under the curve |
| CBR | Clinical benefit rate |
| CT | Computed tomography |
| CI | Confidence interval |
| EOC | Epithelial ovarian cancer |
| eGFR | Estimated glomerular filtration rate |
| FTC | Fallopian tube cancer |
| FIGO | International Federation of Gynecology and Obstetrics |
| ISC | Interval cytoreductive surgery |
| NCI-CTCAE | National Cancer Institute’s Common Terminology Criteria for Adverse Events |
| NACT | Neoadjuvant chemotherapy |
| MRI | Magnetic resonance image |
| ORR | Overall response rate |
| OS | Overall survival |
| PARP inhibitors | Poly(ADP-ribose) polymerase (PARP) inhibitors |
| PPSC | Primary peritoneal serous carcinoma |
| PSC | Primary cytoreductive surgery |
| PFS | Progression-free survival |
| PD | Progressive disease |
| RDI | Relative dose intensity |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| RR | Risk ratio |
| VIF | Variance inflation factor |
Author Contributions
Conceptualization, M.C., W.-L.L., and P.-H.W.; formal analysis, M.C.; investigation, H.H.L.; resources, W.-H.C., N.-R.L., and H.-C.H.; data curation, M.C., H.H.L., W.-H.C., H.-C.H., H.-Y.H. and Y.-J.C.; methodology, M.C., H.H.L., H.-Y.H. and P.-H.W.; writing—original draft, M.C., H.H.L., and P.-H.W.; writing—review and editing, W.-L.L. and P.-H.W.; supervision, W.-L.L. and P.-H.W.; project administration, W.-L.L. and P.-H.W.
Funding
This research was supported by grants from the Taipei Veterans General Hospital (V108C-085) and from the Ministry of Science and Technology, Executive Yuan (MOST: 106-2314-B-075-061-MY3), Taipei, Taiwan. The authors appreciate the financial support from the Female Cancer Foundation, Taipei, Taiwan.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.McGuire W.P., Hoskins W.J., Brady M.F., Kucera P.R., Partridge E.E., Look K.Y., Clarke-Pearson D.L., Davidson M. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N. Engl. J. Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 2.Piccart M.J., Bertelsen K., James K., Cassidy J., Mangioni C., Simonsen E., Stuart G., Kaye S., Vergote I., Blom R., et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: Three-year results. J. Natl. Cancer Inst. 2000;92:699–708. doi: 10.1093/jnci/92.9.699. [DOI] [PubMed] [Google Scholar]
- 3.Piccart M.J., Bertelsen K., Stuart G., Cassidy J., Mangioni C., Simonsen E., James K., Kaye S., Vergote I., Blom R., et al. Long-term follow-up confirms a survival advantage of the paclitaxel-cisplatin regimen over the cyclophosphamide-cisplatin combination in advanced ovarian cancer. Int. J. Gynecol. Cancer. 2003;13:144–148. doi: 10.1111/j.1525-1438.2003.13357.x. [DOI] [PubMed] [Google Scholar]
- 4.Alberts D.S. Carboplatin versus cisplatin in ovarian cancer. Semin. Oncol. 1995;22:88–90. [PubMed] [Google Scholar]
- 5.du Bois A., Neijt J.P., Thigpen J.T. First-line chemotherapy with carboplatin plus paclitaxel in advanced ovarian cancer—a new standard of care? Ann. Oncol. 1999;10:51–53. doi: 10.1023/A:1008355317514. [DOI] [PubMed] [Google Scholar]
- 6.Neijt J.P., Engelholm S.A., Tuxen M.K., Sorensen P.G., Hansen M., Sessa C., de Swart C.A., Hirsch F.R., Lund B., van Houwelingen H.C. Exploratory phase III study of paclitaxel and cisplatin versus paclitaxel and carboplatin in advanced ovarian cancer. J. Clin. Oncol. 2000;18:3084–3092. doi: 10.1200/JCO.2000.18.17.3084. [DOI] [PubMed] [Google Scholar]
- 7.du Bois A., Luck H.J., Meier W., Adams H.P., Mobus V., Costa S., Bauknecht T., Richter B., Warm M., Schroder W., et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J. Natl. Cancer Inst. 2003;95:1320–1329. doi: 10.1093/jnci/djg036. [DOI] [PubMed] [Google Scholar]
- 8.Ozols R.F., Bundy B.N., Greer B.E., Fowler J.M., Clarke-Pearson D., Burger R.A., Mannel R.S., DeGeest K., Hartenbach E.M., Baergen R. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A Gynecologic Oncology Group study. J. Clin. Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 9.Sung P.L., Wen K.C., Horng H.C., Chang C.M., Chen Y.J., Lee W.L., Wang P.H. The role of α2,3-linked sialylation on clear cell type epithelial ovarian cancer. Taiwan. J. Obstet. Gynecol. 2018;57:255–263. doi: 10.1016/j.tjog.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Tewari K.S., Burger R.A., Enserro D., Norquist B.M., Swisher E.M., Brady M.F., Bookman M.A., Fleming G.F., Huang H., Homesley H.D., et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J. Clin. Oncol. 2019;37:2317–2328. doi: 10.1200/JCO.19.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vergote I., Scambia G., O’Malley D.M., Van Calster B., Park S.Y., Del Campo J.M., Meier W., Bamias A., Colombo N., Wenham R.M., et al. Trebananib or placebo plus carboplatin and paclitaxel as first-line treatment for advanced ovarian cancer (TRINOVA-3/ENGOT-ov2/GOG-3001): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20:862–876. doi: 10.1016/S1470-2045(19)30178-0. [DOI] [PubMed] [Google Scholar]
- 12.Coleman R.L., Fleming G.F., Brady M.F., Swisher E.M., Steffensen K.D., Friedlander M., Okamoto A., Moore K.N., Efrat Ben-Baruch N., Werner T.L., et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N. Engl. J. Med. 2019 doi: 10.1056/NEJMoa1909707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Burg M.E., van Lent M., Buyse M., Kobierska A., Colombo N., Favalli G., Lacave A.J., Nardi M., Renard J., Pecorelli S. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. Gynecological Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer. N. Engl. J. Med. 1995;332:629–634. doi: 10.1056/NEJM199503093321002. [DOI] [PubMed] [Google Scholar]
- 14.Vergote I., Trope C.G., Amant F., Kristensen G.B., Ehlen T., Johnson N., Verheijen R.H., van der Burg M.E., Lacave A.J., Panici P.B., et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 2010;363:943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 15.Kusunoki S., Terao Y., Hirayama T., Fujino K., Ujihira T., Ota T., Takeda S. Safety and efficacy of neoadjuvant chemotherapy with bevacizumab in advanced-stage peritoneal/ovarian cancer patients. Taiwan. J. Obstet. Gynecol. 2018;57:650–653. doi: 10.1016/j.tjog.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Wang P.H. Neoadjuvant chemotherapy before definite operative approach for women with advanced-stage epithelial ovarian cancer. Taiwan. J. Obstet. Gynecol. 2018;57:623–624. doi: 10.1016/j.tjog.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Bartels H.C., Rogers A.C., McSharry V., McVey R., Walsh T., O’Brien D., Boyd W.D., Brennan D.J. A meta-analysis of morbidity and mortality in primary cytoreductive surgery compared to neoadjuvant chemotherapy in advanced ovarian malignancy. Gynecol. Oncol. 2019;154:622–630. doi: 10.1016/j.ygyno.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Horner W., Peng K., Pleasant V., Brackmann M., Ebott J., Gutfreund R., McLean K., Reynolds R.K., Uppal S. Trends in surgical complexity and treatment modalities utilized in the management of ovarian cancer in an era of neoadjuvant chemotherapy. Gynecol. Oncol. 2019;154:283–289. doi: 10.1016/j.ygyno.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Klein D.A., Mann A.K., Freeman A.H., Liao C.I., Kapp D.S., Chan J.K. Chemotherapy alone for patients 75 years and older with epithelial ovarian cancer-is interval cytoreductive surgery still needed? Am. J. Obstet. Gynecol. 2019 doi: 10.1016/j.ajog.2019.07.050. [DOI] [PubMed] [Google Scholar]
- 20.Markman M., Bundy B.N., Alberts D.S., Fowler J.M., Clark-Pearson D.L., Carson L.F., Wadler S., Sickel J. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: An intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J. Clin. Oncol. 2001;19:1001–1007. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 21.Markman M., Liu P.Y., Wilczynski S., Monk B., Copeland L.J., Alvarez R.D., Jiang C., Alberts D. Southwest Oncology, Oncology G. Gynecologic, Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: A Southwest Oncology Group and Gynecologic Oncology Group trial. J. Clin. Oncol. 2003;21:2460–2465. doi: 10.1200/JCO.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong D.K., Bundy B., Wenzel L., Huang H.Q., Baergen R., Lele S., Copeland L.J., Walker J.L., Burger R.A. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 23.Coleman R.L., Oza A.M., Lorusso D., Aghajanian C., Oaknin A., Dean A., Colombo N., Weberpals J.I., Clamp A., Scambia G., et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949–1961. doi: 10.1016/S0140-6736(17)32440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Driel W.J., Koole S.N., Sikorska K., Schagen van Leeuwen J.H., Schreuder H.W.R., Hermans R.H.M., de Hingh I., van der Velden J., Arts H.J., Massuger L., et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N. Engl. J. Med. 2018;378:230–240. doi: 10.1056/NEJMoa1708618. [DOI] [PubMed] [Google Scholar]
- 25.Moore K., Colombo N., Scambia G., Kim B.G., Oaknin A., Friedlander M., Lisyanskaya A., Floquet A., Leary A., Sonke G.S., et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 2018;379:2495–2505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 26.Marchetti C., De Felice F., Perniola G., Palaia I., Musella A., Di Donato V., Cascialli G., Muzii L., Tombolini V., Benedetti Panici P. Role of intraperitoneal chemotherapy in ovarian cancer in the platimum-taxane-based era: A meta-analysis. Crit. Rev. Oncol. Hematol. 2019;136:64–69. doi: 10.1016/j.critrevonc.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Vergote I., du Bois A., Floquet A., Rau J., Kim J.W., Del Campo J.M., Friedlander M., Pignata S., Fujiwara K., Colombo N., et al. Overall survival results of AGO-OVAR16: A phase 3 study of maintenance pazopanib versus placebo in women who have not progressed after first-line chemotherapy for advanced ovarian cancer. Gynecol. Oncol. 2019;155:186–191. doi: 10.1016/j.ygyno.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 28.Tsibulak I., Zeimet A.G., Marth C. Hopes and failures in front-line ovarian cancer therapy. Crit. Rev. Oncol. Hematol. 2019;143:14–19. doi: 10.1016/j.critrevonc.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Matanes E., Gotlieb W.H. Immunotherapy of gynecological cancers. Best Pract. Res. Clin. Obstet. Gynaecol. 2019;60:97–110. doi: 10.1016/j.bpobgyn.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Wendel Naumann R., Coleman R.L., Brown J., Moore K.N. Phase III trials in ovarian cancer: The evolving landscape of front line therapy. Gynecol. Oncol. 2019;153:436–444. doi: 10.1016/j.ygyno.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Feng Y., Huang H., Wan T., Zhang C., Tong C., Liu J. Comparison of PARPis with angiogenesis inhibitors and chemotherapy for maintenance in ovarian cancer: A network meta-analysis. Adv. Ther. 2019 doi: 10.1007/s12325-019-01106-1. [DOI] [PubMed] [Google Scholar]
- 32.Liu C.H., Chang Y., Wang P.H. Poly(ADP-ribose) polymerase (PARP) inhibitors and ovarian cancer. Taiwan. J. Obstet. Gynecol. 2017;56:713–714. doi: 10.1016/j.tjog.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 33.Chang C.M., Yang Y.P., Chuang J.H., Chuang C.M., Lin T.W., Wang P.H., Yu M.H., Chang C.C. Discovering the deregulated molecular functions involved in malignant transformation of endometriosis to endometriosis-associated ovarian carcinoma using a data-driven, function-based analysis. Int. J. Mol. Sci. 2017;18:2345. doi: 10.3390/ijms18112345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang C.M., Chiou S.H., Yang M.J., Yen M.S., Wang P.H. Gene set-based integrative analysis of ovarian clear cell carcinoma. Taiwan. J. Obstet. Gynecol. 2016;55:552–557. doi: 10.1016/j.tjog.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Chang C.M., Wang P.H., Horng H.C. Gene se-based analysis of mucinous ovarian carcinoma. Taiwan. J. Obstet. Gynecol. 2017;56:210–216. doi: 10.1016/j.tjog.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 36.Chang C.C., Su K.M., Lu K.H., Lin C.K., Wang P.H., Li H.Y., Wang M.L., Lin C.K., Yu M.H., Chang C.M. Key immunological functions involved in the progression of epithelial ovarian serous carcinoma discovered by the gene ontology-based immunofunctionome analysis. Int. J. Mol. Sci. 2018;19:3311. doi: 10.3390/ijms19113311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simon R., Norton L. The Norton-Simon hypothesis: Designing more effective and less toxic chemotherapeutic regimens. Nat. Clin. Pract. Oncol. 2006;3:406–407. doi: 10.1038/ncponc0560. [DOI] [PubMed] [Google Scholar]
- 38.Su W.H., Ho T.Y., Li Y.T., Lu C.H., Lee W.L., Wang P.H. Metronomic therapy for gynecologic cancers. Taiwan. J. Obstet. Gynecol. 2012;51:167–178. doi: 10.1016/j.tjog.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Katsumata N., Yasuda M., Isonishi S., Takahashi F., Michimae H., Kimura E., Aoki D., Jobo T., Kodama S., Terauchi F., et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): A randomised, controlled, open-label trial. Lancet Oncol. 2013;14:1020–1026. doi: 10.1016/S1470-2045(13)70363-2. [DOI] [PubMed] [Google Scholar]
- 40.Pignata S., Scambia G., Katsaros D., Gallo C., Pujade-Lauraine E., De Placido S., Bologna A., Weber B., Raspagliesi F., Panici P.B., et al. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2014;15:396–405. doi: 10.1016/S1470-2045(14)70049-X. [DOI] [PubMed] [Google Scholar]
- 41.Chan J.K., Brady M.F., Penson R.T., Huang H., Birrer M.J., Walker J.L., DiSilvestro P.A., Rubin S.C., Martin L.P., Davidson S.A., et al. Weekly vs. every-3-week paclitaxel and carboplatin for ovarian cancer. N. Engl. J. Med. 2016;374:738–748. doi: 10.1056/NEJMoa1505067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker J.L., Brady M.F., Wenzel L., Fleming G.F., Huang H.Q., DiSilvestro P.A., Fujiwara K., Alberts D.S., Zheng W., Tewari K.S., et al. Randomized trial of intravenous versus intraperitoneal chemotherapy plus bevacizumab in advanced ovarian carcinoma: An NRG Oncology/Gynecologic Oncology Group Study. J. Clin. Oncol. 2019;37:1380–1390. doi: 10.1200/JCO.18.01568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milani A., Kristeleit R., McCormack M., Raja F., Luvero D., Widschwendter M., MacDonald N., Mould T., Olatain A., Hackshaw A., et al. Switching from standard to dose-dense chemotherapy in front-line treatment of advanced ovarian cancer: A retrospective study of feasibility and efficacy. ESMO Open. 2017;1:e000117. doi: 10.1136/esmoopen-2016-000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marth C., Reimer D., Zeimet A.G. Front-line therapy of advanced epithelial ovarian cancer: Standard treatment. Ann. Oncol. 2017;28:viii36–viii39. doi: 10.1093/annonc/mdx450. [DOI] [PubMed] [Google Scholar]
- 45.Lee M.X., Tan D.S. Weekly versus 3-weekly paclitaxel in combination with carboplatin in advanced ovarian cancer: Which is the optimal adjuvant chemotherapy regimen? J. Gynecol. Oncol. 2018;29:e96. doi: 10.3802/jgo.2018.29.e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takaya H., Nakai H., Murakami K., Tobiume T., Suzuki A., Mandai M., Matsumura N. Efficacy of weekly administration of paclitaxel and carboplatin for advanced ovarian cancer patients with poor performance status. Int. J. Clin. Oncol. 2018;23:698–706. doi: 10.1007/s10147-018-1264-9. [DOI] [PubMed] [Google Scholar]
- 47.Marchetti C., De Felice F., Di Pinto A., D’Oria O., Aleksa N., Musella A., Palaia I., Muzii L., Tombolini V., Benedetti Panici P. Dose-dense weekly chemotherapy in advanced ovarian cancer: An updated meta-analysis of randomized controlled trials. Crit. Rev. Oncol. Hematol. 2018;125:30–34. doi: 10.1016/j.critrevonc.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 48.Shibutani T., Nagao S., Suzuki K., Kaneda M., Yamamoto K., Jimi T., Yano H., Kitai M., Shiozaki T., Matsuoka K., et al. Dose-dense paclitaxel and carboplatin vs. conventional paclitaxel and carboplatin as neoadjuvant chemotherapy for advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer: A retrospective study. Int. J. Clin. Oncol. 2019 doi: 10.1007/s10147-019-01567-y. [DOI] [PubMed] [Google Scholar]
- 49.Huang B.S., Chang W.H., Wang K.C., Huang N., Guo C.Y., Chou Y.J., Huang H.Y., Chen T.J., Lee W.L., Wang P.H. Endometriosis might be inversely associated with developing chronic kidney disease: A population-based cohort study in Taiwan. Int. J. Mol. Sci. 2016;17:1079. doi: 10.3390/ijms17071079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang W.H., Horng H.C., Yeh C.C., Guo C.Y., Chou Y.J., Huang N., Huang H.Y., Chen Y.J., Lee W.L., Wang P.H. Risks of female genital tract related cancers (gynecological cancers) or breast cancer in women with and without chronic kidney disease: A population-based cohort study in Taiwan. Medicine. 2018;97:e0157. doi: 10.1097/MD.0000000000010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fiseha T., Mengesha T., Girma R., Kebede E., Gebreweld A. Estimation of renal function in adult outpatients with normal serum creatinine. BMC Res. Notes. 2019;12:462. doi: 10.1186/s13104-019-4487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schoen M.W., Basch E., Hudson L.L., Chung A.E., Mendoza T.R., Mitchell S.A., St Germain D., Baumgartner P., Sit L., Rogak L.J., et al. Software for administering the National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events: Usability Study. JMIR Hum. Factors. 2018;5:e10070. doi: 10.2196/10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chung A.E., Shoenbill K., Mitchell S.A., Dueck A.C., Schrag D., Bruner D.W., Minasian L.M., St Germain D., O’Mara A.M., Baumgartner P., et al. Patient free text reporting of symptomatic adverse events in cancer clinical research using the National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) J. Am. Med. Inform. Assoc. 2019;26:276–285. doi: 10.1093/jamia/ocy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raimondi A., Randon G., Sepe P., Claps M., Verzoni E., de Braud F., Procopio G. The evaluation of response to immunotherapy in metastatic renal cell carcinoma: Open challenges in the clinical practice. Int. J. Mol. Sci. 2019;20:4263. doi: 10.3390/ijms20174263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi T., Jiang R., Pu H., Yang H., Tu D., Dai Z., Cai Y., Zhang Y., Cheng X., Jia H., et al. Survival benefits of dose-dense early postoperative intraperitoneal chemotherapy in front-line therapy for advanced ovarian cancer: A randomised controlled study. Br. J. Cancer. 2019;121:425–428. doi: 10.1038/s41416-019-0543-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suidan R.S., He W., Sun C.C., Zhao H., Rauh-Hain J.A., Fleming N.D., Lu K.H., Giordano S.H., Meyer L.A. Total and out-of-pocket costs of different primary management strategies in ovarian cancer. Am. J. Obstet. Gynecol. 2019;221:e1–e136. doi: 10.1016/j.ajog.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chelariu-Raicu A., Coleman R.L., Sood A.K. Anti-angiogenesis therapy in ovarian cancer: Which patient is it most likely to benefit? Oncology. 2019;33:629378. [PubMed] [Google Scholar]
- 58.Armstrong D.K., Alvarez R.D., Bakkum-Gamez J.N., Barroilhet L., Behbakht K., Berchuck A., Berek J.S., Chen L.M., Cristea M., DeRosa M., et al. NCCN guidelines insights: Ovarian cancer, version 1.2019. J. Natl. Compr. Cancer Netw. 2019;17:896–909. doi: 10.6004/jnccn.2019.0039. [DOI] [PubMed] [Google Scholar]
- 59.Burger R.A., Brady M.F., Bookman M.A., Fleming G.F., Monk B.J., Huang H., Mannel R.S., Homesley H.D., Fowler J., Greer B.E., et al. Gynecologic Oncology Group. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 60.Perren T.J., Swart A.M., Pfisterer J., Ledermann J.A., Pujade-Lauraine E., Kristensen G., Carey M.S., Beale P., Cervantes A., Kurzeder C., et al. ICON7 Investigators. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 61.Oza A.M., Cook A.D., Pfisterer J., Embleton A., Ledermann J.A., Pujade-Lauraine E., Kristensen G., Carey M.S., Beale P., Cervantes A., et al. ICON7 trial investigators. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16:928–936. doi: 10.1016/S1470-2045(15)00086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.du Bois A., Kristensen G., Ray-Coquard I., Reuss A., Pignata S., Colombo N., Denison U., Vergote I., Del Campo J.M., Ottevanger P., et al. AGO Study Group led Gynecologic Cancer Intergroup/European Network of Gynaecologic Oncology Trials Groups Intergroup Consortium. Standard first-line chemotherapy with or without nintedanib for advanced ovarian cancer (AGO-OVAR 12): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2016;17:78–89. doi: 10.1016/S1470-2045(15)00366-6. [DOI] [PubMed] [Google Scholar]
- 63.du Bois A., Floquet A., Kim J.W., Rau J., del Campo J.M., Friedlander M., Pignata S., Fujiwara K., Vergote I., Colombo N., et al. Incorporation of pazopanib in maintenance therapy of ovarian cancer. J. Clin. Oncol. 2014;32:3374–3382. doi: 10.1200/JCO.2014.55.7348. [DOI] [PubMed] [Google Scholar]
- 64.Boussios S., Karihtala P., Moschetta M., Karathanasi A., Sadauskaite A., Rassy E., Pavlidis N. Combined strategies with poly (ADP-Ribose) polymerase (PARP) inhibitors for the treatment of ovarian cancer: A literature review. Diagnostics. 2019;9:87. doi: 10.3390/diagnostics9030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beaver J.A., Coleman R.L., Arend R.C., Armstrong D.K., Bala S., Mills G.B., Sood A.K., Herzog T.J. Advancing drug development in gynecologic malignancies. Clin. Cancer Res. 2019;25:4874–4880. doi: 10.1158/1078-0432.CCR-19-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]