Abstract
Rationale:
Consensus guidelines on the management of methotrexate-induced nephrotoxicity using glucarpidase (Voraxaze) may be relatively unfamiliar to the nephrology community.
Presenting concerns of the patient:
A 61-year-old man with intravascular large B-cell lymphoma was admitted for cycle #1 of high-dose methotrexate (HDMTX) following 2 cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy. On admission, he was clinically euvolemic and had a creatinine clearance of 98 mL/min. He received standard HDMTX toxicity prophylaxis with volume expansion, urinary alkalinization, and leucovorin rescue.
Diagnoses:
Despite prophylactic efforts, he developed a severe acute kidney injury, creatinine 63 to 226 µmol/L (2.56 mg/dL), following HDMTX, impaired methotrexate clearance, and neurotoxicity manifested by status epilepticus.
Interventions:
He was given glucarpidase to convert extracellular methotrexate into its inactive metabolites, glutamate and DAMPA (4-deoxy-4-amino-N10-methylpteroic acid) at 52 hours post-HDMTX. Cross-reactivity between commercial methotrexate immunoassays with DAMPA led to falsely elevated methotrexate concentrations for much longer than expected based on the current guideline (5 days instead of <48 hours). This required ongoing monitoring of methotrexate concentration by mass spectrometry.
Outcomes:
The patient remained nonoliguric and did not develop acute indications for dialysis. Serum creatinine peaked at 608 µmol/L (6.88 mg/dL) 6 days after HDMTX. He ultimately had a full renal and neurologic recovery.
Lessons learned:
Glucarpidase is an effective option for nonrenal elimination of methotrexate-induced nephrotoxicity. Timing of methotrexate concentration monitoring to assess for toxicity, how to access the drug, and the need for ongoing monitoring by mass spectrometry beyond the guideline recommendation are highlighted for centers where HDMTX therapy may be used.
Keywords: lymphoma, methotrexate, toxicity, glucarpidase, DAMPA
Abrégé
Justification:
Les lignes directrices consensuelles sur la prise en charge de la néphrotoxicité induite par le méthotrexate par l’administration de glucarpidase (VoraxazeMD) sont possiblement mal connues en néphrologie.
Présentation du cas:
Nous présentons le cas d’un patient de 61 ans atteint d’un lymphome intravasculaire à grandes cellules B qui avait été admis pour un cycle de traitement à dose élevée de méthotrexate (HDMTX) après deux cycles de chimiothérapie par R-CHOP. À l’admission, le patient était cliniquement euvolémique et présentait une clairance de la créatinine à 98 mL/min. Le patient a reçu la prophylaxie standard pour une toxicité à HDMTX avec expansion volumique, alcalinisation urinaire et sauvetage par leucovorine.
Diagnostic:
Malgré les mesures prophylactiques, l’état du patient a évolué vers une grave insuffisance rénale aigüe (créatinine initiale de 63 à 226 µmol/L [2,56 mg/dL]) après le traitement au HDMTX, de même qu’une altération de la clairance du méthotrexate et une neurotoxicité manifestée par un status epilepticus.
Interventions:
Le patient a reçu du glucarpidase pour convertir le méthotrexate extracellulaire en ses métabolites inactifs, le glutamate et le DAMPA (acide 4-déoxy-4-amino-N10-méthylptéroïque) 52 heures après le traitement au HDMTX. La réactivité croisée entre les immunoessais commerciaux au méthotrexate et le DAMPA a entraîné des concentrations faussement élevées de méthotrexate pour beaucoup plus longtemps que prévu selon la recommandation actuelle (5 jours plutôt que < 48 heures). Cette situation a nécessité une surveillance continue de la concentration du méthotrexate par spectrométrie de masse.
Résultats:
Le patient est demeuré non oligurique et n’a pas nécessité de dialyse. Le taux de créatinine sérique a culminé à 608 µmol/L (6,88 mg/dL) six jours après l’administration de HDMTX. Les fonctions rénale et neurologique du patient se sont finalement rétablies complètement.
Leçons tirées:
La glucarpidase est une option efficace pour éliminer de façon non rénale la néphrotoxicité induite par le méthotrexate. Le moment de mesurer la concentration de méthotrexate pour évaluer la toxicité, la façon d’accéder au médicament et la nécessité d’une surveillance continue par spectrométrie de masse au-delà de la recommandation actuelle sont clarifiés pour les centres où un traitement par HDMTX pourrait être administré.
What was known before
Glucarpidase can be used for nonrenal elimination of methotrexate in the setting of nephrotoxicity. Its use is supported by recent guidelines published in the oncology literature.
What this adds
Cross-reactivity of commercial methotrexate immunoassays with DAMPA (4-deoxy-4-amino-N10-methylpteroic acid) led to falsely elevated methotrexate concentrations for much longer than expected (4-5 days). Thus, ongoing monitoring of methotrexate concentrations by mass spectrometry beyond the guideline recommendation is suggested.
Introduction
High-dose methotrexate (HDMTX; ≥500 mg/m2) is used as part of chemotherapy regimens for various adult and childhood cancers.1 By interfering with folate metabolism, methotrexate (MTX) impairs thymidine and DNA synthesis in rapidly dividing malignant cells, leading to cell death. Polyglutamation increases the size and charge of MTX, enhancing its antiproliferative effects through intracellular accumulation and decreased efflux.2 Methotrexate is primarily excreted by the kidneys (80%-90%).3 Methotrexate can cause afferent arteriolar vasoconstriction, precipitate in tubules, and cause direct tubular injury. Acute kidney injury (AKI) has been reported in 2% to 12% of patients receiving HDMTX.4 In cases of severe renal dysfunction, glucarpidase (Voraxaze) can be used for nonrenal elimination by converting extracellular MTX into its inactive metabolites.5
Presenting Concerns
A 61-year-old man was admitted for cycle #1 of HDMTX. Three months ago, he was diagnosed with intravascular large B-cell lymphoma (ILCL). He initially presented with seizures and the diagnosis was confirmed via right temporal lobe biopsy. His seizures were controlled with levetiracetam. Prior to admission, he received 2 of 6 cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy with no adverse effects. Other medical history was noncontributory.
Clinical Findings
On admission, he was euvolemic with an unremarkable physical examination. His creatinine was 63 µmol/L (0.71 mg/dL) and his creatinine clearance was 98 mL/min. Serum alanine aminotransferase was normal (38 U/L). Per protocol, he underwent volume expansion and urinary alkalinization with intravenous (IV) sodium bicarbonate 0.15 mEq/mL at 160 mL/h. Urine pH was assessed every 12 hours and maintained at ≥7.0, with urine output ≥ 100 mL/h. After 12 hours of pretreatment, he received 6500 mg (=3500 mg/m2) of IV MTX over 4 hours, which was well tolerated.
Following HDMTX, he developed an AKI with a creatinine of 226 µmol/L (2.56 mg/dL) and a methotrexate concentration ([MTX]) of 175 µmol/L by immunoassay (>35× the toxic threshold) at 18 hours. High-dose leucovorin (folinic acid) was immediately started at 1860 mg (=1000 mg/m2) IV over 30 minutes every 3 hours to allow the formation of reduced intracellular folate in the presence of MTX. Variability in polyglutamation between tumor cells and nonmalignant cells allows leucovorin to selectively rescue nonmalignant cells from the effects of HDMTX while maintaining tumor cell cytotoxicity.2 Table 1 summarizes the patient’s clinical course.
Table 1.
Patient’s Timeline.
| Day | Event | Hours post-HDMTX | Serum creatinine (µmol/L) | Methotrexate concentration |
|---|---|---|---|---|
| Day -41 | Diagnosed with DLBCL with cerebral vessel involvement | |||
| Day -33 | R-CHOP, cycle #1 | |||
| Day -12 | R-CHOP, cycle #2 | |||
| Day 0 | HDMTX, cycle #1 | 0 (=1400h) | 63 (at baseline) | |
| AKI identified High-dose leucovorin started at 1000 mg/m2 IV every 3 hours (10× standard dose) |
18 | 226 | 175 µmol/L (>35× the toxic threshold) | |
| Day 1 | Progressive AKI | 41 | 374 | 31 µmol/L (persistently toxic concentration) |
| Neurotoxicity (status epilepticus), patient transferred to ICU | 42 | |||
| Glucarpidase procured and administered, 50 units/kg IV over 5 minutes | 52 | 434 | ||
| Day 2 | Discrepancy between methotrexate concentration by immunoassay and LC-MS/MS | 60 | 479 | Immunoassay: 7.26 µmol/L LC-MS/MS: <0.05 µmol/L |
| Day 6 | Peak serum creatinine reached; patient remained nonoliguric with no acute indications for dialysis | 608 | Immunoassay: 1.60 µmol/L | |
| Day 31 | Patient transferred to ward | 88 | ||
| Day 38 | R-CHOP, cycle #3 | 63 | ||
| Day 44 | Patient discharged to rehab | 60 |
Note. To convert serum creatinine from µmol/L to mg/dL, multiply by 0.0113. HDMTX = high-dose methotrexate; DLBCL = diffuse large B-cell lymphoma; R-CHOP = rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; AKI = acute kidney injury; IV = intravenous; ICU = intensive care unit; LC-MS/MS = liquid chromatography-tandem mass spectrometry.
Diagnostic Focus and Assessment
Despite undergoing volume expansion, urinary alkalinization, and leucovorin rescue, the patient had progressive AKI from MTX-induced nephrotoxicity with a further increase in creatinine to 374 µmol/L (4.23 mg/dL). Notably, he had no traditional risk factors for MTX toxicity, such as body mass index ≥ 25 kg/m2, urine pH < 7.0, IV fluid intake < 3 L/m2/24 h, diarrhea, or baseline renal or hepatic dysfunction.6 Previous case reports identified a potential interaction between MTX and levetiracetam resulting in delayed MTX elimination, but a retrospective review of 81 patients receiving 280 cycles of HDMTX did not support this interaction.7 Whether he was genetically susceptible to having altered pharmacodynamics of MTX handling was unknown; no testing was done to evaluate this. With severe renal dysfunction, MTX clearance was impaired as evidenced by persistently elevated [MTX] (31.00 µmol/L at 41 hours by immunoassay). This led to neurotoxicity manifested by electroencephalogram (EEG)-confirmed status epilepticus from a right temporal focus. There have been 3 other cases of status epilepticus in adults receiving HDMTX.8-10 The patient had a diagnostic brain biopsy, which likely increased his risk for neurological complications. He also developed a transaminitis 1 week after HDMTX, peaking at 12 times the upper limit of normal after 3 weeks.
Therapeutic Focus and Assessment
Given his severe AKI and clinical sequelae following HDMTX, a decision was made to administer glucarpidase (Voraxaze), the carboxypeptidase G2 enzyme that converts extracellular MTX into its inactive metabolites, glutamate and DAMPA (4-deoxy-4-amino-N10-methylpteroic acid), which are eliminated by the liver. Glucarpidase decreases plasma [MTX] by 98% within 15 minutes if given within 48 to 60 hours of HDMTX.4 An alternative would have been high-flux hemodialysis (HFHD). Methotrexate has a low molecular weight (454 daltons), but its dialyzability is limited by high protein binding (50%) and high volume of distribution. Serum MTX clearance using HFHD is between 1.00 and 2.04 mL/min/kg11 and has a half-life during HFHD of 2.3 to 3.4 hours,12 with a mean reduction in [MTX] between 42% and 94% over 4 to 12 hours.13 Prolonged treatment is often required because hemodialysis clears MTX from the intravascular compartment and a rebound of free MTX may be seen when dialysis is stopped. Continuous venovenous hemodiafiltration with maximum effluent rates has also been effective.14
Per the 2017 consensus guidelines on the use of glucarpidase in the setting of rising creatinine, expert opinion recommends its use in patients with 24-hour [MTX] > 50 µM, 36-hour [MTX] > 30 µM, 42-hour [MTX] > 10 µM, or 48-hour [MTX] > 5 µM.15 The patient’s [MTX] was within the recommended treatment range. As such, glucarpidase was procured and administered (50 units/kg IV bolus over 5 minutes) at 52 hours post-HDMTX. He required 4 vials (1000 units/vial) at a cost of CAD39 200. There were no contraindications per the drug monograph and no dose adjustments needed for renal dysfunction.5 A smaller dose may have also been effective.16 Leucovorin was held 2 hours before and after glucarpidase to prevent its metabolism to 5-formylpteroate and glutamate.15
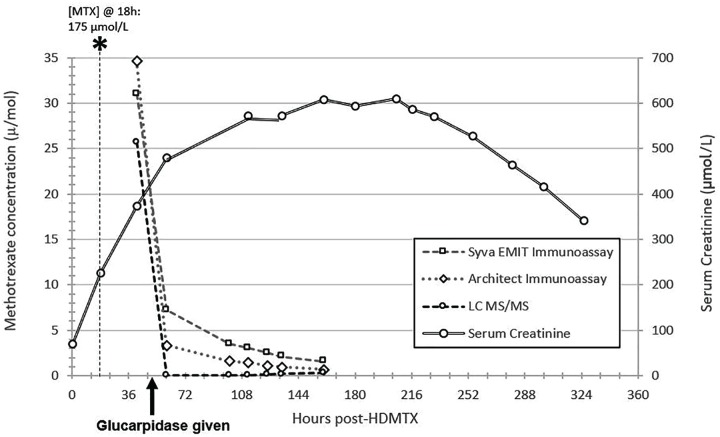
The patient’s [MTX] reassessed 8 hours after glucarpidase remained elevated at 7.26 µmol/L by immunoassay but was <0.05 µmol/L by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Figure 1 shows his [MTX] using 3 different assays. Cross-reactivity with DAMPA ranges from 26% to 100% for common MTX immunoassays,17,18 leading to falsely elevated [MTX]. Consensus guidelines advise on monitoring [MTX] during the first 48 hours by a chromatographic method only, which is based on the pharmacokinetic literature suggesting that DAMPA’s half-life is 9 hours,5 after which monitoring by immunoassay is considered acceptable. Interestingly, his [MTX] by immunoassay remained falsely elevated for more than 4.5 days after glucarpidase, far longer than 48 hours. The patient had not yet developed any significant liver impairment that would have impacted DAMPA’s metabolism.
Figure 1.
Patient’s methotrexate concentrations.
Note. To convert serum creatinine from µmol/L to mg/dL, multiply by 0.0113. MTX = methotrexate; LC-MS/MS = liquid chromatography-tandem mass spectrometry; HDMTX = high-dose methotrexate.
Follow-up and Outcomes
The patient remained nonoliguric with urine output consistently ≥ 100 mL/h and did not develop acute indications for dialysis. Serum creatinine peaked at 608 µmol/L (6.88 mg/dL) 6 days after HDMTX, with full recovery to baseline by 31 days. His renal course was consistent with previous literature.19 Neurologically, his seizures were controlled with clobazam and lacosamide. His course was further complicated by febrile neutropenia and treated with broad-spectrum antibiotics. He was discharged to rehab 45 days after admission. The possibility of rechallenging him with intrathecal MTX was discussed; however, he declined further MTX and continued with R-CHOP chemotherapy.
Discussion
This report highlights a case of MTX-induced nephrotoxicity managed with glucarpidase that was refractory to standard prophylaxis. The 2017 consensus guidelines on the use of glucarpidase may be relatively unfamiliar to the nephrology community.15 This patient had a toxic [MTX] and severe AKI at 18 hours, but there are no recommendations on administering glucarpidase within 24 hours of receiving HDMTX. In retrospect, had [MTX] been reassessed at 24 hours, glucarpidase may have been administered earlier than 52 hours before he experienced further clinical toxicity.
Glucarpidase is only approved in the United States but was accessible through Health Canada’s Special Access Program given the serious nature of this patient’s clinical course despite standard prophylaxis. Since 2017, there have been 19 orders across Canada, 172 orders in the United States, and 222 orders within the European Union, predominantly in France (S. Ward, BTG International Inc, personal communication, February 14, 2019). Glucarpidase is costly, but it is difficult to compare the costs to acute dialysis given the unknown duration of therapy needed. In Canada, the incremental cost of AKI requiring dialysis has been estimated at CAD18 291.20 Cost considerations of using glucarpidase would need to be weighed against the speed and effectiveness at which glucarpidase decreases plasma [MTX] and the inherent risks associated with hemodialysis.
A novel finding from this case is the prolonged duration of falsely elevated [MTX] by immunoassay due to the cross-reactivity with DAMPA (4-5 days). Perhaps, the pharmacokinetic literature on DAMPA is not as robust as expected. In this case, elevated [MTX] by immunoassay beyond 48 hours after glucarpidase caused unnecessary confusion and consideration of the need for repeat glucarpidase or dialysis. Confirmation of undetectable [MTX] by LC-MS/MS supported ongoing expectant management. Future iterations of glucarpidase consensus guidelines should advise for monitoring by LC-MS/MS well beyond 48 hours. Notably, only 1 lab in Canada is equipped to analyze MTX by LC-MS/MS, which is relevant for Canadian physicians who may use this drug. In the future, an immunoassay designed to address this limitation may aid in clinical decision making.
Acknowledgments
The authors acknowledge The Hospital for Sick Children for the measurement of methotrexate by chemiluminescent immunoassay and LC-MS/MS.
Footnotes
List of Abbreviations: AKI, acute kidney injury; CNS, central nervous system; DAMPA, 4-deoxy-4-amino-N10-methylpteroic acid; EEG, electroencephalogram; HDMTX, high-dose methotrexate; HFHD, high-flux hemodialysis; ILCL, intravascular large B-cell lymphoma; LC-MS/MS, liquid chromatography-tandem mass spectrometry; [MTX], methotrexate concentration; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone.
Ethics Approval and Consent to Participate: Research Ethics Board approval was not required for this case report. The authors obtained written patient consent to disseminate this case.
Consent for Publication: Consent for publication has been provided by all authors.
Availability of Data and Materials: The data and materials are not available for this study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ann Young  https://orcid.org/0000-0001-6562-8139
https://orcid.org/0000-0001-6562-8139
References
- 1. Treon SP, Chabner BA. Concepts in use of high-dose methotrexate therapy. Clin Chem. 1996;42(8 Pt 2):1322-1329. [PubMed] [Google Scholar]
- 2. Cohen IJ. Defining the appropriate dosage of folinic acid after high-dose methotrexate for childhood acute lymphatic leukemia that will prevent neurotoxicity without rescuing malignant cells in the central nervous system. J Pediatr Hematol Oncol. 2004;26(3):156-163. [DOI] [PubMed] [Google Scholar]
- 3. Winograd B, Lippens RJ, Oosterbaan MJ, Dirks MJ, Vree TB, van der Kleijn E. Renal excretion and pharmacokinetics of methotrexate and 7-hydroxy-methotrexate following a 24-h high dose infusion of methotrexate in children. Eur J Clin Pharmacol. 1986;30(2):231-238. [DOI] [PubMed] [Google Scholar]
- 4. Widemann BC, Balis FM, Kim A, et al. Glucarpidase, leucovorin, and thymidine for high-dose methotrexate-induced renal dysfunction: clinical and pharmacologic factors affecting outcome. J Clin Oncol. 2010;28(25):3979-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Voraxaze Dosing & Administration—Voraxaze. https://www.voraxaze.com/Dosing-Administration. Accessed February 11, 2019.
- 6. Schwartz S, Borner K, Muller K, et al. Glucarpidase (carboxypeptidase g2) intervention in adult and elderly cancer patients with renal dysfunction and delayed methotrexate elimination after high-dose methotrexate therapy. Oncologist. 2007;12(11):1299-1308. [DOI] [PubMed] [Google Scholar]
- 7. Reeves D, DiDominick S, Finn S, Kim HJ, Shake A. Methotrexate elimination when coadministered with levetiracetam. Ann Pharmacother. 2016;50(12):1016-1022. [DOI] [PubMed] [Google Scholar]
- 8. Naing A, Luong D, Extermann M. Methotrexate-induced status epilepticus. Am J Hematol. 2005;80(1):35-37. [DOI] [PubMed] [Google Scholar]
- 9. Finkelstein Y, Zevin S, Heyd J, Bentur Y, Zigelman Y, Hersch M. Emergency treatment of life-threatening intrathecal methotrexate overdose. Neurotoxicology. 2004;25(3):407-410. [DOI] [PubMed] [Google Scholar]
- 10. Rao RD, Swanson JW, Dejesus RS, Hunt CH, Tefferi A. Methotrexate induced seizures associated with acute reversible magnetic resonance imaging (MRI) changes in a patient with acute lymphoblastic leukemia. Leuk Lymphoma. 2002;43(6):1333-1336. [DOI] [PubMed] [Google Scholar]
- 11. Wall SM, Johansen MJ, Molony DA, DuBose TD, Jr, Jaffe N, Madden T. Effective clearance of methotrexate using high-flux hemodialysis membranes. Am J Kidney Dis. 1996;28(6):846-854. [DOI] [PubMed] [Google Scholar]
- 12. Saland JM, Leavey PJ, Bash RO, Hansch E, Arbus GS, Quigley R. Effective removal of methotrexate by high-flux hemodialysis. Pediatr Nephrol. 2002;17(10):825-829. [DOI] [PubMed] [Google Scholar]
- 13. Widemann BC, Balis FM, Kempf-Bielack B, et al. High-dose methotrexate-induced nephrotoxicity in patients with osteosarcoma. Cancer. 2004;100(10):2222-2232. [DOI] [PubMed] [Google Scholar]
- 14. Vilay AM, Mueller BA, Haines H, Alten JA, Askenazi DJ. Treatment of methotrexate intoxication with various modalities of continuous extracorporeal therapy and glucarpidase. Pharmacotherapy. 2010;30(1):111. [DOI] [PubMed] [Google Scholar]
- 15. Ramsey LB, Balis FM, O’Brien MM, et al. Consensus guideline for use of glucarpidase in patients with high-dose methotrexate induced acute kidney injury and delayed methotrexate clearance. Oncologist. 2018;23(1):52-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scott JR, Zhou Y, Cheng C, et al. Comparable efficacy with varying dosages of glucarpidase in pediatric oncology patients. Pediatr Blood Cancer. 2015;62(9):1518-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Albertioni F, Rask C, Eksborg S, et al. Evaluation of clinical assays for measuring high-dose methotrexate in plasma. Clin Chem. 1996;42(1):39-44. [PubMed] [Google Scholar]
- 18. Bouquie R, Gregoire M, Hernando H, et al. Evaluation of a methotrexate chemiluminescent microparticle immunoassay: comparison to fluorescence polarization immunoassay and liquid chromatography-tandem mass spectrometry. Am J Clin Pathol. 2016;146(1):119-124. [DOI] [PubMed] [Google Scholar]
- 19. Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11(6):694-703. [DOI] [PubMed] [Google Scholar]
- 20. Collister D, Pannu N, Ye F, et al. Health care costs associated with AKI. Clin J Am Soc Nephrol. 2017;12(11):1733-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]