Abstract
The radioprotective effect of amitriptyline, an inhibitor of acid sphingomyelinase (ASMase), on radiation-induced impairment of hippocampal neurogenesis, loss of interneuron, and animal weight changes was investigated in BALB/c mice by immunostaining of biomarkers for cell division (Ki67), immature neurons (doublecortin or DCX), and interneurons (parvalbumin or PV) in the dentate gyrus (DG) of hippocampus. The results indicated that preirradiation (with 10 mg/kg, 2 times per day, for 7 consecutive days) or postirradiation (with 10 mg/kg, 2 times per day, for 14 consecutive days) treatment (pretreatment or posttreatment) with intraperitoneal injection of amitriptyline prevented the loss of newly generated neurons, proliferating cells, and interneurons in the subgranular zone of the DG. At the molecular level, pretreatment or posttreatment inhibited the expression of sphingomyelin phosphodiesterase 1 (SMPD1) gene which codes for ASMase. The pretreatment for 7 days also prevented radiation-induced weight loss from 2 to 3 weeks, but not within 1 week after irradiation. On the other hand, the posttreatment with amitriptyline for 14 days could improve animal weight gain from 4 to 6 weeks after irradiation. The present study suggests that amitriptyline may be a promising candidate radio-neuroprotective drug to improve radiation-induced impairment of hippocampal neurogenesis and relevant neurological and neuropsychological disorders.
Keywords: ionizing radiation (IR), weight loss, neurogenesis, subgranular zone (SGZ), acid sphingomyelinase (ASMase) inhibitor
Introduction
Radiotherapy has been widely used as an effective treatment for brain and head and neck cancers. However, radiotherapy-induced long-term brain side effects such as impairment of cognitive function,1 spatial learning and memory,2,3 and related neurogenesis3,4 may persist for a lifetime and seriously affect the quality of patient’s life, particularly in children.5 Human epidemiological and animal experimental studies have shown that radiation exposure increased the risk of Alzheimer disease,6 dementia,7 and ischemic stroke.8 With increased hospital stockpiling of nuclear waste from medical diagnosis, the use of X-ray computed tomography (CT), isotopes for diagnosis, radiotherapy, occupational exposure, and possible radiological terrorism, the development of novel effective radioprotective drugs with less side effect becomes very imperative. While amifostine has been used as a radioprotective agent, the authorization is only for a few defined and clinical indications, such as the incidence of xerostomia in patients undergoing radiotherapy for head and neck cancer.9,10 It was originally used to reduce the cumulative renal toxicity from cisplatin in non-small cell lung cancer.11,12 Acid sphingomyelinase (ASMase) is a soluble glycoprotein with a relative molecular mass of 64 000, and its coding gene sphingomyelinase phosphodiesterase 1 (SMPD1) is located at the p15.1-15.4 region of chromosome 11, about 5 to 6 kb in length, containing 6 exons.13 Acid sphingomyelinase is ubiquitously expressed and releases ceramide (CE) from sphingomyelin, predominantly in lysosomes and on the plasma membrane. It has been reported that ASMase plays a crucial role in cell function, and deficiency of ASMase results in many diseases, such as Niemann-Pick type A and B and the lysosomal storage disease.14,15 While the early study did now indicate a causal relationship between radiation-induced CE release and apoptosis in the lymphocytes from patients with Niemann-Pick disease and ASMase−/− mice model,16 a recent study showed an increased ASMase activity and induced endothelial cell apoptosis after the fractional radiation exposure to mice with tumor,17 suggesting that ASMase maybe is closely related to the radiation-induced apoptosis.
Acid sphingomyelinase inhibitors can reduce the ASMase expression and downregulate CE levels, which may prevent disease genesis. The currently used functional inhibitors of ASMase are mainly tricyclic antidepressants which include amitriptyline and clomipramine. Amitriptyline has been used not only for the treatment of depression but also for neuropathic pain, anxiety, and hyperactivity disorder.18,19 Amitriptyline competes with the enzyme for binding to the inner lysosomal membrane.20 The release of the ASMase from the membrane results in proteolytic cleavage of the enzyme and thereby a reduction in its cellular activity.20,21 Although a number of promising radiation countermeasure agents are currently under development,12 no study has evaluated whether amitriptyline could be radioprotective, in particular, prevent radiation-induced impairment of neurogenesis.
In the present study, in the mouse model, the effect of amitriptyline on radiation-induced impairment of hippocampal cell division, neurogenesis, and damage of interneurons was evaluated using relevant markers such as Ki67, doublecortin (DCX), and parvalbumin (PV), respectively. Its effect on the expression of SMPD1 gene which codes for ASMase was also investigated using real-time polymerase chain reaction (PCR).
Materials and Methods
Experimental Animals
A total of 80 BALB/c mice aged 8 weeks old were used for this study. Animals were randomly divided into 4 groups including: group I (normal control), normal healthy mice without irradiation but treated with intraperitoneal injection of saline; group II (experimental control), mice irradiated with whole-body X rays at 5 Gy (4.23 Gy/min; Precise Treatment System; Elekta, Crawley, United Kingdom) and treated with saline; group III (pretreatment group), mice pretreated with amitriptyline (10 mg/kg) for 7 consecutive days before whole-body irradiation with 5 Gy; group IV (posttreatment group), mice were whole-body irradiated with 5 Gy followed by intraperitoneal injection with amitriptyline (10 mg/kg, 1 hour after irradiation) for 14 consecutive days. All the injection was done 2 times per day with 6-hour interval. Animal weight was measured the next day postirradiation and continuously for 6 weeks. All BALB/c mice were purchased from the Beijing Laboratory Animal Research Center (Beijing, China). Mice were accommodated in Yangtze University animal house for at least 1 week before the experiment and were maintained under standard conditions of ventilation, light, and humidity. Efforts were made to minimize animal suffering and to use the minimal number of animals throughout the study. All animal treatments were carried out according to the Guidelines for the Care and Use of Laboratory Animals published by the National Institutes of Health and approved by the Institutional Animal Care and Use Committee of Yangtze University.
Real-Time PCR
The SMPD1 gene expression was determined using the real-time PCR. Six weeks after weight monitoring, animals (10 in each group) were decerebrated and the hippocampus was removed for real-time PCR. The total RNA was extracted from the hippocampus using TRIzol reagent (Invitrogen, Carlsbad, CA). To analyze the expression levels of SMPD1 messenger RNA, RNA from each mice was reversely transcribed into complementary DNA using the reverse transcription system (Takara, Shiga, Japan). The sequences of the primer pairs used for SMPD1 were: forward, 5′-ACCTTAACCCTGGCTACCGA-3′ and reverse, 5′-GTTGGCCTGGGTCAGATTCA-3′. The primer pairs used for β-actin were: forward, 5′-CTGAGAGGGAAATCGTGCGT-3′, and reverse, 5′-CCACAGGATTCCATACCCAAGA-3′. The analysis was performed using a real-time PCR detection system (Takara). The reaction were performed at 95°C for 30 seconds, followed by 45 cycles of denaturation at 95°C for 5 seconds, annealing at 53°C for 30 seconds, and extension at 72°C for 30 seconds.
Immunohistochemical Staining
At 42 days after irradiation, animals (n = 10 per group) were anesthetized with 1% pentobarbital sodium at 0.1 mL/10 g and perfused with 4% paraformaldehyde. The brain tissues were removed and postfixed overnight and then transferred to 30% sucrose in 0.1 mol/L phosphate buffer (pH: 7.4). Sagittal brain sections were then cut at 50 µm and processed by immunohistochemistry to investigate the radiation-induced changes in neurogenesis using neurogenesis marker DCX, cell proliferation marker Ki67, and interneuron marker PV.
For immunohistochemistry, serial sections were transferred to 0.1 M phosphate-buffered saline (PBS) (pH: 7.4) in 3 different wells of a 24-well tissue culture dish. For the immunocytochemical study, free-floating sections were treated with 3% H2O2 for 10 minutes and blocked with 2% normal horse serum for 2 hours at room temperature. The sections were then incubated with primary goat antibodies for DCX (1:200; Santa Cruz Biotechnology, Inc., Dallas, TX ), rabbit antibodies for PV (1:4000; Swant, Fribourg, Switzerland), and Ki67 (1:200; GeneTex, Hsinchu, Taiwan) in 0.1 M PBS with 0.1% Triton X-100 (PBS-TX) overnight. The sections were then washed in PBS-TX and placed in biotinylated goat anti-rabbit or horse anti-goat secondary antibodies for 1 hour. After 3 washes in PBS-TX, the sections were placed in avidin–biotin complex reagent (Vector Laboratories, Inc, Burlingame, California) in PBS-TX for 30 minutes and then washed in PBS-TX and reacted in 3,3′-diaminobenzidine peroxidase substrate (Vector Laboratories, Inc) for 10 minutes. After immunostaining, the sections were mounted, counterstained with hematoxylin, and then covered with a coverslip.
Statistical Analysis
The animal weight gain was calculated as ([postirradiation weekly weight − preirradiation animal weight]/preirradiation weight) × 100% in different groups, the repeated measures ANOVA (Figure 1) followed by Student t test was used to analyze animal weight gain. Relative expression of SMPD1 gene was analyzed with one-way ANOVA followed by Student t test, and all values were normalized with β-actin gene. For counting of DCX, Ki67, and PV-labeled immunopositive cells in the subgranular zone (SGZ) in the hilus of the dentate gyrus (DG), 8 sections of hippocampus from each animal were used. The immunopositive cells were counted and indicated as DCX immunopositive cell number/per square millimeter of the area of the hilus. All the data were reported as mean ± standard error and were analyzed by SPSS 21.0 software program. The significance level was set at P ≤ .05.
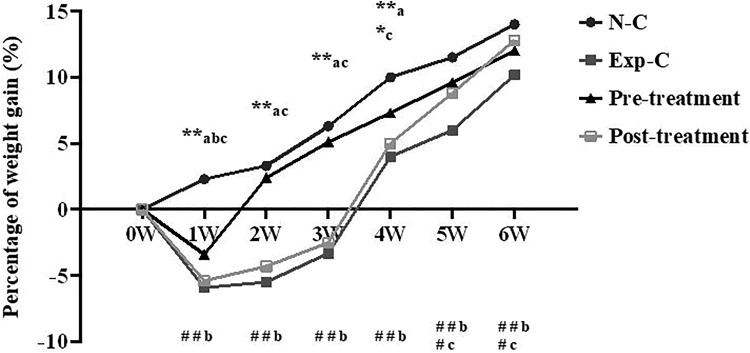
Figure 1.
Acute irradiation with 5 Gy significantly reduces animal weight gain in irradiated mice treated with saline (Exp-C) or amitriptyline (posttreatment group) when compared to the normal control without irradiation (N-C) measured from 1 to 4 weeks postirradiation. At 1 week after irradiation, a significant reduction of animal weight gain also occurs in amitriptyline pretreatment group. Amitriptyline pretreatment significantly improves animal weight gain from 2 to 6 weeks when compared to the experimental control, whereas posttreatment with amitriptyline improves animal weight gain from 4 to 6 weeks after irradiation. *P < .05, **P < .01 versus the normal control group; #P < .05, ##P < .01 versus the experimental control group. a indicates the experimental control group; b, the pretreatment group; c, the posttreatment group. n = 20 per group.
Results
Percentage of Weight Gain
The weight gain of the normal control mice increased continuously from 1 to 6 weeks with saline injection (Figure 1). However, the percentage of weight gain in irradiated mice (−3.3%) with saline injection reduced significantly from 1 to 3 weeks postirradiation compared with the normal control mice (6.8%) and those pretreatment (4.8%) or posttreatment group (−2.5%). Pretreatment or posttreatment significantly improved animal weight gain, although the improvement occurred at different times after irradiation (Figure 1).
The Expression of SMPD1 Gene
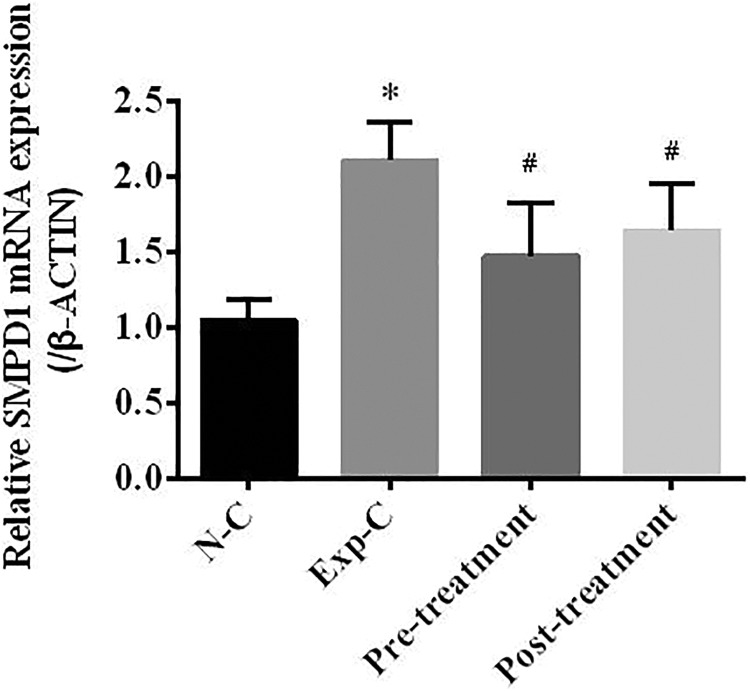
Real-time reverse transcription PCR study of the SMPD1 gene expression showed a significant upregulation of this gene in the irradiated mice with saline injection at 6 weeks after irradiation when compared to the normal control group. Pretreatment or posttreatment with amitriptyline significantly reduces SMPD1 gene expression when compared to the experimental control with saline injection. There was no significant difference in SMPD1 gene expression between the normal control and pretreatment or posttreatment group (Figure 2).
Figure 2.
Real-time polymerase chain reaction study indicates that irradiation significantly upregulates sphingomyelinase phosphodiesterase 1 (SMPD1) gene expression in the experimental control animals with saline injection at 6 weeks after radiation exposure. Pretreatment or posttreatment with amitriptyline significantly reduces SMPD1 gene expression when compared to the experimental control mice. *P < .05 compared to the normal control group, #P < .05 compared to the experimental control group. n = 10 per group.
Hippocampal Neurogenesis
Doublecortin immunohistochemistry
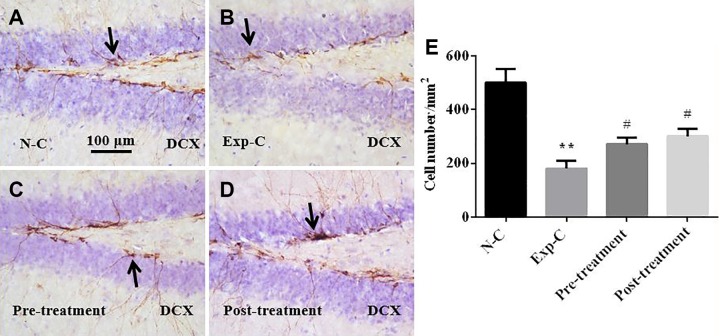
The DCX immunohistochemistry demonstrated a significant loss of newly generated neurons in SGZ of the DG in the experimental control mice with saline injection when compared to the normal control mice without radiation exposure (Figure 3A, B, and E). Pretreatment (Figure 3B, C, and E) or posttreatment (Figure 3B, D, and E) with amitriptyline significantly prevents the loss of newly generated neurons when compared to the experimental control group with saline injection (Figure 3B). There were no significant differences in the number of DCX immunopositive neurons between amitriptyline-treated mice and the normal control mice (Figure 3A, C, D, and E).
Figure 3.
Doublecortin (DCX) immunohistochemistry shows that amitriptyline prevents the loss of DCX immunopositive neurons in subgranular zone of the dentate gyrus after irradiation (A-D). Statistical analysis shows significant reduction of DCX cells in Exp-C mice. Pretreatment and posttreatment with amitriptyline significantly reduces the loss of DCX-labeled immature neurons (E). **P < .01 versus the normal control group, #P < .05 versus the experimental control group.
Ki67 immunohistochemistry
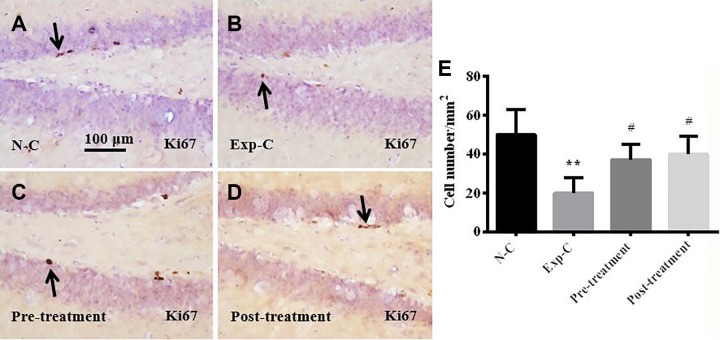
Immunohistochemical staining of cell proliferation markers Ki67 showed significantly reduced dividing cells in SGZ in the experimental control mice when compared to the normal control (Figure 4A, B, and E). Pretreatment (4B, C, E) and posttreatment (Figure 4B, D, and E) with amitriptyline significantly prevent the loss of dividing cells in the SGZ. There were no significant differences in the number of dividing cells between amitriptyline-treated mice and the normal control mice (Figure 4A, C, D, and E).
Figure 4.
Ki67 immunohistochemistry demonstrates that amitriptyline prevents the loss of proliferating cells in subgranular zone and hilus of the dentate gyrus after irradiation (A-D). Statistical analysis shows that the number of Ki67 cells reduced significantly in Exp-C. Pretreatment and posttreatment with amitriptyline prevents the loss of Ki67-labeled proliferating cells (E). **P < .01 versus the normal control group, #P < .05 versus the experimental control group.
Parvalbumin immunohistochemistry
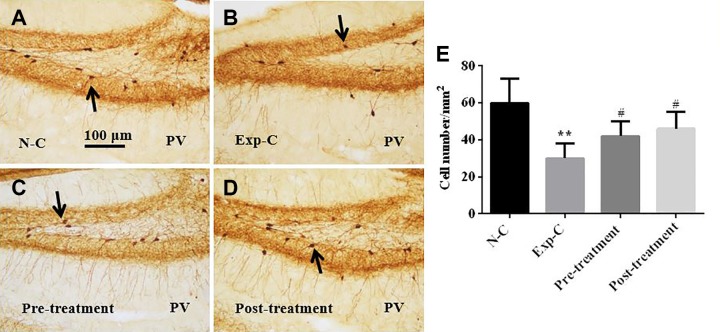
Parvalbumin immunohistochemistry showed that irradiation induced a significant loss of PV immunopositive interneurons in SGZ in the experimental control with a saline injection when compared to the normal control mice (Figure 5A, B, and E). Pretreatment (Figure 5B, C, and E) and posttreatment (Figure 5B, D, and E) with amitriptyline prevented the loss of PV immunopositive interneurons in SGZ. There were no significant differences in the number of PV immunopositive interneurons between amitriptyline-treated mice and the normal control mice (Figure 5A, C, D, and E).
Figure 5.
Parvalbumin (PV) immunohistochemistry shows that amitriptyline prevents the loss of PV immunopositive interneurons in the subgranular zone and the hilus of the dentate gyrus after irradiation (A-D). Statistical analysis shows significant reduction of PV cells in the experimental control group. Pretreatment and posttreatment with amitriptyline prevent the loss of PV-labeled interneurons significantly (E). **P < .01 versus the normal control group, #P < .05 versus the experimental control group.
Discussion
Irradiation Induces the Impairment of Neurogenesis and the Change of Interneurons in the DG
Hippocampus plays an important role in modulating spatial and episodic memory.22 These cognitive processes are affected by multiple oscillating activities (eg, β-, θ- and γ-rhythms) that occur during specific behavioral events.23 In the hippocampus, there are at least 20 distinct types of interneurons which innervate discrete subcellular regions of pyramidal cells in a temporally distinct manner.24 And inhibitory GABAergic interneurons are an important protective factors that could maintain the highly coordinated activity of principal cells.24-26 Brain irradiation has been reported to induce many acute and chronic changes at the cellular and molecular level.27 Some studies have reported that ionizing irradiation affects GABAergic neurotransmission, including PV neurons.28,29 Recently, it was demonstrated that PV immunopositive interneurons in the hippocampus were the key components of intrinsic hippocampal θ-oscillators.30 These interneurons were vulnerable to oxidative stress including radiation exposure.31 A recent research has demonstrated that high-dose irradiation could upregulate the expression of calcium-binding proteins in CA1 area, while in the SGZ of the DG, autophagy was increased.32 It was consistent with radiation-induced impairment of neurogenesis in our previous study3,33 and in the present work, including reduced cell division, newly generated neurons, and PV immunopositive interneurons in SGZ. Parvalbumin immunopositive interneurons are one type of GABAergic interneurons.34,35 The previous study indicated that the reduction in PV immunopositive interneurons following oxidative stress was not due to the loss of these neurons per se but due to the result of decreased expression of PV proteins in GABAergic neurons. In central nervous system after maturation, many PV immunopositive interneurons are surrounded by perineuronal nets (PNNs), which are involved in the protection of these interneurons from oxidative stress.36 Both PV immunopositive interneurons with immature PNNs and without PNNs are susceptible to oxidative stress.37 A recent study reported that PV immunopositive interneuron density was significantly decreased in the secondary motor cortex (M2), secondary visual cortex–mediomedial area (V2MM), and secondary auditory cortex (AuD) regions of irradiated mice. While the density of PV immunopositive interneurons was increased in the retrosplenial granular cortex, a region (RSGa) of irradiated mice, compared with other cortices, this region has a higher percentage of PV immunopositive interneurons surrounded by lectins Wisteria floribunda agglutinin–positive PNNs.38 It suggests that PV immunopositive interneurons are selectively damaged in the brain due to oxidative stress caused by exposure to ionizing irradiation. In the present study, we showed a significant loss of newly generated DCX immunopositive neurons, proliferating cells, and PV immunopositive interneurons in the SGZ and hilus of the DG in the experimental control group, which was consistent with our previous study.4 It suggests that impairment of hippocampal neurogenesis or loss of PV immunopositive interneurons in the DG may be involved in radiation-induced cognitive impairment.4
Amitriptyline Serves as a Functional Inhibitor of ASMase to Prevent Loss of Animal Weight and Newly Generated Cells or Neurons
Previous studies have shown that ionizing irradiation reduces body weight in mice.4,39,40 It was supported by the current study showing a much lighter body weight in irradiated mice than in the normal control mice. In our study, we showed that pretreatment and posttreatment with amitriptyline could significantly prevent radiation-induced animal weight loss. Ionizing radiation activates signaling pathways not only in the nucleus as a result of DNA damage but also in the plasma membrane.41 ASMase/CE pathway is one of the signal pathways in the plasma membrane. Several studies have demonstrated that endothelial apoptosis is the key factor that participates in radiation damage. Exposed at a single high dose of irradiation (>8-10 Gy) could significantly induce endothelial apoptosis via an ASMase-induced CE-dependent mechanism, whereas a low-dose (1.8-3 Gy) fractionated radiotherapy induces endothelial cell damage involving death signaling pathways.42,43 Glucocorticosterone stress activates Jak-3, at least in part, via the ASMase and inhibition of this enzyme using amitriptyline reduces Jak-3 phosphorylation and improves behavior as well as hippocampal neurogenesis.44 Glucocorticosterone stress may also induce p38K phosphorylation/activation in the hippocampus and thereby reduces neurogenesis and induces depression-like symptoms which are prevented by antidepressants via inhibition of the ASMase/CE system. It suggests that inhibition of ASMase by amitriptyline may prevent glucocorticoid-mediated stress and induce phosphorylation/activation of p38K.45 In the present study, the expression of ASMase gene SMPD1 was significantly increased after radiation exposure but reduced after amitriptyline administration. Meanwhile, more newly generated neurons in the SGZ survived after preirradiation or postirradiation treatment with amitriptyline. It suggests that activation of ASMase may be involved in radiation-induced brain damage, in particular, impairment of neurogenesis, and amitriptyline may serve as a potential candidate radio-neuroprotective drug.
Conclusion
As a functional inhibitor of ASMase, amitriptyline prevented radiation-induced impairment of neurogenesis in the SGZ and protected PV immunopositive interneurons in SGZ and the hilus of the DG. It also improved animal weight gain after radiation exposure. The present study suggests that amitriptyline may be a promising candidate drug to be used to prevent brain radiotherapy-induced impairment of neurogenesis and subsequent neuropsychological disorders, in particular, depression, since it has been clinically used as an antidepressant medicine.
Footnotes
Authors’ Note: The work described in the manuscript has not been published previously. Yu Rong Guo and Zi Wei Liu are co-first authors.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China [grant number 81772223] (R.B.X.) and National Research Foundation of Singapore to Singapore Nuclear Research and Safety Initiative (T.F.R.).
ORCID iD: Feng Ru Tang  https://orcid.org/0000-0003-2462-1787
https://orcid.org/0000-0003-2462-1787
References
- 1. Balentova S, Hajtmanova E, Filova B, Borbelyova V, Lehotsky J, Adamkov M. Effects of fractionated whole-brain irradiation on cellular composition and cognitive function in the rat brain. Int J Radiat Biol. 2018;94(3):238–247. [DOI] [PubMed] [Google Scholar]
- 2. Acharya S, Wu S, Ashford JM, et al. Association between hippocampal dose and memory in survivors of childhood or adolescent low-grade glioma: a 10-year neurocognitive longitudinal study. Neuro Oncol. 2019. doi:10.1093/neuonc/noz068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tang FR, Loke WK, Wong P, Khoo BC. Radioprotective effect of ursolic acid in radiation-induced impairment of neurogenesis, learning and memory in adolescent BALB/c mouse. Physiol Behav. 2017;175:37–46. [DOI] [PubMed] [Google Scholar]
- 4. Wang SW, Ren BX, Qian F, et al. Radioprotective effect of epimedium on neurogenesis and cognition after acute radiation exposure. Neurosci Res. 2019;145:46–53. [DOI] [PubMed] [Google Scholar]
- 5. Waxweiler TV, Amini A, Vinogradskiy Y, et al. Hypofractionated re-irradiation to the brainstem in children with recurrent brain tumors. Pediatr Blood Cancer. 2017;64(5). doi:10.1002/pbc.26341. [DOI] [PubMed] [Google Scholar]
- 6. Lehrer S, Rheinstein PH, Rosenzweig KE. Association of radon background and total background ionizing radiation with Alzheimer’s disease deaths in U.S. States. J Alzheimers Dis. 2017;59(2):737–741. [DOI] [PubMed] [Google Scholar]
- 7. Chen JH, Yen YC, Liu SH, et al. Dementia risk in irradiated patients with head and neck cancer. Medicine. 2015;94(45):e1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dorresteijn LD, Kappelle AC, Boogerd W, et al. Increased risk of ischemic stroke after radiotherapy on the neck in patients younger than 60 years. J Clin Oncol. 2002;20(1):282–288. [DOI] [PubMed] [Google Scholar]
- 9. Cakmak G, Miller LM, Zorlu F, Severcan F. Amifostine, a radioprotectant agent, protects rat brain tissue lipids against ionizing radiation induced damage: an FTIR microspectroscopic imaging study. Arch Biochem Biophys. 2012;520(2):67–73. [DOI] [PubMed] [Google Scholar]
- 10. Praetorius NP, Mandal TK. Alternate delivery route for amifostine as a radio-/chemo-protecting agent. J Pharm Pharmacol. 2008;60(7):809–815. [DOI] [PubMed] [Google Scholar]
- 11. Schiller JH, Storer B, Berlin J, et al. Amifostine, cisplatin, and vinblastine in metastatic non-small-cell lung cancer: a report of high response rates and prolonged survival. J Clin Oncol. 1996;14(6):1913–1921. [DOI] [PubMed] [Google Scholar]
- 12. Singh VK, Newman VL, Romaine PL, Wise SY, Seed TM. Radiation countermeasure agents: an update (2011-2014). Expert Opin Ther Pat. 2014;24(11):1229–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. da Veiga Pereira L, Desnick RJ, Adler DA, Disteche CM, Schuchman EH. Regional assignment of the human acid sphingomyelinase gene (SMPD1) by PCR analysis of somatic cell hybrids and in situ hybridization to 11p15.1→p15.4. Genomics. 1991;9(2):229–234. [DOI] [PubMed] [Google Scholar]
- 14. Brady RO, Kanfer JN, Mock MB, Fredrickson DS. The metabolism of sphingomyelin. II. Evidence of an enzymatic deficiency in Niemann-Pick disease. Proc Natl Acad Sci U S A. 1966;55(2):366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schuchman EH, Levran O, Pereira LV, Desnick RJ. Structural organization and complete nucleotide sequence of the gene encoding human acid sphingomyelinase (SMPD1). Genomics. 1992;12(2):197–205. [DOI] [PubMed] [Google Scholar]
- 16. Santana P, Pena LA, Haimovitz-Friedman A, et al. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996;86(2):189–199. [DOI] [PubMed] [Google Scholar]
- 17. Zhu H, Deng K, Zhao YQ, et al. The effects of ASMase mediated endothelial cell apoptosis in multiple hypofractionated irradiations in CT26 tumor bearing mice. Asian Pac J Cancer Prev. 2015;16(11):4543–4548. [DOI] [PubMed] [Google Scholar]
- 18. Melanson SE, Lewandrowski EL, Griggs DA, Flood JG. Interpreting tricyclic antidepressant measurements in urine in an emergency department setting: comparison of two qualitative point-of-care urine tricyclic antidepressant drug immunoassays with quantitative serum chromatographic analysis. J Anal Toxicol. 2007;31(5):270–275. [DOI] [PubMed] [Google Scholar]
- 19. Dworkin RH, Backonja M, Rowbotham MC, et al. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60(11):1524–1534. [DOI] [PubMed] [Google Scholar]
- 20. Kornhuber J, Tripal P, Reichel M, et al. Identification of new functional inhibitors of acid sphingomyelinase using a structure-property-activity relation model. J Med Chem. 2008;51(2):219–237. [DOI] [PubMed] [Google Scholar]
- 21. Hurwitz R, Ferlinz K, Sandhoff K. The tricyclic antidepressant desipramine causes proteolytic degradation of lysosomal sphingomyelinase in human fibroblasts. Biol Chem Hoppe Seyler. 1994;375(7):447–450. [DOI] [PubMed] [Google Scholar]
- 22. Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9(1):65–75. [DOI] [PubMed] [Google Scholar]
- 23. Colgin LL. Rhythms of the hippocampal network. Nat Rev Neurosci. 2016;17(4):239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Sci. 2008;321(5885):53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6(4):347–470. [DOI] [PubMed] [Google Scholar]
- 26. Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378(6552):75–78. [DOI] [PubMed] [Google Scholar]
- 27. Hladik D, Tapio S. Effects of ionizing radiation on the mammalian brain. Mutat Res. 2016;770(pt B):219–230. [DOI] [PubMed] [Google Scholar]
- 28. Wu PH, Coultrap S, Pinnix C, et al. Radiation induces acute alterations in neuronal function. PloS One. 2012;7(5):e37677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dagne BA, Sunay MK, Cayla NS, et al. High dose gamma radiation selectively reduces GABAA-slow inhibition. Cureus. 2017;9(3):e1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amilhon B, Huh CY, Manseau F, et al. Parvalbumin interneurons of hippocampus tune population activity at theta frequency. Neuron. 2015;86(5):1277–1289. [DOI] [PubMed] [Google Scholar]
- 31. Steullet P, Cabungcal JH, Coyle J, et al. Oxidative stress-driven parvalbumin interneuron impairment as a common mechanism in models of schizophrenia. Mol Psychiatry. 2017;22(7):936–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ouyang YB, Ning S, Adler JR, Maciver B, Knox SJ, Giffard R. Alteration of interneuron immunoreactivity and autophagic activity in rat hippocampus after single high-dose whole-brain irradiation. Cureus. 2017;9(6):e1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tang FR, Loke WK, Khoo BC. Postnatal irradiation-induced hippocampal neuropathology, cognitive impairment and aging. Brain Dev. 2017;39(4):277–293. [DOI] [PubMed] [Google Scholar]
- 34. Kepecs A, Fishell G. Interneuron cell types are fit to function. Nature. 2014;505(7483):318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tremblay R, Lee S, Rudy B. GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron. 2016;91(2):260–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cabungcal JH, Steullet P, Morishita H, et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci U S A. 2013;110(22):9130–9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Suttkus A, Rohn S, Weigel S, Glockner P, Arendt T, Morawski M. Aggrecan, link protein and tenascin-R are essential components of the perineuronal net to protect neurons against iron-induced oxidative stress. Cell Death Dis. 2014;5:e1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ueno H, Suemitsu S, Murakami S, et al. Region-specific reduction of parvalbumin neurons and behavioral changes in adult mice following single exposure to cranial irradiation. Int J Radiat Biol. 2019;95(5):611–625. [DOI] [PubMed] [Google Scholar]
- 39. Chapman WH. The weight and mortality response of male and female mice in the lethal x-ray dose range. Radiat Res. 1955;2(5):502–511. [PubMed] [Google Scholar]
- 40. Nunamaker EA, Anderson RJ, Artwohl JE, Lyubimov AV, Fortman JD. Predictive observation-based endpoint criteria for mice receiving total body irradiation. Comp Med. 2013;63(4):313–322. [PMC free article] [PubMed] [Google Scholar]
- 41. Watters D. Molecular mechanisms of ionizing radiation-induced apoptosis. Immunol Cell Biol. 1999;77(3):263–271. [DOI] [PubMed] [Google Scholar]
- 42. Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8(2):89–91. [DOI] [PubMed] [Google Scholar]
- 43. Moeller BJ, Dreher MR, Rabbani ZN, et al. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell. 2005;8(2):99–110. [DOI] [PubMed] [Google Scholar]
- 44. Gulbins A, Grassme H, Hoehn R, et al. Role of Janus-Kinases in major depressive disorder. Neurosignals. 2016;24(1):71–80. [DOI] [PubMed] [Google Scholar]
- 45. Grassme H, Jernigan PL, Hoehn RS, et al. Inhibition of acid sphingomyelinase by antidepressants counteracts stress-induced activation of P38-Kinase in major depression. Neurosignals. 2015;23(1):84–92. [DOI] [PubMed] [Google Scholar]